Abstract
Background
Body weight is associated with colorectal cancer (CRC) risk and survival, but the impact of long-term post-diagnostic weight change is unclear. We investigated whether weight change over the 5 years following CRC diagnosis is associated with survival.
Methods
CRC cases diagnosed from 1997–2008 were identified through four population-based cancer registry sites. Participants enrolled within two years of diagnosis and reported their height and weight two years prior. Follow-up questionnaires were administered ∼5 years after diagnosis. Associations of change in weight (kg) or body mass index (BMI) with overall and CRC-specific survival were estimated using Cox regression adjusted for age, sex, stage, baseline BMI, NSAID use, smoking, time between diagnosis and enrollment, and study site.
Results
At the five-year post-diagnostic survey, 2,049 participants reported higher (53%, median +5kg), unchanged (12%), or lower (35%, median −4kg) weight. Over a median 5.1 years (range 0.3-9.9) of subsequent follow-up, 344 participants died (91 from CRC). Long-term weight loss (per 5kg) was associated with poorer overall (Hazard Ratio [HR]: 1.13, 95% Confidence Interval [CI]: 1.07–1.21) and CRC-specific survival (HR: 1.25, 95% CI: 1.13–1.39). Significantly lower survival was similarly observed for relative weight loss (>5% vs. ≤5% change), BMI reduction (per 1 unit), or BMI category change (overweight to normal vs. remaining overweight).
Conclusions
Weight loss 5-years after CRC diagnosis was significantly associated with decreased long-term survival, suggesting the importance of avoiding weight loss in CRC survivors. Future research should attempt to evaluate this association accounting for whether this weight change was intentional, or represents a marker of declining health.
Keywords: Colorectal neoplasms, body mass index, weight loss, mortality, survivors, epidemiology, follow-up studies
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the fourth leading cause of cancer death worldwide [1]. Improvements in CRC screening and treatment have contributed to a growing population of CRC survivors [2]. As such, efforts to identify modifiable behaviors and risk factors associated with CRC survival are of increasing clinical [3, 4] and public health [5, 6] importance.
Body mass index (BMI) has been associated with both the risk of developing CRC [7–11] and survival after CRC diagnosis [12–16]. Most prior studies have relied upon measurements of BMI taken at a single time point either before, at, or after CRC diagnosis [14], the timing of which may be important for prognosis [12]. As weight loss is a common side-effect of CRC, longitudinal studies of weight change over time may provide a more complete picture of how body weight is associated with cancer survival.
Growing evidence suggests that weight gain is associated with increased CRC risk [17, 18]. Fewer studies have evaluated adult weight change as it relates to CRC survival [12, 13, 19–22], but have suggested that post-diagnostic weight loss may be associated with lower survival. These studies have differed in the duration of the time interval over which weight change is measured and in the relation of timing of weight measurements to time of diagnosis, and have focused on weight change in the 6–24 month period immediately following diagnosis. To our knowledge, no study has investigated long-term post-diagnostic weight change as it relates to CRC survival.
We investigated whether long-term weight change, rather than absolute weight/BMI, in the five-year period following CRC diagnosis is associated with long-term survival.
Methods
Study participants
The Colon Cancer Family Registry (CCFR) is an international consortium focused on CRC research [23, 24]. This study included data from four population-based CCFR study sites: Cancer Care Ontario/Lunenfeld-Tanenbaum Research Institute (Toronto, Ontario, Canada), Fred Hutchinson Cancer Research Center (Seattle, Washington, USA), Mayo Clinic (Rochester, Minnesota, USA), and University of Melbourne (Melbourne, Victoria, Australia).
Details of the CCFR have been previously described [13, 24–28], and are available online [23]. Briefly, incident CRC cases diagnosed from 1997–2008 were identified through population-based cancer registries. Enrolled participants completed questionnaires at baseline and at five-year intervals. Local study approval was provided by the institutional review boards at each study center, and all participants provided written informed consent.
Only participants who completed both the baseline and the 5-year post-diagnosis follow-up questionnaire were included in this analysis. Of these 3,001 cases, we excluded those: whose baseline survey was completed >2 years after diagnosis (n=283), whose follow-up survey was completed >6 years after enrollment (n=245), those with <3 months of follow-up time after the follow-up survey (n=410), and participants with missing information on weight (n=212), height (n=16), smoking status (n=8) or NSAID use (n=48). Thus, data on 2,049 participants remained for analyses.
Data collection
Participants completed a structured telephone interview or self-administered questionnaire at a median 9 months (range 0–24 months) after diagnosis (Figure 1) in which they self-reported demographic, epidemiologic, and dietary characteristics two years prior. These responses thus correspond to participant characteristics at a median of 15.0 months (range 1–24 months) before diagnosis. Participants were actively followed via periodic contact, and completed follow-up questionnaires a median 4.9 years (range 3.5-6.0 years) after enrollment (i.e., median 5.9 years after CRC diagnosis).
Figure 1.
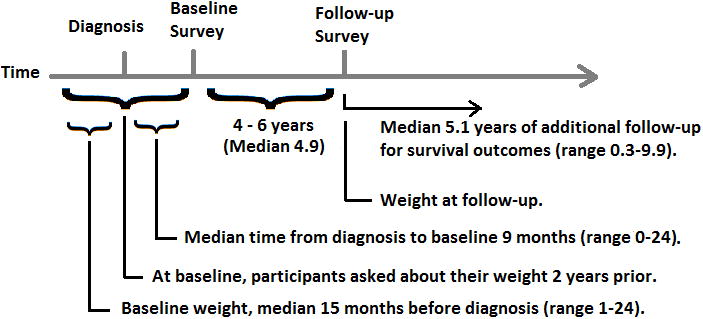
Study timeline, representing relative timing of diagnosis, baseline survey, follow-up survey, and subsequent follow-up for survival outcomes.
Cancer stage at diagnosis was derived from information on TNM stage and SEER summary stage into American Joint Committee on Cancer (AJCC) stages I-IV [29]. Follow-up for vital status and cause of death was completed through periodic linkage to population-based registries, contact with relatives, and the collection of death certificates. Vital status information was available through June 2014. ICD−10 codes retrieved from death records were used to classify whether deaths were due to CRC (C18.0, C18.2-C20.9, or C26.0) or any other cause [30].
Data definitions and statistical analyses
Participants self-reported height and weight at baseline (weight 2 years prior) and 5-year follow-up (current weight) [24]. Change in weight was calculated by subtracting weight at the 5-year follow-up from baseline weight. Relative weight change was calculated as weight change divided by weight at baseline, multiplied by 100. Body mass index (BMI) was categorized according to WHO criteria [31] as underweight (<18.5 kg/m2), normal weight (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0). Smoking history and non-steroidal anti-inflammatory drug (NSAID) use were defined as never/ever regular users.
Delayed-entry Cox regression models were used to estimate the Hazard Ratio (HR) and 95% Confidence Interval (95% CI) for the association between weight change and overall or CRC-specific survival. Survival time was calculated starting at the date of the 5-year follow-up survey and ending at either death or date of last follow-up. Models adjusted for the following potential confounders [32]: age at diagnosis, sex, smoking history, NSAIDs use, stage at diagnosis, days between diagnosis and baseline survey, and baseline BMI category. In order to more fully capture potential associations with survival, associations with weight change were assessed for several continuous and categorical parameterizations: linear weight change (per 5kg); linear BMI change (per 1kg/m2); categorical weight change (gained >2kg, or lost >2kg, versus ≤2kg difference); and categorical relative weight change (gained >5%, or lost >5%, versus ≤5% difference). Additional analyses examined categorical BMI change (moved into a higher BMI category, or lower BMI category, versus no change). Proportional hazards assumptions were verified by testing for a non-zero slope of the scaled Schoenfeld residuals on ranked failure times [33]. All P-values are two-sided, with P<0.05 denoting statistical significance. Analyses were performed using Stata version 14 [34].
Results
Among the 2,049 individuals included in the analytic dataset, 344 died (91 from CRC) over a median 5.1 years of follow-up (range 0.3-9.9 years) after the 5-year follow-up survey. Participant characteristics are presented in Table 1, both overall and by category of relative weight change (>5% loss, ≤5% change, or >5% gain). At follow-up, 1,090 participants (53%) had gained weight (median +5kg), 244 (12%) reported the same weight, and 715 (35%) had lost weight (median −4kg). The majority of participants (72%) were in the same BMI category at both time points (Table 2).
Table 1.
Study population characteristics.
| Categories | Total | Lost weight (> 5%) | Maintained weight (≤ 5% change) | Gained weight (> 5%) | ||||
|---|---|---|---|---|---|---|---|---|
| Participants, Total and by site, n (%) | 2,049 | 386 | 19 | 985 | 48 | 678 | 33 | |
| Cancer Care Ontario | 499 | 24% | 99 | 20 | 232 | 46 | 168 | 34 |
| Fred Hutchinson Cancer Research Center | 962 | 47% | 188 | 20 | 425 | 47 | 322 | 33 |
| Mayo Clinic | 246 | 13% | 42 | 17 | 135 | 55 | 69 | 28 |
| University of Melbourne | 347 | 17% | 57 | 17 | 166 | 49 | 119 | 35 |
| Deaths, n (%) | 344 | 17% | 117 | 30 | 139 | 14 | 88 | 13 |
| CRC deaths, n (%) | 91 | 4% | 40 | 10 | 31 | 3 | 20 | 3 |
| Years between diagnosis and baseline survey, mean (SD) | 0.8 | 0.4 | 0.9 | 0.4 | 0.9 | 0.4 | 0.8 | 0.4 |
| Years between baseline and follow-up surveys, mean (SD) | 5.0 | 0.5 | 5.0 | 0.5 | 5.0 | 0.5 | 5.0 | 0.5 |
| Years of follow-up from diagnosis, mean (SD) | 10.4 | 2.1 | 10.0 | 2.2 | 10.6 | 2.0 | 10.4 | 2.1 |
| Years of follow-up from 5-year survey, mean (SD) | 4.6 | 1.9 | 4.2 | 2.0 | 4.7 | 1.8 | 4.6 | 1.9 |
| Age at diagnosis (years), mean (SD) | 54.8 | 11.1 | 57.2 | 11.2 | 55.6 | 10.9 | 52.2 | 10.9 |
| Sex (Female), n (%) | 1,028 | 50% | 193 | 50 | 451 | 46 | 384 | 57 |
| Baseline weight (kg), mean (SD) | 80.1 | 18.6 | 87.1 | 20.8 | 79.5 | 17.2 | 76.9 | 18.3 |
| Follow-up weight (kg), mean (SD) | 81.7 | 19.5 | 76.4 | 18.5 | 79.6 | 17.2 | 87.6 | 21.6 |
| Weight change (kg), mean (SD) | 1.6 | 9.7 | -10.7 | 7.6 | 0.2 | 2.2 | 10.7 | 8.6 |
| Height (cm), mean (SD) | 170.1 | 10.0 | 170.3 | 10.2 | 171.2 | 9.8 | 169.7 | 10.0 |
| Baseline BMI (kg/m2), mean (SD) | 27.4 | 5.4 | 29.9 | 6.1 | 27.0 | 4.9 | 26.5 | 5.2 |
| Follow-up BMI (kg/m2), mean (SD) | 28.0 | 5.8 | 26.2 | 5.3 | 27.1 | 4.9 | 30.3 | 6.4 |
| BMI change (kg/m2), mean (SD) | 0.6 | 3.4 | -3.7 | 2.6 | 0.1 | 0.8 | 3.8 | 3.1 |
| Cancer stage at diagnosis, n (%) | ||||||||
| I | 571 | 28% | 98 | 25 | 308 | 31 | 165 | 24 |
| II | 565 | 28% | 118 | 31 | 256 | 26 | 191 | 28 |
| III | 415 | 20% | 67 | 17 | 204 | 21 | 144 | 21 |
| IV | 130 | 6% | 26 | 7 | 68 | 7 | 36 | 5 |
| Missing | 368 | 18% | 77 | 20 | 149 | 15 | 142 | 21 |
| Smoking history, n (%) | ||||||||
| Never | 928 | 45% | 164 | 42 | 460 | 47 | 304 | 45 |
| Ever | 1,121 | 55% | 222 | 58 | 525 | 53 | 374 | 55 |
| NSAID use, n (%) | ||||||||
| Never | 1,187 | 58% | 224 | 58 | 559 | 57 | 404 | 60 |
| Ever | 862 | 42% | 162 | 42 | 426 | 43 | 274 | 40 |
Table 2.
Participant BMI category over time.
| BMI (follow-up) * | ||||||
|---|---|---|---|---|---|---|
| Underweight | Normal | Overweight | Obese | Total | ||
| BMI (baseline) | Underweight | 9 | 16 | 1 | 3 | 29 |
| Normal | 15 | 514 | 169 | 15 | 713 | |
| Overweight | 3 | 110 | 531 | 147 | 791 | |
| Obese | 1 | 9 | 84 | 422 | 516 | |
| Total | 28 | 649 | 785 | 587 | 2,049 | |
Shading refers to cases that moved to a higher (red) or lower (green) BMI category from baseline to follow-up.
Weight loss was consistently associated with lower survival (Table 3). Modeled continuously, weight loss (per 5kg) after CRC diagnosis was significantly associated with lower overall (HR=1.13, 95% CI: 1.07–1.21) and CRC-specific survival (HR=1.25, 95% CI: 1.13–1.39), as was a decrease in BMI (per 1kg/m2; overall HR=1.07, 95% CI: 1.03–1.11, CRC-specific HR=1.13, 95% CI: 1.06–1.20). Modeled categorically, relative weight loss of >5% (vs. ≤5%) was significantly associated with decreased overall (HR=2.58, 95% CI: 1.97–3.38) and CRC-specific survival (HR=4.31, 95% CI: 2.63–7.06), as was absolute weight loss of >2kg (vs. ≤2kg; overall HR=2.03, 95% CI: 1.52–2.70, CRC-specific HR=4.22, 95% CI: 2.38–7.49). Weight gain was not associated with survival.
Table 3.
Association of long-term changes in weight or BMI with overall and CRC-specific survival, modeled continuously or categorically.
| Overall mortality | CRC-specific mortality | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n(deaths) | HR*† | 95% CI | p-value | n(deaths) | HR* | 95% CI | p-value | |
| Per 5kg loss | 2049(344) | 1.13 | 1.07–1.21 | <0.001 | 2049(91) | 1.25 | 1.13–1.39 | <0.001 |
| Per 1kg/m2 loss | 2049(344) | 1.07 | 1.03–1.11 | <0.001 | 2049(91) | 1.13 | 1.06–1.20 | <0.001 |
| Gain >5% | 678(88) | 1.01 | 0.76–1.35 | 0.95 | 678(20) | 0.9 | 0.51–1.60 | 0.73 |
| Same (+/-5%) | 985(139) | Ref. | 985(31) | Ref. | ||||
| Loss >5% | 386(117) | 2.58 | 1.97–3.38 | <0.001 | 386(40) | 4.31 | 2.63–7.06 | <0.001 |
| Gain >2kg | 821(114) | 1.11 | 0.83–1.48 | 0.50 | 821(27) | 1.22 | 0.67–2.24 | 0.52 |
| Same (+/-2kg) | 711(92) | Ref. | 711(18) | Ref. | ||||
| Loss >2kg | 517(138) | 2.03 | 1.52–2.70 | <0.001 | 517(46) | 4.22 | 2.38–7.49 | <0.001 |
Adjusted for age at diagnosis, sex, smoking history, NSAID use, stage at diagnosis, time from diagnosis to baseline survey, baseline BMI category, and study.
Stratified by age at diagnosis (proportional hazards violation).
Moving from overweight to normal BMI over the post-diagnosis period (vs. overweight at both time points) was significantly associated with lower overall (HR=3.67, 95% CI: 2.41–5.60) and CRC-specific survival (HR=6.68, 95% CI: 3.06–14.55) (Table 4). Moving from obese to overweight was also associated with lower overall survival (HR=1.69, 95% CI: 1.00-2.85).
Table 4.
Association of long-term BMI category change with long-term overall and CRC-specific survival.
| Overall mortality | CRC-specific mortality | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| n(deaths) | HR* | 95% CI | p-value | n(deaths) | HR* | 95% CI | p-value | |
| Normal to Obese | 15(4) | † | 15(1) | † | ||||
| Normal to Overweight | 169(15) | 0.84 | 0.47–1.53 | 0.58 | 169(6) | 0.84 | 0.31–2.26 | 0.73 |
| Maintained Normal | 514(75) | Ref. | 514(19) | Ref. | ||||
| Normal to Underweight | 15(5) | 2.23 | 0.87–5.71 | 0.09 | 15(2) | † | ||
| Overweight to Obese | 147(16) | 0.88 | 0.50–1.55 | 0.65 | 147(2) | † | ||
| Maintained Overweight | 531(76) | Ref. | 531(17) | Ref. | ||||
| Overweight to Normal | 110(41) | 3.67 | 2.41–5.60 | < 0.001 | 110(15) | 6.68 | 3.06–14.55 | <0.001 |
| Overweight to Underweight | 3(0) | † | 3(0) | † | ||||
| Maintained Obese | 422(75) | Ref. | 422(18) | Ref. | ||||
| Obese to Overweight | 84(22) | 1.69 | 1.00–2.85 | 0.049 | 84(6) | 1.86 | 0.67–5.18 | 0.24 |
| Obese to Normal | 9(3) | † | 9(2) | † | ||||
| Obese to Underweight | 1(0) | † | 1(0) | † | ||||
Adjusted for age at diagnosis, sex, smoking history, NSAID use, stage at diagnosis, time from diagnosis to baseline survey, baseline BMI, and study.
Estimate not reported due to <5 events.
Several post-hoc exploratory analyses were performed to further explore these associations. Sensitivity analyses excluding those with stage IV CRC, missing stage information, or both, did not substantially alter the results in Table 3 or Table 4 (Supplementary Table 1). In sensitivity analyses of continuous weight change stratified by BMI category at baseline, weight loss remained statistically significant among those overweight and obese at baseline for overall survival, and among those overweight at baseline for CRC-specific survival (Supplementary Table 2). In sensitivity analyses of categorical weight change stratified by BMI category at baseline, the associations of loss of either >5% or >2kg body weight with survival remained statistically significant among those of normal or overweight, but not obese, BMI at baseline.
Additional exploratory analyses evaluated participant sex or cancer stage at diagnosis as potential effect modifiers of the association between weight change and survival. Likelihood ratio tests of nested models containing interaction terms with weight change were not statistically significant for stage at diagnosis, but suggested a potential difference by sex for CRC-specific survival (Supplementary Table 3). In sex-stratified analyses, associations between weight loss and survival had larger effect sizes and remained statistically significant in males, but had reduced effect sizes and were not statistically significant in females.
Discussion
Weight loss in the ∼5-year period after CRC diagnosis was significantly associated with lower overall and CRC-specific survival. This association was consistently observed whether weight loss was modeled continuously or categorically, or in relative or absolute terms. These findings support the importance of weight loss as a potential indicator of declining health in cancer survivors, even years after diagnosis.
Emerging evidence suggest a J-shaped association between body weight and CRC survival, where overweight survivors may have the lowest risk of mortality [16, 35, 36]. This relationship may vary by cancer stage, with higher BMI associated with lower survival for early-stage CRC but higher survival for later-stage CRC [15]. Meta-analyses of clinical trials suggest that underweight and obese (but not overweight) BMI may be detrimental for stage II-III CRC [37], and that lower BMI (<28kg/m2) may be detrimental for stage IV CRC [38]. The timing of weight assessment in relation to diagnosis appears important when considering prognosis, with pre-diagnostic BMI perhaps more informative for prognosis than post-diagnostic BMI [12]. Most previous research, however, has utilized static BMI measurements to evaluate the association of body weight with survival. As weight often changes over time, there is a recognized need investigating the impact of weight change in cancer survivors [3, 39, 40].
Weight loss related to CRC symptoms and treatment is common, and may negatively impact subsequent survival. Previous studies have demonstrated that weight control is important to CRC survivorship, observing lower survival with pre-diagnostic weight gain [13], pre- to post-diagnosis weight loss [12, 21], and short-term post-diagnostic weight loss [20, 22]. While an early study reported no association with short-term post-diagnostic weight change [19], a recent meta-analysis suggests that weight maintenance after diagnosis may be advantageous for cancer survivors who are overweight after diagnosis [35]. Of note, these previous studies have generally evaluated shorter time intervals for weight change after diagnosis (1-2 years), and have had near-term periods of follow-up (up to 5 years). Ours is the first study to evaluate the association of long-term (4-6 years) weight change after CRC diagnosis with subsequent long-term (>5 years) survival.
Consistent with studies of short-term weight changes, we observe that weight loss is associated with lower survival, and that weight maintenance may be beneficial even if a patient is initially overweight. Notably, these findings are in conflict with current survivorship guidelines, which recommend that patients obtain and maintain a normal weight [36, 41]. This conflict is particularly striking as our findings relate to long-term weight change 4-6 years after diagnosis, when a cancer survivor is likely moving past the period of active cancer treatment. While CRC-related issues and symptoms are most prominent during the first three years post-diagnosis, long-term effects can persist [42]. More research is needed to identify whether additional factors, such as long-term effects or metabolic dysfunction, may contribute to the observed lower survival after weight loss.
Associations of post-diagnostic weight loss with lower survival has been reported in several other cancer types [43], including breast [44, 45], endometrial [46], lung [47], prostate [48], and esophageal cancer [49]. Involuntary weight and muscle loss leading to cachexia and sarcopenia, respectively, are common in cancer patients and are indicators of poor prognosis [3, 20, 50]. Together, these results suggest the importance of avoiding weight loss after diagnosis, and may point towards weight maintenance as a prudent goal for survivors of some cancers [44]. Potential biological rationale for better survival with higher weight in cancer survivors includes extra nutritional reserves, greater muscle mass, a lower likelihood of dose-limiting toxicity, or potentially less aggressive tumors [3, 51, 52].
BMI is a crude measure of adiposity, and does not capture differences in body composition (muscle vs. fat) or fat distribution (subcutaneous vs. visceral) [53]. These differences also vary by sex, with females generally carrying proportionately more body fat and males having more central adiposity [54], which may be contributing to the differential associations of weight loss and survival observed in the sex-stratified exploratory analyses. Body habitus is also likely influenced by many factors that change over time, both lifestyle (caloric intake, diet, physical activity, etc.) and medical (access to medical care, treatments, co-morbidities, adverse events, and late effects). As such, it is difficult to determine the proportional contribution of weight change on long-term survival. We are unable to determine whether the weight loss reported in our study was intentional (e.g., through diet and/or exercise) or unintentional (e.g., cachexia or sarcopenia). Given that unintentional weight loss likely relates to treatment effects or disease progression/recurrence, and could be a general marker of declining health, this limitation should be considered before encouraging clinical implementation of our findings.
We were unable to evaluate how diet and exercise may have contributed to energy balance and weight change. Exercise may be particularly important in cancer survivors, as weight loss independent of exercise may promote sarcopenia or loss of lean mass [36, 55] and increased physical activity after CRC diagnosis is associated with improved survival [56, 57]. Guidelines for weight management in adults recommend a three-pronged approach of dietary energy restriction, regular physical activity, and behavioral modification [58]. Additional investigation is needed to identify how these factors influence weight change in CRC survivors, and to determine how sarcopenia or cachexia may be involved in the observed association with survival.
This study has several limitations. Our results are based on long-term changes in weight among participants who survived ≥5 years after their diagnosis. This inherent survival bias meant that our sample comprised proportionately more participants who were younger, had an early-stage cancer at diagnosis, and likely had fewer comorbidities, potentially limiting the generalizability of these findings. Another limitation is that body weight was self-reported at each time point, perhaps resulting in measurement error with regard to weight change. However, questionnaire-based body weight recall tends to be fairly accurate [59]. Participants are also likely to have internal consistency in their reporting, such that change in weight may be less prone to such bias than individual weight measurements. Finally, participants may have undergone large fluctuations in weight during the post-diagnosis period that would not have been captured by our surveys. Although we included established and suspected factors that might confound the relationship under study, there may nonetheless be other unmeasured confounding factors influencing these findings, such as participants' area of residence or access to treatment [60]. Future studies with longer and more frequent follow-up of participants who develop CRC are needed to evaluate how the pace, trajectory, or pattern of weight change may affect the association of body weight with CRC survival.
Our study has several strengths that improve confidence in our results. These include a large sample size, long follow-up time, well-characterized outcomes, and detailed information on both the primary exposure and potential confounders. Another major strength is the population-based design, which allows for an estimate of the association between weight change and CRC survival that is more likely to be generalizable. These findings are also consistent with findings from shorter-term studies, and warrant replication and exploration in larger and more frequently-characterized study populations. Exploratory analyses suggesting that these associations may be particularly relevant for males and those who are overweight at diagnosis also warrant future investigation.
Conclusion
These findings suggest the importance of avoiding weight loss among long-term CRC survivors, even among those who are overweight or obese. This conflicts with current recommendations to achieve and maintain a normal weight. Patients and physicians should be aware of the potential decreased survival among those with weight loss after CRC, even many years after diagnosis. Additional research is warranted to evaluate the additional contribution of diet, physical activity, and body composition for CRC survival within the context of weight change following diagnosis.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Cancer Institute (NCI; K05 CA152715, K07 CA172298, and UM1 CA167551) and the National Center for Advancing Translational Sciences (KL2 TR000421) at the National Institutes of Health (NIH), and through cooperative agreements with the following centers: Australasian Colorectal Cancer Family Registry (U01 CA074778 and U01/U24 CA097735), Mayo Clinic Cooperative Family Registry for Colon Cancer Studies (U01/U24 CA074800), Ontario Familial Colorectal Cancer Registry (U01/U24 CA074783), and Seattle Colorectal Cancer Family Registry (U01/U24 CA074794). Seattle CCFR research was also supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center (FHCRC), which was funded by Control Nos. N01-CN-67009 (1996-2003) and N01-PC-35142 (2003-2010) and Contract No. HHSN2612013000121 (2010-2017) from the Surveillance, Epidemiology and End Results (SEER) Program of the NCI with additional support from the FHCRC. The content of this manuscript does not necessarily reflect the views or policies of the NIH, authors' affiliated institutions, or any of the collaborating centers of the CCFR, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CCFR.
Footnotes
Conflicts of Interest: None.
Author Contributions: Conceptualization (JMK,SAC,PAN), Data Acquisition (XH,NL,AKW,JH,JDP,PAN), Analysis/Interpretation (JMK,XH,SH,JR,SVA,SAC,AIP,PAN), Original Draft (JMK), Review/Editing (JMK,XH,SH,JR,NML,AKW,JH,JCF,JDP,PTC,SG,MC,SVA,SAC,AIP,PAN).
References
- 1.Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] doi: 10.1002/ijc.29210. http://globocan.iarc.fr. [DOI] [PubMed]
- 2.de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–70. doi: 10.1158/1055-9965.EPI-12-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown JC, Meyerhardt JA. Obesity and Energy Balance in GI Cancer. J Clin Oncol. 2016;34(35):4217–4224. doi: 10.1200/JCO.2016.66.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodwin PJ, Chlebowski RT. Obesity and Cancer: Insights for Clinicians. J Clin Oncol. 2016;34(35):4197–4202. doi: 10.1200/JCO.2016.70.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayvergia N, Denlinger CS. Lifestyle Factors in Cancer Survivorship: Where We Are and Where We Are Headed. J Pers Med. 2015;5(3):243–63. doi: 10.3390/jpm5030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith JL, Pollack LA, Rodriguez JL, et al. Assessment of the status of a National Action Plan for Cancer Survivorship in the USA. J Cancer Surviv. 2013;7(3):425–38. doi: 10.1007/s11764-013-0276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–47. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund / American Institute for Cancer Research Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007. [Google Scholar]
- 9.Campbell PT, Jacobs ET, Ulrich CM, et al. Case-control study of overweight, obesity, and colorectal cancer risk, overall and by tumor microsatellite instability status. J Natl Cancer Inst. 2010;102(6):391–400. doi: 10.1093/jnci/djq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Win AK, Dowty JG, English DR, et al. Body mass index in early adulthood and colorectal cancer risk for carriers and non-carriers of germline mutations in DNA mismatch repair genes. Br J Cancer. 2011;105(1):162–9. doi: 10.1038/bjc.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell PT, Cotterchio M, Dicks E, et al. Excess body weight and colorectal cancer risk in Canada: associations in subgroups of clinically defined familial risk of cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1735–44. doi: 10.1158/1055-9965.EPI-06-1059. [DOI] [PubMed] [Google Scholar]
- 12.Campbell PT, Newton CC, Dehal AN, et al. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30(1):42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 13.Campbell PT, Newton CC, Newcomb PA, et al. Association between body mass index and mortality for colorectal cancer survivors: overall and by tumor molecular phenotype. Cancer Epidemiol Biomarkers Prev. 2015;24(8):1229–38. doi: 10.1158/1055-9965.EPI-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkin E, O'Reilly DA, Sherlock DJ, et al. Excess adiposity and survival in patients with colorectal cancer: a systematic review. Obes Rev. 2014;15(5):434–51. doi: 10.1111/obr.12140. [DOI] [PubMed] [Google Scholar]
- 15.Kocarnik JM, Chan AT, Slattery ML, et al. Relationship of pre-diagnostic body mass index with survival after colorectal cancer: Stage-specific associations. Int J Cancer. 2016;139(5):1065–72. doi: 10.1002/ijc.30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroenke CH, Neugebauer R, Meyerhardt J, et al. Analysis of Body Mass Index and Mortality in Patients With Colorectal Cancer Using Causal Diagrams. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.0732. 10.1001/jamaoncol.2016.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. Am J Epidemiol. 2015;181(11):832–45. doi: 10.1093/aje/kwu357. [DOI] [PubMed] [Google Scholar]
- 18.Keum N, Greenwood DC, Lee DH, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst. 2015;107(2) doi: 10.1093/jnci/djv088. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26(25):4109–15. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Kroenke CH, Prado CM, et al. Association of Weight Change after Colorectal Cancer Diagnosis and Outcomes in the Kaiser Permanente Northern California Population. Cancer Epidemiol Biomarkers Prev. 2017;26(1):30–37. doi: 10.1158/1055-9965.EPI-16-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baade PD, Meng X, Youl PH, et al. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1410–20. doi: 10.1158/1055-9965.EPI-11-0079. [DOI] [PubMed] [Google Scholar]
- 22.Vergidis J, Gresham G, Lim HJ, et al. Impact of Weight Changes After the Diagnosis of Stage III Colon Cancer on Survival Outcomes. Clin Colorectal Cancer. 2016;15(1):16–23. doi: 10.1016/j.clcc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Colon Cancer Family Registry. http://www.coloncfr.org/
- 24.Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb PA, Zheng Y, Chia VM, et al. Estrogen plus progestin use, microsatellite instability, and the risk of colorectal cancer in women. Cancer Res. 2007;67(15):7534–9. doi: 10.1158/0008-5472.CAN-06-4275. [DOI] [PubMed] [Google Scholar]
- 26.Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: the Seattle Colon Cancer Family Registry. Cancer. 2011;117(21):4948–57. doi: 10.1002/cncr.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardikar S, Newcomb PA, Campbell PT, et al. Prediagnostic Physical Activity and Colorectal Cancer Survival: Overall and Stratified by Tumor Characteristics. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1130–7. doi: 10.1158/1055-9965.EPI-15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phipps AI, Robinson JR, Campbell PT, et al. Prediagnostic alcohol consumption and colorectal cancer survival: The Colon Cancer Family Registry. Cancer. 2016 doi: 10.1002/cncr.30446. 10.1002/cncr.30446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edge S, Byrd D, Compton C, et al. AJCC cancer staging manual. 7th. New York, NY: Springer; 2010. [Google Scholar]
- 30.WHO. International classification of diseases. 10th. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 31.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 32.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosmer DW, Lemeshow S, May S. Applied survival analysis: Regression modeling of time-to-event data. 2nd. Hoboken, New Jersey: John Wiley & Sons; 2008. [Google Scholar]
- 34.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 35.Wu S, Liu J, Wang X, et al. Association of obesity and overweight with overall survival in colorectal cancer patients: a meta-analysis of 29 studies. Cancer Causes Control. 2014;25(11):1489–502. doi: 10.1007/s10552-014-0450-y. [DOI] [PubMed] [Google Scholar]
- 36.Demark-Wahnefried W, Rogers LQ, Alfano CM, et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 2015;65(3):167–89. doi: 10.3322/caac.21265. [DOI] [PubMed] [Google Scholar]
- 37.Sinicrope FA, Foster NR, Yothers G, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119(8):1528–36. doi: 10.1002/cncr.27938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renfro LA, Grothey A, Kerr D, et al. Survival following early-stage colon cancer: an ACCENT-based comparison of patients versus a matched international general populationdagger. Ann Oncol. 2015;26(5):950–8. doi: 10.1093/annonc/mdv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ligibel JA, Alfano CM, Hershman D, et al. Recommendations for Obesity Clinical Trials in Cancer Survivors: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;33(33):3961–7. doi: 10.1200/JCO.2015.63.1440. [DOI] [PubMed] [Google Scholar]
- 40.Ligibel JA, Wollins D. American Society of Clinical Oncology Obesity Initiative: Rationale, Progress, and Future Directions. J Clin Oncol. 2016;34(35):4256–4260. doi: 10.1200/JCO.2016.67.4051. [DOI] [PubMed] [Google Scholar]
- 41.Denlinger CS, Ligibel JA, Are M, et al. Survivorship: nutrition and weight management, Version 2.2014. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(10):1396–406. doi: 10.6004/jnccn.2014.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw. 2009;7(8):883–93. doi: 10.6004/jnccn.2009.0058. quiz 894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenlee H, Unger JM, LeBlanc M, et al. Association between Body Mass Index and Cancer Survival in a Pooled Analysis of 22 Clinical Trials. Cancer Epidemiol Biomarkers Prev. 2017;26(1):21–29. doi: 10.1158/1055-9965.EPI-15-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caan BJ, Kwan ML, Shu XO, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1260–71. doi: 10.1158/1055-9965.EPI-12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cespedes Feliciano EM, Kroenke CH, Bradshaw PT, et al. Postdiagnosis Weight Change and Survival Following a Diagnosis of Early-Stage Breast Cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(1):44–50. doi: 10.1158/1055-9965.EPI-16-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsuo K, Moeini A, Cahoon SS, et al. Weight Change Pattern and Survival Outcome of Women with Endometrial Cancer. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5237-9. 10.1245/s10434-016-5237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90(10):1905–11. doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonn SE, Wiklund F, Sjolander A, et al. Body mass index and weight change in men with prostate cancer: progression and mortality. Cancer Causes Control. 2014;25(8):933–43. doi: 10.1007/s10552-014-0393-3. [DOI] [PubMed] [Google Scholar]
- 49.Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. 2016;13(3):185–98. doi: 10.1038/nrclinonc.2015.200. [DOI] [PubMed] [Google Scholar]
- 50.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 51.Caan BJ, Kroenke CH. Next Steps in Understanding the Obesity Paradox in Cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(1):12. doi: 10.1158/1055-9965.EPI-16-0764. [DOI] [PubMed] [Google Scholar]
- 52.Strulov Shachar S, Williams GR. The Obesity Paradox in Cancer-Moving Beyond BMI. Cancer Epidemiol Biomarkers Prev. 2017;26(1):13–16. doi: 10.1158/1055-9965.EPI-16-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothman KJ. BMI-related errors in the measurement of obesity. Int J Obes (Lond) 2008;32(3):S56–9. doi: 10.1038/ijo.2008.87. [DOI] [PubMed] [Google Scholar]
- 54.Karastergiou K, Smith SR, Greenberg AS, et al. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68(7):375–88. doi: 10.1111/j.1753-4887.2010.00298.x. [DOI] [PubMed] [Google Scholar]
- 56.Campbell PT, Patel AV, Newton CC, et al. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876–85. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 57.Je Y, Jeon JY, Giovannucci EL, et al. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2013;133(8):1905–13. doi: 10.1002/ijc.28208. [DOI] [PubMed] [Google Scholar]
- 58.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olivarius NF, Andreasen AH, Loken J. Accuracy of 1-, 5- and 10-year body weight recall given in a standard questionnaire. Int J Obes Relat Metab Disord. 1997;21(1):67–71. doi: 10.1038/sj.ijo.0800365. [DOI] [PubMed] [Google Scholar]
- 60.Sankaranarayanan J, Qiu F, Watanabe-Galloway S. A registry study of the association of patient's residence and age with colorectal cancer survival. Expert Rev Pharmacoecon Outcomes Res. 2014;14(2):301–13. doi: 10.1586/14737167.2014.891441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


