Abstract
PURPOSE OF REVIEW
Volume management in hemodialysis patients is often challenging. Assessing volume status and deciding how much fluid to remove during hemodialysis, the so-called ultrafiltration rate (UFR), has remained a conundrum.
RECENT FINDINGS
To date there is no objective assessment tool to determine the needed UFR during each hemodialysis session. Higher volume overload or higher UFR is associated with poor outcomes including worse mortality and unfavorable clinical outcomes. We suggest combined use of the following criteria to determine UFR or post-dialysis target dry weight: pre-hemodialysis blood pressure and its intradialytic changes, muscle cramps, dyspnea from pulmonary vascular congestion, peripheral edema, tachycardia or palpitation, headache or lightheadedness, perspiration, post-dialysis fatigue. Restricting fluid and salt intake – and high-dose loop diuretic use in case of residual kidney function – can be helpful in controlling fluid gains. More frequent and more severe hypotensive episodes are associated with poor outcomes including higher death risk.
Keywords: Hemodialysis, mortality, volume overload, ultrafiltration rate, fluid retention, residual kidney function
Introduction
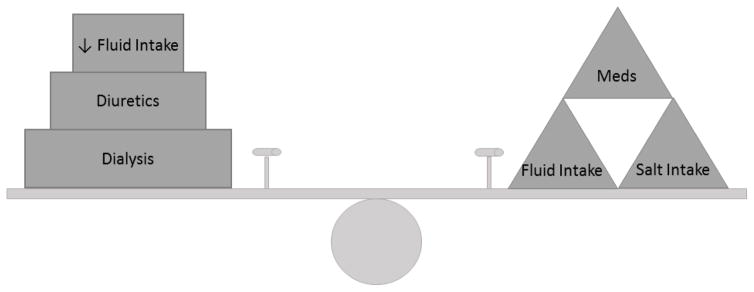
Volume control and management in long-term or maintenance hemodialysis (HD) patients has been an on-going struggle for both patients and nephrologists alike. Between HD treatments, the inter-dialytic period, most maintenance HD patients will accumulate volume with their daily meals and fluid intake as most long-term HD patients eventually lose their residual kidney function. Therefore, the majority of volume control or removal with dialysis, known as ultrafiltration, will need to be done during the patient’s short HD treatments (Figure 1), typically thrice-weekly. This often requires HD treatment times and/or frequency to be increased which is often met with resistance from the patient’s perspective.
Figure 1.
Depicting factors related to hemodialysis patients in fluid balance management. See also table 1 for list of clinical criteria
Clinical Presentation
In hemodialysis patients, fluid gains are expected during the inter-dialytic dialysis period. HD patients know this as “how much are you above your dry weight (DW)” or the weight at which patients cannot tolerate further fluid removal.1 This inter-dialytic weight gain (IDWG) is mainly from food and fluid intake in the maintenance HD patient as what goes in, stays in. And with each HD treatment, one significant goal is to remove these fluid gains by ultrafiltration and maintain the patients DW. Chronic fluid overload develops as patient’s fluid gains exceed the ability of dialysis to ultrafiltrate to the set DW and the patient’s volume status exceeds euvolmia. This can occur by high fluid intake and/or by inaccurate assessments of the patient’s volume status by the nephrologist and HD staff.
Volume status assessments are often performed by clinicians and based on the patient’s symptoms and physical examination findings with possible supplementation of some more objective measurements. Volume assessment often involves medical staff assessing patients with useful clinical criteria in determining the need for fluid removal with each HD treatment (Table 1). Blood pressure parameters are often helpful in guiding fluid removal. Baseline or pre-HD systolic BP (SBP) that are high (e.g. >160 mmHg) may suggest a higher volume state that may be responsive to fluid removal. Conversely, low pre-HD SBP (e.g. <120 mmHg) may suggest euvolemia or possibly hypovolemia to guide HD staff to target less fluid removal or an adjustment in dry weight. Additionally, intradialytic BP changes can also be suggestive of volume status. SBP increases during dialysis, known as intradialytic hypertension, may be indicative of hypervolemia necessitating further fluid removal with dialysis. Notably, the change in pre-HD and post-HD SBP should not be greater than 20–30 mmHg as larger changes in SBP have been associated with increased mortality.2
Table 1.
Suggested clinical criteria for assessment and adjustment of ultrafiltration rate (UFR) in hemodialysis patients
| Remove more fluid or decrease target dry weight | Remove less fluid or increase target dry weight | Comments | |
|---|---|---|---|
| Baseline systolic BP | High, e.g. >160 mmHg | Low, e.g. <120 mmHg | If sBP increases 1–2 hrs after HD initiation (intradialytic hypertension), may need more fluid removal |
| Intradialytic BP change | Not known | Not known | The difference between pre- and post-HD sBP should not be larger than 20–30 mmHg2 |
| Cardiac symptoms | Pulmonary edema (see below) | Tachycardia, palpitation, chest pain | Stope UFR if chest pain is reported |
| Pulmonary Symptoms | Pulmonary congestion or SOB | - | |
| CNS symptoms | - | Lightheadedness | Headache towards the end of HD may suggest need to lower UFR |
| Musculoskeletal Symptoms | If no cramps | If worsening cramps | Cramps may happen towards the end of HD |
| Perspiration | Sweating towards the end of HD session | ||
| Peripheral edema | Lower extremity or sacral edema | Not even trace edema at the start of dialysis | |
| Inter-dialytic weight gain | High | Low | Generally, weight gain between 2 HD session should be less than <1.5 kg |
| Residual kidney function (RKF) | If minimal to no residual kidney function | If urine volume >500 cc/day or Kru>3 ml/min | Least UFR is recommended in patients with substantial RKF in order to prolong preservation of RKF |
| Appetite and food intake | Greater appetite and higher protein intake | Diminished appetite | |
| GI symptoms | Diarrhea or diabetic gastroparesis bouts | Least UFR during gastroenteritis is recommended |
Patient symptoms are also helpful in guiding volume management. Shortness of breath (dyspnea) associated with pulmonary edema is often assessed for by HD staff to determine when increased fluid removal is necessary. Additionally, peripheral edema can be easily assessed for prior to HD to guide ultrafiltration. But symptoms in guiding HD staff for decreasing or slowing fluid removal are more subtle including: tachycardia, palpitations, chest pain, lightheadedness, perspiration (especially in final hours of HD therapy), and the development of muscle cramping. These symptoms typically develop towards the end of a dialysis treatment and are often an indication of too much fluid removal.
Additional considerations for volume management include monitoring inter-dialytic weight gains, the weight gain between 2 HD sessions. In general, IDWG should be less than 1.5 kg (or <20 ml/kg). High IDWG will often guide HD staff to increase fluid removal with each treatment. Supplementary to IDWG, greater reported food intake and a strong appetite also guide clinicians in increasing fluid removal with each HD session. Conversely, if patients report poor intake, diminished appetite, diarrhea, or bouts of diabetic-related gastroparesis adjustments to decrease ultrafiltration are often made by HD staff.
Other methodologies for assessing volume include: inferior vena cava diameter, biochemical parameters, continuous blood volume monitoring, ultrasound of lung, and bioimpedance.3–8 While these methodologies may offer more “objective” measurements for volume status, it is well known that they are still fraught with errors from calibration and operator interpretation of the findings.
The estimation of each patient’s volume status is an important window into guiding nephrologist with volume management goals for each dialysis treatment. And there is now a growing body of research into the association of volume status and dialysis patient outcomes.
Clinical Consequences and Patient Outcome
Volume overload in ESRD patients has been a clinical challenge that is associated with morbid conditions such as lower extremity edema, anasarca, ascites, pulmonary congestion/edema, hypertension and worsening heart failure.9–11 In fact, ESRD patients in general share similar risks as heart failure patients. Both populations share high mortality risks (20 to 25% in the United States) mostly due to cardiovascular etiologies12, experience chronic wasting syndrome13–15, and exhibit survival paradoxes including the obesity and cholesterol.16–18 Furthermore, volume overload or fluid retention in ESRD patients has been observed to be associated with increased risk of mortality. Kalantar et al. has previously shown that in 34,107 hemodialysis patients that had higher interdialytic weight gains, >1.5 kg of body fluid between 2 consecutive HD treatments, had higher risk of 2-year mortality for both all-cause and cardiovascular mortality robust for multivariate adjustment and in subgroup analysis. Whereas HD patients with IDWG of <1.0 kg between dialysis treatments had a survival advantage with the lowest cardiovascular death risk. In this study, 86% of HD patients were found to have >1.5 kg IDWG and patients younger in age, male sex, having longer dialysis vintage, diabetic status, and larger body habitus were also found to have higher IDWG. Additionally, improved nutritional status that included higher protein intake was also associated with larger IDWG.19
In a recent study by Zoccali et al., chronic exposure to fluid overload was assessed and quantified by body fluid measurements via bioimpedance spectroscopy. Here the authors found that chronic fluid overload was associated with increased death risk in incident hemodialysis patients. Death risk was analyzed by baseline fluid overload and cumulative 1 year fluid overload exposure to avoid a survival-bias and examine the cumulative nature of fluid overload, respectively. And in both analysis Zoccali et al. found fluid overload to be associated with mortality robust to multivariate adjustment. Additionally, cumulative fluid overload was found to have a higher association with death risk with a HR of 1.50 (95% CI 1.38 to 1.64) versus baseline fluid overload HR of 1.26 (95% CI 1.19 to 1.33).20
Volume overload may also contribute to increase cardiac output and hypertension which may lead to increase use of anti-hypertensives. In the absence of clinical features of volume overload, hypertension requiring more and more medications may itself be the indicator of volume overload.21 And increasing anti-hypertensives, in the setting of volume overload, may fail in controlling blood pressure. In fact, in one study, the increasing use of anti-hypertensives was found to be a determinant of poor volume control and patients receiving more medications were likely to be hypertensive.22
Volume overload also contributes to the preload-related factors that may promote left ventricular hypertrophy (LVH) via myocardial cell lengthening and eccentric and asymmetric LV remodeling.23, 24 The degree and persistence of LVH in ESRD patients has been strongly associated with increased mortality risk25 and a decrease in LVH has been associated with a subsequent decrease cardiovascular mortality risk26 and reduction in non-ischemic cardiac failure rates.27 It has also been reported that reducing LV mass has associated improvements with anemia and phosphorus levels.28 Though there are associated benefits of improving LVH, it is unclear if more aggressive ultrafiltration could prevent and/or regress LVH.29
Additionally, volume overload may also contribute to pulmonary dysfunction including interstitial edema, airway obstruction30–33 and pleural effusions.34, 35 Chronic volume overload may also contribute to the development of pulmonary hypertension where central fluid overload may be the most likely contributing factor.36
As we can see, volume overload as measured by persistently high IDWG or fluid measured by bioimpedance has significant associations with mortality outcomes and clinical consequences. Thus accurate assessment of volume status in dialysis patients and the treatment of volume overload are of paramount concern for all of those caring for HD patients.
Approach to Fluid Management in HD Patients
So, what can we do to combat the effects of volume overload? The first step is to control “what goes in”. It may seem obvious to dialysis patient care providers that decreasing intake of salt and fluids would be an “easy” solution to control volume overload. But as all dialysis care providers know, this solution is anything but easy to achieve in the HD population. In dialysis patients with significant residual kidney function, the option of diuretics may have an added benefit. Bragg-Gresham et al. had shown the observation that diuretic use was associated with lower all-cause and cardiac mortality risk.37 Now this may be the fact that HD patients with residual kidney function do seem to have a survival advantage.38 But the authors propose that diuretics use may help preserve RKF by minimizing hypotensive episodes during dialysis by managing volume gradually with diuretics rather than intermittently with HD treatments.37
The final treatment option for controlling volume status in ESRD patients would be ultrafiltration with HD which is often the only available treatment for volume control in the majority of HD patients (who no longer have any RKF). But the question remains, how much fluid can we safely pull off with HD? This question has recently come to national stage in the determination of dialysis care guidelines.39, 40 The ultrafiltration rate (UFR), fluid volume removed per hour per patient DW (mL/hr/kg), has been studied in several observational studies to elucidate the optimal fluid removal rate during HD. Saran et al. found that UFR of >10 mL/hr/kg were associated with higher all-cause mortality risk (HR 1.09, p=0.02) but not with cardiovascular (CV) related mortality41 in the international DOPPs cohort. And more recently in a prevalent HD cohort, Flythe et al. found that UFR of >13 mL/hr/kg was associated with and increased all-cause and CV mortality risk of 59% and 71%, respectively (p<0.001 for both).42 Finally, Kim et al. recently found in a cohort of incident HD patients that UFR showed a linear association with all-cause and CV mortality where UFR of ≥10 ml/hr/kg was associated with HR (95% CI) of 1.15 (1.10–1.19) and 1.23 (1.16–1.31), respectively.
Ultrafiltration may lead to these poor outcomes by causing intravascular depletion with resulting hypotension and reducing coronary blood flow resulting in ischemia or myocardial stunning.43, 44 Repetitive insults and stunning may then lead to ventricular remodeling and the effects of heart failure44–46 when ejection fraction declines. This ultrafiltration induce hypotension may also lead to ischemia to other organs including the brain47, GI tract48, 49 and kidney. Intradialytic hypotension with ischemia to the kidney may also be a contributing factor to further loss of residual kidney function50 which would result in more difficulties with volume management for HD patients.
DISCUSSION
If higher UFRs are associated with worse outcomes and HD patients still require the same amount of fluid removed with each HD treatment, what is the solution to this conundrum? For patients already established on dialysis, the easiest choices would be to either increase HD treatment run times and/or frequency which would both effectively reduce the UFR. But as most nephrologist have experienced, this strategy for lowering UFR is met with significant resistance from the patients. In a study by Flythe et al., in 600 HD patients surveyed, 12% of patients were willing to have an “extra” fourth weekly treatment. In fact, some patients valued additional fluid intake so much that 21% of patients surveyed were willing to increase treatment times by 30 minutes to accommodate the additional fluid intake.51
And now, volume management may become even more challenging as UFR itself is starting to find “cutoffs” to be used as a dialysis quality metric.39, 52, 53 With a potential UFR threshold of ≤13 mL/hr/kg, many patients, who are not willing to extend their treatment times or change their daily fluid intakes, will potentially leave their HD treatments volume overloaded perpetuating a chronic volume overloaded state. The potential effects and consequences of a UFR limitation are unknown to us at this time as our UFR-related studies to date are observation in nature. But it is reasonable to anticipate an increased frequency of uncontrolled hypertension and volume-related hospitalizations utilizing greater quantities of anti-hypertensive medications and more hospital days, respectively, if volume control cannot be adequately achieved.
Preventative strategies for managing volume in HD patients may be optimal method for both avoiding mortality risks with higher UFRs and adequately controlling volume. From our studies in home (short daily) and nocturnal hemodialysis, we have learned these modalities are associated with a lower prevalence of LVH54 which may be related to reduced ultrafiltration-induced hypotension and myocardial stunning as these frequent HD modalities would remove fluid more slowly. Additionally, improved BP control has also been reported to be related to the reduction in the extracellular fluid volume55, 56 in short daily HD. More frequent HD, such as short daily and nocturnal modalities, may offer a more physiologic model for fluid removal, avoiding large shifts in fluid volume, in patients choosing HD as their treatment modality of choice.
An additional preventative strategy may be the implementation of incremental HD from the onset of renal replacement therapy. One of the main tenants of incremental HD is to maximize the utility of RKF. RKF has the benefit of improved fluid homeostasis. It allows HD patients to require less total ultrafiltration volumes and thus lower UFRs which may lead to a reduction in the occurrence of intradialytic hypotension and its associated complications57, 58 and UFRs associated mortality risk.59, 60 Utilizing RKF, such as in PD, patients can also continue to use diuretics for further control of their volume status. With RKF, patients will have the most physiologic method for maintaining adequate volume management. Incremental dialysis, which may seem to be in opposition to more frequent HD modalities, has the similar principle benefit of reducing the need for additional volume removal with each dialysis treatment.
Conclusion
What we can deduct from the volume management of HD patients is that rapid fluid removal can have harmful consequences. As such, adequate volume management in this patient population should occur at a slower rate for optimizing outcomes. Strategies such as fluid and salt restriction and continued use of diuretics in patients with significant RKF have shown some benefit. But the HD-centered strategies of more frequent HD (e.g. short daily and nocturnal) and incremental HD with a focus on preserving RKF will require further study in their relationship to ESRD volume management.
Acknowledgments
The study was supported by Dr. Kalantar-Zadeh’s research grants from the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) of the National Institute of Health (NIH) (K24-DK091419 and R01-DK095668), and philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang and AVEO.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Jason A. Chou declares no conflict of interest.
Kamyar Kalantar-Zadeh has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, ZS-Pharma, and was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA during 2007–2012.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Agarwal R, Weir MR. Dry-weight: a concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1255–1260. doi: 10.2215/CJN.01760210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J, Rhee CM, Sim JJ, Kim YL, Ricks J, Streja E, Vashistha T, Tolouian R, Kovesdy CP, Kalantar-Zadeh K. A comparative effectiveness research study of the change in blood pressure during hemodialysis treatment and survival. Kidney international. 2013;84:795–802. doi: 10.1038/ki.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chazot C, Vo-Van C, Zaoui E, Vanel T, Hurot JM, Lorriaux C, Mayor B, Deleaval P, Jean G. Fluid overload correction and cardiac history influence brain natriuretic peptide evolution in incident haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26:2630–2634. doi: 10.1093/ndt/gfq804. [DOI] [PubMed] [Google Scholar]
- 4.Franz M, Pohanka E, Tribl B, Woloszczuk W, Horl WH. Living on chronic hemodialysis between dryness and fluid overload. Kidney Int Suppl. 1997;59:S39–42. [PubMed] [Google Scholar]
- 5.Ishibe S, Peixoto AJ. Methods of assessment of volume status and intercompartmental fluid shifts in hemodialysis patients: implications in clinical practice. Seminars in dialysis. 2004;17:37–43. doi: 10.1111/j.1525-139x.2004.17112.x. [DOI] [PubMed] [Google Scholar]
- 6.Lopot F, Kotyk P, Blaha J, Forejt J. Use of continuous blood volume monitoring to detect inadequately high dry weight. The International journal of artificial organs. 1996;19:411–414. [PubMed] [Google Scholar]
- 7.Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19:356–363. doi: 10.1016/j.echo.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney international. 2014;86:489–496. doi: 10.1038/ki.2014.207. [DOI] [PubMed] [Google Scholar]
- 9.Katzarski KS. Monitoring of blood volume during haemodialysis treatment of acute renal and multiple organ failures. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(Suppl 8):20–23. doi: 10.1093/ndt/11.supp8.20. [DOI] [PubMed] [Google Scholar]
- 10.Kraemer M, Rode C, Wizemann V. Detection limit of methods to assess fluid status changes in dialysis patients. Kidney international. 2006;69:1609–1620. doi: 10.1038/sj.ki.5000286. [DOI] [PubMed] [Google Scholar]
- 11.Charra B, Laurent G, Chazot C, Calemard E, Terrat JC, Vanel T, Jean G, Ruffet M. Clinical assessment of dry weight. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996;11(Suppl 2):16–19. doi: 10.1093/ndt/11.supp2.16. [DOI] [PubMed] [Google Scholar]
- 12.Kalantar-Zadeh K, Abbott KC, Kronenberg F, Anker SD, Horwich TB, Fonarow GC. Epidemiology of dialysis patients and heart failure patients. Semin Nephrol. 2006;26:118–133. doi: 10.1016/j.semnephrol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Anker SD, Horwich TB, Fonarow GC. Nutritional and anti-inflammatory interventions in chronic heart failure. The American journal of cardiology. 2008;101:89E–103E. doi: 10.1016/j.amjcard.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwich TB, Kalantar-Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155:883–889. doi: 10.1016/j.ahj.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, Morley JE. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–442. doi: 10.1097/MCO.0b013e3281a30594. [DOI] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. Journal of the American College of Cardiology. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 18.Horwich TB, Fonarow GC. Reverse epidemiology beyond dialysis patients: chronic heart failure, geriatrics, rheumatoid arthritis, COPD, and AIDS. Seminars in dialysis. 2007;20:549–553. doi: 10.1111/j.1525-139X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, Wabel P, Stuard S. Chronic Fluid Overload and Mortality in ESRD. Journal of the American Society of Nephrology : JASN. 2017 doi: 10.1681/ASN.2016121341. Zoccali et al.’s recent JASN paper highlight mortality outcomes related to chronic fluid overload as measured by bioimpedance. This paper, in addition to Kalantar-Zadeh et al.’s 2009 “Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis” have well shown the association of chronic fluid overload with worse outcomes in HD patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal R. What are the Consequences of Volume Expansion in Chronic Dialysis Patients?: Hypertension as a Manifestation of Volume Overload in Hemodialysis Patients. Seminars in dialysis. 2015;28:231–232. doi: 10.1111/sdi.12349. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R. Epidemiology of interdialytic ambulatory hypertension and the role of volume excess. American journal of nephrology. 2011;34:381–390. doi: 10.1159/000331067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left Ventricular Hypertrophy in Chronic Kidney Disease Patients: From Pathophysiology to Treatment. Cardiorenal Med. 2015;5:254–266. doi: 10.1159/000435838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin LC, Franco RJ, Gavras I, Matsubara BB, Garcia S, Caramori JT, Barretti BB, Balbi AL, Barsanti R, Padovani C, Gavras H. Association between hypervolemia and ventricular hypertrophy in hemodialysis patients. Am J Hypertens. 2004;17:1163–1169. doi: 10.1016/j.amjhyper.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney international. 2004;65:1492–1498. doi: 10.1111/j.1523-1755.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 26.London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metivier F, Adda H, Safar ME. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. Journal of the American Society of Nephrology : JASN. 2001;12:2759–2767. doi: 10.1681/ASN.V12122759. [DOI] [PubMed] [Google Scholar]
- 27.Foley RN, Parfrey PS, Kent GM, Harnett JD, Murray DC, Barre PE. Serial change in echocardiographic parameters and cardiac failure in end-stage renal disease. Journal of the American Society of Nephrology : JASN. 2000;11:912–916. doi: 10.1681/ASN.V115912. [DOI] [PubMed] [Google Scholar]
- 28.Covic A, Mardare NG, Ardeleanu S, Prisada O, Gusbeth-Tatomir P, Goldsmith DJ. Serial echocardiographic changes in patients on hemodialysis: an evaluation of guideline implementation. J Nephrol. 2006;19:783–793. [PubMed] [Google Scholar]
- 29.Galetta F, Cupisti A, Franzoni F, Femia FR, Rossi M, Barsotti G, Santoro G. Left ventricular function and calcium phosphate plasma levels in uraemic patients. J Intern Med. 2005;258:378–384. doi: 10.1111/j.1365-2796.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- 30.Elsayed ME, Stack AG. What are the Consequences of Volume Expansion in Chronic Dialysis Patients?: Pulmonary Manifestations of Volume Expansion. Seminars in dialysis. 2015;28:235–239. doi: 10.1111/sdi.12351. [DOI] [PubMed] [Google Scholar]
- 31.Muir AL, Flenley DC, Kirby BJ, Sudlow MF, Guyatt AR, Brash HM. Cardiorespiratory effects of rapid saline infusion in normal man. Journal of applied physiology. 1975;38:786–775. doi: 10.1152/jappl.1975.38.5.786. [DOI] [PubMed] [Google Scholar]
- 32.Prisk GK, Olfert IM, Arai TJ, Wagner PD, Hopkins SR. Rapid intravenous infusion of 20 ml/kg saline does not impair resting pulmonary gas exchange in the healthy human lung. J Appl Physiol (1985) 2010;108:53–59. doi: 10.1152/japplphysiol.00787.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farney RJ, Morris AH, Gardner RM, Armstrong JD., Jr Rebreathing pulmonary capillary and tissue volume in normals after saline infusion. Journal of applied physiology: respiratory, environmental and exercise physiology. 1977;43:246–253. doi: 10.1152/jappl.1977.43.2.246. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Kobayashi T. Pleural calcification: a type of “metastatic calcification” in chronic renal failure. Br J Radiol. 1983;56:93–98. doi: 10.1259/0007-1285-56-662-93. [DOI] [PubMed] [Google Scholar]
- 35.Bakirci T, Sasak G, Ozturk S, Akcay S, Sezer S, Haberal M. Pleural effusion in long-term hemodialysis patients. Transplant Proc. 2007;39:889–891. doi: 10.1016/j.transproceed.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Havlucu Y, Kursat S, Ekmekci C, Celik P, Serter S, Bayturan O, Dinc G. Pulmonary hypertension in patients with chronic renal failure. Respiration. 2007;74:503–510. doi: 10.1159/000102953. [DOI] [PubMed] [Google Scholar]
- 37.Bragg-Gresham JL, Fissell RB, Mason NA, Bailie GR, Gillespie BW, Wizemann V, Cruz JM, Akiba T, Kurokawa K, Ramirez S, Young EW. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;49:426–431. doi: 10.1053/j.ajkd.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Obi Y, Rhee CM, Mathew AT, Shah G, Streja E, Brunelli SM, Kovesdy CP, Mehrotra R, Kalantar-Zadeh K. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. Journal of the American Society of Nephrology : JASN. 2016 doi: 10.1681/ASN.2015101142. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Flythe JE, Assimon MM, Wenger JB, Wang L. Ultrafiltration Rates and the Quality Incentive Program: Proposed Measure Definitions and Their Potential Dialysis Facility Implications. Clinical journal of the American Society of Nephrology : CJASN. 2016;11:1422–1433. doi: 10.2215/CJN.13441215. Flythe et al.’s CJASN paper highlights the changing landscape in dialysis metrics regarding ultrafiltration rate and its associated outcomes. As currently in the United States, national HD metrics are starting to implement UFR cut-offs as part of patient quality initiatives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flythe JE. Ultrafiltration Rate Clinical Performance Measures: Ready for Primetime? Seminars in dialysis. 2016;29:425–434. doi: 10.1111/sdi.12529. [DOI] [PubMed] [Google Scholar]
- 41.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney international. 2006;69:1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 42.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney international. 2011;79:250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 46.Gao XM, Wong G, Wang B, Kiriazis H, Moore XL, Su YD, Dart A, Du XJ. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J Hypertens. 2006;24:1663–1670. doi: 10.1097/01.hjh.0000239304.01496.83. [DOI] [PubMed] [Google Scholar]
- 47.Eldehni MT, McIntyre CW. Are there neurological consequences of recurrent intradialytic hypotension? Seminars in dialysis. 2012;25:253–256. doi: 10.1111/j.1525-139X.2012.01057.x. [DOI] [PubMed] [Google Scholar]
- 48.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2011;6:133–141. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jefferies HJ, Crowley LE, Harrison LE, Szeto CC, Li PK, Schiller B, Moran J, McIntyre CW. Circulating endotoxaemia and frequent haemodialysis schedules. Nephron Clinical practice. 2014;128:141–146. doi: 10.1159/000366519. [DOI] [PubMed] [Google Scholar]
- 50.Marants R, Grant C, Lee T, McIntyre CW. Renal Perfusion Falls during Hemodialysis: An Explanation for the Loss of Residual Renal Function in Dialysis Patients. Journal of the American Society of Nephrology : JASN. 2016;27 Abstract Edition: 327A. [Google Scholar]
- 51.Flythe JE, Mangione TW, Brunelli SM, Curhan GC. Patient-stated preferences regarding volume-related risk mitigation strategies for hemodialysis. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:1418–1425. doi: 10.2215/CJN.03280314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agar JW. Personal viewpoint: Limiting maximum ultrafiltration rate as a potential new measure of dialysis adequacy. Hemodialysis international International Symposium on Home Hemodialysis. 2016;20:15–21. doi: 10.1111/hdi.12288. [DOI] [PubMed] [Google Scholar]
- 53*.Arbor Research Collaborative for Health and the University of Michagan Kidney Epidemiology and Cost Center. End stage renal disease (ESRD) quality measure development and maintenance hemodialysis adequacy clinical technical expert panel summary report. 2013. This expert panel summary report also highlights the movement of UFR into dialysis quality metrics which will change HD Volume management across the United States. [Google Scholar]
- 54.Weinreich T, De los Rios T, Gauly A, Passlick-Deetjen J. Effects of an increase in time vs. frequency on cardiovascular parameters in chronic hemodialysis patients. Clinical nephrology. 2006;66:433–439. doi: 10.5414/cnp66433. [DOI] [PubMed] [Google Scholar]
- 55.Zilch O, Vos PF, Oey PL, Cramer MJ, Ligtenberg G, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in haemodialysis patients is reduced by short daily haemodialysis. J Hypertens. 2007;25:1285–1289. doi: 10.1097/HJH.0b013e3280f9df85. [DOI] [PubMed] [Google Scholar]
- 56.Nesrallah G, Suri R, Moist L, Kortas C, Lindsay RM. Volume control and blood pressure management in patients undergoing quotidian hemodialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2003;42:13–17. doi: 10.1016/s0272-6386(03)00532-8. [DOI] [PubMed] [Google Scholar]
- 57.Dorairajan S, Chockalingam A, Misra M. Myocardial stunning in hemodialysis: what is the overall message? Hemodialysis international International Symposium on Home Hemodialysis. 2010;14:447–450. doi: 10.1111/j.1542-4758.2010.00495.x. [DOI] [PubMed] [Google Scholar]
- 58.McIntyre CW. Haemodialysis-induced myocardial stunning in chronic kidney disease - a new aspect of cardiovascular disease. Blood purification. 2010;29:105–110. doi: 10.1159/000245634. [DOI] [PubMed] [Google Scholar]
- 59.Chou JA, Streja E, Nguyen DV, Rhee CM, Obi Y, Inrig JK, Amin A, Kovesdy CP, Sim JJ, Kalantar-Zadeh K. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2017 doi: 10.1093/ndt/gfx037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. Journal of the American Society of Nephrology : JASN. 2015;26:724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]