Abstract
Objectives
Depression following pregnancy is common, but its extent and association with maternal morbidity in the first six months postpartum have not been well described in low resource settings such as rural Bangladesh.
Methods
We used data from a population-based, community trial of approximately 39,000 married rural Bangladeshi women aged 13-44 between 2001 and 2007 to examine the relation between women's reported morbidity symptoms from childbirth to three months postpartum, and subsequent depressive symptoms assessed at six months postpartum. We calculated crude and adjusted risk ratios for depressive symptoms following women's reports of reproductive, urinary, neurologic, nutrition and other illness measures constructed based on symptomatic reporting.
Results
In models adjusted for sociodemographic factors and co-morbidities, all postpartum illnesses were associated with an increased relative risk (RR, with 95% confidence intervals [CI] excluding 1) of depressive symptoms by six months postpartum. These morbidities included uterine prolapse (RR=1.20, 95% CI:1.04-1.39), urinary tract infection (RR=1.24, 95% CI:1.11-1.38), stress related incontinence (SRI) (RR 1.49, 95% 1.33-1.67), simultaneous SRI and continuously dripping urine (RR=1.60-2.96), headache (RR=1.20 (95% CI:1.12-1.28), convulsions (RR=1.67, 95%CI 1.36-2.06), night blindness (RR=1.33, 95% CI:1.19-1.49), anemia (RR=1.38, 95% CI:1.31-1.46), pneumonia (RR 1.24, 95% CI:1.12-1.37), gastroenteritis (RR=1.24, 95% CI 1.17-1.31) and hepatobiliary disease (RR=2.10, 96% CI:1.69-2.60).
Conclusions for Practice
Illnesses during the first three postpartum months were risk factors for depressive symptoms, with the strongest associations noted for convulsions and hepatobiliary disease. Symptoms of depression may be of particular concern among women suffering from physical illnesses.
Keywords: depressive symptoms, maternal morbidity, maternal illness, mental health, Bangladesh
Introduction
Postpartum depression is a common and debilitating condition globally (Institute of Health Metrics and Evaluation, 2015). Major depression is the primary determinant of years lived with a disability among women of reproductive age worldwide (Institute of Health Metrics and Evaluation, 2015). According to a recent meta-analytic review, approximately 20% of postpartum women in low- and middle-income countries (LMICs) suffer from depressive symptoms and anxiety (Fisher et al., 2012). In addition to important negative effects for women, depressive symptoms have been linked with poorer child health and development (Stein et al., 2014; Wachs, Black, & Engle, 2009). Poor physical and mental health, evident symptomatically, often coexist (Behan, Doyle, Masterson, Shiers, & Clarke, 2015; Doherty & Gaughran, 2014).
Women of reproductive age in LMICs bear a high burden of morbidity (Filippi et al., 2006; Tuncalp, Hindin, Souza, Chou, & Say, 2012) and many lack access to adequate care (Ronsmans, Graham, & Lancet Maternal Survival Series Steering Group, 2006). Estimates from Bangladesh show that 7-53% of women report experiencing nausea, vomiting, vaginal discharge, anemia or infections in pregnancy (Kim et al., 2012). Furthermore, regardless of the type of illness (e.g. urinary tract infection, pneumonia, gastroenteritis) less than 50% seek treatment from any type of provider (Kim et al., 2012) and 24% of Bangladeshi women report poor health after delivery (National Institute of Population Research and Training (NIPORT), ORC Macro, Johns Hopkins University, & ICDDR, 2003). The types of morbidity experienced by women, however, that may lead to postpartum depression in low-income settings have not been well characterized, despite the fact that postnatal depressive symptoms appear to be more frequent in LMICs than in high-income countries (Parsons, Young, Rochat, Kringelbach, & Stein, 2012). Few studies on this topic have been conducted among women in low-resource settings, and most available research has been small or clinic-based, as noted below.
The aim of our study was to provide an initial estimate of the magnitude of depressive symptoms among women in the 1st year postpartum, identify risk factors and their strength of association with several health conditions following childbirth and depressive symptoms. It is based on prospectively collected symptomatic morbidity data from a large, rural population cohort of women who, on becoming pregnant, participated in a placebo-controlled vitamin A or β-carotene supplementation trial and were followed through six months postpartum, in northwestern Bangladesh (West et al., 2011). In this analysis we relate symptoms of morbidity during the first three months postpartum to subsequent depressive symptoms reported by women at six months postpartum.
Methods
We analyzed data for 39,434 married women ages 13-44 years living in 19 rural administrative unions in adjacent districts of Gaibandha and Rangpur in northwest Bangladesh who participated, during pregnancy and the early postpartum period in the JiVitA-1 trial and gave birth to singletons. JiVitA-1 was a cluster randomized, double-masked, placebo-controlled, community trial with the original goal of evaluating the effects of vitamin supplementation on maternal mortality (West et al., 2011). (Registered with Clinical Trials -NCT0019882)
Bangladesh is an agricultural, largely riverine-delta in the South Asia region. With a population of about 165 million people, it has the highest population density among non-city countries. Most Bangladeshis are ethno-linguistically Bengali, with about 78% of the population being Muslim, 21% Hindu and the remainder divided among Buddhist, Christian and tribal, non-religious groups. Literacy rates are low, with about 55.1% women and 62.5% men above the age of 15 reporting being able to read, although the government's push to for girl's education has recently reversed the ratio of girls to boys in primary school (UNICEF, 2010).
Women's labor force participation continues to remain approximately 15% below men's (World Bank, 2017). The maternal mortality rate is high at ∼320 per 100,000 (UNICEF, 2010), but has dropped dramatically in the last decade, with skilled birth attendants present at 42% births in 2015 compared to 11% in 2012 (World Bank, 2017), complete antenatal care coverage, however, remains low at only 50% of women (UNICEF, 2016). Adolescent marriage and pregnancy rates are exceedingly high with 74% of women being married before the age of 18 and over 82% of women giving birth before the age of 20 (UNICEF, 2010; World Bank, 2017).
The contiguous area included in this study covered 435 square kilometers, divided into 596 clusters (“sectors”) of 250-350 households serving as units for field work and randomization. The study area is situated between the confluence of the Teesta and Brahmaputra rivers and Dhaka-Rangpur trunk road and is typical of rural Bangladesh with households lacking electricity (85%) and housing typically constructed of tin sheets, bamboo or mud/thatch. The principal sources of income in the area are small landholding agriculture, wage labor or small business (Labrique et al., 2011).
Data collection for JiVitA-1 took place from August 2001 to October 2007. Women of reproductive age living in the study area were initially enumerated and eligible for enrollment into a pregnancy surveillance system if they were 13-44 years of age, married and residing with their husband, and not menopausal or sterilized. Women who married after the initial census were, within 4 months of marriage, also invited to participate in the pregnancy surveillance system. Pregnancy was detected on the basis of a report of amenorrhea in the previous month and a confirmative positive human chorionic gonadotropin (hCG) urine test. Consenting pregnant women were interviewed privately in the home by trained staff at the time of pregnancy detection (typically at 8-10 weeks gestation). Information about socioeconomic and demographic factors was included in this initial interview, among other topics. At 3 months postpartum, home interviews were repeated in which women were asked to recall the occurrence and duration (in days) of morbidity symptoms over the past 3 months; that is, since giving birth. At 6 months postpartum, women were asked to recall depressive symptoms experienced since giving birth. Women were included in the present analysis if they provided any data on morbidity at three months postpartum and information about depressive symptoms at 6 months postpartum. This study was approved by the Institutional Review Board.Bangladesh Medical Research Council and the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Measures
Women's morbidity was assessed using a standardized questionnaire based on self-report of twenty-eight symptoms and validated with respect to their expected association with thinness in early pregnancy as measured by arm circumference (Kim et al., 2012). For each morbidity symptom, women were asked about its occurrence and duration within the past 3 months. Postpartum illnesses were defined by the presence of one or more reported symptoms adapted from the WHO definitions of each illness (World Health Organization), as reported previously in research from Bangladesh (Kim et al., 2012). Illnesses, grouped by system, with reported symptom definitions are summarized in Table 1. Systems groupings included the reproductive system (i.e. reproductive tract infection, uterine prolapse), urinary system (i.e. urinary tract infection, stress related incontinence, and continuous drip of urine), neurological system (i.e. severe headache, convulsion), nutritional deficiency (i.e. night blindness, anemia), and other systems (i.e. pneumonia, gastroenteritis, and hepatobiliary disease).
Table 1. Definitions of maternal postpartum illness by reported symptoms during the first three months postpartum.
| Maternal Postpartum Illnesses Grouped by System | Definitions of Illnesses According to Reported Symptoms in Past Three Months |
|---|---|
| Reproductive | |
| Reproductive tract infection | 1) Vaginal discharge and lower abdominal pain
and high fever; or 2) Vaginal discharge and painful urination |
| Uterine prolapse | Uterus descended |
| Urinary | |
| Urinary tract infection | 1) Painful urination and lower abdominal pain;
or 2) Painful urination and high fever |
| Stress-related incontinence | Episodic incontinence caused by coughing, sneezing, laughing, or lifting heavy objects |
| Continuous drip of urine | Continuous drip of urine lasting at least one day |
| Neurologic | |
| Severe headache | Severe headache lasting at least one day |
| Convulsion | Uncontrolled shaking of the body lasting at least one day |
| Nutritional Deficiency | |
| Night blindness | Inability to see at night with adequate vision in the day occurring over at least one 24-hour period |
| Anemia | Both symptoms present in the past 30
days: 1) Breathlessness at rest, and 2) Weakness resulting in an inability to work |
| Other | |
| Pneumonia | Any 2 of the following symptoms:1) Productive
cough, 2) Rapid breathing, or 3) High fever |
| Gastroenteritis | Any one of the following symptoms: 1) Diarrhea (watery stools ≥4×/d); or 2) Bloody/mucoid stools and vomiting; or 3) Bloody/mucoid stools and lower abdominal pain. |
| Hepatobiliary Disease | All 3 of the following symptoms: 1) Right upper quadrant (liver) pain; and 2) At least one symptom of elevated bilirubin or biliary obstruction: jaundice, yellow eyes, ash colored stools, tea colored urine; and 3) At least one constitutional symptom: low grade fever, poor appetite, nausea, vomiting. |
All postpartum illnesses were defined as binary outcomes (yes or no), with the exception of stress-related incontinence and continuous drip of urine which were combined into four categories of:neither, either one alone or both conditions. The reference category for each was no symptoms meeting the definition of the condition.
Depressive Symptoms
Postpartum depressive symptoms were reported by the women based on questions about their experiences in the prior six months. Because no validated depressive symptom scale existed in Bangladesh at the time of the study, we created a five-item scale based on items modified from the Patient Health Questionnaire (PHQ-9) and the Center for Epidemiologic Studies Depression Scale (CES-D). A standard suicidal ideation question was also added. Independent translation and back translation was used and the items were piloted in focus group discussions to ensure their adequate translation, cultural relevance, and understandability. The five-item scale included: feeling sad all the time; becoming more forgetful; crying all the time; having thoughts of hurting oneself; and not wanting to bathe or eat for several days (Cronbach alpha= 0.72). Responses to the scale were then summed and classified as 0-2 and 3-5 symptoms. While cutoffs were only descriptive, the classification for high depressive symptom category (≥ 3 symptoms) was experienced by 14% of the study women, making this cutoff more stringent than other prevalence estimates from women in rural Bangladesh. The Edinburgh Postpartum Depression Scale was validated in Bangladeshi after the current data were collected, and showed a higher percentage of women with depressive symptoms, about 32 and 18 percent in two separate studies (Gausia, Fisher, Ali, & Oosthuizen, 2009; Nasreen, Kabir, Forsell, & Edhborg, 2011).
Demographic variables included: women's age (≤19, 20-29, ≥30), parity (zero vs. ≥1), education (0, 1-9 yrs vs. ≥10 yrs), religion (Muslim vs. non-Muslim), and living standard index in quartiles (1st=low, 2nd=middle low, 3rd=middle high, vs. 4th=high). The living standard index included household assets (e.g. toilet facilities, beds, radios) and was based on Principal Component Analysis (Gunnsteinsson et al., 2010).
Statistical Analysis
The analysis examined the relation of a defined illness, described above, between childbirth and three months postpartum and other risk factors with depressive symptoms during the first 6 months postpartum among women with singleton live births. Descriptive statistics using bivariate analyses were first calculated to determine the proportion of women with high and low depressive symptoms according to morbidities and socio-demographic characteristics, using chi-square tests.
Generalized linear models with binomial error structure and a logarithmic link function were used to estimate crude risk ratios and 95% confidence intervals. Two adjustments were used to estimate the adjusted risk ratios of depressive symptoms for each morbidity illness. The first models examined each illness independently, adjusting for socio-demographic covariates that were significant in the bivariate analyses (women's age, parity, education, living standard index, religion). A second model adjusted for the same covariates as the first model while also including all morbidities simultaneously. Both models were also adjusted for geographical cluster and assignment to vitamin supplementation group in the original study (to account for sampling and the study design).
The adjusted risk ratios were calculated using generalized linear models with a log-link and Poisson distribution because the binomial error structure failed to converge. To resolve the issue of non-convergence, a Poisson distribution error structure was used so that the coefficient from this distribution approximated the risk ratio. The Huber-White robust variance estimator was used to correct for inflation in the standard errors when using this method (Cummings, 2009).
Results
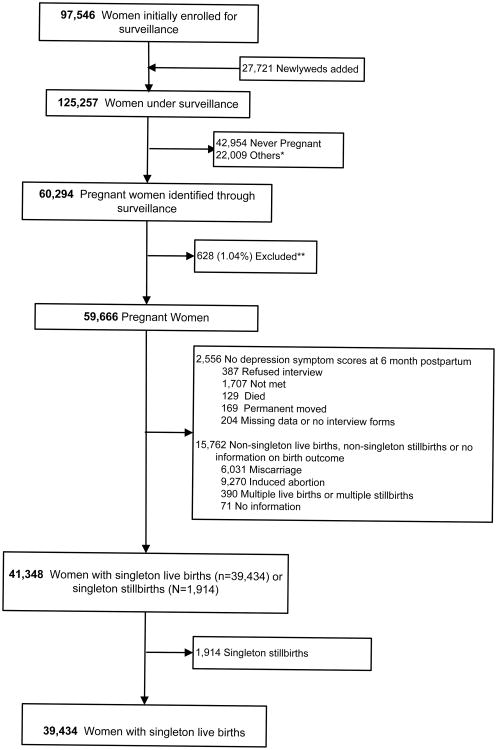
A total of 125,257 women were enrolled for surveillance in the JiVitA-1 trial of which 60,294 were pregnant at the time of enrollment or through the course of the trial. A total of 39,434 women delivered singleton live births and provided data on depressive symptoms. Women were excluded who did not consent to participate or were lost to follow-up (1.04%, 628), who had no data on depressive symptoms at six months postpartum (4.2%, 2,556), who had multiple births or multiple stillbirths, miscarriages, abortions or had no information on birth outcome (26.1%, 15,762). Finally, among singleton births following those exclusions, singleton stillbirths were additionally excluded (3.2%, 1,914). (Figure 1)
Figure 1. Study Sample.
*Other reasons included permanently moved from the study area (2027), sterilized (3148), reported menopause (3914), husband died or divorce (3435), refused to participate (34), died before detecting a pregnancy (493), had a pregnancy outcome after October 12, 2006 (5243), reported a last menstrual period after January 5th, 2006 (1929) or had an unknown date of a last menstrual period (16).
** Reasons for exclusions were: refused consent (594), outcome occurred before consent (60), vital status unknown (51), pregnancy outcome unknown (4), died before consent (5).
The majority of women in the study sample was less than 30 years old (91.8%), had one or more children (60.4%), less than 10 years of education (92.7%) and were Muslim (91.9%). The percentage of women who experienced high depressive symptoms (3 to 5 symptoms) was 13.5%. The prevalence of postpartum illness ranged from 0.33% for continuous drip of urine to 22.1% for gastroenteritis (Table 2).
Table 2. Women's socio-demographic characteristics, early postpartum illness, and postpartum depressive symptoms among rural Bangladeshi women with singleton live births, N=39,434.
| Depressive Symptoms | ||||
|---|---|---|---|---|
| Low (0-2) n=34,129 | High (3-5) n=5,305 | |||
| Total, N (%) | N (%) | N% | p-value | |
| Socio-demographic characteristics | ||||
| Maternal age (yrs) | ||||
| ≤ 19 | 20,390 (51.74) | 18,039 (88.47) | 2,351 (11.53) | |
| 20-29 | 15,819 (40.14) | 13,514 (85.43) | 2,305 (14.57) | <0.001 |
| ≥ 30 | 3,202 (8.12) | 2,556 (86.55) | 646 (13.45) | <0.001 |
| Parity | ||||
| Zero | 15,611 (39.62) | 13,890 (88.98) | 1,721 (11.02) | |
| ≥ 1 | 23,788 (60.38) | 20,209 (84.95) | 3,579 (15.05) | <0.001 |
| Maternal education (yrs) | ||||
| None | 18,914 (48.07) | 15,984 (84.51) | 2,930 (15.49) | <0.001 |
| 1-9 | 17,594 (44.72) | 15,474 (87.95) | 2,120 (12.05) | <0.001 |
| ≥10 | 2,836 (7.21) | 2,588 (91.26) | 248 (8.74) | |
| Maternal employment | ||||
| No | 33,674 (85.46) | 29,174 (86.64) | 4,500 (13.36) | |
| Yes | 5,730 (14.54) | 4,930 (86.04) | 800 (13.96) | 0.22 |
| Living standard index | ||||
| 1st quartile (poor) | 10,089 (25.60) | 8,462 (83.87) | 1,627(16.13) | <0.001 |
| 2nd | 10,004 (25.39) | 8,591 (85.88) | 1,413 (14.12) | <0.001 |
| 3rd | 9,617 (24.41) | 8,406 (87.41) | 1,211 (12.59) | <0.001 |
| 4th quartile (rich) | 9,694 (24.60) | 8,645 (89.18) | 1,049 (10.82) | |
| Religion | ||||
| Non-Muslim | 3,187 (8.09) | 2,812 (88.23) | 375 (11.77) | |
| Muslim | 36,217 (91.91) | 31,292 (86.40) | 4,925 (13.60) | <0.01 |
| Maternal Postpartum Illness | ||||
| Reproductive | ||||
| Reproductive tract infection | ||||
| No | 38,002 (97.48) | 32,956 (86.72) | 5,046 (13.28) | |
| Yes | 1,432 (2.52) | 1,173 (81.91) | 259 (18.09) | <0.001 |
| Uterine prolapse | ||||
| No | 38,277 (97.77) | 33,216 (86.78) | 5,061 (13.22) | |
| Yes | 875 (2.23) | 695 (79.43) | 180 (20.57) | <0.001 |
| Urinary | ||||
| Urinary tract infection | ||||
| No | 37,054 (93.96) | 32,216 (86.94) | 4,838 (13.06) | |
| Yes | 2,380 (6.04) | 1,913 (80.38) | 467 (19.62) | <0.001 |
| Stress-related incontinence | ||||
| No | 37,384 (96.83) | 32,547 (87.06) | 4,837 (12.94) | |
| Yes | 1,224 (3.17) | 932 (76.14) | 292 (23.86) | <0.001 |
| Continuously dripping urine | ||||
| No | 39,136 (99.67) | 33,901 (86.62) | 5,235 (13.38) | |
| Yes | 129 (0.33) | 94 (72.87) | 35 (27.13) | <0.001 |
| Neurologic | ||||
| Severe Headache | ||||
| No | 33,265 (85.84) | 29,145 (87.61) | 4,120 (12.39) | |
| Yes | 5,487 (14.16) | 4,499 (81.99) | 988 (18.01) | <0.001 |
| Convulsions | ||||
| No | 39,020 (99.35) | 33,819 (86.67) | 5,201 (13.33) | |
| Yes | 254 (0.65) | 183 (72.05) | 71 (27.95) | <0.001 |
| Nutritional Deficiency | ||||
| Night Blindness | ||||
| No | 37,944 (96.74) | 32,973 (86.90) | 4,971 (13.10) | |
| Yes | 1,278 (3.26) | 990 (77.46) | 288 (22.54) | <0.001 |
| Anemia | ||||
| No | 25,446 (82.33) | 22,751 (89.41) | 2,695 (10.59) | |
| Yes | 7,363 (18.67) | 5,701 (77.43) | 1,662 (22.57) | <0.001 |
| Other Conditions | ||||
| Pneumonia | ||||
| No | 37,501(95.10) | 32,599 (86.93) | 4,902 (13.07) | |
| Yes | 1,933 (4.90) | 1,530 (79.15) | 403 (20.85) | <0.001 |
| Gastroenteritis | ||||
| No | 31,002 (77.89) | 27,177 (87.66) | 3,825 (12.34) | |
| Yes | 8,432 (22.11) | 6,952 (82.45) | 1,480 (17.55) | <0.001 |
| Hepatobiliary Disease | ||||
| No | 39,261 (99.5) | 34,021 (86.65) | 5,240 (13.35) | |
| Yes | 173 (0.45) | 108 (62.43) | 65 (37.57) | <0.001 |
| Vitamin Supplementation Group | ||||
| Beta | 13,174 (33.41) | 11,433(86.78) | 1,741 (13.22) | 0.61 |
| Placebo | 13,152 (33.35) | 11,363(86.40) | 1,789 (13.60) | |
| Vitamin A | 13,108 (33.24) | 11,333 (86.46) | 1,775 (13.54) | |
In the crude analyses, older age, higher parity, less education and lower living standard index were significantly associated with high depressive symptoms (p<0.05) while women's employment and vitamin supplementation group were not related (Table 2). In bivariate analyses of illness experienced within 3 months postpartum and depressive symptoms measured at 6 months postpartum, all postpartum illnesses were significantly associated with high depressive symptoms (p<0.05)(Table 2).
In models adjusted for only socio-demographic variables, all postpartum illnesses in the first three months following delivery except for continuously dripping urine (CDU) were associated with between a 1.3 and 2.8 increased risk of high depressive symptomatology at 6 months postpartum, compared to women who didn't experience these illnesses (all p<0.05). (Model 1, Table 3). In the fully adjusted model including all the postpartum illnesses and socio-demographic variables, women who experienced postpartum illnesses within 3 months postpartum had between a 1.2 to 2.2 elevated risk of high depressive symptoms at 6 months postpartum compared to women who did not experience these illnesses (all p<0.05). Only reproductive tract infection and CDU were not statistically significantly associated with risk of high depressive symptoms (p≥0.05) in the fully adjusted model (Model 2, Table 3). The magnitude of the adjusted relative risk for high postpartum depressive symptoms was largest, over two-fold, for women who experienced both stress-related incontinence and continuous drip of urine and for women who experienced hepatobiliary disease (Table 3).
Table 3. Bivariate and multivariate associations between maternal postpartum morbidities at three months and postpartum depressive symptoms at six months among rural Bangladeshi women with singleton live births.
| Risk of Depressive Symptoms | ||||||
|---|---|---|---|---|---|---|
| Crude | Model 1: Semi-adjusted (includes socio-demographics) | Model 2: Fully adjusted (includes all maternal illnesses) | ||||
| Relative Risk (95% CI) | p-value | Relative Risk (95% CI) | p-value | Relative Risk (95% CI) | p-value | |
| Maternal Postpartum Illness | ||||||
| Reproductive | ||||||
| Reproductive tract infection | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.36 (1.22-1.53) | <0.001 | 1.29 (1.13-1.49) | <0.001 | 0.90 (0.76-1.06) | 0.21 |
| Uterine prolapse | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.56 (1.36-1.78) | <0.001 | 1.46 (1.28-1.8) | <0.001 | 1.20 (1.04-1.39) | 0.01 |
| Urinary | ||||||
| Urinary tract infection | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.50 (1.38-1.64) | <0.001 | 1.47 (1.35-1.60) | <0.001 | 1.24 (1.11-1.38) | <0.001 |
| Stress-related incontinence (SRI) and continuously dripping urine (CDU) | ||||||
| No SRI or CDU | 1.00 | 1.00 | 1.00 | |||
| SRI only | 1.80 (1.62-2.00) | <0.001 | 1.76 (1.59-1.96) | <0.001 | 1.49 (1.33-1.67) | <0.001 |
| CDU only | 1.10 (0.62-1.96) | 0.73 | 1.05 (0.58-1.88) | 0.79 | 0.77 (0.40-1.50) | 0.45 |
| Both SRI and CDU | 2.94 (2.06-4.19) | <0.001 | 2.78 (2.00-3.89) | <0.001 | 2.18 (1.60-2.96) | <0.001 |
| Neurologic | ||||||
| Severe Headache | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.45 (1.36-1.55) | <0.001 | 1.38 (1.30-1.47) | <0.001 | 1.20 (1.12-1.28) | <0.001 |
| Convulsions | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 2.10 (1.72-2.56) | <0.001 | 2.02 (1.66-2.46) | <0.001 | 1.67 (1.36-2.06) | <0.001 |
| Nutritional Deficiency | ||||||
| Night Blindness | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.72 (1.55-1.91) | <0.001 | 1.57 (1.42-1.75) | <0.001 | 1.33 (1.19-1.49) | <0.001 |
| Anemia | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 2.13 (2.02-2.25) | <0.001 | 1.43 (1.36- 1.51) | <0.001 | 1.38 (1.31-1.46) | <0.001 |
| Other Conditions | ||||||
| Pneumonia | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.59 (1.46-1.75) | <0.001 | 1.55 (1.43-1.71) | <0.001 | 1.24 (1.12-1.37) | <0.001 |
| Gastroenteritis | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 1.42 (1.35-1.50) | <0.001 | 1.37 (1.30-1.45) | <0.001 | 1.24 (1.17-1.31) | <0.001 |
| Hepatobiliary Disease | ||||||
| No | 1.00 | 1.00 | 1.00 | |||
| Yes | 2.81 (2.32-3.42) | <0.001 | 2.78 (2.30-3.38) | <0.001 | 2.10 (1.69-2.60) | <0.001 |
Model 1 examines each morbidity measure independently, adjusting for maternal age, parity, maternal education, living standard index, religion, village cluster, and vitamin supplementation group
The total sample size for analyses in Model 1 was 39,315, except in the case of uterine prolapse (N=39,038), stress-related incontinence (N=38, 482), severe headache (N=38,639), convulsion (N=39,159), and night blindness (39,107).
In Model 2, all the maternal morbidities are included simultaneously in one model, also adjusting for maternal age, parity, maternal education, living standard index, religion, village cluster, and vitamin supplementation group. Total sample size for Model 2 is 37,811.
The number of missing observations or unknown response were: maternal age=90, parity=29, living standard index=30, religion=30, uterine prolapse=82, stress-related incontinence and continuous dripping=839, night blindness=212, severe headache=682; convulsion=160. No missing or unknown responses were present for reproductive tract infection, urinary tract infection, anemia, gastroenteritis, pneumonia, and hepatobiliary disease.
Discussion
In our study population, approximately 1 to 22% of rural Bangladeshi women reported one symptomatic illness, defined in accordance with WHO criteria, within three months of childbirth, with anemia and gastroenteritis being most common. Nearly all investigated morbidities, including reproductive, urinary and gastrointestinal tract, neurological, respiratory, hepatobiliary and nutritional disorders, were significantly associated with an increased risk of postpartum depressive symptoms reported between delivery and 6 months postpartum. Illnesses associated with an approximate two-fold higher risk of postpartum depressive symptoms included stress-related incontinence co-occurring with continuously dripping urine, convulsion and presumptive hepatobiliary disease. The only two conditions for which we did not find an increased risk of depressive symptoms after adjustment for potential confounders and other morbidities were continuous drip of urine that occurred alone (without stress-related incontinence) and reproductive tract infection.
These results of elevated depressive symptoms in women who are ill in the first few months following childbirth expand emerging research on this topic from other countries within the South Asian region. To date, most research on physical morbidities in the postpartum period has been based on clinical samples, data from one time point, or small samples. The results of one cross-sectional study of 102 women referred to a hospital in South India showed that having a medically diagnosed illness was related to postpartum depressive symptoms; however no information was available on type of illness (Shivalli & Gururaj, 2015). Other research has suggested that severity of depressive symptoms increases with the number of morbid symptoms (Webb et al., 2008) and that each additional postpartum physical symptom had a multiplicative effect on risk of depressive symptoms (Howell, Mora, DiBonaventura, & Leventhal, 2009). These studies suggest that postpartum illnesses that are common in low-income societies such as rural Bangladesh may predispose women to postpartum depression.
Links between poor postpartum health and depressive symptomatology may be related to the experience of pain, behavioral or functional impairment associated with these illnesses (Webb et al., 2008). A qualitative analysis from rural South India indicated that women's perception of the cause of ill-health may also play a role in producing depressive symptoms (Savarimuthu et al., 2010). Causal explanatory views of illness and the number of non-medical causes that women attributed to a particular health problem have been associated with increased likelihood of depressive symptoms (Savarimuthu et al., 2010). Given that non-medical explanations are common in other parts of South Asia (Saravanan, David, Bhugra, Prince, & Jacob, 2005) and rural Bangladeshi women hold supernatural explanations for postpartum physical illnesses (Goodburn, Gazi, & Chowdhury, 1995), it is possible that these findings are transferable to our study setting. Other research from India has suggested that causal attributions for depressive symptoms should be interpreted in the context of social adversity, where cultural attitudes may contribute to its manifestation (Rodrigues, Patel, Jaswal, & de Souza, 2003).
Strengths of our study included a large, population-based, cohort design, standardized morbidity and depressive symptom questions about salient and likely well-recalled conditions, a well-defined event and exposure time frame (i.e., pregnancy through 6 months) and execution of fieldwork by highly trained and supervised interviewers. While we benefited from a prospective design with short-recall periods, a limitation is that the time window for assessing morbidity and depressive symptoms, as defined and implemented, overlapped somewhat; that is, the recall period for the postpartum morbidities corresponded to the first three months postpartum (asked at ∼3 months postpartum), while the time of recall for depressive symptoms was anytime during the first six postpartum months (asked at ∼6 months postpartum), making it not possible to discern strict temporality between morbidity and depressive symptom occurrence. Secondly, while every effort was made to align symptoms with WHO-guided criteria for illnesses, a woman's morbidity was, nonetheless, self-reported rather than abstracted from medical records or clinician diagnoses. In our study, data quality may have been high due to rapport built with study staff through repeated visits with participating women, compared to surveys or studies for which there is more limited contact with participants. Furthermore, in rural Bangladesh, many women are under-diagnosed and treated (National Institute of Population Research and Training (NIPORT) et al., 2003), which could lead to potential biases in clinic-based studies.
Although the present study included a wide range of diverse physical disorders, the illnesses that were defined and investigated were not inclusive of all illnesses that occur after childbirth or that may predict depressive symptoms. Research primarily in high-income countries has documented other physical health problems associated with postpartum depressive symptoms, such as back pain (Gutke, Josefsson, & Oberg, 2007; Webb et al., 2008) and vaginal pain (Webb et al., 2008). Additionally, while the items we used to refer to morbidities were pretested locally, we do not have information on the reliability of their self-report. However, in the context of rural Bangladesh, where it has been estimated that 74% of women have no postnatal care (National Institute of Population Research and Training (NIPORT), Mitra and Associates, & Macro International, 2009), self-report may be a more accurate way to assess many of these illnesses than a formal diagnosis. In addition to research on postpartum conditions, literature is accumulating about the effects of birth complications on depressive symptoms. In Asia, pregnancy complications, negative childbirth experiences, and severe morbidity at the time of childbirth have been associated with subsequent postpartum depressive symptomatology (Gausia et al., 2012; Giri et al., 2015; Lyengar, Yadav, & Sen, 2012; Shivalli & Gururaj, 2015). Some studies, however, have not found a significant relation between severe medical complications during or immediately after pregnancy with postpartum depressive symptoms, perhaps because the occurrence of severe morbidity is quite rare (Norhayati, Nik Hazlina, Aniza, & Asrenee, 2016).
The results of our study suggest that women who are physically ill in the first months postpartum face an increased risk of postpartum depressive symptoms. This finding may be especially important for women in low- and middle-income societies who often have inadequate access to health care, poor overall health, and who disproportionately live in conditions of chronic poverty. They are, thus, prone to suffer from postpartum morbidities compared to their counterparts in high-income countries. Assessment of both postpartum morbidity and depressive symptom experience appear to be clear unmet needs in impoverished rural South Asia. Where resources for mental healthcare are limited in low-resource settings like rural Bangladesh (Saxena, Thornicroft, Knapp, & Whiteford, 2007), an alternative strategy may be to strengthen primary health care systems to better enable the assessment and follow-up of physical and mental health concerns in the first half-year following childbirth.
Our findings point to the potential for improved treatment and care for women in the first months postpartum to reduce depressive symptoms. Evidence linking postpartum depressive symptoms to poorer parenting, less secure attachment (Lovejoy, Graczyk, O'Hare, & Neuman, 2000), and poorer child development (Petterson & Albers, 2001; Surkan, Kennedy, Hurley, & Black, 2011), suggests that a reduction of depressive symptoms could benefit child health and development as well. Statistics from a nationally representative survey among Bangladeshi women suggest that less than half seek care for non-life threatening medical conditions occurring around the time of pregnancy (National Institute of Population Research and Training (NIPORT) et al., 2003). Nonetheless, many of these postpartum physical morbidities can be effectively treated, and reducing these morbidities may have the potential of also improving postpartum depressive symptoms. Our results suggest that national or local policies to improve access to primary care for postpartum women in rural Bangladesh should be prioritized, as these efforts may also have positive spillover effects on depressive symptoms, in addition to providing relief from the postpartum illness. In the instances when women are seen for postpartum illness in Bangladesh, primary care providers may also consider screening these women for elevated depressive symptoms.
Significance.
What is already known on this subject?
Studies focusing on low- and middle- income countries (LMICs), particularly in the South Asian region, have examined the effects of socioeconomic factors, partner relationships, and other psychosocial factors on postpartum depression. However, to date, most research on morbidities in the postpartum period in LMICs has been clinic based, used data from a single time point, with small samples, or ambiguously defined measurements of physical illness.
What this study adds?
For health conditions spanning all domains (including those associated with the reproductive, urinary, neurological and other systems as well as nutritional deficiencies), we found early postpartum morbidities posed significant risks for elevated postpartum depressive symptoms. The study design adds strength to our findings, through use of a large, population-based, cohort study and standardized morbidity questions about salient and likely well-recalled conditions.
Acknowledgments
The trial was supported by the Bill and Melinda Gates Foundation (GH614, Global Control of Micronutrient Deficiency, Project Officer: Ellen Piwoz), Seattle, WA; The analyses for this paper was supported by the National Institute of Child Health and Development, NIH [1 RO3 HD069731-01A1], Bethesda, MD; Other funding for data collection included the Office of Health, Infectious Diseases and Nutrition, USAID (Micronutrients for Health Cooperative Agreement HRN-A-00-97-00015-00 and Global Research Activity GHS-A-00-03-00019-00), Washington DC; USAID Mission Bangladesh, Dhaka; Ministry of Health and Family Welfare, Government of the Peoples' Republic of Bangladesh, Dhaka; The Sight and Life Global Nutrition Research Institute, Baltimore, MD; and the National Institute of Drug Abuse (T32DA13911), Bethesda, MD.
Footnotes
Clinical trial registration: ClinicalTrials.gov; NCT0019882
Conflict of interest statement: The authors declare that they have no conflict of interest.
References
- Behan C, Doyle R, Masterson S, Shiers D, Clarke M. A double-edged sword: review of the interplay between physical health and mental health. Irish Journal of Medical Science. 2015;184(1):107–112. doi: 10.1007/s11845-014-1205-1. [DOI] [PubMed] [Google Scholar]
- Cummings P. Methods for estimating adjusted risk ratios. STATA Journal. 2009;9(2):175–196. [Google Scholar]
- Doherty AM, Gaughran F. The interface of physical and mental health. Social Psychiatry and Psychiatric Epidemiology. 2014;49(5):673–682. doi: 10.1007/s00127-014-0847-7. [DOI] [PubMed] [Google Scholar]
- Filippi V, Ronsmans C, Campbell OM, Graham WJ, Mills A, Borghi J, et al. Osrin D. Maternal health in poor countries: the broader context and a call for action. Lancet. 2006;368(9546):1535–1541. doi: 10.1016/S0140-6736(06)69384-7. [DOI] [PubMed] [Google Scholar]
- Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, Holton S, Holmes W. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bulletin of the World Health Organization. 2012;90(2):139G–149G. doi: 10.2471/BLT.11.091850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gausia K, Fisher C, Ali M, Oosthuizen J. Antenatal depression and suicidal ideation among rural Bangladeshi women: a community-based study. Archives of Women's Mental Health. 2009;12(5):351–358. doi: 10.1007/s00737-009-0080-7. [DOI] [PubMed] [Google Scholar]
- Gausia K, Ryder D, Ali M, Fisher C, Moran A, Koblinsky M. Obstetric complications and psychological well-being: experiences of Bangladeshi women during pregnancy and childbirth. Journal of Health, Population and Nutrition. 2012;30(2):172–180. doi: 10.3329/jhpn.v30i2.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri RK, Khatri RB, Mishra SR, Khanal V, Sharma VD, Gartoula RP. Prevalence and factors associated with depressive symptoms among post-partum mothers in Nepal. BMC Research Notes. 2015;8:111. doi: 10.1186/s13104-015-1074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodburn EA, Gazi R, Chowdhury M. Beliefs and practices regarding delivery and postpartum maternal morbidity in rural Bangladesh. Studies in Family Planning. 1995;26(1):22–32. [PubMed] [Google Scholar]
- Gunnsteinsson S, Labrique AB, West KP, Christian P, Sucheta M, Shamim AA, et al. Klemm RDW. Constructing indices of rural living standards in Northwest Bangladesh. Journal of Health, Population and Nutrition. 2010;28:509–519. doi: 10.3329/jhpn.v28i5.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutke A, Josefsson A, Oberg B. Pelvic girdle pain and lumbar pain in relation to postpartum depressive symptoms. Spine. 2007;32(13):1430–1436. doi: 10.1097/BRS.0b013e318060a673. [DOI] [PubMed] [Google Scholar]
- Howell EA, Mora PA, DiBonaventura MD, Leventhal H. Modifiable factors associated with changes in postpartum depressive symptoms. Archives of Women's Mental Health. 2009;12(2):113–120. doi: 10.1007/s00737-009-0056-7. [DOI] [PubMed] [Google Scholar]
- Institute of Health Metrics and Evaluation. Global Burden of Disease Arrow Diagram. 2015 Retrieved from http://vizhub.healthdata.org/irank/arrow.php.
- Kim JM, Labrique A, West KP, Rashid M, Shamim AA, Ali H, et al. Christian P. Maternal morbidity in early pregnancy in rural northern Bangladesh. International Journal of Gynecology & Obstetrics. 2012;119(3):227–233. doi: 10.1016/j.ijgo.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Labrique AB, Christian P, Klemm RD, Rashid M, Shamim AA, Massie A, et al. West KP., Jr A cluster-randomized, placebo-controlled, maternal vitamin A or beta-carotene supplementation trial in Bangladesh: design and methods. Trials. 2011;12:102. doi: 10.1186/1745-6215-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O'Hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clinical Psychology Review. 2000;20(5):561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Lyengar K, Yadav R, Sen S. Consequences of maternal complications in women's lives in the first postpartum year: a prospective cohort study. Journal of Health, Population and Nutrition. 2012;30(2):226–240. doi: 10.3329/jhpn.v30i2.11318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreen HE, Kabir ZN, Forsell Y, Edhborg M. Prevalence and associated factors of depressive and anxiety symptoms during pregnancy: a population based study in rural Bangladesh. BMC Women's Health. 2011;11:22. doi: 10.1186/1472-6874-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Population Research and Training (NIPORT), Mitra and Associates, & Macro International. Bangladesh Demographic and Health Survey 2007 Chapter 9 Maternal and Newborn Health. Retrieved from Dhaka, Bangladesh and Calverton, Maryland (USA): 2009. [Google Scholar]
- National Institute of Population Research and Training (NIPORT), ORC Macro, Johns Hopkins University & ICDDR, B. Bangladesh Maternal Health Services and Maternal Mortality Survey 2001. Retrieved from Dhaka, Bangladesh and Calverton, Maryland (USA): 2003. [Google Scholar]
- Norhayati MN, Nik Hazlina NH, Aniza AA, Asrenee AR. Severe Maternal Morbidity and Postpartum Depressive Symptomatology: A Prospective Double Cohort Comparison Study. Research in Nursing & Health. 2016 doi: 10.1002/nur.21741. [DOI] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Rochat TJ, Kringelbach ML, Stein A. Postnatal depression and its effects on child development: a review of evidence from low-and middle-income countries. British Medical Bulletin. 2012;101(1):57–79. doi: 10.1093/bmb/ldr047. [DOI] [PubMed] [Google Scholar]
- Petterson SM, Albers AB. Effects of poverty and maternal depression on early child development. Child Development. 2001;72(6):1794–1813. doi: 10.1111/1467-8624.00379. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Patel V, Jaswal S, de Souza N. Listening to mothers: qualitative studies on motherhood and depression from Goa, India. Social Science & Medicine. 2003;57(10):1797–1806. doi: 10.1016/s0277-9536(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Ronsmans C, Graham WJ Lancet Maternal Survival Series Steering Group. Maternal mortality: who, when, where, and why. Lancet. 2006;368(9542):1189–1200. doi: 10.1016/S0140-6736(06)69380-X. [DOI] [PubMed] [Google Scholar]
- Saravanan B, David A, Bhugra D, Prince M, Jacob KS. Insight in people with psychosis: the influence of culture. International Review of Psychiatry. 2005;17(2):83–87. doi: 10.1080/09540260500073596. [DOI] [PubMed] [Google Scholar]
- Savarimuthu RJ, Ezhilarasu P, Charles H, Antonisamy B, Kurian S, Jacob KS. Post-partum depression in the community: a qualitative study from rural South India. International Journal of Social Psychiatry. 2010;56(1):94–102. doi: 10.1177/0020764008097756. [DOI] [PubMed] [Google Scholar]
- Saxena S, Thornicroft G, Knapp M, Whiteford H. Resources for mental health: scarcity, inequity, and inefficiency. Lancet. 2007;370(9590):878–889. doi: 10.1016/S0140-6736(07)61239-2. [DOI] [PubMed] [Google Scholar]
- Shivalli S, Gururaj N. Postnatal depression among rural women in South India: do socio-demographic, obstetric and pregnancy outcome have a role to play? PLoS One. 2015;10(4):e0122079. doi: 10.1371/journal.pone.0122079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Pariante CM. Effects of perinatal mental disorders on the fetus and child. Lancet. 2014;384(9956):1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bulletin of the World Health Organization. 2011;89(8):608–615. doi: 10.2471/BLT.11.088187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncalp O, Hindin MJ, Souza JP, Chou D, Say L. The prevalence of maternal near miss: a systematic review. BJOG. 2012;119(6):653–661. doi: 10.1111/j.1471-0528.2012.03294.x. [DOI] [PubMed] [Google Scholar]
- UNICEF. Women and girls in Bangladesh. 2010 Retrieved from https://www.unicef.org/bangladesh/Women_and_girls_in_Bangladesh.pdf.
- UNICEF. Maternal and Newborn Health Disparities: Bangladesh. 2016 Retrieved from https://data.unicef.org/resources/maternal-newborn-health-disparities-country-profiles/
- Wachs TD, Black MM, Engle PL. Maternal Depression: A Global Threat to Children's Health, Development, and Behavior and to Human Rights. Child Development Perspectives. 2009;3(1):51–59. [Google Scholar]
- Webb DA, Bloch JR, Coyne JC, Chung EK, Bennett IM, Culhane JF. Postpartum physical symptoms in new mothers: their relationship to functional limitations and emotional well-being. Birth. 2008;35(3):179–187. doi: 10.1111/j.1523-536X.2008.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West Keith P, Christian Parul, Labrique Alain B, Rashid Mahbubur, Shamim Abu Ahmed, Klemm Rolf D W, et al. Ali Hasmot. Effects of vitamin A or beta carotene supplementation on pregnancy-related mortality and infant mortality in rural Bangladesh: a cluster randomized trial. JAMA. 2011;305(19):1986–1995. doi: 10.1001/jama.2011.656. [DOI] [PubMed] [Google Scholar]
- World Bank. Gender Data Portal: Bangladesh. 2017 Retrieved from http://datatopics.worldbank.org/gender/country/bangladesh.
- World Health Organization Reproductive Health and Research. Pregnancy, Childbirth, Postpartum, and Newborn Care: A Guide for Essential Practice. 2nd. Geneva, Switzerland: World Health Organization; 2003. [PubMed] [Google Scholar]