Abstract
Voltage-gated sodium channels initiate action potentials in brain neurons. In the 1970’s, much was known about the function of sodium channels from measurements of ionic currents using the voltage clamp method, but there was no information about the sodium channel molecules themselves. As a postdoctoral fellow and staff scientist at the National Institutes of Health, I developed neurotoxins as molecular probes of sodium channels in cultured neuroblastoma cells. During those years, Bruce Ransom and I crossed paths as members of the laboratories of Marshall Nirenberg and Philip Nelson and shared insights about sodium channels in neuroblastoma cells from my work and electrical excitability and synaptic transmission in cultured spinal cord neurons from Bruce’s pioneering electrophysiological studies. When I established my laboratory at the University of Washington in 1977, my colleagues and I used those neurotoxins to identify the protein subunits of sodium channels, purify them, and reconstitute their ion conductance activity in pure form. Subsequent studies identified the molecular basis for the main functions of sodium channels—voltage-dependent activation, rapid and selective ion conductance, and fast inactivation. Bruce Ransom and I re-connected in the 1990’s, as ski buddies at the Winter Conference on Brain Research and as faculty colleagues at the University of Washington when Bruce became our founding Chair of Neurology and provided visionary leadership of that department. In the past decade my work on sodium channels has evolved into structural biology. Molecular modeling and X-ray crystallographic studies have given new views of sodium channel function at atomic resolution. Sodium channels are also the molecular targets for genetic diseases, including Dravet Syndrome, an intractable pediatric epilepsy disorder with major co-morbidities of cognitive deficit, autistic-like behaviors, and premature death that is caused by loss-of-function mutations in the brain sodium channel NaV1.1. Our work on a mouse genetic model of this disease has shown that its multi-faceted pathophysiology and co-morbidities derive from selective loss of electrical excitability and action potential firing in GABAergic inhibitory neurons, which disinhibits neural circuits throughout the brain and leads directly to the epilepsy, premature death and complex co-morbidities of this disease. It has been rewarding for me to use our developing knowledge of sodium channels to help understand the pathophysiology and to suggest potential therapeutic approaches for this devastating childhood disease.
Introduction
It is a great pleasure for me to write this summary chapter on our work on sodium channels in recognition of the outstanding contributions of Professor Bruce Ransom to neuroscience and neurology. Bruce and I were postdoctoral fellows at the same time at the National Institutes of Health in the 1970’s—Bruce was a Clinical Associate in the laboratory of Dr. Philip Nelson in the National Institute of Child Health & Human Development and I was a postdoctoral research fellow and later a staff scientist in the laboratory of Dr. Marshall Nirenberg in the National Heart, Lung, & Blood Institute. Although they were administratively in different Institutes, these two laboratories were relatively close to each other in Building 36, along with many other neuroscience programs at NIH, and there were active collaborations between the Nelson and Nirenberg laboratories. At that time, Bruce was carrying out some of the first, and most extensive, studies of the electrophysiological properties of cultured neurons from brain and spinal cord [1–5]. My research project focused on neurotoxins that acted on voltage-gated sodium channels. My training as a Ph. D. student had been in membrane biochemistry, and I was excited about the possibility of using specific neurotoxins as biochemical probes to identify the protein components of sodium channels and begin to study their biochemical properties, using the cultured neuroblastoma cell lines that had been developed in the Nirenberg Lab [6]. After these first interactions at the National Institutes of Health, Bruce and I took separate paths. I moved directly to the University of Washington and established an independent research laboratory in the Department of Pharmacology in 1977. Bruce moved to Stanford University for further clinical training in Neurology and then to Yale University to begin his independent research program in the Department of Neurology there. We often found time to ski together and discuss research at the Winter Conference on Brain Research. Some years later, our paths merged again when I served as a member of the Search Committee for the Chair of Neurology at the University of Washington, under the leadership of Dr. Wayne Crill, Professor and Chair of our Department of Physiology & Biophysics and Professor of Neurology. We were fortunate to recruit Bruce Ransom to become the founding Chair of Neurology at the University of Washington. It has been a great pleasure to interact closely with Bruce again, in research collaborations on sodium channels in glial cells [7–10], in joint academic initiatives in neuroscience and molecular medicine, and in skiing adventures at many locations, during our overlapping tenures as Chair of Pharmacology and Chair of Neurology. Bruce’s visionary leadership has made our Department of Neurology, which he founded, one of the very best worldwide.
Neurotoxins and Sodium Channels
Voltage-gated sodium channels initiate action potentials in neurons and other excitable cells [11], and their dysfunction causes inherited epilepsy, chronic pain, and other diseases of hyperexcitability [12, 13]. The physiological role of sodium channels in action potential generation was well established by the classic work of Hodgkin and Huxley in 1952 [14]. Many of the basic functional properties of sodium channels were analyzed in extensive voltage clamp studies of nerve axons [11]. However, in the 1970’s, there was no experimental information on the protein molecules that formed sodium channels or on how they worked at the molecular or structural level. This brief review touches on some of the highlights of our research on sodium channels that has led from the early experiments as a postdoctoral fellow at the NIH to an increasingly complete understanding of sodium channel structure, function, pharmacology, and roles in disease.
My work as a postdoctoral fellow at NIH and as a new faculty member at the University of Washington showed that different classes of neurotoxins act on distinct receptor sites on sodium channels and serve as activators, inhibitors, or allosteric modulators of their voltage-dependent gating processes. We developed these potent neurotoxins as molecular probes for ligand binding and photoaffinity labeling studies and also as tools to activate sodium channels in biochemical experiments and study their functional properties in nontraditional cell and membrane preparations. These studies, and related work in other laboratories, were reviewed comprehensively in an article in the Annual Review of Pharmacology & Toxicology in 1980 [15].
The Sodium Channel Protein
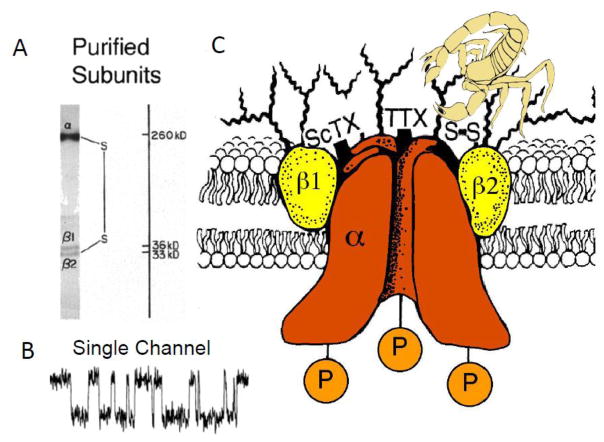
Based on these early studies of toxin action, my colleagues and I were able to use specific neurotoxins in complementary ways to identify, purify, and functionally characterize the sodium channel protein. As a first step, we identified the large α subunits of 260 kDa and smaller β subunits of 30–40 kDa by photoaffinity labeling with a derivative of the polypeptide α-toxin from the North African scorpion Leiurus quinquestriatus [16]. This scorpion toxin was one of the first gating modifier toxins to be characterized, and we assumed that it would covalently label the voltage-dependent gating apparatus of sodium channels. After much work to design experimental conditions to solubilize the sodium channel while retaining its high-affinity binding of the pore-blocker saxitoxin, we found that sodium channels purified from rat brain are composed of an α subunit with a noncovalently associated β1 subunit and a disulfide-linked β2 subunit (Fig. 1A; [17–19]). Because the purified protein contained receptor sites for the gating modifier scorpion toxins and the pore-blockers tetrodotoxin and saxitoxin, we assumed that it comprised a complete sodium channel with functional gating apparatus and pore. Importantly, we showed that this purified protein complex was sufficient to reconstitute voltage-gated sodium channel function with the correct pharmacology in single-walled phospholipid vesicles; moreover, activation of the purified and reconstituted sodium channel protein with the lipid-soluble neurotoxin batrachotoxin revealed single purified channels with the expected single-channel conductance and voltage sensitivity after insertion into planar phospholipid bilayers (Fig. 1B; [20–22]). These studies led to a biochemical model of the sodium channel protein as illustrated in Fig. 1C.
Figure 1. Sodium channels as originally purified from mammalian brain.
A. The α and β1 subunits of brain sodium channels analyzed by SDS-PAGE. B. Single channel currents from purified and reconstituted brain sodium channels. Sodium channels purified from rat brain were first reconstituted into phospholipid vesicles under conditions that yielded an average of one sodium channel molecule per vesicle. These vesicles fused with pre-formed planar phospholipid bilayers and sodium currents were recorded. Single channel currents with the voltage dependence and conductance of sodium channels in situ were recorded. C. A model of the purified rat brain sodium channel derived from biochemical experiments. This model depicts the state of the field in 1986 when it was drawn [32]. Since that time, cloning of sodium channel β subunits has revealed a family of four related genes, each of which encodes a single membrane-spanning protein with a large, glycosylated extracellular N-terminal domain composed primarily of an immunoglobulin-like fold and a small intracellular C-terminal domain [26, 27, 29].
Primary Structures of Sodium Channel Subunits
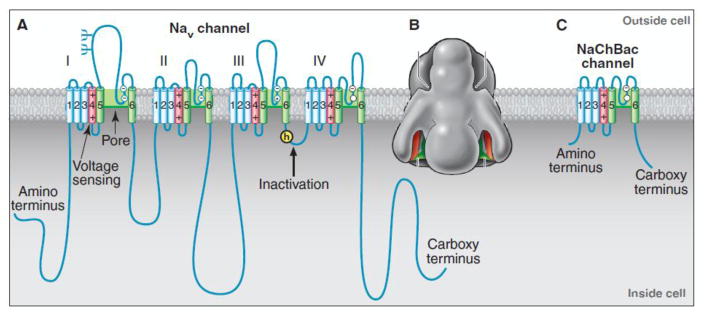
Cloning and sequencing cDNA encoding the α subunits of sodium channels established their primary structures and showed that mRNA encoding the α subunit is sufficient for expression of functional sodium channels [23–25]. Sodium channel α subunits are composed of approximately 2000 amino acid residues organized in four homologous domains, which each contain six transmembrane segments (Fig. 2A). Biochemical analyses and cDNA cloning showed that sodium channel β subunits are composed of an N-terminal extracellular immunoglobulin-like fold, a single transmembrane segment, and a short intracellular segment [26, 27]. These subunits are thought to form complexes composed of a single α subunit and one or two β subunits in neuronal membranes, and co-expression of β subunits modulates the kinetics and voltage dependence of sodium channel activation and inactivation [28, 29]. Almost two decades after purification and biochemical characterization of Na V channels, a low-resolution structural model of a sodium channel from electric eel electroplax based on cryo-electron microscopy revealed the size and shape of the sodium channel protein (Fig. 2B), which closely resembled models drawn from early biochemical studies of brain sodium channels (Fig. 1C).
Figure 2. Sodium channels in eukaryotes and prokaryotes.
A. The α subunit of NaV1.2 channels is illustrated as a transmembrane folding diagram in which cylinders represent transmembrane alpha helices and lines represent connecting amino acid sequences in proportion to their length. Structural components responsible for voltage sensing, pore formation, and fast inactivation are indicated. B. A low-resolution image of the sodium channel from electric eel electroplaque. C. A transmembrane folding diagram of the bacterial sodium channel NaChBac. Reprinted from [79].
Functional Modules of Sodium Channels
The three key steps in sodium channel function are voltage-dependent activation, rapid and selective ion conductance, and fast inactivation on the millisecond time scale. Voltage-dependent activation of sodium channels depends on movement of “electrically charged particles” across the cell membrane through the transmembrane electric field [14]. The transmembrane movement of these “gating charges” was detected as a small capacitative gating current in squid giant axon [30]. Based on the original amino acid sequence, Robert Guy and I independently proposed that the S4 transmembrane segments of sodium channels, which contain four conserved motifs of a positively charged amino acid residue flanked by two hydrophobic residues, carry the gating charges in the sliding helix or helical screw model of voltage sensing [31–34]. In this model, the S4 segment is in a transmembrane position in both resting and activated states, the gating charges are stabilized by forming ion pairs with neighboring negatively charged and hydrophilic residues, and their outward movement is catalyzed by exchange of ion pair partners [31–35]. Mutagenesis showed that the S4 arginines are indeed the gating charges [36]; their transmembrane position in resting and activated states was confirmed by scorpion toxin binding studies [37, 38]; outward movement was detected by chemical labeling experiments under voltage clamp control [39–41]; and exchange of ion pairs partners was demonstrated by disulfide locking of substituted cysteine residues [42–44]. These results on sodium channels, and much parallel work on voltage-gated potassium channels, led to a consensus that the sliding helix model is an accurate description of voltage-sensing [45].
Amino acid residues in the short segments between S5 and S6 (Fig. 2A) form the receptor site for the pore blocker tetrodotoxin [46, 47]. Analysis of site-directed mutations showed that a set of four residues (DEKA), each in an equivalent position in one of the four domains, control selectivity for sodium vs. calcium, consistent with the idea that they come together to form a key ion coordination site in the pore [48]. Moreover, substitutions in this signature DEKA motif cause progressive changes in Na/K selectivity. These results established the S5 and S6 segments and the conserved P loop between them as the pore-forming module.
Sodium channels in vertebrates open in response to depolarization and then inactivate within 1–2 ms [49]. This fast inactivation process is required for repetitive firing of action potentials in neural circuits and for control of excitability in nerve and muscle cells. Studies with site-directed antibodies showed that the short intracellular loop connecting homologous domains III and IV of the sodium channel α subunit mediates fast inactivation by folding into the intracellular mouth of the pore and blocking it (Fig. 2A; [50]). Severing the channel protein within this loop by expression of the sodium channel in two separate pieces greatly slowed inactivation [36]. The key amino acid motif IFM is required to maintain closure of the inactivation gate [51], and peptides containing this inactivation gate sequence motif can restore fast inactivation to mutant sodium channels [52]. Analysis of the structure of the inactivation gate by NMR showed that it contains a rigid alpha helix preceded by two loops of protein. This structure arrays the IFM motif and a neighboring Thr residue in position for interaction with the open pore and block of sodium conductance (Fig. 2A; [53]).
Ancestral Sodium Channels
Sodium channel α subunits are encoded by ten genes in mammals [54]. Surprisingly, the sodium channel family is ancient in evolution. The bacterial sodium channel NaChBac and many prokaryotic relatives are composed of homotetramers of a single subunit whose structure resembles one of the domains of a vertebrate sodium channel (Fig. 2C; [55, 56]). It is likely that these bacterial sodium channels are the evolutionary ancestors of the larger, four-domain sodium channels in eukaryotes.
Sodium Channel Structure at Atomic Resolution
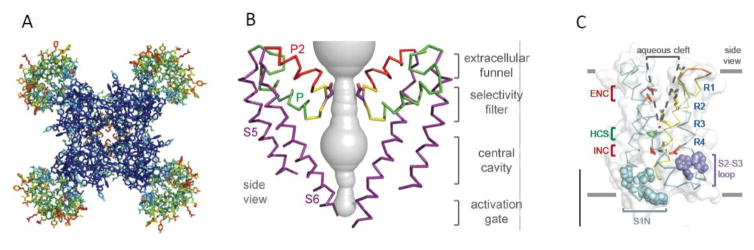
Sodium channel architecture was revealed at atomic resolution by determination of the crystal structure of the bacterial sodium channel NaVAb at high resolution (2.7Å) in 2011 (Fig. 3A; [57]). As viewed from the top, the central pore is surrounded by four pore-forming modules composed of S5 and S6 segments and the intervening P loop (Fig. 3A, blue). Four voltage-sensing modules composed of S1–S4 segments are symmetrically associated with the outer rim of the pore module (Fig. 3A, green and red). A view from the side of NavAb reveals a large external vestibule, a narrow ion selectivity filter, a large central cavity lined by the S6 segments, and an intracellular activation gate the S6 segments cross at the intracellular surface of the membrane (Fig. 3B; [57]). The ion selectivity filter has a high-field-strength site at its extracellular end, which is formed four negatively charged glutamate residues [57]. This site is crucial for ion selectivity in vertebrate sodium and calcium channels [48]. Considering its dimensions of approximately 4.6 Å square, Na+ with up to four planar waters of hydration could fit in this high-field-strength site. This outer site is followed on the intracellular side by two ion coordination sites formed by backbone carbonyls [57]. These two carbonyl sites are perfectly designed to bind Na+ with four planar waters of hydration but would be much too large to bind Na+ directly. Thus, Na+ is selected and conducted as a hydrated cation interacting with the pore through its inner shell of bound water molecules.
Figure 3. A. Structure of the NaVAb bacterial sodium channel at 2.7Å resolution.
A. Top view. Colors represent temperature factors such that increased mobility in the crystals coded in warmer colors. Blue, pore-forming module; green to red, voltage-sensing module. B. Ion permeation pathway. The pore-forming modules of two NaVAb subunits are shown surrounding the pore. S5, P helix, P2 helix, and S6 segments are labeled. Contour of the water-filled pore, gray. C. Three-dimensional structure of the voltage-sensing module of NavAb. Arginine gating charges, blue (R1–R4). Extracellular negative cluster (ENC), red; intracellular negative cluster (INC), red. Hydrophobic constriction site (HCS), green.
Drug Receptor Sites in Sodium Channels
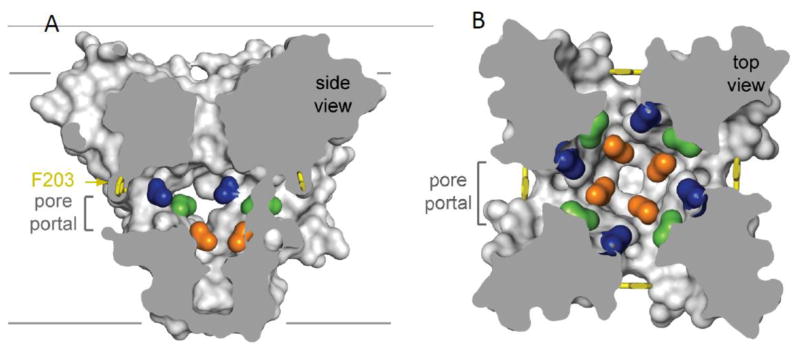
Sodium channels are blocked by drugs used clinically as local anesthetics, antiarrhythmics, and antiepileptics [58]. The receptor site for local anesthetics and related drugs is formed by amino acid residues in the S6 segments in domains I, III, and IV of mammalian sodium channels [59–63]. These drugs bind to a common receptor site in the pore and impede ion permeation. The amino acid residues that form the receptor sites for sodium channel blockers line the inner surface of the S6 segments and create a three-dimensional drug receptor site in NavAb, where drug binding would fully block the pore (Fig. 4A [28, 57, 59, 61]). Access to this receptor site by large or hydrophilic drugs would require opening of the intracellular activation gate, which is tightly closed. This tight closure of the activation gate provides a structural basis for use-dependent block of sodium channels by local anesthetics and related drugs [64], as they would bind much more rapidly when the channel is frequently opened. Remarkably, as predicted by the modulated receptor hypothesis [64], fenestrations lead from the lipid phase of the membrane sideways into the drug receptor site, providing an access pathway for binding of small hydrophobic drugs in the resting state (Fig. 4A, B, pore portals; [57]).
Figure 4. Drug receptor site in NaVAb.
A. Side view of NaVAb with amino acid residues analogous to the NaV1.2 drug receptor site colored. Pore portal denotes the fenestration leading to the membrane lipid phase in each of the four subunits of NaVAb. B. Top view of NaVAb colored as in A.
Structure of a Eukaryotic Sodium Channel
Recent studies with the cryo-electron microscopy method has greatly advances research on sodium channels by revealing the three-dimensional structure of a four-domain sodium channel for the first time [65]. The sodium channel from cockroach has the same fold of its core transmembrane segments as the bacterial sodium channels. The four voltage-sensing modules have slightly different structures, but all are in activated conformations. The backbone structure of the ion selectivity filter is the same as bacterial sodium channels, but the high field-strength site has a rectangular array of Asp-Glu-Ala-Lys rather than an array of four glutamate residues. The fast inactivation gate in the intracellular linker between domains III and IV is withdrawn from the intracellular mouth of the pore and is interacting with the C-terminal domain. The amino acid sequence characteristic of the inactivation particle in mammalian sodium channels (Ile-Phe-Met) is not conserved in the cockroach sodium channel, so it may not have a fast inactivation process. The folding patterns of the intracellular and extracellular loops connecting the transmembrane segments are revealed for the first time. This landmark structure opens the way to future structure-function studies of mammalian sodium channels guided by a eukaryotic sodium channel structure.
Pathophysiology of Sodium Channel Mutations
Because of its crucial function in excitable cells, a large number of genetic diseases are caused by mutations of sodium channels, including inherited forms of periodic paralysis, cardiac arrhythmia, epilepsy, and chronic pain [12, 13]. The genes most frequently associated with inherited forms of epilepsy encode sodium channels NaV1.1 and NaV1.2, which are highly expressed in the brain. Our research has focused on Dravet Syndrome (DS; also known as Severe Myoclonic Epilepsy of Infancy), which is a severe form of childhood epilepsy with frequent premature deaths and many co-morbidities. DS is primarily caused by loss of function due to stop codons, deletions, or inactivating single residue mutations in the SCN1A gene encoding NaV1.1 channels, demonstrating that haploinsufficiency of this sodium channel is pathogenic.
Effects of NaV1.1 Mutations that Cause Epilepsy in Dravet Syndrome
DS begins during the first year of life, with seizures associated with elevated body temperature due to fever or bathing, and progresses to prolonged, clustered, or continuous seizures that are drug-resistant [66, 67]. During the second to fifth years of life, patients develop co-morbidities including hyperactivity, psychomotor delay, ataxia, sleep disorder, autistic-like behaviors, and cognitive impairment. It was a surprise that haploinsufficiency of a NaV channel causes epilepsy, because reduced sodium current should lead to hypoexcitability rather than hyperexcitability. We generated a mouse genetic model of DS by targeted deletion of the Scn1a gene in mice [68, 69]. DS mice have spontaneous and thermally evoked seizures like DS patients [70], and they die prematurely after postnatal day 21 when they begin to have spontaneous seizures [68, 71]. We found that interneurons in the hippocampus of DS mice have a substantial defect in sodium currents and action potential firing, whereas excitatory pyramidal neurons are unaffected [68]. These results pointed to disinhibition of neural circuits as the probable cause of epilepsy and premature death in DS.
To definitively test the hypothesis that selective loss of Na+ channels in GABAergic inhibitory neurons is the causative change in DS, we inserted Lox P sites on both sides of an exon encoding a large part of domain IV in the Scn1a gene, and the Floxed mice were mated with a mouse strain expressing the Cre recombinase in GABAergic inhibitory neurons in the cerebral cortex and hippocampus under the Dlx1,2 promoter, which is selectively expressed in migrating inhibitory neuron precursors destined for these brain areas. Cre+ mice die prematurely and have spontaneous seizures that are as severe as Scn1a+/− mice [72], confirming that loss of NaV1.1 channels in forebrain inhibitory neurons is sufficient to cause epilepsy and premature death in DS.
The most devastating outcome of DS is sudden unexpected death in epilepsy (SUDEP). Up to 70% of DS mice die by the time of sexual maturity at 60 postnatal days [73]. SUDEP in this mouse model is always preceded by a severe tonic-clonic seizure and an associated period of severe bradycardia, which leads to ventricular fibrillation [73]. Bradycardia is caused by hyperactivity of the parasympathetic cholinergic outflow from the central nervous system to the heart during and immediately following the seizure, as it is reduced by the general muscarinic antagonist atropine and the peripherally restricted muscarinic antagonist N-methylscopolamine [73]. These results show that epilepsy and premature death in DS are caused directly by loss-of-function of NaV1.1 channels and resulting reduced excitability of GABAergic inhibitory neurons.
Reduced Excitability of GABAergic Interneurons Causes Co-morbidities in DS
Co-morbidities contribute substantially to the poor quality of life, burden of care, and premature deaths in DS [Dravet, www.ilae-epilepsy.org/ctf/dravet.html, 74, 75]. We have linked all of these co-morbidities to failure of firing of GABAergic interneurons (Table 1). DS mice have mild ataxia [76]. Sodium currents and firing rates of cerebellar Purkinje neurons are substantially reduced in DS mice, consistent with causing their ataxia [76]. Both children and mice with DS have a significant impairment of sleep and circadian rhythm [77]. Reduced excitability of the GABAergic neurons of the suprachiasmatic nucleus causes the circadian rhythm defect [78]. Failure of normal rebound firing of action potentials by the GABAergic neurons of the reticular nucleus of the thalamus reduces the frequency and amplitude of sleep spindles and non-REM sleep [79].
Table 1.
| Patient Phenotype | Symptoms in DS Mice | Physiological Correlates | Causal Evidence for Mechanism |
|---|---|---|---|
| Febrile Seizures | Thermally induced seizures | Failure of AP firing in forebrain GABAergic neurons | Observed in forebrain-specific KO of NaV1.1 |
| Epilepsy | Spontaneous seizures after P20 | Racine 5 tonic-clonic seizures | Observed in forebrain-specific KO of NaV1.1 |
| Premature Death | SUDEP after P21 | Excess parasympathetic outflow, bradycardia, and ventricular failure | Observed in forebrain-specific but not cardiac-specific KO |
| Ataxia | Abnormal foot placement in walking | Failure of AP firing in GABAergic cerebellar Purkinje neurons | |
| Circadian Rhythm | Long circadian cycle; weak light-induced phase shift; increased negative masking | Failure of AP firing in GABAergic neurons in the ventral SCN | Not observed in forebrain-specific KO mouse; reversed by clonazepam |
| Sleep Impairment | Reduced non-REM sleep, delta wave power, sleep spindles | Failure of rebound AP firing in GABAergic neurons in the RNT | Observed in forebrain-specific KO mouse |
| Cognitive Deficit | Failure of spatial learning and memory | Failure of AP firing by GABAergic neurons in hippocampus and cerebral cortex | Observed in forebrain-specific KO mouse; reversed by clonazepam |
| Autistic-like Behavior | Impaired social interaction; repetitive behaviors | Failure of AP firing by PV-expressing GABAergic neurons in hippocampus and cerebral cortex | Observed in forebrain-specific and PV-specific KO mouse strains; reversed by clonazepam |
| Hyperactivity | Increased distance explored in open field | Failure of AP firing by SST-expressing GABAergic neurons in hippocampus and cerebral cortex | Observed in forebrain-specific and SST-specific KO mouse strains; reversed by clonazepam |
AP, action potential; SCN, suprachiasmatic nucleus of the hypothalamus; RNT, reticular nucleus of the thalamus; REM, rapid-eye-movement.
DS mice have severe cognitive impairment, observed as a deficit in spatial learning and memory in the context-dependent fear-conditioning test and the Barnes circular maze test [80]. In addition, DS mice have striking autistic-like traits, including repetitive grooming and circling behaviors, as well as failure of social interaction [80]. These cognitive and behavioral deficits are also observed in DS mice in which the NaV1.1 channels have been selectively deleted in forebrain GABAergic interneurons [80]. Therefore, our results show that failure of firing of action potentials in forebrain GABAergic interneurons is responsible for these co-morbidities of DS in mice.
Pathogenic Roles of Major Interneuron Classes in DS
In the cerebral cortex, interneurons can be separated into three non-overlapping classes that express specific marker proteins: parvalbumin-expressing (PV), somatostatin-expressing (SST), and 5-HT3a receptor-expressing (5-HT3aR) [81]. We used the Cre-Lox method to delete NaV1.1 channels specifically in each of these interneuron classes [82]. We found that these interneurons are selectively involved in the different DS phenotypes (Table 1; [82]). Deletion in PV interneurons causes pro-epileptic effects and autistic-like behavior, but not other phenotypes [82]. Deletion in SST interneurons causes pro-epileptic effects and hyperactivity, but not other phenotypes [82]. Deletion in both PV and SST interneurons causes synergistic effects on the severity of epilepsy and on cognitive deficit [82]. Deletion in 5-HT3aR interneurons causes only autistic-like effects without epilepsy or cognitive deficit (Cheah et al., unpublished). These results with specific deletion in 5-HT3aR interneurons are important because they show that autistic-like behaviors are a result of the genetic mutation and do not arise as a consequence of epilepsy. Overall, our experiments with specific gene deletion methods show that the complex phenotypes of DS arise directly from disinhibition of multiple neural circuits by impairment of electrical excitability of GABAergic interneurons. These results are encouraging for therapy of this devastating disease, because they imply that enhancement of excitability of GABAergic interneurons by specific pharmacotherapy or local gene therapy has the possibility to rescue the multifaceted deficits of this disease. As a basic neuroscientist, it has been a pleasure to be a participant in these advancements in understanding the pathophysiology of this devastating disease and investigating next-generation therapies.
Conclusions and Perspectives
Sodium channels have been a constant theme of my research over more than forty years. In that time, we and the ion channel field as a whole have advanced from knowledge of ionic currents that generate action potentials toward a full understanding at the molecular, chemical, and atomic levels of the mechanisms underlying ion channel function and electrical signaling. It has been a special pleasure to have been part of the major advances of this field and to have had the scientific and academic interactions and the many personal connections with Bruce Ransom, one of the founders of our current understanding of cellular physiology and one of the leaders of academic neurology.
Acknowledgments
The work on sodium channels from my laboratory has depended on many outstanding colleagues—graduate students, postdoctoral fellows, and research technicians. Without their insight, hard work, dedication, and professionalism, we would not have made significant advances. Our work also has depended on the continuous support of research grants from the National Institute of Neurological Disorders & Stroke and the National Heart, Lung, & Blood Institute of the National Institutes of Health. Their generous support made this research on sodium channels possible, as well as most other advances in biomedical sciences in the U.S.
References
- 1.Ransom BR, Barker JL, Nelson PG. Two mechanisms for poststimulus hyperpolarisations in cultured mammalian neurones. Nature. 1975;256:424–425. doi: 10.1038/256424a0. [DOI] [PubMed] [Google Scholar]
- 2.Ransom BR, Bullock PN, Nelson PG. Mouse spinal cord in cell culture. III. Neuronal chemosensitivity and its relationship to synaptic activity. J Neurophysiol. 1977;40:1163–1177. doi: 10.1152/jn.1977.40.5.1163. [DOI] [PubMed] [Google Scholar]
- 3.Ransom BR, Christian CN, Bullock PN, Nelson PG. Mouse spinal cord in cell culture. II. Synaptic activity and circuit behavior. J Neurophysiol. 1977;40:1151–1162. doi: 10.1152/jn.1977.40.5.1151. [DOI] [PubMed] [Google Scholar]
- 4.Nelson PG, Ransom BR, Henkart M, Bullock PN. Mouse spinal cord in cell culture. IV. Modulation of inhibitory synaptic function. J Neurophysiol. 1977;40:1178–1187. doi: 10.1152/jn.1977.40.5.1178. [DOI] [PubMed] [Google Scholar]
- 5.Ransom BR, Neale E, Henkart M, Bullock PN, Nelson PG. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977;40:1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- 6.Catterall WA, Nirenberg M. Sodium uptake associated with activation of action potential ionophores of cultured neuroblastoma and muscle cells. Proc Natl Acad Sci U S A. 1973;70:3759–3763. doi: 10.1073/pnas.70.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black JA, Yokoyama S, Waxman SG, Oh Y, Zur KB, Sontheimer H, Higashida H, Ransom BR. Sodium channel mRNAs in cultured spinal cord astrocytes: in situ hybridization in identified cell types. Brain Res Mol Brain Res. 1994;23:235–245. doi: 10.1016/0169-328x(94)90230-5. [DOI] [PubMed] [Google Scholar]
- 8.Black JA, Westenbroek R, Minturn JE, Ransom BR, Catterall WA, Waxman SG. Isoform-specific expression of sodium channels in astrocytes in vitro: immunocytochemical observations. Glia. 1995;14:133–144. doi: 10.1002/glia.440140208. [DOI] [PubMed] [Google Scholar]
- 9.Brown AM, Westenbroek RE, Catterall WA, Ransom BR. Axonal L-Type Ca2+ Channels and Anoxic Injury in Rat CNS White Matter. J Neurophysiol. 2001;85:900–911. doi: 10.1152/jn.2001.85.2.900. [DOI] [PubMed] [Google Scholar]
- 10.Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, Malhotra JD, Jones D, Avery C, Gillespie PJ, 3rd, Kazen-Gillespie KA, Kazarinova-Noyes K, Shrager P, Saunders TL, Macdonald RL, Ransom BR, Scheuer T, Catterall WA, Isom LL. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel β2-subunits. Proc Natl Acad Sci U S A. 2002;99:17072–17077. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hille B. Ionic Channels of Excitable Membranes. 3. Sinauer Associates Inc; Sunderland, MA: 2001. [Google Scholar]
- 12.Lehman-Horn F, Jurkat-Rott K. Voltage-gated ion channels and hereditary disease. Physiol Rev. 1999;79:1317–1372. doi: 10.1152/physrev.1999.79.4.1317. [DOI] [PubMed] [Google Scholar]
- 13.Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28:11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- 16.Beneski DA, Catterall WA. Covalent labeling of protein components of the sodium channel with a photoactivable derivative of scorpion toxin. Proc Natl Acad Sci U S A. 1980;77:639–643. doi: 10.1073/pnas.77.1.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartshorne RP, Catterall WA. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981;78:4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartshorne RP, Messner DJ, Coppersmith JC, Catterall WA. The saxitoxin receptor of the sodium channel from rat brain. Evidence for two nonidentical β subunits. J Biol Chem. 1982;257:13888–13891. [PubMed] [Google Scholar]
- 19.Hartshorne RP, Catterall WA. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984;259:1667–1675. [PubMed] [Google Scholar]
- 20.Talvenheimo JA, Tamkun MM, Catterall WA. Reconstitution of neurotoxin-stimulated sodium transport by the voltage-sensitive sodium channel purified from rat brain. J Biol Chem. 1982;257:11868–11871. [PubMed] [Google Scholar]
- 21.Tamkun MM, Talvenheimo JA, Catterall WA. The sodium channel from rat brain. Reconstitution of neurotoxin-activated ion flux and scorpion toxin binding from purified components. J Biol Chem. 1984;259:1676–1688. [PubMed] [Google Scholar]
- 22.Hartshorne RP, Keller BU, Talvenheimo JA, Catterall WA, Montal M. Functional reconstitution of the purified brain sodium channel in planar lipid bilayers. Proc Natl Acad Sci U S A. 1985;82:240–244. doi: 10.1073/pnas.82.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noda M, Shimizu S, Tanabe T, Takai T, Kayano T, Ikeda T, Takahashi H, Nakayama H, Kanaoka Y, Minamino N, et al. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984;312:121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- 24.Noda M, Ikeda T, Suzuki H, Takeshima H, Takahashi T, Kuno M, Numa S. Expression of functional sodium channels from cloned cDNA. Nature. 1986;322:826–828. doi: 10.1038/322826a0. [DOI] [PubMed] [Google Scholar]
- 25.Goldin AL, Snutch T, Lubbert H, Dowsett A, Marshall J, Auld V, Downey W, Fritz LC, Lester HA, Dunn R, Catterall WA, Davidson N. Messenger RNA coding for only the a subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. ProcNatlAcadSciUSA. 1986;83:7503–7507. doi: 10.1073/pnas.83.19.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isom LL, De Jongh KS, Patton DE, Reber BFX, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- 27.Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA. Structure and function of the β2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 28.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 29.Brackenbury WJ, Isom LL. Na Channel β Subunits: Overachievers of the Ion Channel Family. Front Pharmacol. 2011;2:53. doi: 10.3389/fphar.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong CM, Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- 31.Catterall WA. Voltage-dependent gating of sodium channels: correlating structure and function. Trends Neurosci. 1986;9:7–10. [Google Scholar]
- 32.Catterall WA. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- 33.Yarov-Yarovoy V, Baker D, Catterall WA. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K+ channels. Proc Natl Acad Sci U S A. 2006;103:7292–7297. doi: 10.1073/pnas.0602350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guy HR, Seetharamulu P. Molecular model of the action potential sodium channel. Proc Natl Acad Sci U S A. 1986;83:508–512. doi: 10.1073/pnas.83.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarov-Yarovoy V, DeCaen PG, Westenbroek RE, Pan CY, Scheuer T, Baker D, Catterall WA. Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc Natl Acad Sci U S A. 2012;109:E93–102. doi: 10.1073/pnas.1118434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuhmer W, Conti F, Suzuki H, Wang XD, Noda M, Yahagi N, Kubo H, Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989;339:597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- 37.Rogers JC, Qu Y, Tanada TN, Scheuer T, Catterall WA. Molecular determinants of high affinity binding of a-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel a subunit. JBiolChem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- 38.Cestele S, Qu Y, Rogers JC, Rochat H, Scheuer T, Catterall WA. Voltage sensor-trapping: enhanced activation of sodium channels by β-scorpion toxin bound to the S3-S4 loop in domain II. Neuron. 1998;21:919–931. doi: 10.1016/s0896-6273(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 39.Yang N, Horn R. Evidence for voltage-dependent S4 movement in sodium channel. Neuron. 1995;15:213–218. doi: 10.1016/0896-6273(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 40.Yang N, George AL, Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16:113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- 41.Yang N, George AL, Jr, Horn R. Probing the outer vestibule of a sodium channel voltage sensor. Biophys J. 1997;73:2260–2268. doi: 10.1016/S0006-3495(97)78258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeCaen PG, Yarov-Yarovoy V, Zhao Y, Scheuer T, Catterall WA. Disulfide locking a sodium channel voltage sensor reveals ion pair formation during activation. Proc Natl Acad Sci U S A. 2008;105:15142–15147. doi: 10.1073/pnas.0806486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeCaen PG, Yarov-Yarovoy V, Sharp EM, Scheuer T, Catterall WA. Sequential formation of ion pairs during activation of a sodium channel voltage sensor. Proc Natl Acad Sci U S A. 2009;106:22498–22503. doi: 10.1073/pnas.0912307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeCaen PG, Yarov-Yarovoy V, Scheuer T, Catterall WA. Gating charge interactions with the S1 segment during activation of a Na+ channel voltage sensor. Proc Natl Acad Sci U S A. 2011;108:18825–18830. doi: 10.1073/pnas.1116449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vargas E, Yarov-Yarovoy V, Khalili-Araghi F, Catterall WA, Klein ML, Tarek M, Lindahl E, Schulten K, Perozo E, Bezanilla F, Roux B. An emerging consensus on voltage-dependent gating from computational modeling and molecular dynamics simulations. J Gen Physiol. 2012;140:587–594. doi: 10.1085/jgp.201210873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noda M, Suzuki H, Numa S, Stuhmer W. A single point mutation confers tetrodotoxin and saxitoxin insensitivity on the sodium channel II. FEBS Lett. 1989;259:213–216. doi: 10.1016/0014-5793(89)81531-5. 0014-5793(89)81531-5 [pii] [DOI] [PubMed] [Google Scholar]
- 47.Terlau H, Heinemann SH, Stuhmer W, Pusch M, Conti F, Imoto K, Numa S. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett. 1991;293:93–96. doi: 10.1016/0014-5793(91)81159-6. [DOI] [PubMed] [Google Scholar]
- 48.Heinemann SH, Terlau H, Stuhmer W, Imoto K, Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- 49.Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952;116:497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vassilev PM, Scheuer T, Catterall WA. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science. 1988;241:1658–1661. doi: 10.1126/science.241.4873.1658. [DOI] [PubMed] [Google Scholar]
- 51.West JW, Patton DE, Scheuer T, Wang Y, Goldin AL, Catterall WA. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc Natl Acad Sci U S A. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eaholtz G, Scheuer T, Catterall WA. Restoration of inactivation and block of open sodium channels by an inactivation gate peptide. Neuron. 1994;12:1041–1048. doi: 10.1016/0896-6273(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 53.Rohl CA, Boeckman FA, Baker C, Scheuer T, Catterall WA, Klevit RE. Solution structure of the sodium channel inactivation gate. Biochemistry. 1999;38:855–861. doi: 10.1021/bi9823380. [DOI] [PubMed] [Google Scholar]
- 54.Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 55.Ren D, Navarro B, Xu H, Yue L, Shi Q, Clapham DE. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- 56.Koishi R, Xu H, Ren D, Navarro B, Spiller BW, Shi Q, Clapham DE. A superfamily of voltage-gated sodium channels in bacteria. J Biol Chem. 2004;279:9532–9538. doi: 10.1074/jbc.M313100200. [DOI] [PubMed] [Google Scholar]
- 57.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Catterall WA. Common modes of drug action on Na+ channels: Local anesthetics, antiarrhythmics and anticonvulsants. Trends Pharmacol Sci. 1987;8:57–65. [Google Scholar]
- 59.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of sodium channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 60.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the sodium channel alpha subunit in voltage-dependent gating and drug block. J Biol Chem. 2002 doi: 10.1074/jbc.M206126200. In review. [DOI] [PubMed] [Google Scholar]
- 62.Yarov-Yarovoy V, Brown J, Sharp EM, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel α subunit. J Biol Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- 63.Wang GK, Quan C, Wang S. A common local anesthetic receptor for benzocaine and etidocaine in voltage-gated mu1 Na+ channels. Pflugers Arch. 1998;435:293–302. doi: 10.1007/s004240050515. [DOI] [PubMed] [Google Scholar]
- 64.Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen H, Zhou Q, Pan X, Li Z, Wu J, Yan N. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution. Science. 2017:355. doi: 10.1126/science.aal4326. [DOI] [PubMed] [Google Scholar]
- 66.Engel J, Jr International League Against E. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 67.Dravet C, Bureau M, Guerrini R, Giraud N, Roger J. Severe myoclonic epilepsy in infants. In: Roger J, Dravet C, Bureau M, Dreifus FE, Perret A, Wolf P, editors. Epileptic sndromes in infancy, childhood and adolescence. 2. John Libbey; London: 1992. pp. 75–102. [Google Scholar]
- 68.Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 69.Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oakley JC, Cho AR, Cheah CS, Scheuer T, Catterall WA. Synergistic GABA-enhancing therapy against seizures in a mouse model of Dravet syndrome. J Pharmacol Exp Ther. 2013;345:215–224. doi: 10.1124/jpet.113.203331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, Scheuer T, Catterall WA. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 2013;123:1798–1808. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheah CS, Yu FH, Westenbroek RE, Kalume FK, Oakley JC, Potter GB, Rubenstein JL, Catterall WA. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2012;109:14646–14651. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kalume F, Westenbroek RE, Cheah CS, Oakley JC, Scheuer T, Catterall WA. Sudden unexpected death in a mouse model of Dravet Syndrome. Journal of Clinical Investigation. 2013 doi: 10.1172/JCI66220. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dravet C. Dravet’s syndrome (Severe myoclonic epilepsy in infancy) 2003 doi: 10.1016/B978-0-444-52891-9.00065-8. http://www.ilae-epilepsy.org/ctf/dravet.html. [DOI] [PubMed]
- 75.Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71–102. [PubMed] [Google Scholar]
- 76.Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nolan KJ, Camfield CS, Camfield PR. Coping with Dravet syndrome: parental experiences with a catastrophic epilepsy. Dev Med Child Neurol. 2006;48:761–765. doi: 10.1017/S0012162206001629. [DOI] [PubMed] [Google Scholar]
- 78.Han S, Yu FH, Schwartz MD, Linton JD, Bosma MM, Hurley JB, Catterall WA, de la Iglesia HO. NaV1.1 channels are critical for intercellular communication in the suprachiasmatic nucleus and for normal circadian rhythms. Proc Natl Acad Sci U S A. 2012;109:E368–377. doi: 10.1073/pnas.1115729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalume F, Oakley JC, Westenbroek RE, Gile J, de la Iglesia HO, Scheuer T, Catterall WA. Sleep impairment and reduced interneuron excitability in a mouse model of Dravet Syndrome. Neurobiol Dis. 2015;77:141–154. doi: 10.1016/j.nbd.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, Potter GB, Rubenstein JL, Scheuer T, de la Iglesia HO, Catterall WA. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubinstein M, Han S, Tai C, Westenbroek RE, Hunker A, Scheuer T, Catterall WA. Dissecting the phenotypes of Dravet syndrome by gene deletion. Brain. 2015;138:2219–2233. doi: 10.1093/brain/awv142. [DOI] [PMC free article] [PubMed] [Google Scholar]