Abstract
Single particle ICP-MS has evolved rapidly as a quantitative method for determining nanoparticle size and number concentration at environmentally relevant exposure levels. Central to the application of spICP-MS is a commonly used, but not rigorously validated, calibration approach based on the measured transport efficiency and the response of ionic standards. In this work, we present a comprehensive and systematic study of the accuracy, precision and robustness of spICP-MS using the rigorously characterized reference material (RM) 8017 (Polyvinylpyrrolidone Coated Nominal 75 nm Silver Nanoparticles), recently issued by the National Institute of Standards and Technology (NIST). We report for the first time, statistically significant differences in frequency-based and size-based measures of transport efficiency with NIST RM 8013 Gold Nanoparticles and demonstrate that the size-based measure of transport efficiency is more robust and yields accurate results for the silver nanoparticle RM relative to TEM-based reference values. This finding is significant, because the frequency-based method is more widely applied. Furthermore, we demonstrate that the use of acidified ionic standards improves measurement of ICP-MS Ag response, but does not degrade the accuracy of the results for AgNP suspensions in water or various other diluents. Approaches for controlling AgNP dissolution were investigated and are shown to effectively improve particle stability in dilute suspensions required for spICP-MS analysis, while minimally affecting the measured intensity and allowing for more robust analysis. This study is an important and necessary advancement toward full validation and adoption of spICP-MS by the broader research community.
Keywords: Size characterization, single particle inductively coupled plasma mass spectrometry, silver nanoparticle, matrix effect, sample treatment
Graphical Abstract

Introduction
The increasing application of manufactured nanomaterials (MNMs) in consumer and medical products has raised concerns about the eventual release of ENMs and their potential adverse effects on the environment and human health [1, 2]. Risk assessments are needed to evaluate the implication of ENM usage and to advance sustainable development of nanotechnology [2–4]. Of particular demand are innovative and validated analytical methodologies that can detect and examine the quantity, size and physical/chemical properties of ENMs under realistic exposure and release scenarios, and throughout their life cycle [5, 6]. Inductively coupled plasma mass spectrometry operated under single particle mode (spICP-MS) is one such recent innovation. With spICP-MS, mass measurements are performed on a “particle by particle” basis, providing comprehensive information about number concentration, size distribution (if morphology, chemical composition and density are known), ion/particle partitioning for metal containing nanoparticles (NPs) and even apparent core density and state of aggregation (if coupled with an appropriate orthogonal technique) [7–9]. More importantly, the advantages of environmentally relevant number concentration detection limits, elemental selectivity, number based measurement and rapid output make spICP-MS a promising method for overcoming many challenges for the characterization of ENMs in natural environments [7]. In recent years, this method and its application have evolved rapidly, with progress in metrological criteria [10–14], data acquisition [15] and processing [16–19], experimental methodology [20–23], as well as instrumentation [24, 25]. However, challenges remain with respect to obtaining accurate and consistent measurements of size, size distribution, and number concentration using spICP-MS [26], while appropriate validation studies are scarce.
A major challenge is the analyte mass calibration that establishes a correlation between measured intensity and elemental mass quantity. The most straightforward approach is to use a NP standard with known geometry, size and composition that contains the same element as the targeted NP. However, use of this method is limited by the scarcity of NP reference materials (RM) [27]. More commonly, an alternative method utilizing measured transport efficiency (ηn) and analyte response in ionic standards is adopted [20]. It has greatly expanded the application of spICP-MS to a number of ENMs, including Ag [17, 18, 25, 28–34], Cu [35], TiO2 [28], ZnO [36, 37], CeO2 [37], and La2O3 [38]. This approach relies on ηn, which is determined experimentally via particle size, or frequency methods, using available NP standards (e.g., gold nanoparticle RM 8012 or RM 8013 from the National Institute of Standards and Technology (NIST)). The two methods were reported to yield similar ηn [20, 39], but in our laboratories we have repeatedly observed differences [40]. ηn is one of the major factors influencing the accuracy of spICP-MS measurement for both particle size and concentration [22], but evaluation and comparison of ηn determined by these two methods using certified RMs has been limited. Most studies applied the frequency method [17, 18, 25, 29, 32, 34, 35] and many did not specify how ηn was determined [28, 36–38]. For example, in a single particle calculation tool published by RIKILT Wageningen University & Research [41], the transport efficiency calculation uses the frequency method alone. The European Commission funded NanoDefine project is conducting an international interlaboratory comparison using the RIKILT tool. In a recent international interlaboratory study of spICP-MS analysis of commercial AgNPs using ηn by the frequency method, large variations between labs in both particle size and number concentration were reported (relative standard deviations between laboratories are 28 % and 107 %, respectively, for 40 nm AgNPs in water) [34]. However, without a rigorously characterized AgNP material in the size range measurable by spICP-MS, contributions from individual factors (e.g., NP heterogeneity, sample handling, instrumentation, measurement and data analysis protocol) are difficult to evaluate. NIST recently issued a nominal 75 nm AgNP reference material (RM 8017) with value assignments for key properties including size, size distribution, and total mass of Ag in a vial, providing a means for validating the spICP-MS method through orthogonal comparison with other well-established sizing techniques [42].
For spICP-MS to evolve into a mature and widely adopted tool for characterization of environmental samples, it is necessary to improve its robustness in dynamic systems. AgNPs represent a distinctly unique group of ENMs that are subject to rapid changes in chemical composition and size in highly dilute suspensions. Oxidative dissolution is expected to modify the size distribution and ion/particle partitioning of AgNPs [43], but limited attention has been given to its impact on spICP-MS analysis [21]. Potential influences include (1) bias in particle size, size distribution, number concentration and dissolved fraction, (2) increase in size detection limit, and (3) reduction in reproducibility. Improvements in sample handling to enhance the stability from the time of dilution to analysis are thus important to increase the robustness of the spICP-MS method.
The aim of this work was to identify potential pitfalls and provide practical recommendations for spICP-MS methodology through evaluation with a rigorously characterized AgNP RM (NIST RM 8017) as a test bed. The contributions of ηn and ionic calibration to measurement trueness and precision are examined theoretically and by experimental comparison with a reference TEM value. This study is the first, to our knowledge, to thoroughly evaluate the widely-adopted transport efficiency based spICP-MS calibration strategy using a well characterized NP RM. Methods for assigning the central tendency of the size distribution are evaluated. The evolution of particle size in the concentrated stock suspension and in analyte suspensions diluted to the appropriate level for spICP-MS analysis are monitored to provide information about the stability of AgNPs at different stages. Finally, modifications for sample handling prior to analysis are presented to improve measurement confidence and reduce measurement uncertainty.
Materials and methods1
Chemicals
The AgNP used in this study was NIST RM 8017, lyophilized polyvinyl-pyrrolidone (PVP, molar mass 40 kDa) capped AgNPs with a nominal core diameter of 75 nm. Detailed information on RM 8017 is provided in the Electronic Supplementary Material (ESM) and the Report of Investigation [42]. AgNP suspension was reconstituted by adding 2 mL of water, and was then stored at 4 °C in the dark for future use. A AuNP RM (nominal 60 nm, NIST RM 8013) was used to determine the transport efficiency. In addition, NIST SRM 3151 (Ag standard solution) and SRM 3121 (Au standard solution) were used to prepare ionic calibration standards. Additional measurements of the transport efficiency were obtained using NIST RM 8012, nominal 30 nm Au NPs. Optima grade nitric acid and hydrochloric acid, thiourea, and 1 mol L−1 tris(hydroxymethyl)aminomethane (tris) base solution were purchased from Fisher Scientific Inc. (Pittsburgh, PA); sodium carbonate, sodium bicarbonate, sodium tetraborate decahydrate, sodium citrate dihydrate, sodium hydroxide, sodium sulfide nonahydrate, and reduced L-glutathione (GSH) were purchased from Sigma-Aldrich (St. Louis, MO).
Instrumentation
A ThermoFisher X series II quadrupole ICP-MS (Waltham, MA), equipped with a microflow perfluoroalkoxy concentric nebulizer (PFA-ST, Elemental Scientific, Omaha, NE) and a glass impact bead spray chamber cooled to 2 °C, was used. In addition, the sample uptake system used a PFA-encapsulated carbon fiber support autosampler probe with integrated PFA transfer tubing (0.25 mm i.d., ES-5037-3250-150, Elemental Science, Omaha, NE), and peristaltic pump used a PVC 2-stop tubing with 0.27 mm i.d and 151 mm length (ES-4397-1027, Elemental Science, Omaha, NE). Instrument operating and data acquisition parameters are listed in Table S1. 2 % volume fraction HNO3 (2 % HNO3) was used as a rinse solution between samples. Data were collected in time resolved analysis (TRA) mode with a dwell time of 10 ms and acquisition time of 180 s. The limit of detection for AuNP and AgNPs were 10 nm and 20 nm, respectively.
Sample preparation
Nalgene™ low density polyethylene bottles and high purity water, prepared in-house by sub-boiling distillation of deionized water in a conditioned quartz still, were used to prepared all aqueous samples. For spICP-MS analysis, the AgNP RM and AuNP RM stock suspensions were gravimetrically diluted in water to a concentration of 0.04 ng Ag g−1 and 0.03 ng Au g−1, respectively (1.7 × 104 particles mL−1). The concentration was optimized to mitigate undesired particle coincidence while maintaining adequate particle flow (at least 400 particles were registered). In order to examine the matrix effect on AgNPs, samples were also diluted in moderately hard reconstituted water (MHRW) [44] and 2 % HNO3. In addition, diluents (Table 1) containing chemical agents that were reported to limit the dissolution of AgNP were used to prepare the spICP-MS analyte suspensions. Stock solutions of buffers (0.1 mol L−1 for carbonate and tris, and 0.05 mol L−1 for borate) were first prepared and pH adjusted using 0.1 mol L−1 NaOH or 0.1 mol L−1 HNO3 if needed, and then were diluted to 1 mmol L−1 for carbonate and tris or 0.5 mmol L−1 for borate prior to use. GSH and Na2S solutions were prepared fresh to avoid the degradation of sulfur compounds. Ag+ standards of (0 to 10) ng g−1 were prepared in 2 % HNO3 and the diluents specified in Table 1 to matrix match AgNP suspensions. Specifically, the first dilution of mass fraction of 1.8 µg g−1 and 5.0 µg g−1 for AgNPs and Ag+, respectively, were prepared in water; subsequent dilutions utilized the specified diluents. In addition, Au+ standards of (0.5 to 28) ng g−1 were prepared in a thiourea solution (2.4 % volume fraction HCl, 0.04 % volume fraction HNO3, and 0.5 % mass fraction thiourea).
Table 1.
Diluents used for making spICP-MS analyte suspensions
| Diluent | Chemical composition (mmol L−1) | Measured pH* |
|---|---|---|
| Water | Water | 5.8 |
| Carbonate buffer 1 | 0.1 Na2CO3, 0.9 NaHCO3 | 9.3 |
| Carbonate buffer 2 | 0.6 Na2CO3, 0.4 NaHCO3 | 10.2 |
| Carbonate buffer 3 | 0.9 Na2CO3, 0.1 NaHCO3 | 10.5 |
| Borate buffer | 0.5 Na2B4O7 pH adjusted to 10 | 9.8 |
| Tris buffer | 1 tris pH adjusted to 9 | 9.0 |
| Sodium citrate | 1 sodium citrate dihydrate | 7.8 |
| NaCl | 1 NaCl | 5.8 |
| Glutathione (GSH) | 1 GSH | 3.4 |
| Na2S | 0.1 Na2S·9 H2O | 10.0 |
Data acquisition and process
Intensities of 107Ag or 197Au were recorded by ICP-MS. Sample flow rate was measured daily (at least three replicates) by quantifying the mass of sample uptake for 5 min on a five-place analytical balance. Measurement variation between replicates was < 2 % relative standard deviation. Sample flow rates measured for NP suspensions and ionic standards prepared in the diluents in Table 1 showed no difference from sample flow rates measured for preparations in water. The transport efficiency was determined in triplicate via both the particle size and frequency methods using freshly diluted RM 8013 [16, 20]. In AgNP experiments, a typical sample series included water blank, AgNP analyte suspensions and Ag+ standards. In addition, freshly diluted AgNP suspensions in water were measured at the beginning and the end of the queue as quality control (QC) to check instrument performance. The relative difference of the mean particle intensity of QC samples was < 10 % in most experiments, and experiments were repeated after retuning instrument if intensity drift was > 20 %. Measurement of AgNPs were conducted within 30 min after dilution unless otherwise specified. Selected AgNP samples were monitored over time to test the stability. The recorded temporal intensity data were then exported to Microsoft Excel and processed based on an established protocol to calculate particle size and number concentration [20, 40]. In addition, the central tendency of the size distribution was estimated by OriginPro using lognormal fitting. Detailed information on data processing is provided in the ESM.
Results and Discussion
Measurement challenges
An approach [20] that correlates observed intensity to elemental mass using an intensity-mass coefficient established with ionic standards and the measured ηn was the adopted calibration scheme to examine the “unknown” AgNP RM. This approach assumes that the ICP-MS response for ionic species is identical to particulate species and that the ηn of the unknown NP samples is the same as the ηn determined with the NP standard composed of a different analyte. If one or the other assumption is not valid, errors in diameter and/or number concentration of the unknown sample will result.
Estimation of bias in sizing and counting unknowns
Consider the best-case scenario where a diameter (D) and a number concentration (CNP) in close agreement with RM values are obtained for a spherical metal NP; the spICP-MS protocol is validated and in this situation, the sensitivity (SM) and ηn are deemed accurate. Suppose however, SM and ηn are biased; errors in the calculated D and CNP of the unknown NP could occur (detailed derivation in the ESM). The percent bias (% Δ) in D as a function of the percent bias in SM and ηn is given by:
| Eq-1 |
The percent bias in CNP as a function of percent bias in ηn is:
| (Eq-2) |
Eq-1 and Eq-2 are illustrated in Fig. 1, where % Δ = 0 represents the unbiased condition. For a negative bias in SM, overestimation of particle size is predicted (and vice versa); whereas for a negative bias in ηn, underestimation of particle size and overestimation of number concentration are expected (and vice versa). Note that in the unlikely case that both SM and ηn are biased by the same relative degree, D would theoretically be unbiased, while CNP would still be biased.
Fig. 1.
Theoretical calculation of bias in particle size (D) and number concentration (CNP) by spICP-MS analysis of spherical metal NPs: (a) effect of sensitivity (SM), and (b) effect of transport efficiency (ηn). % Δ is the percent bias from the ideal condition (% Δ = 0), where SM and ηn are accurately determined and lead to unbiased D and CNP. The red dashed line in (a) corresponds to a −47 % measured difference in ICP-MS response for Ag+ in MHRW vs. water. The red dashed line in (b) gives an average difference of −24 % between ηn determined by the particle frequency (ηn−F) vs. size (ηn−S) method using the NIST AuNP RM.
Matrix effect
The sensitivity to analyte mass is determined by calibration with ionic standards. While ionic calibration standards are almost always acidified, NP suspensions are unstable in acid. As such, spICP-MS samples are typically prepared by dilution in DI water or at times, environmental samples in natural matrices are directly measured. In light of the assumption that the generation and transport of ions from a dissolved standard and a NP suspension are the same, the effect of matrix on the ICP-MS response for particulate and ionic species, specifically whether the ionic standards should be matrix matched to NP samples, warrants investigation. DI water, 2 % HNO3 and MHRW were selected to represent the simplest, acidified and environmental matrices, respectively. For AgNPs, the intensity distributions were consistent in water and MHRW, but as expected, AgNP mass decreased in 2 % HNO3 (shown as shift in intensity distribution) due to AgNP dissolution [43] (Fig. S1). In contrast, ionic Ag standards in 2 % HNO3 (Ag+) produced steady intensities proportional to Ag mass fraction, but signal instability/suppression and nonlinear response were observed for Ag+ in water and MHRW (Fig. S2). The initial signal drift in water was improved by increasing the sample stabilization time from 30 s to 90 s, but at Ag mass fractions ≤ 1 ng g−1 the response for Ag+ in water was reduced compared to acidified standards (Fig. S3), possibly due to nonspecific analyte adsorption onto the sample vessel or sample introduction system of the ICP- MS [45, 46]. The response of Ag+ in water was comparable to that in 2 % HNO3 for mass fractions 2 ng g−1 Ag and greater. A matrix effect was evident in MHRW where sensitivity across the studied Ag mass fraction range ((0 – 5) ng g−1) decreased 47 % comparing to Ag+ in 2 % HNO3. In addition, NP related spikes were recorded, suggesting Ag+ reacts with MHRW components (e.g. Cl−, SO42−, CO32−), leading to apparent mass loss. Clearly, the diluent matrix has a substantial effect on Ag+ but not on AgNPs (intensity change in the case of AgNPs in 2% HNO3 is the result of a redox reaction), thus matrix matching ionic standards and particulate samples is not advisable for spICP-MS analysis of Ag as significant error can result due to the differing response of the two species. AgNPs (RM8017) in MHRW serve as an example; use of the response factor measured with Ag+ in MHRW to calculate the mass and size of RM 8017 in MHRW lead to positive biases of 89 % and 24 %, in mass and size, respectively (Fig. 1a). However, the consistent intensity distribution of AgNPs in MHRW as compared to water (Fig. S1) suggests that the particle mass and size should remain nearly identical in the two matrices. Moreover, the unspecific adsorption of dissolved Ag species at low mass fractions in nonacidified matrices makes quantification of these species by spICP-MS infeasible.
Transport efficiency discrepancies
Transport efficiency was determined daily using freshly diluted AuNP suspensions from multiple vials of NIST RM 8013 via both particle frequency and size methods [20], which are denoted as ηn−F and ηn−S, respectively. The ηn−F calculates the ratio of the number of detected particles to the theoretical number of particles delivered to the ICP-MS, while ηn−S correlates the pulse intensity of a known size (mass) NP standard with the intensity of ionic standards containing the same element. Differences between the two ηn values were observed, where ηn−F is consistently lower than ηn−S (Fig. 2). The ratios of ηn−F / ηn−S ranged from 0.64 to 0.89 and over the course of a 2-year study, a mean ratio of 0.76 ± 0.08 (one standard deviation, 1σ, n = 18) was observed. In addition, greater short-term (within day) variability in ηn−F (1σ = 0.02 to 0.59) relative to ηn−S (1σ = 0.01 to 0.20) was observed. Similar results were observed with 30 nm AuNPs (NIST RM 8012, data not shown) and indicate that neither size dependent changes in transport efficiency [47] nor degradation of the RM 8013 AuNP suspension are likely to have caused the observed differences. As predicted by Fig. 1b, if ηn−F is used to compute particle size and number concentration, on average, results will be biased relative to those calculated using ηn−S by −9 % and 32 %, respectively.
Fig. 2.
Comparison of ηn determined via particle frequency (ηn−F) and size (ηn−S) methods using freshly diluted AuNP RM: (a) ηn and (b) ratio of ηn−F / ηn−S. Calculation of ηn was based on particle number and intensity after correction of split events and false positives [16]. The x-axis indicates measurements (from 1 to 18) during 2 years. Data in (a) show mean ± 1σ (n=3). Data in (b) give mean ηn−F / mean ηn−S and the propagated uncertainty.
Several factors can lead to a low bias in the ηn as measured by the frequency method. First, if the sample flow rate is not accurate the computed expected number of events will be biased. In our case, flow rate measurements were accomplished with sufficient precision (less than 2 % variation between replicates and were demonstrated to be independent of solution matrix). Second, if samples are not diluted properly (i.e., particle number concentration is too high), the probability of coincident events occurring in the same measurement period increases, resulting in a low bias in the measured frequency. However, for the number concentrations used in this study, the probability of coincidence was reduced to < 1% [16]. Finally, if the effective particle number concentration transported to the spray chamber differs from the computed number concentration (derived from the dilution factor, Au reference mass fraction, reference size (TEM), density, and morphology), the computed ηn−F will be in error. Quantification of total Au in stored RM stocks and freshly diluted spICP-MS suspensions after aqua regia digestion showed that measured Au mass fractions were consistent with the ROI Au mass fraction reference value, though the measurement uncertainty for quantification of the dilute spICP-MS suspensions was substantial (30 % relative) due to the low Au concentration. In a separated experiment to investigate the stability of stored dilute suspensions, measured Au mass fractions were observed to decrease with increasing time of storage,, suggesting that loss of AuNPs through surface adsorption in the container does occur at the low number concentrations required for spICP-MS analysis. Measurement results of ηn−F using the same dilute suspension over time showed a negative trend, whereas ηn−S remained more or less consistent (despite expected variation due to changes in tune conditions which were optimized daily, data not shown), indicating that ηn−F is prone to variability and bias from AuNP loss, and serve to demonstrate that it is imperative to use freshly diluted NP suspensions to measure ηn−F. Interestingly, when the values for ηn−F were computed using the Au mass fraction measured in the dilute suspension, the obtained values were still lower than ηn−S, suggesting other unidentified factors must have contributed to the discrepancy in measurement of ηn by particle frequency and size methods.
On the other hand, bias in the particle size measure of transport can arise if the ICP-MS sensitivity to NP and ionic analytes is not identical. Studies using monodisperse microdroplet generation to deliver well-defined droplets at approximately 100 % transport efficiency to the ICP-MS show that this possibility is less likely. These studies reveal that sensitivity to NPs and ions per unit mass of the analyte is similar in the case of Au and Ag [48, 49]. In this study, a thiourea/mixed acid solution was deliberately used as a diluent for Au+ standards to reduce the known memory effect for Au. Use of thiourea as a chelating ligand for Au+ was important to eliminate signal drift caused by nonspecific adsorption of Au+ on components of the ICP-MS sample introduction system because prior experience showed that using dilute aqua regia alone, was insufficient. In this case, the signal intensity for Au+ may be affected by the thiourea/mixed acid diluent which in turn, would affect ηn−S. Our prior research has shown that the ICP-MS response to Au+ in the thiourea/mixed acid was within 5% of that in water, thus the use of thiourea-based diluent for Au+ standards is an unlikely source of the observed differences between ηn−F and ηn−S [40]. Others have reported an increase in Au+ sensitivity in the presence of HCl [27], which suggests that matrix-induced biases differ depending on the ICP-MS sample introduction system, i.e. the nebulizer and spray chamber combination.
Validation of spICP-MS method for sizing and quantifying the AgNP RM
In theory, the approach proposed by Pace et al. [20] is a versatile spICP-MS method for sizing and quantifying NPs when a well-defined NP standard containing the same analyte of interest is not available. However, our results demonstrate that implementation of this approach can be experimentally challenging due to factors influencing the response of the ionic calibration standards and the measurement of ηn. The contribution of these individual factors toward bias in the spICP-MS results was evaluated through direct comparison of experimental results with the reference TEM and mass fraction data for RM 8017 [42].
Selecting transport efficiency
Three freshly reconstituted RM 8017 vials were diluted in water and analyzed within 30 min. The instrument response for Ag was determined with Ag+ in 2 % HNO3. The ηn−S and ηn−F measured on the same day with freshly prepared AuNPs were (3.73 ± 0.01) % and (2.91 ± 0.11) %, respectively (replicate 16, Fig. 2). Calculation using ηn−S yielded a mean AgNP diameter of 70.4 nm ± 1.8 nm (U expressed at a 95 % confidence intervals, n=3 vials) and a number concentration of (5.2 ± 0.6) × 1011 mL−1, which lie within the expanded uncertainty intervals defined by the reference TEM diameter (74.6 nm ± 3.8 nm) and the number concentration (4.7 ± 0.7) × 1011 mL−1 derived from the TEM diameter and reference Ag mass (Fig. S4). However, use of ηn−F resulted in a mean diameter of 65.4 nm ± 2.1 nm and a number concentration of (6.5 ± 0.9) × 1011 mL−1 (Fig. S4), of which both values lie outside the TEM-defined uncertainty intervals. The substantial deviation from reference values for results computed using ηn−F indicates that this measure is underestimating the Ag transport efficiency (Fig. 1b). This was confirmed by using RM 8017 (AgNP) to measure ηn for comparison to RM 8013 (AuNP)-measured values for ηn. Using RM 8017, ηn−F was (3.98 ± 0.04) % and ηn−S was (3.94 ± 0.30) % (1σ, n=3 vials). Both values are in agreement with ηn−S measured using RM 8013, justifying the use of RM 8013 measured ηn−S to size and quantify AgNP samples. It is possible that the different surface functionality of the AuNPs and AgNPs plays a role in the extent of particle loss, thus use of ηn−F measured with citrate coated AuNPs to compute the number concentration of PVP coated AgNPs yeilds bias. This result implies that the principles of both particle size and frequency methods are valid; however, factors (e.g., AuNP loss) that may affect the quantification of variables needed for computing ηn could result in an error in the measured ηn and thus lead to bias in the size and number concentration of NP samples.
It is noteworthy that over the course of this study, ηn−S varied between 2.65 % and 4.42 % (Fig. 2a) due to changes in operating conditions, but the calculated mean diameter of freshly diluted AgNP suspensions remained nearly constant at 68.8 nm ± 1.9 nm (1σ, n≥3 subsamples, n=7 vials), demonstrating that ηn−S provides a robust measure of transport. We are aware that similar ηn can be derived under some conditions [20, 39]; however, our results demonstrate that careful evaluation of both ηn−S and ηn−F with freshly prepared NP standards is needed to avoid possible pitfalls when the ηn based calibration protocol is used. Here, we find that the particle size protocol is not affected by the particle loss issue, and yields a more robust measure of ηn for calibration of both measured AgNP mass and number. As a result, the size protocol was used for the sizing data reported below.
Assigning particle size
Using ionic response in 2 % HNO3 and ηn−S, intensities of individual AgNP pulses were converted to diameters and then binned to generate a distribution plot (Fig. S5). The central tendency of a size distribution is generally reported as the arithmetic mean. However, bias could arise if the inevitable measurement artifacts, such as split events, false positives and coincident particle events [11, 39] are not taken into consideration. The split events and false positives reduce the apparent mass (size) of NPs and increase the observed particle number, resulting in underestimation of size and overestimation of concentration. Particle coincidence gives rise to a tail of particle oligomers (shown as large particles), leading to overestimation of size and underestimation of concentration. Thus, measures of central tendency that can mitigate their influence are expected to yield a better estimation of particle size.
The arithmetic mean of the size distribution yields a size 6 % smaller than the TEM value (Table 2). Alternatively, trimmed mean, median and lognormal fitting [50] were used to determine the central tendency. Similar sizes in closer agreement with the RM TEM value (difference ≤ 3 %) were obtained, suggesting more accurate size assignment by these methods. The trimmed mean used a 10 % threshold (excludes upper and lower 10% of population size) to eliminate the bias from false positives (INP ≤ 10 counts) and split events, which accounted for 6 % of observed particle events (Fig. S5b). However, size assignment of unknowns could be difficult due to the complexity in the size distribution of natural samples. Appropriate mathematical fittings are recommended in this situation. In the present case study using a monodispersed AgNP RM, no significant differences in size are observed by median, trimmed mean or lognormal fitting, but use of the uncorrected mean should be avoided. Compared to the median (essentially a 50 % trimmed mean), the 10 % trimmed mean uses more of the data to derive the central tendency of the distribution. As such, size data reported hereinafter are estimated using a 10 % trimmed mean.
Table 2.
Comparison of different measures of central tendency and a correction protocol for spICP-MS measurement of AgNP RM 8017 particle size and number concentration
| Method | Method for assigning size | Sizeb (nm) | Number concentration (mL−1)c |
|---|---|---|---|
| TEM [42] | Mean | 74.6 ± 3.8 | (4.7 ± 0.7) × 1011 |
|
| |||
| spICP-MSa | Mean | 70.4 ± 1.8 | (5.2 ± 0.6) × 1011d |
| 10 % trimmed mean | 72.3 ± 1.8 | ||
| Median | 72.4 ± 1.9 | ||
| Lognormal fitting | 72.7 ± 1.9 | ||
|
|
|||
| Corrected mean | 72.5 ± 1.9 | (4.9 ± 0.6) × 1011e | |
Data include measurements of three freshly reconstituted RM 8017 vials. lognormal fitting (adjusted R-square 0.95 to 0.97) is the peak location of distribution fitted by a lognormal model; corrected mean is the mean of the size distribution after correction for split/false positive events [16].
For TEM, data represents mean ± U at 95 % uncertainty intervals of 9 vials; for spICP-MS, data represents mean ± U of the central tendencies of size distributions from three vials, where U is computed via a previously published Kragten spreadsheet approach [40] u sing the value of k = 2 to obtain an approximately 95 % confidence interval.
For TEM, the number concentration is calculated from the mass of Ag in a vial, the reconstituted mass, and the TEM diameter, assuming spherical geometry and density of bulk Ag; for spICP-MS, data gives the mean ± U, where U is computed via Kragten spreadsheet [40] using the value of k = 2 to obtain an approximately 95 % confidence interval.
Number concentration derived from all observed particle events.
Number concentration corrected for false positives and split events.
The spICP-MS measured number concentration is 10 % greater than the expected value for RM 8017. This can be improved by intensity correction for split events and false positives (Table 2, Fig. S5). However, for unknown samples when such correction is impractical, bias in the particle number is expected. Recently, with the development of fast scanning ICP-MS systems (dwell time of µs), improved measurement of particle size and number concentration has been reported [15, 24, 25].
Effect of stability on robustness of spICP-MS measurement for AgNPs
While early experiments focused on method validation using newly reconstituted stocks and fresh dilutions, we observed that AgNP size decreased rather rapidly in the dilute spICP-MS suspensions (0.04 ng g−1 Ag) stored under ambient lab conditions. Continuous decrease in size was observed, along with a substantial increase of Ag+ background (Fig. 3a). Specifically, the particle size decreased from (71.1 ± 0.3) nm to (67.3 ± 0.1) nm and (54.9 ± 0.6) nm (1σ, n = 3 subsamples from one reconstituted vial) after 0.5 h and 6 h of storage at room temperature in the dark, respectively. The corresponding mass reductions are 15 % and 54 %, respectively, resulting in an elevated ionic background (Fig. 3a insert) that increased the size detection limit from 14 nm to 23 nm. This result is consistent with previously reported oxidative dissolution of Ag0 NPs [43]. Size (mass) reduction follows the first-order kinetic law, dmAgnp/mAgnp = −kdt, in the first 6 h, and appears to slow between (6 to 24) h (Fig. S6). The rate constant k in the initial 6-h stage is 0.14 h−1 (Excel Solver was used to minimize the sum of squared error), which is comparable with AgNP dissolution under similar conditions [31] but is considerably higher than reported rates at higher number concentrations [51] (Table S2). Decrease of AgNP size in dilute suspensions used for spICP-MS analysis has been reported, but to a lower extent than what we have observed [21]. Our results were validated on different ICP-MS systems in our lab (data not shown). Because the dissolution of AgNPs is a dynamic process that is influenced by particle size, surface coating, concentration, and solution environment [31, 43, 51, 52], the difference between our results and previous studies are likely due to dissimilarities between AgNP samples and exposure conditions (e.g., capping agent, size, solution pH et al.). In our lab, the time at which the particle size decreases to 95 % of the initial value (t95%) for RM8017 at 0.04 ng g−1 was estimated to be (0.5 −1) h.
Fig. 3.
Temporal size measurement of AgNPs in dilute spICP-MS suspension versus reconstituted stock. (a) Size distribution of AgNPs in the spICP-MS suspension (0.04 ng g−1), showing a rapid decrease of particle size. The dilute suspensions (n = 3) were prepared from a newly reconstituted RM 8017 in water and stored under ambient lab conditions. The insert compares the temporal change of the background intensity (Idis, black symbols) with the dissolved Ag+ concentration (blue symbols). The Idis reports the mean ± 1σ, where the black line is used as a guide to the eye. Because the response for Ag+ in water at low concentration was problematic, the dissolved fraction was estimated from the size decrease. The dashed blue line shows data fit with a first order kinetic law . (b) Average size of AgNPs in reconstituted stocks (1 mg g−1) stored at 4 °C in the dark, showing nearly unchanging size over a course of 220 days. Data represent the mean ± 1σ of multiple size measurements (n ≥ 3 subsamples on n ≥ 3 RM 8017 vials). The red dashed line shows the mean size of (70.9 ± 1.0) nm over 220 days. Representative size distributions of AgNPs on day 0 and day 220 are displayed in the insert.
We further examined the stability of AgNPs in the concentrated reconstituted stock (1 mg g−1 Ag) stored at 4 °C in the dark. Aliquots were taken for dilution and measured within 30 min. As shown in Fig. 3b, AgNP size remains nearly constant over a course of a 220-day experiment, and the size distributions of freshly reconstituted and chronically aged stocks appear to be unchanged. Furthermore, AgNPs stored in the refrigerator at 1.7 µg g−1 showed insignificant change in size over 90 days (Δ D = 0.3 %). We surmised that the PVP coating on the particle surface and excess PVP (mass ratio of PVP/Ag = 10) in solution provide a reducing environment that greatly decreased the extent of oxidative dissolution. In addition, while the measured number concentration decreased dramatically in the dilute analyte suspensions after 24 h, only minor variation was observed in the concentrated stocks over time (Fig. S7).
Together, these results clearly demonstrate that the storage condition significantly influences the stability of AgNPs in aqueous suspensions. In the dilute spICP-MS suspensions, rapid dissolution takes place over a time frame that would compromise the accuracy and robustness of the spICP-MS measurement for AgNPs. It is recommended to store AgNP samples at the highest practical concentration [53] and lowest possible temperature (but not less than 4 °C) if possible, and that special attention is needed to minimize the time lapse between sample dilution and measurement. In the case of the RM 8017, we recommend that measurements should be completed within 0.5 h after sample dilution to limit the dissolution induced size reduction to < 5 %.
Improving AgNP stability for robust spICP-MS measurements
The rapid dissolution of AgNPs in water at the low mass concentrations used for spICP-MS analysis can lead to significant artifacts in size and dissolved fraction, which may limit the measurement confidence and increase uncertainty. Improving sample preparation procedures to ensure sufficient stability of AgNP size (mass) from the time of sample dilution to the time of analysis will advance the robustness and reliability of the spICP-MS method. As such, various approaches for controlling AgNP dissolution were investigated.
Low temperature
The rate constant for AgNP dissolution is inversely dependent on temperature [43], suggesting the potential to increase stability through temperature control. Three conditions were tested: (1) dilute and store at room temperature; (2) dilute in pre-refrigerated (4 °C) water and store at room temperature; (3) dilute in pre-refrigerated water and store at 4 °C. Size reduction is suppressed in samples at lower temperature (Fig. S8). Use of conditions (2) and (3) decreased the rate constant to 0.12 h−1 and 0.05 h−1, and extended the t95% to 1.2 h and 3.0 h, respectively, demonstrating that temperature control is a simple, yet effective way to improve AgNP stability. Note however, neither the stock nor the spICP-MS suspension should be subjected to freezing or near freezing temperatures to avoid possible destabilization [42]. Moreover, since the temperature control approach requires minimal sample manipulation, the native status of all Ag species is preserved.
High pH
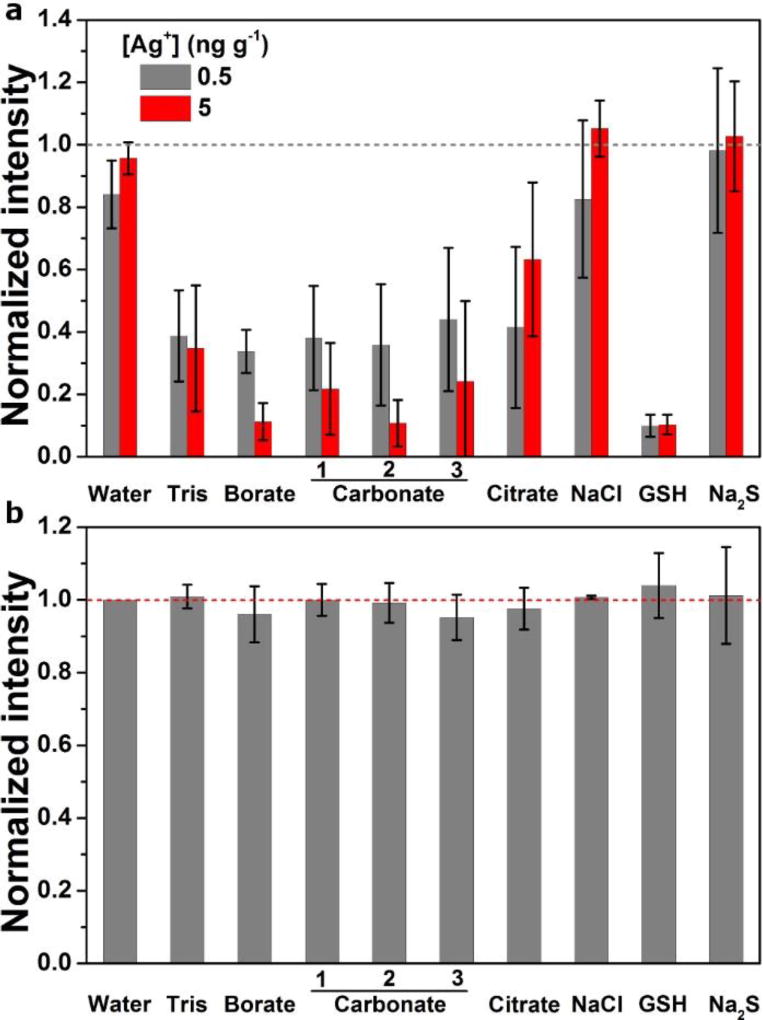
The second approach tested control of suspension pH. Because AgNP dissolution (2Ag0 + O2 + 2H+ ↔ 2Ag+ + 2OH−) is an oxidation reaction that requires both dissolved oxygen and protons [43], increase of solution pH inhibits size reduction. We substituted basic buffers, including tris (pH 9.0), borate (pH 9.8) and carbonate (pH 9.2 to 10.8), for DI water to prepare dilute suspensions. Freshly prepared AgNP and Ag+ in buffers were first analyzed. Despite the differences in diluent chemistry, the measured intensity of the AgNPs remained nearly unchanged (Fig. 4) and computed AgNP size distributions (using Ag+ standards in 2 % HNO3) were in good agreement with the AgNP size distribution measured in water (Fig. S9). In contrast, severely depressed signals were observed for Ag+ in all buffers (Fig. 4 and S10). Matrix-induced signal suppression is a well-known phenomenon in ICP-MS, but chemical interaction between Ag+ and diluent components could also contribute to the depressed signal. Because the intensity of AgNPs was shown to be independent of the diluent matrix, calibration of the measured mass using Ag+ standards in 2 % HNO3 gave accurate results. The evolution of AgNP size was tracked over 48 h, showing slower size reduction at pH ≥ 9 (Fig. 5a). The percentage of size reduction at 6 h was in the range from −2.4 % to −4.6 %. We discovered that AgNP stability in the first 24 h in basic pH buffers (pH 9 – 11) is nearly independent of concentration (carbonate 2, 1 mmol L−1 vs 5 mmol L−1, data not shown) and composition, providing flexibility in buffer selection.
Fig. 4.
Comparison of 107Ag response to Ag+ and AgNP in various diluents. (a) Intensities of Ag+ standards normalized to the intensity of 5 ng g−1 Ag+ in 2 % HNO3 (shown as the gray line). Data represent the mean ± 1σ (n = 3). (b) Intensities of AgNPs normalized to the intensity in water (shown as the red dashed line). Data represent the mean ± 1σ of normalized 10 % trimmed mean intensities for multiple RM 8017 vials (n ≥ 3). All solutions were freshly prepared and measured within 30 min after dilution.
Fig. 5.
Temporal size measurement of AgNPs in diluents: (a) effect of pH, and (b) role of surface modification. Following storage of diluted spICP-MS suspensions in the dark at 4 °C, aliquots were directly used for spICP-MS measurements. Data represent the mean ± 1σ of particle size measured from multiple RM 8017 vials (n ≥ 3).
Modification of AgNP surface
Surface modification with surface active agents that were reported to alter AgNP surface chemistry and control the oxidation process, including sodium citrate [52, 53], NaCl [31], glutathione (GSH) [52] and Na2S [54, 55], were tested. NaCl and Na2S are not typically used to stabilize AgNPs due to transformation of the silver chemical state and/or colloidal state [54–57]. Here we utilize the ability of Cl− and S2− to inhibit AgNP dissolution and therefore preserve Ag mass of AgNPs [31, 55, 58]. Intensity suppression of Ag+ was observed in GSH, sodium citrate and at 0.5 ng g−1 Ag in NaCl (Fig. 4a, Fig. S10). The Ag+ response in Na2S and at 5 ng g−1 Ag in NaCl is comparable with that in 2 % HNO3, but measurement uncertainty is generally high. Evidence also suggests formation of nanocrystalline Ag2S in Na2S (Fig. S10). In contrast, the intensity of AgNPs is nearly consistent across diluents (Fig. 4b), again allowing for calibration using Ag+ in 2% HNO3. The temporal size change of AgNPs in these diluents shows an overall slower size reduction (Fig. 5b). Specifically, t95% is 6 h in sodium citrate, 24 h in NaCl and Na2S, and the size remained relatively constant in GSH for the course of a 7-day experiment. Sodium citrate, NaCl and GSH inhibit the dissolution process in a similar manner through surface binding and blockage of active sites for O2 adsorption [59]. The extent of inhibition is consistent with agent affinity for the Ag surface (GSH > Cl− > citrate). In addition, the antioxidative strength of GSH is anticipated to contribute through scavenging of dissolved O2 [52]. The role of Na2S involves a surface reaction in which active Ag0 is converted to highly insoluble Ag2S, which in turn passivates the AgNP surface and prevents dissolution [52, 55, 56]. Although the density change from Ag0 to Ag2S suggests an increase in particle size via formation of the Ag2S layer, the apparent AgNP size measured by spICP-MS is unlikely to be affected due to preservation of the silver mass. The results however, show that particle size decreases sharply after aging for 2 d in Na2S, concomitant with a continuous increase in the measured particle number concentration (data not shown), suggesting formation of progeny silver-containing particles. Such a dynamic transformation of sulfidized AgNPs in a Na2S-containing system is a unique finding and may have important implications for the fate of Ag2S in the environment. However, this finding is outside the scope of the present study, and so will not be discussed in detail.
Matrix effect on AgNPs and Ag+
In this study, diluent components were deliberately used at low dose (≤ 1mmol L−1) to prevent particle aggregation and limit matrix effects on AgNP intensity. In fact, the change in ICP-MS response to AgNPs was < 4 % in all the matrices tested, which is consistent with a study on AuNPs showing minimal effect on particle intensity when concentration of matrix components was kept low [18]. However, substantial matrix effect on NPs can be problematic if NP suspensions for spICP-MS measurement are dispersed in biological or environmental media containing a high content of matrix (e.g., phosphate-buffered saline, sea water, etc.), which may require sensitivity correction using isotopic dilution analysis [60] or matrix matched ionic standards [27]. For samples that need additional dilution in water or the proposed diluents from this study, the matrix effect on NPs is expected to be low. Ag+, on the other hand, experienced strong interference from diluent chemistry, which we presume was due to the combined contributions of soluble Ag loss through reaction with solution components (e.g., Cl−, S2−, CO32−, etc.) and suppression of Ag+ sensitivity by the matrix. Thus, because a strong matrix effect on Ag+ but not on AgNPs was observed, (Fig. 4 and Fig. S1, S2), use of Ag+ calibration in 2 % HNO3 giving comparable sensitivity as Ag+ in water is crucial for accurate calculation of AgNP size. It should be noted that while the chemical approaches are beneficial for size measurement, the quantitative analysis of dissolved fraction could be problematic due to the observed matrix effect on Ag+.
The effect of the various diluents on measured number concentration was tested since it is possible for the matrix to influence the nebulization process and alter colloidal stability through modification of the NP surface [18]. Fig. 6 shows the particle number concentration of AgNPs diluted in the studied matrices. In some cases, the diluent significantly affected the measured number concentration. For newly diluted samples, an 8% increase was observed in Tris, and prominent change was observed for diluents containing surface active agents. The measured number concentration decreased in citrate, NaCl and GSH, but increased in Na2S. A change in the sample flow was ruled out as a possible cause. Changes in AgNP surface chemistry which can alter the interaction between AgNPs and containers and/or sample introduction system likely contributes to a greater effect from the surface active agents. The 24 h aged samples generally showed a decrease in particle number, which is attributable to particle adsorption on the container. Future work to assessing surface property of the container and sample introduction system to mitigate loss of NPs will improve quantification of particle number by spICP-MS.
Fig. 6.
Matrix effect on the measured number concentration of AgNPs in diluents. Data represent the mean ± 1σ (n ≥ 3) of results normalized to the measured number concentration of fresh AgNP suspension in water. Sample were measured within 30 min after dilution and after 24 h ageing at 4 °C in the dark.
Conclusions
We have described the evaluation of a widely utilized spICP-MS protocol using a well characterized AgNP RM (NIST RM 8017). We find that challenges related to calibration of the ICP-MS response using Ag+ standards and quantification of transport efficiency are the two primary sources of uncertainty that can significantly impact the accuracy of results for particle size, number concentration and dissolved fraction. Use of diluents other than 2 % HNO3 for Ag+ standards resulted in unstable intensity profiles, nonlinear response, and severe signal intensity depression in solutions with chemical compositions (e.g., Cl−, CO32−, S2−, GSH, etc.) relevant to environmental and biological systems. In contrast, AgNPs yield nearly constant intensity, independent of solution chemistry for, when matrix concentration was in the mmol L−1 range. We find that the transport efficiencies determined via particle frequency and size methods using citrate stabilized AuNPs (RM 8013, NIST) are frequently in disagreement, with evidence suggesting the size method yields the more robust measure of transport efficiency. Theoretical calculation demonstrates that significant errors in both particle size and number concentration could arise if a generalized protocol using transport efficiency determined by the frequency method and ICP-MS response measured with Ag+ standards matrix-matched to NP samples are used. Using an orthogonal comparison of spICP-MS results with TEM-measured reference values, a calibration procedure using Ag+ in 2 % HNO3 and the transport efficiency measured by the size method is shown to yield significantly more accurate results for AgNP size and number concentration. Following the protocols outlined here, spICP-MS AgNP results were within 3 % and 10 % of reference values for size and number concentration, respectively.
Furthermore, RM 8017 serves as a proxy for oxidatively unstable NPs subject to dynamic size change, that could be a contributing factor to measurement bias and uncertainty. The size distribution and number concentration of AgNPs remained nearly unchanged in the reconstituted stock suspension for 220 days. However, upon dilution of the stock to the concentration level appropriate for spICP-MS analysis, a rapid decrease in size occurs due to oxidation resulting in an increase in the size detection limit and modifications in size, size distribution and dissolved fraction. To limit the influence of oxidation on spICP-MS measurement, it is recommended that (1) AgNP samples be stored at the highest possible concentration and the lowest possible temperature (above the freezing point), and (2) the time lapse between sample dilution and measurement is minimized (30 min or less). Furthermore, the independence of AgNP intensity on solution chemistry allows improvement of size stability through chemical modification of the AgNP surface or the environment that the AgNP is exposed to, and supports the implementation of Ag+ standards in 2 % HNO3 for calibration of the measured mass. Use of basic buffers (pH ≥ 9) and surface active agents were shown to inhibit size reduction to varying degrees. Specifically, particle size remained nearly constant in GSH over 7 days.
This study demonstrates the potential pitfalls when a generalized spICP-MS protocol is applied to characterize oxidatively active NPs in dynamic systems, and highlights the importance of method development and validation based on instrumentation, experimental conditions, and analyte of interest using available NP standards. The results of this study provide a rigorous framework for achieving robust and accurate spICP-MS analysis, particularly in the presence of redox unstable metallic NPs.
Supplementary Material
Acknowledgments
The authors would like to thank Arnab K. Mukherjee (NIST Materials Measurement Science Division, NIST), Antonio R. Montoro Bustos and Lee L. Yu (Chemical Sciences Division, NIST) for their thorough reviews of the manuscript.
Footnotes
Certain trade names and company products are mentioned in the text or identified in illustrations in order to specify adequately the experimental procedure and equipment used. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the products are necessarily the best available for the purpose.
Compliance with ethical standards
Conflict of interest The authors declare no conflict of interest
References
- 1.Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF, Rejeski D, Hull MS. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015;6:1769–80. doi: 10.3762/bjnano.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P. Assessing the risks of manufactured nanomaterials. Environ Sci Technol. 2006;40:4336–45. doi: 10.1021/es062726m. [DOI] [PubMed] [Google Scholar]
- 3.Ju-Nam Y, Lead JR. Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications. Sci Total Environ. 2008;400:396–414. doi: 10.1016/j.scitotenv.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 4.Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21:1166–70. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 5.Mitrano DM, Motellier S, Clavaguera S, Nowack B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int. 2015;77:132–47. doi: 10.1016/j.envint.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Laborda F, Bolea E, Cepria G, Gomez MT, Jimenez MS, Perez-Arantegui J, Castillo JR. Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples. Anal Chim Acta. 2016;904:10–32. doi: 10.1016/j.aca.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Laborda F, Bolea E, Jimenez-Lamana J. Single particle inductively coupled plasma mass spectrometry: A powerful tool for nanoanalysis. Anal Chem. 2014;86:2270–78. doi: 10.1021/ac402980q. [DOI] [PubMed] [Google Scholar]
- 8.Degueldre C, Favarger PY. Colloid analysis by single particle inductively coupled plasma-mass spectroscopy: a feasibility study. Colloid Surface A. 2003;217:137–42. [Google Scholar]
- 9.Tan J, Liu J, Li M, Hadri HE, Hackley VA, Zechariah MR. Electrospray-differential mobility hyphenated with single particle inductively coupled plasma mass spectrometry for characterization of nanoparticles and their aggregates. Anal Chem. 2016;88:8548–55. doi: 10.1021/acs.analchem.6b01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laborda F, Jimenez-Lamana J, Bolea E, Castillo JR. Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS. J Anal Atom Spectrom. 2013;28:1220–32. [Google Scholar]
- 11.Tuoriniemi J, Cornelis G, Hassellov M. Size discrimination and detection capabilities of single-particle ICPMS for environmental analysis of silver nanoparticles. Anal Chem. 2012;84:3965–72. doi: 10.1021/ac203005r. [DOI] [PubMed] [Google Scholar]
- 12.Olesik JW, Gray PJ. Considerations for measurement of individual nanoparticles or microparticles by ICP-MS: determination of the number of particles and the analyte mass in each particle. J Anal Atom Spectrom. 2012;27:1143–55. [Google Scholar]
- 13.Ho KS, Lui KO, Lee KH, Chan WT. Considerations of particle vaporization and analyte diffusion in single-particle inductively coupled plasma-mass spectrometry. Spectrochim Acta B. 2013;89:30–9. [Google Scholar]
- 14.Lee WW, Chan WT. Calibration of single-particle inductively coupled plasma-mass spectrometry (SP-ICP-MS) J Anal Atom Spectrom. 2015;30:1245–54. [Google Scholar]
- 15.Strenge I, Engelhard C. Capabilities of fast data acquisition with microsecond time resolution in inductively coupled plasma mass spectrometry and identification of signal artifacts from millisecond dwell times during detection of single gold nanoparticles. J Anal Atom Spectrom. 2016;31:135–44. [Google Scholar]
- 16.Liu J, Murphy KE, MacCuspie RI, Winchester MR. Capabilities of single particle inductively coupled plasma mass spectrometry for the size measurement of nanoparticles: A case study on gold nanoparticles. Anal Chem. 2014;86:3405–14. doi: 10.1021/ac403775a. [DOI] [PubMed] [Google Scholar]
- 17.Peters RJB, Rivera ZH, van Bemmel G, Marvin HJP, Weigel S, Bouwmeester H. Development and validation of single particle ICP-MS for sizing and quantitative determination of nano-silver in chicken meat. Anal Bioanal Chem. 2014;406:3875–85. doi: 10.1007/s00216-013-7571-0. [DOI] [PubMed] [Google Scholar]
- 18.Peters R, Herrera-Rivera Z, Undas A, van der Lee M, Marvin H, Bouwmeester H, Weigel S. Single particle ICP-MS combined with a data evaluation tool as a routine technique for the analysis of nanoparticles in complex matrices. J Anal Atom Spectrom. 2015;30:1274–85. [Google Scholar]
- 19.Cornelis G, Hassellov M. A signal deconvolution method to discriminate smaller nanoparticles in single particle ICP-MS. J Anal Atom Spectrom. 2014;29:134–44. [Google Scholar]
- 20.Pace HE, Rogers NJ, Jarolimek C, Coleman VA, Higgins CP, Ranville JF. Determining transport rfficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal Chem. 2011;83:9361–69. doi: 10.1021/ac201952t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telgmann L, Metcalfe CD, Hintelmann H. Rapid size characterization of silver nanoparticles by single particle ICP-MS and isotope dilution. J Anal Atom Spectrom. 2014;29:1265–72. [Google Scholar]
- 22.Tuoriniemi J, Cornelis G, Hassellov M. Improving the accuracy of single particle ICPMS for measurement of size distributions and number concentrations of nanoparticles by determining analyte partitioning during nebulization. J Anal Atom Spectrom. 2014;29:743–52. [Google Scholar]
- 23.Hadri HE, Petersen EJ, Winchester MR. Impact of and correction for instrument sensitivity drift on nanoparticle size measurements by single-particle ICP-MS. Anal Bioanal Chem. 2016;408:5099–108. doi: 10.1007/s00216-016-9397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montano MD, Badiei HR, Bazargan S, Ranville JF. Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times. Environ Sci Nano. 2014;1:338–46. [Google Scholar]
- 25.Abad-Alvaro I, Pena-Vazquez E, Bolea E, Bermejo-Barrera P, Castillo JR, Laborda F. Evaluation of number concentration quantification by single-particle inductively coupled plasma mass spectrometry: microsecond vs. millisecond dwell times. Anal Bioanal Chem. 2016;408:5089–97. doi: 10.1007/s00216-016-9515-y. [DOI] [PubMed] [Google Scholar]
- 26.Bustos ARM, Petersen EJ, Possolo A, Winchester MR. Post hoc interlaboratory comparison of single particle ICP-MS size measurements of NIST gold nanoparticle reference materials. Anal Chem. 2015;87:8809–17. doi: 10.1021/acs.analchem.5b01741. [DOI] [PubMed] [Google Scholar]
- 27.Montano MD, Olesik JW, Barber AG, Challis K, Ranville JF. Single particle ICP-MS: Advances toward routine analysis of nanomaterials. Anal Bioanal Chem. 2016;408:5053–74. doi: 10.1007/s00216-016-9676-8. [DOI] [PubMed] [Google Scholar]
- 28.Donovan AR, Adams CD, Ma Y, Stephan C, Eichholz T, Shi H. Single particle ICP-MS characterization of titanium dioxide, silver, and gold nanoparticles during drinking water treatment. Chemosphere. 2016;144:148–53. doi: 10.1016/j.chemosphere.2015.07.081. [DOI] [PubMed] [Google Scholar]
- 29.Loeschner K, Navratilova J, Kobler C, Molhave K, Wagner S, von der Kammer F, Larsen EH. Detection and characterization of silver nanoparticles in chicken meat by asymmetric flow field flow fractionation with detection by conventional or single particle ICP-MS. Anal Bioanal Chem. 2016;405:8185–95. doi: 10.1007/s00216-013-7228-z. [DOI] [PubMed] [Google Scholar]
- 30.Gray EP, Coleman JG, Bednar AJ, Kennedy AJ, Ranville JF, Higgins CP. Extraction and analysis of silver and gold nanoparticles from biological tissues using single particle inductively coupled plasma mass spectrometry. Environ Sci Technol. 2013;47:14315–23. doi: 10.1021/es403558c. [DOI] [PubMed] [Google Scholar]
- 31.Mitrano DM, Ranville JF, Bednar A, Kazor K, Hering AS, Higgins CP. Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS) Environ Sci Nano. 2014;1:248–59. [Google Scholar]
- 32.Ramos K, Gomez-Gomez MM, Camara C, Ramos L. Silver speciation and characterization of nanoparticles released from plastic food containers by single particle ICPMS. Talanta. 2016;151:83–90. doi: 10.1016/j.talanta.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 33.Murphy KE, Liu J, Guthrie WF, Gorham JM, Bonevich JE, Allen AJ, Winchester MR, Hackley VA, MacCuspie RI. Nanotechnology 2014: Graphene, CNTs, Particles, Films & Composites Technical Proceedings of the 2014 NSTI Nanotechnolgy Conference and Expo. CRC Press; 2014. Use of single particle inductively coupled plasma mass spectrometry to characterize a new silver nanoparticle reference material; pp. 501–4. [Google Scholar]
- 34.Linsinger TPJ, Peters R, Weigel S. International interlaboratory study for sizing and quantification of Ag nanoparticles in food simulants by single-particle ICPMS. Anal Bioanal Chem. 2014;406:3835–43. doi: 10.1007/s00216-013-7559-9. [DOI] [PubMed] [Google Scholar]
- 35.Navratilova J, Praetorius A, Gondikas A, Fabienke W, von der Kammer F, Hofmann T. Detection of engineered copper nanoparticles in soil using single particle ICP-MS. Int J Environ Res Public Health. 2015;12:15756–68. doi: 10.3390/ijerph121215020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hadioui M, Merdzan V, Wilkinson KJ. Detection and characterization of ZnO nanoparticles in surface and waste waters using single particle ICPMS. Environ Sci Technol. 2015;49:6141–48. doi: 10.1021/acs.est.5b00681. [DOI] [PubMed] [Google Scholar]
- 37.Donovan AR, Adams CD, Ma Y, Stephan C, Eichholz T, Shi H. Detection of zinc oxide and cerium dioxide nanoparticles during drinking water treatment by rapid single particle ICP-MS methods. Anal Bioanal Chem. 2016;408:5137–45. doi: 10.1007/s00216-016-9432-0. [DOI] [PubMed] [Google Scholar]
- 38.Frechette-Viens L, Hadioui M, Wilkinson KJ. Practical limitations of single particle ICP-MS in the determination of nanoparticle size distributions and dissolution: case of rare earth oxides. Talanta. 2017;163:121–6. doi: 10.1016/j.talanta.2016.10.093. [DOI] [PubMed] [Google Scholar]
- 39.Pace HE, Rogers NJ, Jarolimek C, Coleman VA, Gray EP, Higgins CP, Ranville JF. Single particle inductively coupled plasma-mass spectrometry: A performance evaluation and method comparison in the determination of nanoparticle size. Environ Sci Technol. 2012;46:12272–80. doi: 10.1021/es301787d. [DOI] [PubMed] [Google Scholar]
- 40.Murphy KE, Liu J, Bustos ARM, Johnson ME, Winchester MR. Characterization of nanoparticle suspensions using single particle inductively coupled plasma mass spectrometry. NIST Special Publication-1200-2. 2015 doi: 10.6028/NIST.SP.1200-21. [DOI] [Google Scholar]
- 41.Single Particle Calculation tool. Wageningen University & Research; 2017. [Accessed 28 June 2017]. Spreadsheet Single Particle Calculation Tool. https://wwwwurnl/en/show/Single-Particle-Calculation-toolhtm. [Google Scholar]
- 42.National Institute of Standards and Technology. Gaithersburg, MD: 2015. Report of Investigation for Reference Material 8017, Polyvinylpyrrolidone Coated Silver Nanoparticles, Nominal Diameter 75 nm. [Google Scholar]
- 43.Liu J, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol. 2010;44:2169–75. doi: 10.1021/es9035557. [DOI] [PubMed] [Google Scholar]
- 44.Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. U.S. Environmental Protection Agency; Washington, DC 20460: 2002. EPA-821-R-02-012. [Google Scholar]
- 45.Sekine R, Khurana K, Vasilev K, Lombi E, Donner E. Quantifying the adsorption of ionic silver and functionalized nanoparticles during ecotoxicity testing: Test container effects and recommendations. Nanotoxicology. 2015;9:1005–12. doi: 10.3109/17435390.2014.994570. [DOI] [PubMed] [Google Scholar]
- 46.Chen W, Wee P, Brindle ID. Elimination of the memory effects of gold, mercury and silver in inductively coupled plasma atomic emission spectroscopy. J Anal Atom Spectrom. 2000;15:409–13. [Google Scholar]
- 47.Ramkorun-Schmidt B, Pergantis SA, Esteban-Fernandez D, Jakubowski N, Gunther D. Investigation of a combined microdroplet generator and pneumatic nebulization system for quantitative determination of metal-containing nanoparticles using ICPMS. Anal Chem. 2015;87:8687–94. doi: 10.1021/acs.analchem.5b01604. [DOI] [PubMed] [Google Scholar]
- 48.Gschwind S, Hagendorfer H, Frick DA, Gunther D. Mass quantification of nanoparticles by single droplet calibration using inductively coupled plasma mass spectrometry. Anal Chem. 2013;85:5875–83. doi: 10.1021/ac400608c. [DOI] [PubMed] [Google Scholar]
- 49.Garcia CC, Murtazin A, Groh S, Horvatic V, Niemax K. Characterization of single Au and SiO2 nano- and microparticles by ICP-OES using monodisperse droplets of standard solutions for calibration. J Anal Atom Spectrom. 2010;25:645–53. [Google Scholar]
- 50.Laborda F, Jimenez-Lamana J, Bolea E, Castillo JR. Selective identification, characterization and determination of dissolved silver (I) and silver nanoparticles based on single particle detection by inductively coupled plasma mass spectrometry. J Anal Atom Spectrom. 2011;26:1362–71. [Google Scholar]
- 51.Kittler S, Greulich C, Diendorf J, Koller M, Epple M. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater. 2010;22:4548–54. [Google Scholar]
- 52.Liu J, Sonshine DA, Shervani S, Hurt RH. Controlled release of biologically active silver from nanosilver surfaces. Acs Nano. 2010;4:6903–13. doi: 10.1021/nn102272n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorham JM, Rohlfing AB, Lippa KA, MacCuspie RI, Hemmati A, Holbrook RD. Storage wars: how citrate-capped silver nanoparticle suspensions are affected by not-so-trivial decisions. J Nanopart Res. 2014;16:2339–53. [Google Scholar]
- 54.Liu J, Pennell KG, Hurt RH. Kinetics and mechanisms of nanosilver oxysulfidation. Environ Sci Technol. 2011;45:7345–53. doi: 10.1021/es201539s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levard C, Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: Impact on dissolution rate. Environ Sci Technol. 2011;45:5260–66. doi: 10.1021/es2007758. [DOI] [PubMed] [Google Scholar]
- 56.Thalmann B, Voegelin A, Sinnet B, Morgenroth E, Kaegi R. Sulfidation kinetics of silver nanoparticles reacted with metal sulfides. Environ Sci Technol. 2014;48:4885–92. doi: 10.1021/es5003378. [DOI] [PubMed] [Google Scholar]
- 57.Kent RD, Oser JG, Vikesland PJ. Controlled evaluation of silver nanoparticle sulfidation in a full-scale wastewater treatment plant. Environ Sci Technol. 2014;48:8564–72. doi: 10.1021/es404989t. [DOI] [PubMed] [Google Scholar]
- 58.Pettibone JM, Liu J. In situ methods for monitoring silver nanoparticle sulfidation in simulated waters. Environ Sci Technol. 2016;50:11145–53. doi: 10.1021/acs.est.6b03023. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt M, Masson A, Brechignac C. Oxygen and silver clusters: Transition from chemisorption to oxidation. Phys Rev Lett. 2003;91:243401. doi: 10.1103/PhysRevLett.91.243401. [DOI] [PubMed] [Google Scholar]
- 60.Sotebier CA, Kutscher DJ, Rottmann L, Jakubowski N, Panne U, Bettmer J. Combination of single particle ICP-QMS and isotope dilution analysis for the determination of size, particle number and number size distribution of silver nanoparticles. J Anal Atom Spectrom. 2016;31:2045–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.