Abstract
Objective
To determine the incidence and risk factors of chronic critical illness (CCI) after severe blunt trauma.
Design
Prospective observational cohort study (NCT01810328).
Setting
Two Level-1 trauma centers in the United States.
Patients
135 adult blunt trauma patients with hemorrhagic shock who survived beyond 48-hours after injury.
Interventions
None
Measurements and Main Results
CCI was defined as an ICU stay lasting ≥14-days with evidence of persistent organ dysfunction. Three subjects (2%) died within the first seven-days, 107 (79%) exhibited rapid recovery and 25 (19%) progressed to CCI. Patients who developed CCI were older (55 vs 44-years-old; p=0.01), had more severe shock (base deficit −9.2 vs −5.5, p=0.005), greater organ failure severity (Denver MOF score, 3.5±2.4 vs 0.8±1.1, p<0.0001) and developed more infectious complications (84% vs 35%, p<0.0001). CCI patients were more likely to be discharged to a long-term care setting (56% vs 34%, p=0.008) than to a rehabilitation facility/home. At four-months, CCI patients had higher mortality (16.0% vs 1.9%; p<0.05), with survivors scoring lower in general health measures (p<0.005). Multivariate analysis revealed age ≥55-years, systolic hypotension ≤70-mmHg, transfusion ≥5-units packed red blood cells within 24-hours, and Denver MOF score at 72-hours as independent predictors of CCI (AUC 0.87, 95% CI [0.75, 0.95]).
Conclusions
While early mortality is low after severe trauma, CCI is a common trajectory in survivors and is associated with poor long-term outcomes. Advancing age, shock severity and persistent organ dysfunction are predictive of CCI. Early identification may facilitate targeted interventions to change the trajectory of this morbid phenotype.
Keywords: critical care, injury, multiple organ failure, shock, chronic critical illness, persistent inflammation immunosuppression and catabolism syndrome
INTRODUCTION
Traumatic injury remains one of the most common causes of death in all age groups, and a significant financial burden in the United States (1, 2). Over the past two decades, clinical advances have significantly reduced in-hospital mortality in critically injured trauma patients (1, 3). Between 2003 and 2009, compliance with evidence-based standard operating procedures decreased 28-day mortality in a severely injured cohort from 22 to 11% (3). However, increasing numbers of severely injured patients that survive develop a state of prolonged intensive care utilization, persistent low-grade organ dysfunction, and dismal post-discharge outcomes (4–6). The term chronic critical illness (CCI) has been used to describe this subset of critically ill patients. However, the diversity of conditions that can culminate in CCI has impeded the development of a consensus definition. Instead, numerous definitions have been developed over the past two decades across multiple populations creating ambiguity in the existing literature (7–17)(Table S1).
Recent efforts at describing the epidemiology and burden of CCI provide some alarming findings. Kahn et al. estimated that 7.6% of patients admitted to the ICU develop CCI, accounting for more than 380,000 cases, 107,000 in-hospital deaths, and over $25 billion in health care expenses (5). Similarly, Iwashyna et al. demonstrated that CCI accounts for 5% of ICU admissions, but over 30% of ICU utilization (12). Both authors report that these patients are less likely to be discharged home and have higher inpatient mortality (5, 12). Additionally, CCI appears to disproportionately affect vulnerable elderly populations (5, 16, 18). The aim of this study is to describe the incidence of and risk factors associated with the development of CCI in severely injured blunt trauma patients with hemorrhagic shock.
MATERIALS AND METHODS
Study Design
We conducted a prospective, observational cohort study over a three-year period (October 2013 – August 2016) at two United States Level 1-trauma centers: University of Florida Health Hospital, Gainesville, Florida and Harborview Medical Center, Seattle, Washington. The Institutional Review Board of each institution granted approval prior to study initiation. Key aspects of study design are listed here, with additional in-depth methodologic description regarding study sites, subject enrollment, outcomes definitions and biostatistical analysis in the expanded materials and methods section of Appendix 1. The study was prospectively registered with clinicaltrials.gov (NCT01810328).
Subjects were initially enrolled under a 96-hour waiver of informed consent protocol previously approved and implemented by both institutions for the Inflammation and Host Response to Injury Program (‘Trauma Glue Grant’)(3). Inclusion criteria included patients aged ≥18-years, confirmation of severe blunt traumatic injury with hemorrhagic shock (systolic blood pressure <90 mmHg or base deficit of ≥6 meq/L within 60-minutes of arrival). Patients expected to survive <48-hours and those with severe traumatic brain injury (TBI; Glasgow Coma Scale <8 and abnormal head computed tomography) were excluded. These inclusion criteria were consistent with the Trauma Glue Grant and were utilized to select for patients likely to survive their initial injuries but at significant risk for multiple organ failure, as previously described (19). Further explanation and justification of inclusion/exclusion criteria are delineated in Appendix 1. All consecutive patients meeting these criteria in which consent was obtained within 96 hours were enrolled.
Demographic, clinical, physiologic, and outcomes data were prospectively collected for the first 28-days after injury, or until ICU discharge. Patients were contacted by telephone four-months after hospital discharge and were interviewed using the 36-Item Short Form Survey (SF-36). For those patients lost to post-discharge follow-up, we queried the Social Security Death Index and Washington State Death Registry to determine mortality at 4-months post-discharge.
Definition of Outcomes
The incidence of CCI was the primary outcome variable. Secondary outcomes included in-hospital and four-month mortality, multiple organ failure (MOF), time-to-recovery, nosocomial infections, and discharge disposition. Currently, there is no consensus definition for CCI. Given this ambiguity, we elected to define CCI as prolonged intensive care unit (ICU) admission (≥14 days) with evidence of ongoing organ dysfunction. This definition is based upon the Trauma Glue Grant experience that patients meeting this criteria demonstrate a prolonged, dysregulated genomic response to injury, persistent organ dysfunction and adverse outcomes (20, 21). We defined persistent organ dysfunction using the Modified Marshal Score criteria requiring either ≥2 in the renal (serum creatinine >1.9 mg/dl [without dialysis]) or pulmonary (PaO2/FiO2 ≤300) categories, or ≥1 in the cardiac category (systolic blood pressure <90 mm Hg, or use of vasopressors). We defined multiple organ failure (MOF) as a maximum Denver MOF score ≥3. ‘Time-to-recovery’ was defined as the number of days after injury to resolution of organ dysfunction, without subsequent recurrence (Table S2). Patients with an ICU LOS <14-days without persistent organ dysfunction were classified as ‘rapid recovery’.
Statistical Analysis
Data are presented as means with standard deviation for continuous variables compared using Student t-test, while those not satisfying normality were compared using the Kruskal-Wallis test. Categorical variables are presented as frequency and percentage and compared using the Pearson χ2 test or Fisher exact test. We used the log-rank test to compare Kaplan-Meier product limit estimates of organ dysfunction recovery between CCI and rapid recovery groups.
For all multivariate analyses, we selected explanatory variables based on their significance in an a priori univariate analysis and reported associations in the literature. We report adjusted odds ratios (OR) with 95% confidence intervals (95% CI) for the final reduced set of variables selected by stepwise model selection at a significance level of 0.10. Area under the receiver operating curve values (AUC) and Hosmer-Lemeshow goodness-of-fit test were used to assess model discrimination and fit.
We compared four-month scaled scores between CCI and rapid recovery groups from the eight domains of SF-36 measures (physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional well-being, social functioning, pain, and general health)(22). To account for loss to follow-up at four-months, inverse probability weighting was used, as previously described (23). Probabilities of loss to follow-up were estimated through logistic regression using the covariates in the 24-hour baseline model.
All significance tests were two-sided, with p-value ≤0.05 considered statistically significant. All statistical analyses were performed with SAS (v.9.4, Cary, NC).
RESULTS
Study Population and CCI Cohort Characteristics
A total of 135 patients were enrolled over the course of three years; 52 patients enrolled at the University of Florida Health Hospital and 83 patients at Harborview Medical Center. The cohort was predominantly comprised of white (87%) males (68%) with a mean age of 46-years. The majority of injuries (n=116, 86%) were motor vehicle-associated. These were severely injured patients with a mean (± SD) ISS of 32.1 ± 13.1 and APACHE II score of 22.8 ± 8.3. Patients showed physiologic evidence of hemorrhagic shock, as measured by worst base deficit (mean, −6.3 ± 7.4 meq/L) and highest lactate (mean, 5.1 ± 4.5 mmol/L) within six hours of injury. Within the first 24-hours, patients were resuscitated with an average of 6.2 ± 12.2 units of packed red blood cells (PRBC), 3.1 ± 7.8 units of fresh frozen plasma (FFP), and 9,330 ± 4,530 ml of crystalloids. Seventy-six percent of the patients received transfusion of at least one unit of PRBC within the first 24-hours.
Three patients who survived the first 48-hours died within seven days from injury (early deaths), three died after the first week but prior to 28-days, and one patient died 52-days after injury, for an inpatient mortality rate of 5.2%. Overall, 18.5% of the study cohort developed CCI. There was no difference in mechanism or severity of injury between those who developed CCI, and those who recovered (Table 1). Patients who developed CCI were older, had evidence of more severe shock and physiologic derangement, and significantly higher transfusion requirements (Table 1). There were no significant differences between CCI and rapid recovery groups with regard to sex, BMI, or number of chronic comorbidities (Table 1).
Table 1. CCI and rapid recovery cohort baseline and injury characteristics*.
Cohort baseline and injury characteristics at time of presentation to the Emergency Department. Early deaths are excluded.
| Characteristics | Rapid recovery (n=107) 79.3% |
CCI (n=25) 18.5% |
p-value |
|---|---|---|---|
|
| |||
| Age (mean, SD) | 44±17 | 55±17 | 0.01 |
| Male sex (n, %) | 73 (68.2) | 19 (76) | 0.63 |
| Race (n, %) | 0.32 | ||
| White | 93 (86.9) | 23 (92) | |
| Hispanic | 5 (4.7) | 2 (8) | |
| African American | 9 (8.4) | 0 (0) | |
| American Indian | 1 (0.9) | 1 (4) | |
| Pacific Islander | 1 (0.9) | 0 (0) | |
| Asian | 2 (1.9) | 1 (4) | |
| Unknown | 1 (0.9) | 1 (4) | |
| BMI (mean, SD) | 28.5±6.6 | 29.6±6.2 | 0.50 |
| Number of comorbidities (n, %) | 0.41 | ||
| 0 | 40 (37.4) | 7 (28) | |
| 1 | 35 (32.7) | 7 (28) | |
| ≥2 | 32 (29.9) | 11 (44) | |
| APACHE II (mean, SD) | 21.2±7.2 | 28.3±9.6 | 0.0002 |
| ISS (mean, SD) | 31.2±13.3 | 34.9±12.7 | 0.19 |
| Maximum AIS score (mean, SD) | |||
| Head | 2.9±1.1 | 2.8±0.9 | 0.93 |
| Neck | 2.8±0.5 | 2.7±0.6 | 0.79 |
| Thorax | 3.3±0.8 | 3.5±0.8 | 0.51 |
| Abdomen | 3.1±0.9 | 3.1±1.0 | 0.64 |
| Spine | 2.1±0.5 | 2.5±0.8 | 0.020 |
| Upper Extremity | 2.2±0.7 | 2.3±0.6 | 0.36 |
| Lower Extremity | 3.5±1.1 | 3.3±1.0 | 0.32 |
| Injury mechanism (n, %) | 0.47 | ||
| Fall | 10 (9.3) | 1 (4) | |
| Motor vehicle collision | 90 (84.1) | 23 (92) | |
| Other | 7(6.5) | 1 (4) | |
| Total transfusion within 24 hours (mean, SD) | |||
| PRBC (units) | 4.3±5.1 | 10.2±13.3 | 0.0004 |
| FFP (units) | 1.9±3.1 | 5.2±8.9 | 0.020 |
| Total crystalloid (ml) within 24 hours (mean, SD) | 8768±4200 | 11597±5413 | 0.020 |
| Worst base deficit within 6 hours (meq/L, mean, SD) | −5.5±6.4 | −9.2±7.5 | 0.005 |
| Highest lactate within 6 hours (mmol/L, mean, SD) | 5.0±5.4 | 5.4±2.7 | 0.17 |
| Lowest ED SBP (mmHg, mean, SD) | 90±27 | 71±28 | 0.010 |
| Initial ED SBP (mm Hg, mean, SD) | 119±35 | 111±25 | 0.11 |
| ER systolic <90 mmHg (n, %) | 19 (17.8) | 3(12) | 0.77 |
Early deaths are excluded (n=3).
Abbreviations: SD, standard deviation; APACHE, acute physiology and chronic health evaluation score; ISS, injury severity score; PRBC, packed red blood cells; FFP, fresh frozen plasma; SBP, systolic blood pressure; ED, emergency department.
Clinical Outcomes of Trauma Patients with Chronic Critical Illness
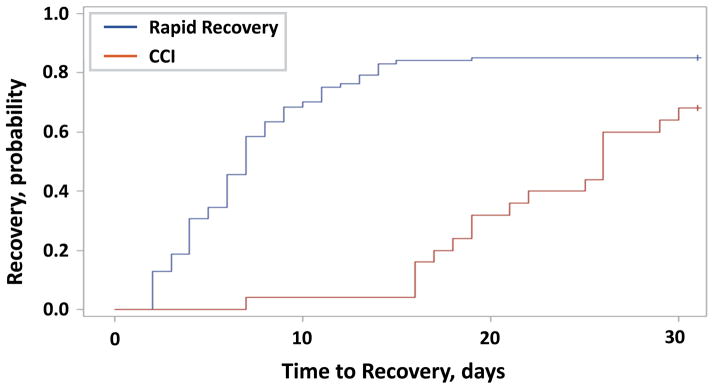
Patients who developed CCI had a higher frequency and greater severity of MOF as measured by the Denver MOF score (Table 2). While there was no significant difference between groups in the rate of mechanical ventilation, CCI patients had significantly fewer ventilator and ICU-free days (Table 2). The trajectory of organ dysfunction recovery between CCI and recovery groups was markedly differed (Figure 1).
Table 2. CCI and rapid recovery outcomes, complications and disposition*.
Cohort in-patient outcomes and complications and final disposition at discharge from index hospitalization. Early deaths are excluded
| Outcomes | Rapid recovery (n=107) 79.3% |
CCI (n=25) 18.5% |
p-value |
|---|---|---|---|
|
| |||
| Mechanically ventilated (n, %) | 89 (83.2) | 24 (96) | 0.24 |
| Ventilator-free days (28-day) | 24.3±3.8 | 9.6±7.9 | <0.0001 |
| MOF (n, %) | 7 (6.5) | 11 (44) | <0.0001 |
| Max. Denver MOF score (mean, SD) | 0.8±1.1 | 3.5±2.4 | <0.0001 |
| Time to recovery† (days, mean, SD) | 9.6±9.3 | 23.6±6.1 | <0.0001 |
| Noninfectious complications (n, %) | 21 (19.6) | 15 (60) | 0.0001 |
| Infectious complications (n, %) | 37 (34.6) | 21 (84) | <0.000 1 |
| Infection source (n, %) | |||
| Pneumonia | 17 (15.9) | 12 (48) | 0.0012 |
| Surgical site infections | 9 (8.4) | 8 (32) | 0.0006 |
| Pseudomembranous colitis | 10 (9.4) | 4 (16) | 0.30 |
| UTI | 7 (6.5) | 4 (16) | 0.22 |
| Blood stream infection | 1 (0.9) | 2 (8) | 0.09 |
| Empyema | 0 (0) | 1 (4) | 0.19 |
| Other | 1 (0.9) | 4 (16) | |
| Time to 1st nosocomial infection (days, mean, SD) | 6.9±5.6 | 5.9±2.9 | 0.94 |
| # of nosocomial infections per patient (mean, SD) | 0.36±0.59 | 1.20±1.44 | <0.0001 |
| Number of nosocomial infections per patient (n, %) | 0.0002 | ||
| 0 | 75 (70.1) | 8 (32) | |
| 1 | 26 (24.3) | 9 (36) | |
| ≥2 | 6 (5.6) | 8 (32) | |
| Length of stay (mean, SD) | |||
| Hospital days | 21.1±28.0 | 41.2±27.8 | <0.0001 |
| ICU days | 7.5±4.9 | 26.5±10.2 | <0.0001 |
| ICU-free days (28-day) | 20.5±4.9 | 3.4±3.9 | <0.0001 |
| 28 day-mortality (n, %) | 0 (0) | 3 (12) | 0.01 |
| Discharge disposition (n, %) | 0.0011 | ||
| ”Good” Disposition | 71 (66.4) | 11 (44) | 0.0078 |
| Inpatient rehabilitation facility | 19 (17.8) | 5 (20) | |
| Home with services | 16 (15) | 3 (12) | |
| Home | 36 (33.6) | 3 (12) | |
| ”Poor” Disposition | 36 (33.6) | 14 (56) | 0.0078 |
| SNF | 34 (31.8) | 7 (28) | |
| LTAC | 2 (1.9) | 3 (12) | |
| Death (in-hospital) | 0 (0) | 4 (16) | |
Early deaths are excluded (n=3).
Abbreviations: SD, standard deviation; MOF, multiple organ failure, Denver MOF score ≥3; UTI, urinary tract infection; SNF, skilled nursing facility; LTAC, long-term acute care facility.
Time to recovery is defined as time to organ dysfunction recovery, in days after injury, see Table S2.
Figure 1. Organ dysfunction recovery after severe trauma.
Time to resolution of organ dysfunction after traumatic injury in days as defined by organ time to recovery (TTR) (See Table S2). Data is censored for death. Log-rank test, p<0.0001.
Patients who developed CCI also had a greater number of infectious and non-infectious complications (Table 2). There was over twice the rate of infectious complications (84% vs 35%; p<0.0001) in those with CCI as compared to the rapid recovery group. The most common sources of infection were pneumonia, empyema, surgical site infections, Clostridium difficile colitis and urinary tract infection. Of these, pneumonia and surgical site infections occurred with significantly greater frequency in CCI patients (Table 2). To examine whether early infectious complications after injury were associated with the subsequent development of CCI, we examined the frequency of infections during the first week of hospitalization. For patients who remained hospitalized for greater than seven days (n=120, 89%), infections during the first seven days of hospitalization were markedly more frequent in patients who developed CCI (64% vs 28%, p=0.0019).
Finally, there were significant differences in the quality of discharge disposition when comparing those with CCI to those that exhibited recovery. Patients with CCI had significantly higher rates of ‘poor’ discharge disposition (skilled nursing facility [SNF], long-term acute care facility [LTAC] or inpatient death, Table 2). The 28-day all-cause mortality rate for CCI patients was 12%, while there were no deaths in the rapid recovery group (Table 2).
Clinical Prediction Models for CCI
Two multivariate clinical risk factor models were developed to facilitate the prediction of CCI based on available data at 24 and 72-hours. Age ≥55-years, severe hypotension (SBP ≤70-mmHg), severity of multiple organ dysfunction as measured by Denver MOF score, and 24-hour transfusion totals ≥5-units of PRBCs, were strong independent predictors of CCI when measured at 24-hours after injury (Table 3). The addition of ongoing organ dysfunction assessment at 72-hours improved the predictive ability of the model (Table 3).
Table 3. Multivariate clinical prediction models for CCI.
Multivariate clinical prediction models revealed that age ≥ 55 years, systolic hypotension ≤ 70 mmHg, transfusion ≥ 5 units PRBC within 24-hours, and Denver MOF score at 72-hours as independent predictors of CCI.
| Model | O.R. | 95% C.I. |
|---|---|---|
|
| ||
| Baseline (24-hour) model† | ||
| Age ≥55 | 3.7 | (1.3, 10.9) |
| Lowest admission systolic blood pressure ≤ 70 mmHg | 4.0 | (1.3, 11.6) |
| PRBC administrated within 24 hours ≥ 5 unit | 5.3 | (1.7, 16.5) |
| Denver MOF score at 24 hours | 2.9 | (1.5, 5.5) |
| Post resuscitation (72-hour) model†† | ||
| Age ≥55 | 3.5 | (1.2, 10.4) |
| Lowest admission systolic blood pressure ≤ 70 mmHg | 3.0 | (1.0, 9.4) |
| PRBC administered within 24 hours ≥ 5 units | 3.2 | (1.0, 9.7) |
| Denver MOF score at 72 hours | 3.0 | (1.7, 5.2) |
Abbreviations: O.R. = odds ratio; C.I. = confidence interval; PRBC = Packed red blood cells (unit; 300cc) transfusion administered within the fırst 24 hours; MOF, multiple organ failure.
Area under receiver operator curve = 0.84 (0.75, 0.93)
Area under receiver operator curve = 0.87 (0.79, 0.95)
Outpatient Function and Mortality
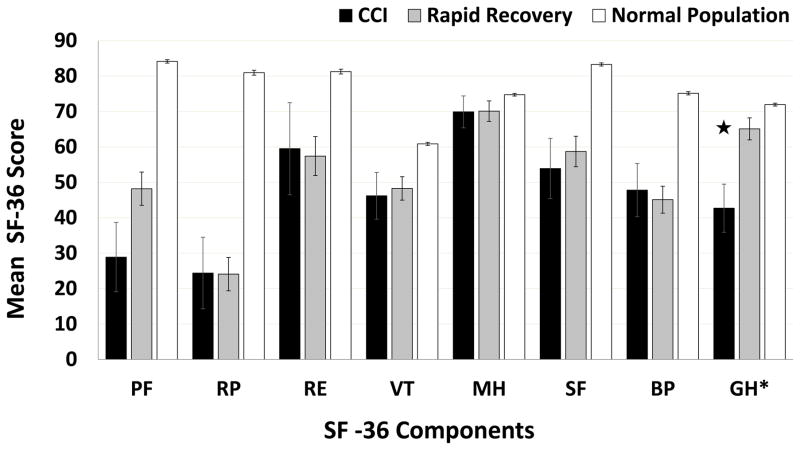
Overall loss to outpatient follow-up was 38%. After cross-check via SSDI and Washington State Death Registry information, overall 4-month mortality was determined to be 6.1%, with significant differences between CCI and rapid recovery cohorts (16% vs 1.9%; p=0.01). After inverse probability weighting to correct for loss to follow-up, mean scores for eight domains of the SF-36 assessment are shown for CCI and rapid recovery groups, with reference comparison to those for a normal population (Figure 2)(22). At four months, CCI patients had significantly lower mean general health score (42.7 vs. 65.1, p=0.004) compared to rapid recovery patients. Energy/fatigue, emotional well-being, social functioning, physical functioning score, pain, and role limitations due to physical health or emotional problems were not statistically different between groups.
Figure 2. Summary of SF-36 scores at 4-month follow-up.
SF-36 component domains: PF, physical function; RP, role limitations due to physical health; RE, role limitations due to emotional problems; VT, energy/fatigue; MH, emotional well-being; SF, social functioning domain; BP, pain; GH, general health; CCI, chronic critical illness. Error bars represent standard error of the mean (SEM). ★, p<0.05 comparing CCI and rapid recovery groups.
DISCUSSION
In this prospective, observational study, we have shown that CCI is currently a common and highly morbid clinical trajectory in patients who initially survive severe trauma. Nearly one-fifth (19%) of patients who survived greater than 48-hours after severe trauma developed CCI. These patients had significantly higher rates of in-hospital complications, infections and had prolonged resource utilization. Additionally, 56% of those developing CCI either died prior to discharge, or had a ‘poor’ discharge disposition (SNF or LTAC), known to be associated with poor long-term outcomes (24).
The clinical phenotype of CCI has been described under a variety of terms including the “neuropathy of critical illness”, “myopathy of critical illness”, “ICU acquired weakness” and “post intensive care syndrome” (25–28). These reports have largely originated from medical ICUs describing individuals with a wide set of admission diagnoses, most commonly acute exacerbations of chronic diseases, who require prolonged mechanical ventilation and are discharged to long-term care facilities. In trauma and surgical ICUs, there is an emerging population of patients who are now surviving severe traumatic insults (and whose outcomes are not primarily driven by severe TBI), who progress into the clinical trajectory of CCI. Because of improvements in trauma systems, hemorrhage control, hemostatic resuscitation and organ support modalities, fewer severely injured patients are dying from refractory hemorrhagic shock or progressing to fulminant early MOF deaths. However, many of these survivors develop persistent, but manageable, low-grade organ dysfunction as a result of a dysfunctional, overly robust innate immune response (19). Evidence of this changing epidemiology of post-injury MOF was first noted in the early 2000’s (29). Cuschieri et al. later reported progressive declines in in-hospital mortality while identifying that 25% of their study patients remained in the ICU for >14-days with prolonged dysfunction of least one organ (3, 20, 21). These patients had higher in-patient mortality, a higher rate of complications, were less likely to be discharged home, and failed to restore immunologic homeostasis at the transcriptomic level when compared to those who recovered (3, 20, 21, 30).
In this study, we also showed that the in-hospital mortality rate for severely injured trauma patients surviving the first 48-hours after injury has declined to approximately 5%, as compared to 11% for the similarly designed Trauma Glue Grant cohort. The present study cannot resolve whether the further reductions in mortality were due to additional improvements in compliance, or refinement of evidenced-based clinical protocols. However, it is important to note the difference in exclusion criteria in this study, i.e. patients not expected to survive more than 48-hours were excluded (versus 24-hours in the Trauma Glue Grant). It is likely that lower mortality of the current cohort is due in part to exclusion of acute hemorrhagic deaths occurring in the 24 to 48-hour period.
Many conditions can lead to prolonged utilization of intensive care resources; thus, understanding the underlying pathophysiology of CCI is essential. We have proposed that many surgical ICU patients suffering from CCI experience recurrent inflammatory insults and exhibit a newly-described phenotype called the persistent inflammation, immunosuppression, and catabolism syndrome (PICS)(31). PICS provides a framework of mechanisms to explain the underlying pathophysiology for CCI. The fundamental theme of this hypothesis is that a dysregulated immune response, likely associated with expansion of myeloid-derived suppressor cells, dysfunctional terminal innate effector cells (dendritic cells in particular), persistent inflammation with concomitant suppression of the adaptive immune response and ongoing protein catabolism are predominant in these patients and lead to poor-long term outcomes (6, 31–33).
The fact that CCI occurred more frequently in elderly subjects and individuals with greater initial physiological disturbances (APACHE II), despite a similar injury severity (ISS), argues that both age (or more likely physiologic frailty; not directly measured in this study) and the magnitude of the early dysfunctional innate immune response to injury contribute to complicated clinical outcomes. Thus, it is not simply the severity of injury and shock, but also the magnitude of aberrancy of the subsequent inflammatory response to injury, and the inability of the aged to recover from these changes that determine whether or not they enter a trajectory of CCI.
An interesting finding in this study is that the frequency of infections in the first seven days after trauma, as well as the frequency of non-infectious complications were significantly greater in CCI patients than in those that did not develop CCI. Particularly, patients that developed CCI had more than twice the frequency of early infections (64% vs 28%, p=0.0019; within seven days of admission) than those with a rapid recovery. Although a causal relationship between the two events cannot be inferred, this is consistent with the hypothesis that secondary infections or complications function as recurrent inflammatory insults contributing to the ongoing dysregulated immune response and persistent organ dysfunction.
We acknowledge several limitations of this study. First, sample sizes are modest, and larger studies will be required to replicate and confirm these findings across a broad range of trauma centers. The results presented here are from two high-volume Level 1-trauma centers in the United States that have had standardized clinical care protocols implemented for over a decade. However, we believe our results show that this study population is consistent with other published series from a wide range of Level 1 and Level 2-trauma centers in the United States describing severely injured blunt trauma patient in hemorrhagic shock (3). Additionally, long-term outcomes described here must be considered preliminary in nature because of overall loss to follow-up rate of 38%. While we acknowledge these limitations, this study demonstrates prospective outpatient follow-up rates superior to almost all previously published series in publication in this challenging cohort of patients, which often exceed 50%(34, 35). Finally, our definition and epidemiologic description of CCI will need further validation in other critically ill populations (i.e., sepsis, pancreatitis, etc) to ensure its applicability and utility across broad and heterogeneous patient populations.
CONCLUSIONS
The current findings provide a benchmark of incidence and outcomes of CCI in severely injured, blunt trauma patients. Although our data suggests that in-hospital mortality continues to decline, CCI develops in a significant proportion of trauma patients who initially survive severe injury and hemorrhagic shock. Additionally, the incidence of infectious complications appears to be associated with the development of CCI. The risk of CCI significantly increases with age, and with the progressive aging of our population, the incidence of CCI will likely also increase. Finally, our results also suggest that the development of CCI has lasting consequences on physical function, chronic morbidity and post-discharge mortality. Future investigations will need to focus on the underlying mechanism(s) that drive the development and persistence of CCI, which we believe to be the persistent inflammation, immunosuppression, and catabolism syndrome (30–32).
Supplementary Material
Expanded materials and methods section.
Numerous definitions have been developed over the past two decades to define patients with prolonged hospitalizations, high resource utilization, and ongoing organ dysfunction.
Parameters used to define Time to Recovery (TTR) as defined by Cuschieri et al [3].
Acknowledgments
Supported in part by grants: R01 GM-104481 and R01 GM-040586 (LLM), R01 GM-113945 (PAE), and P50 GM-111152 (FAM, LLM, SCB, PAE) awarded by the National Institute of General Medical Sciences (NIGMS), and by a postgraduate training grant T32 GM-008721 (JCM, TJL, JAS) in burns, trauma, and perioperative injury by the NIGMS. The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, and the conclusions put forth do not necessarily represent the views of the N.I.H., U.S.P.H.S. The authors declare no competing interests.
The authors would like to acknowledge the invaluable contributions and efforts of Ruth Davis, Dina C. Nacionales, Ricardo F. Ungaro, Patrick T. Verdugo, and Tyler L. Murphy to the execution of this study.
Footnotes
Copyright form disclosure: Drs. Mira, Cuschieri, Ozrazgat-Baslanti, Wang, Loftus, Stortz, 46 Raymond, Lanz, Hennessy, Brumback, Efron, Moore, Maier, Moldawer, and Brakenridge 47 received support for article research from the National Institutes of Health (NIH). Dr. Ozrazgat-48 Baslanti’s institution received funding from National Institute of General Medical Sciences 49 (NIGMS). Mr. Wang’s institution received funding from the NIH. Dr. Loftus’s institution received 50 funding from the NIH. Dr. Raymond’s institution received support from the NIGMS. Ms. Lanz’s 51 institution received funding from the NIH. Ms. Hennessy’s institution received funding from the 52 NIH. Dr. Brumback’s institution received funding from the NIH, and she received funding from 53 Biogen. Dr. Moore’s institution received funding from the NIH. Dr. Maier’s institution received 54 funding from the NIH/NIGMS. Dr. Moldawer’s institution received funding from the NIGMS. The 55 remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.DiMaggio C, Ayoung-Chee P, Shinseki M, et al. Traumatic injury in the United States: In-patient epidemiology 2000–2011. Injury. 2016 doi: 10.1016/j.injury.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochanek KD, Murphy SL, Xu J, et al. Mortality in the United States, 2013. NCHS Data Brief. 2014;(178):1–8. [PubMed] [Google Scholar]
- 3.Cuschieri J, Johnson JL, Sperry J, et al. Benchmarking outcomes in the critically injured trauma patient and the effect of implementing standard operating procedures. Ann Surg. 2012;255(5):993–999. doi: 10.1097/SLA.0b013e31824f1ebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamas D. Chronic critical illness. N Engl J Med. 2014;370(2):175–177. doi: 10.1056/NEJMms1310675. [DOI] [PubMed] [Google Scholar]
- 5.Kahn JM, Le T, Angus DC, et al. The epidemiology of chronic critical illness in the United States*. Crit Care Med. 2015;43(2):282–287. doi: 10.1097/CCM.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox CE. Persistent systemic inflammation in chronic critical illness. Respiratory care. 2012;57(6):859–864. doi: 10.4187/respcare.01719. discussion 864–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly BJ, Douglas SL, Kelley CG, et al. Trial of a disease management program to reduce hospital readmissions of the chronically critically ill. Chest. 2005;128(2):507–517. doi: 10.1378/chest.128.2.507. [DOI] [PubMed] [Google Scholar]
- 8.Nasraway SA, Button GJ, Rand WM, et al. Survivors of catastrophic illness: outcome after direct transfer from intensive care to extended care facilities. Crit Care Med. 2000;28(1):19–25. doi: 10.1097/00003246-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Carson SS, Cox CE, Wallenstein S, et al. Effect of Palliative Care-Led Meetings for Families of Patients With Chronic Critical Illness: A Randomized Clinical Trial. JAMA. 2016;316(1):51–62. doi: 10.1001/jama.2016.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandilov AMIM, Morley M, Coomer NM, Dalton K, Gage B, Superina C, Kennell D. Chronically Critically Ill Population Payment Recommendations (CCIP-PR) Reasearch Triangle Institute, NC; 2014. Mar, [Google Scholar]
- 11.Van den Berghe GH. Acute and prolonged critical illness are two distinct neuroendocrine paradigms. Verh K Acad Geneeskd Belg. 1998;60(6):487–518. discussion 518–420. [PubMed] [Google Scholar]
- 12.Iwashyna TJ, Hodgson CL, Pilcher D, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4(7):566–573. doi: 10.1016/S2213-2600(16)30098-4. [DOI] [PubMed] [Google Scholar]
- 13.Kahn JM, Werner RM, David G, et al. Effectiveness of long-term acute care hospitalization in elderly patients with chronic critical illness. Med Care. 2013;51(1):4–10. doi: 10.1097/MLR.0b013e31826528a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loss SH, Marchese CB, Boniatti MM, et al. Prediction of chronic critical illness in a general intensive care unit. Rev Assoc Med Bras (1992) 2013;59(3):241–247. doi: 10.1016/j.ramb.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 15.MacIntyre NR, Epstein SK, Carson S, et al. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–3954. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 16.Carson SS, Bach PB. The epidemiology and costs of chronic critical illness. Crit Care Clin. 2002;18(3):461–476. doi: 10.1016/s0749-0704(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JE, Meier DE, Litke A, et al. The symptom burden of chronic critical illness. Crit Care Med. 2004;32(7):1527–1534. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 18.Nelson JE, Cox CE, Hope AA, et al. Chronic critical illness. Am J Respir Crit Care Med. 2010;182(4):446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tompkins RG. Genomics of injury: The Glue Grant experience. J Trauma Acute Care Surg. 2015;78(4):671–686. doi: 10.1097/TA.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuenca AG, Gentile LF, Lopez MC, et al. Development of a genomic metric that can be rapidly used to predict clinical outcome in severely injured trauma patients. Crit Care Med. 2013;41(5):1175–1185. doi: 10.1097/CCM.0b013e318277131c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castellanos-Ortega A, Suberviola B, Garcia-Astudillo LA, et al. Impact of the Surviving Sepsis Campaign protocols on hospital length of stay and mortality in septic shock patients: results of a three-year follow-up quasi-experimental study. Crit Care Med. 2010;38(4):1036–1043. doi: 10.1097/CCM.0b013e3181d455b6. [DOI] [PubMed] [Google Scholar]
- 23.Lippman SA, Shade SB, Hubbard AE. Inverse probability weighting in sexually transmitted infection/human immunodeficiency virus prevention research: methods for evaluating social and community interventions. Sex Transm Dis. 2010;37(8):512–518. doi: 10.1097/OLQ.0b013e3181d73feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson GH, Hamlat CA, Rivara FP, et al. Long-term survival of adult trauma patients. JAMA. 2011;305(10):1001–1007. doi: 10.1001/jama.2011.259. [DOI] [PubMed] [Google Scholar]
- 25.Latronico N, Peli E, Botteri M. Critical illness myopathy and neuropathy. Curr Opin Crit Care. 2005;11(2):126–132. doi: 10.1097/01.ccx.0000155357.24360.89. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich O, Reid MB, Van den Berghe G, et al. The Sick and the Weak: Neuropathies/Myopathies in the Critically Ill. Physiol Rev. 2015;95(3):1025–1109. doi: 10.1152/physrev.00028.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–1635. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 28.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 29.Ciesla DJ, Moore EE, Johnson JL, et al. A 12-year prospective study of postinjury multiple organ failure: has anything changed? Arch Surg. 2005;140(5):432–438. doi: 10.1001/archsurg.140.5.432. discussion 438–440. [DOI] [PubMed] [Google Scholar]
- 30.Vanzant EL, Lopez CM, Ozrazgat-Baslanti T, et al. Persistent inflammation, immunosuppression, and catabolism syndrome after severe blunt trauma. J Trauma Acute Care Surg. 2014;76(1):21–29. doi: 10.1097/TA.0b013e3182ab1ab5. discussion 29–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mira JC, Gentile LF, Mathias BJ, et al. Sepsis Pathophysiology, Chronic Critical Illness, and Persistent Inflammation-Immunosuppression and Catabolism Syndrome. Crit Care Med. 2017;45(2):253–262. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentile LF, Cuenca AG, Efron PA, et al. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72(6):1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchioni A, Fantini R, Antenora F, et al. Chronic critical illness: the price of survival. European journal of clinical investigation. 2015;45(12):1341–1349. doi: 10.1111/eci.12547. [DOI] [PubMed] [Google Scholar]
- 34.Laser A, Bruns BR, Kufera JA, et al. Long-term follow-up of blunt cerebrovascular injuries: Does time heal all wounds? J Trauma Acute Care Surg. 2016;81(6):1063–1069. doi: 10.1097/TA.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 35.Kaske S, Lefering R, Trentzsch H, et al. Quality of life two years after severe trauma: a single-centre evaluation. Injury. 2014;45(Suppl 3):S100–105. doi: 10.1016/j.injury.2014.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded materials and methods section.
Numerous definitions have been developed over the past two decades to define patients with prolonged hospitalizations, high resource utilization, and ongoing organ dysfunction.
Parameters used to define Time to Recovery (TTR) as defined by Cuschieri et al [3].