Abstract
The authors sought to describe the association between human immunodeficiency virus (HIV) and blood pressure (BP) levels, and determined the extent to which this relationship is mediated by body weight in a cross‐sectional study of HIV‐infected and HIV‐uninfected controls matched by age, sex, and neighborhood. Mixed‐effects models were fit to determine the association between HIV and BP and amount of effect of HIV on BP mediated through body mass index. Data were analyzed from 577 HIV‐infected and 538 matched HIV‐uninfected participants. HIV infection was associated with 3.3 mm Hg lower systolic BP (1.2‐5.3 mm Hg), 1.5 mm Hg lower diastolic BP (0.2‐2.9 mm Hg), 0.3 m/s lower pulse wave velocity (0.1‐0.4 mm Hg), and 30% lower odds of hypertension (10%‐50%). Body mass index mediated 25% of the association between HIV and systolic BP. HIV infection was inversely associated with systolic BP, diastolic BP, and pulse wave velocity. Comprehensive community‐based programs to routinely screen for cardiovascular risk factors irrespective of HIV status should be operationalized in HIV‐endemic countries.
1. BACKGROUND
Survival of human immunodeficiency virus (HIV)‐infected persons over the years has increased as a result of the successful scale‐up of antiretroviral therapy (ART)1 with the life expectancy of people living with HIV approaching that of HIV‐uninfected individuals.2, 3, 4, 5 This has created an aging HIV population with elevated risk of hypertension and cardiovascular disease.6, 7, 8 In fact, several studies have shown that risk of cardiovascular disease is higher in HIV‐infected patients than HIV‐uninfected persons.9, 10
Epidemiological studies report conflicting associations between HIV and hypertension/blood pressure (BP) levels, with some studies reporting lower BP levels,11, 12, 13 a few reporting comparable 8, 14, 15, 16 and others reporting higher BP levels in HIV‐infected individuals.17, 18 A recent systematic review of studies in sub‐Saharan Africa reported a lower prevalence of hypertension in HIV‐infected individuals compared with general populations.12 Other investigators have examined pulse wave velocity (PWV) as a novel measure of subclinical atherosclerosis and a precursor to clinical hypertension.19 Here again, the reported associations between PWV and HIV are mixed.20, 21
However, these studies have had several major limitations. First, most of the prior studies adjusted for traditional cardiovascular risk factors only, such as age and sex, but had limited data on lifestyle factors such as physical activity, smoking, alcohol use, socioeconomic status, and stress, raising the potential for unmeasured confounding.22, 23, 24 Second, some of these studies included body weight as a potential confounder, whereas it is more likely to be a mediator of the effect of HIV on BP.11 Finally, most of these studies were conducted in rural areas, and are therefore not representative of the rapidly growing urban populations where cardiovascular disease risk tends to be much higher.25
Therefore, we conducted a cross‐sectional study of HIV and cardiovascular risk factors in an urban population in Uganda to better understand the relationships between HIV and BP and PWV. We also investigated the potential role of body weight in mediating the effect of HIV on BP.
2. METHODS
2.1. Study population
We conducted a matched cross‐sectional study in Mbarara municipality, Uganda, between June and October 2015 to recruit HIV‐infected persons and age‐, sex‐, and neighborhood‐matched HIV‐uninfected controls known as the ACHIEvA (Aging and Cardiovascular Diseases in HIV Patients of East Africa) study. These matching factors were chosen a priori as important determinants of both HIV and BP.
We used the electronic databases of the Makerere University Joint AIDS Program (MJAP)/Mbarara Municipal Council (MMC) Health Center IV and the Mbarara University/Mbarara Regional Referral Hospital Immune Suppression Syndrome (ISS) clinic to identify adults aged 40 years or older who had visited the clinic within the past 6 months and lived in one of the three divisions of Mbarara municipality (Kakoba, Kamukuzi, and Nyamitanga). We abstracted identifier and contact information and expected dates for subsequent clinic visits to maximize the chances of finding selected HIV‐infected person. Once contact was established, a visit in their households to perform interviews and measurements was arranged based on their earliest convenience.
To recruit matched HIV‐uninfected controls, we randomly chose a household in the same neighborhood as the index HIV patient and selected an adult resident who was of the same sex and in the same 5‐year age category as the HIV‐infected participant. In Mbarara, the distinction of household members from nonresidents is blurred. We therefore considered a household member as anyone who had had at least 3 meals in a household on at least three consecutive days in the past 6 months. If there was more than one potential match in the same household, we used the Kish method26 to select one of them. In the event that a selected matched individual was not home at the time of the visit, three attempts on separate days, including evenings on week days and weekends were made before sampling another household for a match.
If a selected household had no eligible individual, we visited the immediate neighboring household until an eligible participant was found. We excluded pregnant women because pregnancy causes physiological derangement in metabolic parameters, mentally incapacitated persons because of the inability to properly respond to survey questions, and limb or spinal physically incapacitated persons because of the inability to stand for anthropometric measurements.
2.2. Ethics statement
All participants provided informed consent before enrollment into the study, and separate consent was obtained for release of HIV care data from HIV‐infected participants. The head of the MJAP/MMC clinic, director of the ISS clinic, office of the town clerk, and respective administrative heads of Mbarara municipality granted permission to perform this study.
The ethics review committees at Mbarara University of Science and Technology, Harvard T. H. Chan School of Public Health, and the Uganda National Council of Science and Technology approved the study.
2.3. Data collection
2.3.1. Measurement of covariates
Trained research assistants conducted interviews using electronic devices to capture household asset ownership, stress in life and work using the perceived stress tool,27 and physical activity using a validated physical activity questionnaire for sub‐Saharan Africa.28 The questionnaire also captured information on smoking history (age of starting, duration and intensity of smoking, and efforts to quit), alcohol intake using the Alcohol Use Disorders Identification Test (AUDIT‐C) questionnaire,29 history of diagnosis and/or management of cardiovascular disease and its risk factors (hypertension, diabetes mellitus, dyslipidemia), and a list of current medications.
Waist and hip circumferences were measured by standard procedures30 with the participant standing using a tape (seca 201), weight was measured using standardized scales (seca 762), and height was measured using a roll‐up measuring stadiometer (seca 206). Height and waist and hip circumferences were measured to the nearest 0.1 cm and weight was measured to the nearest kilogram. The plausible ranges for the anthropometric measurements were set as 0.5 to 1.5 for waist to hip ratio, 100 to 200 cm for height, and 30 to 150 kg for weight. Values outside of these ranges were set to missing. We used height and weight to calculate body mass index (BMI) as weight (in kilograms) divided by the square of height (in meters), and categorized BMI as underweight (<18.5 kg/m2), normal weight (18.5‐24.9 kg/m2), overweight (25‐29.9 kg/m2), or obese (>30 kg/m2).
We collected spot urine specimens and measured urine sodium and creatinine (Humastar200, Human Diagnostics Worldwide) and estimated 24‐hour urinary sodium excretion using the Kawasaki formula.31 Laboratory tests were performed at Mbarara Regional Referral Hospital laboratory, which has standardized internal quality‐control protocols and participates in external quality‐control programs by the National Health Laboratory Service.
2.3.2. BP and PWV measurements
BP was measured using a digital upper arm sphygmomanometer (Omron BP710N 3 series, Omron Healthcare Inc.) with small (<21 cm), normal (22‐32 cm), and large cuffs (35‐44 cm). The participant was seated in a chair and allowed to rest for 5 minutes before three measurements were performed with 3‐minute intervals. We used the average of the second and third measurements to determine the BP of each participant. We set a range of 70 to 270 mm Hg as plausible values for systolic BP (SBP) and 30 to 150 mm Hg for diastolic BP (DBP). We defined hypertension as having an SBP ≥140 mm Hg or DBP ≥90 mm Hg or self‐reported use of antihypertensive medications.32
We performed two measurements of carotid‐femoral PWV using a handheld device (Arteriograph, TensioMed Arteriograph TL2) with the participant in the supine position. The device estimates PWV by measuring the time lag between the two peaks in the BP wave and combining this information with the distance between jugular notch and symphysis pubis that the operator measures with a tape. During each measurement, PWV was estimated for each pulse wave during 8 seconds and if the standard deviation (SD) of the estimated PWVs was larger than 1 m/s, the recording was discarded and a repeat measurement was performed as recommended by the manufacturer.33 Up to five attempts were made to obtain a valid measurement.
2.4. Statistical analysis
We used principle component analysis (Table S1 and Table S2) to generate an assets index score based on household utilities and assets34 and perceived stress questionnaire responses to derive composite measures with highest discriminatory capabilities. Participants were divided into quintiles of these scores.
We described population characteristics, exposures, and outcomes by HIV status and compared the distributions between HIV‐infected and matched HIV‐uninfected participants in univariate analyses using t test for continuous variables, chi‐square test for categorical variables, and trend test using median values of categories for ordinal variables. We categorized participants with hypertension into undiagnosed; diagnosed and untreated; diagnosed and treated but not controlled; diagnosed, treated, and controlled, with the latter defined as having an SBP <140 mm Hg and a DBP <90 mm Hg.
We used a priori knowledge to select potential confounders (Figure 1): age, sex, asset index, marital status, smoking, alcohol consumption, and perceived stress score that were postulated to affect the association of HIV and BP. Hierarchical linear mixed effects regression models were used to determine the participant‐level association between HIV and BP (SBP and DBP separately) and PWV, adjusted for participant matching, and interviewer‐related differences in measurement of subjective risk factors.35 In addition, we fit a conditional logistic regression model to determine the association between HIV and hypertension status. We also performed a subgroup analysis to estimate the adjusted odds of prevalent hypertension by separating the HIV‐infected participants into those with shorter (<3 years) or longer (≥3 years) duration of ART.
Figure 1.
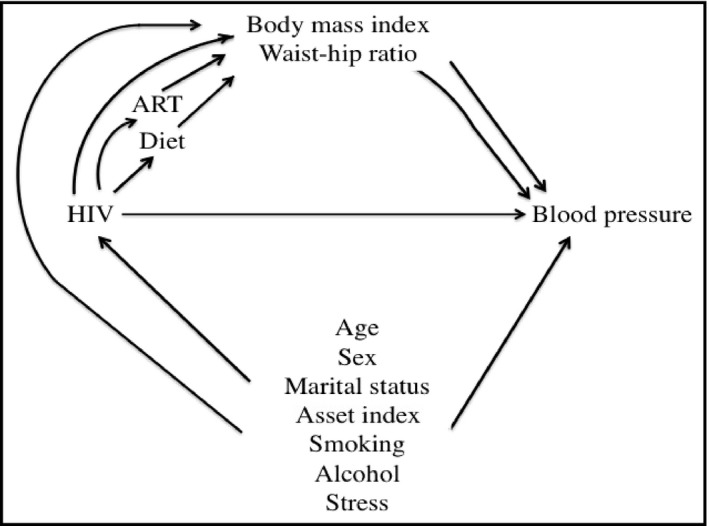
Directed acyclic graph for the relationship between human immunodeficiency virus (HIV) and blood pressure—ACHIEvA (Aging and Cardiovascular Diseases in HIV Patients of East Africa) study 2015
We examined whether BMI (categorized as ≥25 kg/m2 and <25 kg/m2 as a reference value) and waist‐hip ratio (continuous scale) mediated the effects of HIV on SBP and PWV. Direct and indirect effects were estimated using parametric regression models.36, 37 We fit two models, one for BMI as a mediator conditional on HIV and potential confounders and one for SBP conditional on HIV, BMI, and potential confounders with interaction between HIV and BMI. We used bootstrapping to compute bias‐corrected confidence intervals.37 The percentage of mediation was quantified as: (indirect effect/total effect) × 100.
All statistical analyses were conducted using Stata version 13.0 (StataCorp).
3. RESULTS
At time of study, 1659 records of persons with HIV in the clinic databases were eligible for inclusion. We randomly abstracted 909 records and, among these, 184 patients had no telephone contacts, 54 had moved out of the study area by the time of the survey, and 28 had changed their telephone numbers.
A total of 1347 households were visited, 24 people declined to participate, nine had mental incapacitation, two were physically incapacitated, 10 were found to be younger than 40 years, four were pregnant, and 10 had time constraints.
We enrolled 697 HIV‐infected persons and 591 matched HIV‐uninfected persons. All participants with HIV were taking ART except 24 who were not previously diagnosed. After excluding 21 participants who had missing data on confounders (smoking [n = 1], BMI [n = 4], waist circumference [n = 3], and physical activity [n = 13]; Figure 2), the final study population consisted of 1115 participants (577 HIV‐infected and 538 matched HIV‐uninfected). The prevalence of hypertension was higher among the HIV‐uninfected compared with the HIV‐infected participants (28.8% vs 21.5%). In addition, there was no statistical difference in median age in both groups, with HIV‐infected participants having a median age of 45 years (interquartile range, 42‐49 years) and HIV‐uninfected participants having a median age of 46 years (interquartile range, 43‐50 years) (Table 1).
Figure 2.
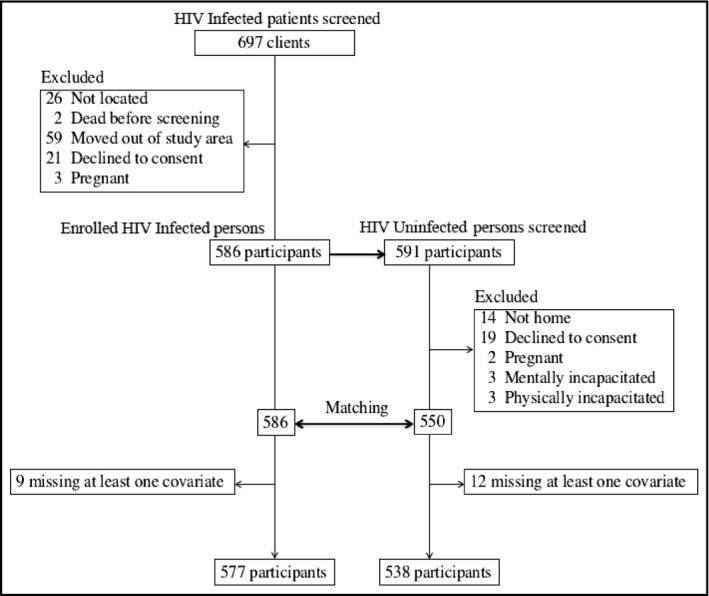
Participant selection process—ACHIEvA (Aging and Cardiovascular Diseases in HIV Patients of East Africa) study 2015
Table 1.
Study characteristics by HIV status—ACHIEvA study 2015 (N = 1115)
| Characteristic | HIV‐infected persons, No. (%) | Matched HIV‐uninfected persons, No. (%) | P value |
|---|---|---|---|
| Women | 326 (57) | 305 (56) | 0.958 |
| Age, median (IQR) | 45 (42‐49) | 46 (43‐50) | 0.0821 |
| Age groups, y | 0.020a | ||
| 40–44 | 246 (42.6) | 178 (33.1) | |
| 45‐49 | 190 (32.9) | 211 (39.2) | |
| 50‐54 | 72 (12.5) | 80 (14.9) | |
| 55‐60 | 45 (7.8) | 36 (6.7) | |
| >60 | 25 (4.2) | 33 (6.1) | |
| Employment status | <0.001 | ||
| Self‐employed | 337 (58.4) | 348 (64.7) | |
| Government or nonprofessional | 73 (12.6) | 62 (11.5) | |
| Private employer | 84 (14.6) | 37 (6.9) | |
| Unemployed | 83 (14.4) | 91 (16.9) | |
| Marital status | <0.001 | ||
| Married or cohabiting | 303 (52.5) | 415 (77.1) | |
| Separated or divorced | 144 (25.0) | 71 (13.2) | |
| Widowed or widower | 119 (20.6) | 48 (8.9) | |
| Single | 11 (1.9) | 4 (0.7) | |
| Religion | 0.009 | ||
| Catholic | 178 (30.8) | 152 (28.2) | |
| Anglican | 276 (47.8) | 232 (43.1) | |
| Muslim | 73 (12.6) | 107 (19.9) | |
| Pentecostal | 46 (8.0) | 42 (7.8) | |
| Other | 4 (0.7) | 5 (0.9) | |
| Smoking status | 0.069a | ||
| Never smoker | 423 (73.3 | 415 (77.1) | |
| Current smoker | 61 (10.6) | 58 (10.8) | |
| Former smoker | 93 (16.1) | 65 (12.1) | |
| Alcohol consumption, g/wk | 0.855a | ||
| 0 | 371 (64.3) | 367 (68.2) | |
| <20 (women) and <40 (men) | 90 (15.6) | 57 (10.6) | |
| 20‐39 (women) and 40‐59 (men) | 20 (3.5) | 16 (3.0) | |
| ≥40 (women) and ≥60 (men) | 96 (16.6) | 98 (18.2) | |
| Perceived stress score (quintiles) | 0.067a | ||
| First | 96 (16.6) | 127 (23.6) | |
| Second | 112 (19.4) | 111 (20.6) | |
| Third | 136 (23.6) | 88 (16.4) | |
| Fourth | 121 (21.0) | 104 (19.3) | |
| Fifth | 112 (19.4) | 108 (20.1) | |
| Asset index | <0.001a | ||
| Poorest | 131 (22.7) | 90 (16.7) | |
| Poorer | 118 (20.4) | 106 (19.7) | |
| Average | 126 (21.8) | 97 (18.0) | |
| Richer | 113 (19.6) | 110 (20.5) | |
| Richest | 89 (15.4) | 135 (24.1) | |
| METS, h/wk | 0.142a | ||
| 0 | 237 (41.1) | 220 (40.9) | |
| 1‐3 | 102 (17.7) | 75 (13.9) | |
| 3‐10 | 127 (22.0) | 114 (21.2) | |
| 10‐20 | 61 (10.6) | 65 (12.1) | |
| >20 | 50 (8.7) | 64 (11.9) | |
| Clinical | |||
| Body mass index category | |||
| Underweight (<18.5) | 56 (9.7) | 26 (4.8) | <0.001a |
| Normal (18.5 to <25) | 301 (52.2) | 210 (39.0) | |
| Overweight (25 to <30) | 131 (22.7) | 169 (31.4) | |
| Obese (≥30) | 89 (15.4) | 133 (24.7) | |
| Waist circumference (both sexes) | 87 (12.6) | 91 (12.4) | <0.001 |
| Waist circumference, men (≥102 cm)b | 11 (4.4) | 29 (12.4) | <0.004 |
| Waist circumference, women (≥88 cm)b | 190 (58.3) | 202 (66.2) | 0.040 |
| Waist‐hip ratio (both sexes) | 0.88 (0.1) | 0.89 (0.1) | 0.059 |
| Waist‐hip ratio, men (≥0.90)b | 97 (38.6) | 120 (51.5) | 0.004 |
| Waist‐hip ratio, women (≥0.85)b | 218 (66.9) | 205 (67.2) | 0.927 |
| Systolic BP, mean (SD)c | 123 (18.3) | 127 (17.8) | <0.001 |
| Diastolic BP, mean (SD)c | 76 (11.5) | 78 (12.3) | 0.027 |
| Antihypertensive medication | 49 (8.5) | 45 (8.4) | 0.685 |
| Prevalent hypertensione | 124 (21.5) | 155 (28.8) | |
| Pulse wave velocity, m/s | 7.8 (1.4) | 8.0 (1.4) | 0.013 |
| Arterial stiffnessd (age stratified) | 10 (1.9) | 5 (1.0) | 0.241 |
| Laboratory | |||
| Serum glycated hemoglobin, mean (SD), % | 5.8 (4.3) | 6.7 (5.1) | 0.005 |
| Serum glycated hemoglobin (>6.5%), No. (%) | 200 (36.4) | 164 (28) | 0.002 |
| Serum HDL cholesterol (all), mean (SD), mg/dL | 58.5 (19.9) | 55.3 (15.0) | 0.01 |
| Serum LDL cholesterol (all), mean (SD), mg/dL | 103 (34.5) | 110 (35.1) | 0.004 |
| Serum triglycerides (all), mean (SD), mg/dL | 175 (99.9) | 181 (91.6) | 0.341 |
| Serum total cholesterol (all), mean (SD), mg/dL | 188 (73.9) | 182 (52.2) | 0.207 |
| Urine sodium excretion, mean (SD), g/24 h | 8.40 (3.3) | 7.96 (3.1) | 0.040 |
aTrend test: waist circumference and waist‐hip ratio. bWorld Health Organization thresholds for substantially increased cardiovascular disease risk. cAverage of same sitting second and third blood pressure (BP) measurements. dEuropean47 cutoffs for arterial stiffness: pulse wave velocity >11 m/s for patients aged 20 to 40 years; pulse wave velocity >12 m/s for patients aged 41 to 59 years. eHypertension defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, and/or self‐reported antihypertensive drug use.
Pulse wave velocity measurements (n = 1026; 530 human immunodeficiency virus [HIV]‐infected and 496 uninfected), glycated hemoglobin (n = 865; 448 HIV‐infected and 417 HIV‐uninfected persons), serum low‐density lipoprotein (LDL) cholesterol and triglycerides (n = 899; 464 HIV‐infected and 435 HIV‐uninfected persons), serum cholesterol and high‐density lipoprotein (HDL) cholesterol (n = 811; 420 HIV‐infected and 391 HIV‐uninfected persons), and urine sodium excretion estimation (n = 890; 462 HIV‐infected and 428 HIV‐uninfected persons).
Abbreviations: ACHIEvA, the Aging and Cardiovascular Diseases in HIV Patients of East Africa study; IQR, interquartile range; METS, metabolic equivalents; SD, standard deviation.
A total of 91% (524/577) of HIV‐infected participants had been taking ART for a median duration of 4.8 years (interquartile range, 2.6‐7.6) with a median nadir CD4 cell count of 206 cells/mL (interquartile range, 104‐307) at time of ART initiation. Although none of the HIV‐infected participants had been initiated on a protease inhibitor (PI)‐based ART regimen, we found that 3% (n = 17) were taking a PI‐based regimen at the time of the study (Table 2).
Table 2.
Characteristics of HIV‐infected participants—ACHIEvA study 2015
| Characteristic | N = 577 |
|---|---|
| Taking ART, No. (%) | 528 (91.5) |
| HIV, not initiated ART | 24 (4.2) |
| Previously undiagnosed, No. (%) | 25 (4.3) |
| Follow‐up duration before ART initiation, median (IQR), mo | 4 .3 (1.4‐24.9) |
| Follow‐up duration after ART initiation, median (IQR), y | 4.8 (2.6‐7.6) |
| Age at ART initiation, median (IQR), y | 40 (37‐45) |
| Systolic BP at ART initiation, mean (SD) | 116 (18) |
| Diastolic BP at ART initiation, mean (SD) | 73 (10) |
| Body mass index at ART initiation, mean (SD) | 22.9 (9.1) |
| CD4 cell counts at ART initiation, median (IQR), cells/mL | 163 (62‐251) |
| CD4 cell counts at time of study, median (IQR), cells/mL | 479 (338‐656) |
| Changed ART regimen during follow‐up, No. (%) | 149 (28) |
| Duration on baseline regimen, median (IQR), ya | 2.4 (0.5‐3.3) |
| Participants initiated on protease inhibitors at ART initiation, No. (%) | 0 (0) |
| Participants taking protease inhibitors at time of study, No. (%) | 17 (3) |
| Participants initiated on stavudine at ART initiation, No. (%) | 87 (16) |
| Participants taking stavudine at time of study, No. (%) | 9 (2) |
Abbreviations: ART, antiretroviral therapy; BP, blood pressure; IQR, interquartile range; SD, standard deviation.
Among patients who had at least one change in drug regimen.
Twenty‐five participants were diagnosed with human immunodeficiency virus (HIV) during the study and had no HIV data and 28 had been diagnosed with HIV but were not receiving care during the ACHIEvA (Aging and Cardiovascular Diseases in HIV Patients of East Africa) study.
A total of 36% of HIV‐uninfected controls and 28% of HIV‐infected participants had serum glycated hemoglobin >6.5% (P = 0.002), defined as diabetes mellitus. HIV‐infected participants had a mean serum low‐density lipoprotein cholesterol level of 103 mg/dL (SD ± 34.5) vs 110 mg/dL (SD ± 35.1) for uninfected controls (P = 0.004; Table 1). The mean SBP was 123 mm Hg (SD ± 18.3) among HIV‐infected participants and 127 mm Hg (SD ± 17.8) among HIV‐uninfected controls. A total of 276 participants were found to have hypertension (119 men [50 HIV‐infected and 69 HIV‐uninfected controls] and 157 women [71 HIV‐infected and 86 HIV‐uninfected controls]).
After adjusting for potential confounders, HIV infection was associated with a 3.3‐mm Hg lower SBP (95% confidence interval [CI], 1.2‐5.3), 1.5‐mm Hg lower DBP (95% CI, 0.2‐2.9), 0.3‐m/s slower PWV (95% CI, 0.1‐0.4), and 30% lower odds of hypertension (95% CI, 10%‐50%). In addition, increasing alcohol consumption was associated with higher SBP (P = 0.003) and DBP (P = 0.002) whereas increasing physical activity was associated with lower SBP (P = 0.040) and PWV (P = 0.001) (Table 3).
Table 3.
Adjusted mean difference for systolic and diastolic BP and pulse wave velocity and adjusted odds ratios (95% confidence interval) for hypertension prevalence—ACHIEvA study 2015 (N = 1115)
| Characteristic | aSystolic BP, mm Hg | c P value | aDiastolic BP, mm Hg | c P value | Pulse wave velocity, m/s | c P value | bHypertension | c P value |
|---|---|---|---|---|---|---|---|---|
| HIV‐uninfected | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| HIV‐infected | −3.3 (−5.3 to −1.2) | 0.002 | −1.5 (−2.9 to −0.2) | 0.027 | −0.3 (−0.4 to −0.1) | 0.002 | 0.7 (0.5‐0.9) | 0.022 |
| Marital status | 0.337 | 0.511 | 0.060 | 0.744 | ||||
| Married | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| Separated/divorced | −3.1 (−5.9 to 0.2) | −0.8 (−2.7 to 1.0) | 0.1 (−0.1 to 0.4) | 0.6 (0.3‐1.3) | ||||
| Widowed | −0.9 (−4.0 to 2.2) | −1.0 (−3.1 to 1.1) | 0.2 (−0.1 to 0.5) | 0.9 (0.4‐1.8) | ||||
| Single | −1.7 (−10.4 to 7.1) | 0.7 (−5.1 to 6.6) | 0.2 (−0.5 to 0.9) | 2.0 (0.3‐14.4) | ||||
| Employment | 0.113 | 0.138 | 0.046 | 0.047 | ||||
| Self‐employed | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| Government/nongovernmental organization | 1.6 (−1.7 to 5.0) | −0.03 (−2.3 to 2.2) | 0.1 (−0.1 to 0.4) | 1.3 (0.6‐3.1) | ||||
| Private employer | 1.9 (−1.5 to 5.4) | 1.7 (−0.5 to 4.0) | 0.0 (−0.2 to 0.3) | 1.4 (0.6‐3.4) | ||||
| Unemployed | 1.6 (−1.4 to 4.7) | 0.8 (−1.2 to 2.8) | 0.3 (0.1‐0.6) | 1.7 (0.8‐3.5) | ||||
| Religion | 0.528 | 0.353 | 0.856 | 0.904 | ||||
| Catholic | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| Anglican | 1.6 (−0.9 to 4.0) | 0.4 (−1.2 to 2.0) | 0.1 (−0.1 to 0.3) | 1.5 (0.8‐2.8) | ||||
| Muslim | 3.1 (−0.3, 6.5) | 0.7 (−1.6, 2.9) | 0.2 (0.1, 0.5) | 1.1 (0.5‐2.5) | ||||
| Pentecostal | −0.7 (−4.9, 3.5) | −2.5 (−5.3, 0.3) | −0.2 (−0.5, 0.2) | 1.2 (0.4‐3.6) | ||||
| Other | 1.1 (−10.7, 12.9) | −1.4 (−9.2, 6.5) | −0.3 (−1.3, 0.6) | 0.8 (0.1‐7.0) | ||||
| Smoking status | 0.098d | 0.686d | 0.355d | 0.580d | ||||
| Never smoker | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| Current smoker | −2.7 (−6.3 to 0.9) | −2.8 (−52 to 0.4) | −0.1 (−0.4 to 0.2) | 0.3 (0.1‐0.9) | ||||
| Former smoker | 2.7 (−0.4 to 5.8) | −0.3 (−2.3 to 1.8) | −0.1 (−0.4 to 0.1) | 0.9 (0.4‐2.1) | ||||
| Alcohol consumption, g/wke | 0.003d | <0.002d | 0.549d | 0.610d | ||||
| 0 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| <20(women) and <40(men) | −0.6 (−3.9 to 2.6) | −0.4 (−2.5 to 1.8) | −0.2 (−0.5 to 0.1) | 1.0 (0.5‐2.1) | ||||
| >20(women) and >40(men) | 5.3 (2.4‐8.2) | 3.6 (1.7‐5.6) | −0.0 (−0.2 to 0.2) | 1.7 (0.8‐3.4) | ||||
| Perceived stress (quintiles) | 0.842d | 0.083d | 0.802d | 0.545d | ||||
| First | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| Second | −0.9 (−4.2 to 2.3) | 0.5 (−1.7 to 2.6) | −0.0 (−0.3 to 0.2) | 0.5 (0.3‐1.2) | ||||
| Third | 0.4 (−2.9 to 3.7) | 0.2 (−1.9 to 2.4) | −0.0 (−0.3 to 0.2) | 0.7 (0.3‐1.6) | ||||
| Fourth | 0.2 (−3.1 to 3.5) | 1.2 (−1.9 to 2.4) | 0.1 (−0.2 to 0.4) | 0.8 (0.3‐1.8) | ||||
| Fifth | −1.0 (−4.3 to 2.2) | 1.8 (−0.4 to 3.9) | −0.0 (−0.3 to 0.2) | 1.0 (−0.3 to 2.4) | ||||
| Asset index | 0.275d | 0.173d | 0.014d | 0.055d | ||||
| Average | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| Poorest | 0.5 (−2.9 to 3.8) | 0.5 (−1.8 to 2.8) | −0.1 (−0.4 to 0.2) | 0.6 (0.3‐1.7) | ||||
| Poorer | 0.6 (−2.6 to 3.8) | 0.2 (−1.9 to 2.4) | −0.0 (−0.3 to 0.2) | 0.7 (0.3‐1.6) | ||||
| Richer | 0.1 (−3.1 to 3.4) | −0.2 (−2.3 to 2.0) | 0.1 (−0.2 to 0.3) | 0.9 (0.4‐2.1) | ||||
| Richest | 1.6 (−1.7 to 4.9) | 2.0 (−0.2 to 4.2) | 0.2 (−0.1 to 0.4) | 1.4 (0.6‐3.2) | ||||
| METS h/wk | 0.040d | 0.321d | 0.001d | 0.507d | ||||
| 0 | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | 1.0 (Reference) | ||||
| 1‐3 | −1.5 (−4.5 to 1.6) | −0.1 (−2.9 to 2.0) | 0.1 (−0.2 to 0.3) | 1.0 (0.5‐2.0) | ||||
| 3‐10 | −1.1 (−3.9 to 1.6) | −1.3 (−3.2 to 0.5) | −0.2 (−0.4 to 0.1) | 1.0 (0.5‐1.9) | ||||
| 10‐20 | −5.1 (−8.7 to −1.6) | −2.7 (−5.1 to −0.4) | −0.6 (−0.9 to −0.4) | 1.0 (0.4‐2.6) | ||||
| >20 | −1.9 (−5.6 to 1.8) | 0.7 (−1.8 to 3.1) | −0.2 (−0.5 to 0.1) | 0.5 (0.2‐1.5) |
Abbreviations: ACHIEvA, Aging and Cardiovascular Diseases in HIV Patients of East Africa study; HIV, human immunodeficiency virus; METS, metabolic equivalents.
Average of same sitting second and third blood pressure (BP) measurements.
Hypertension defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, and/or self‐reported antihypertensive drug use.
Chi‐square test unless otherwise specified.
Test of trend based on median value within each category.
Definition based on number of days consumed alcohol over the past 30 days and the average number of drinks per drinking session.
In subgroup analyses, we found that HIV‐infected participants taking ART ≥3 years had a slightly lower prevalence of hypertension (18.4% [81/440]) compared with those taking ART for <3 years (21% [26/124]). We also estimated the adjusted odds of prevalent hypertension by separating the HIV‐infected participants into those with shorter (<3 years) or longer (≥3 years) duration of ART. We found that both groups had lower odds of hypertension compared with HIV‐uninfected participants and the difference between the two groups were not statistically significant as indicated by a point estimate for one group falling within the 95% CI for the other.
The indirect effect of HIV infection through BMI led to a 0.8‐mm Hg lower SBP (95% CI, 0.3‐1.5), equivalent to 25% of the total association between HIV and SBP (Figure 3, panel A). The corresponding proportion mediated by waist‐hip ratio was only 6% (Figure 3, panel B).
Figure 3.
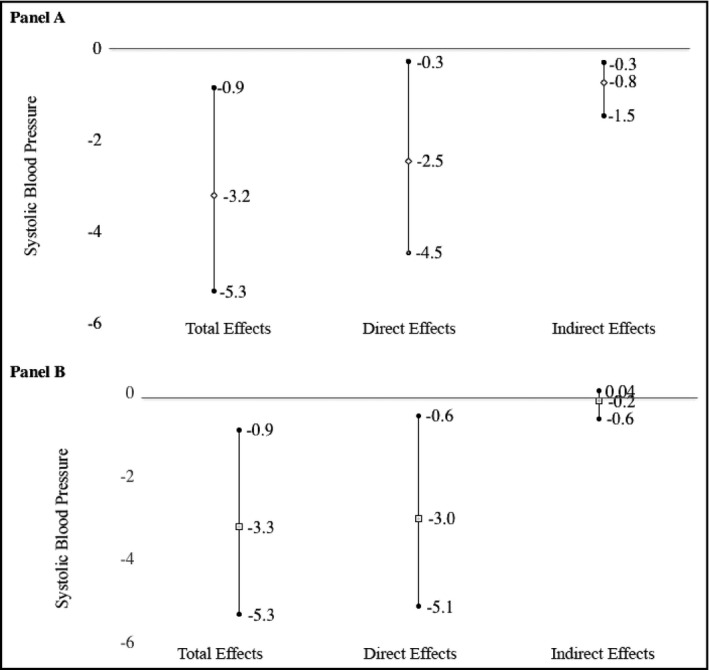
Total, direct, and indirect effects of human immunodeficiency virus (HIV) on systolic blood pressure with respect to body mass index (panel A) and waist‐hip ratio (panel B)—ACHIEvA (Aging and Cardiovascular Diseases in HIV Patients of East Africa) study 2015
The relationship between HIV infection and hypertension care was complex and varied by sex. HIV‐infected participants with hypertension had a higher rate of being previously diagnosed (44% vs 28% in men, 56% vs 48% in women). Among men, HIV‐infected participants who were diagnosed with hypertension had a higher rate of being treated (77% vs 47%) and among those treated, HIV‐infected participants had a higher chance of being controlled (29% vs 11%) compared with HIV‐uninfected counterparts. Among women, HIV‐infected participants with hypertension were less likely to be treated (80% vs 88%) and among those treated, HIV‐infected participants were less likely to have controlled BP (34% vs 61%; Figure 4).
Figure 4.
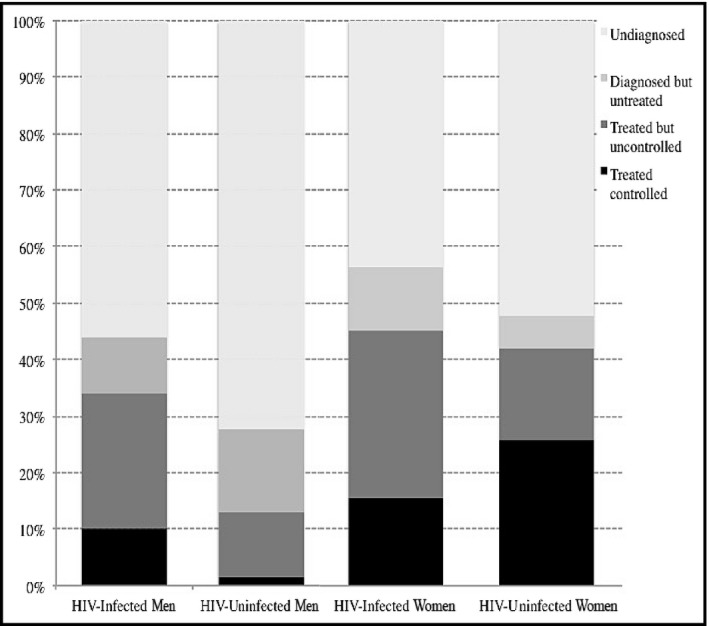
Hypertension awareness, treatment, and control by human immunodeficiency virus (HIV) status and sex—ACHIEvA (Aging and Cardiovascular Diseases in HIV Patients of East Africa) study 2015. ART indicates antiretroviral therapy
4. DISCUSSION
We found an inverse relationship between HIV and BP, PWV, and odds of hypertension in this population of adults from an urban site in Uganda. Our results are similar to those reported in a meta‐analysis of studies in sub‐Saharan Africa, which found that HIV infection was associated with lower SBP and DBP.12 We postulate that this result could be explained by a phenomenon of cardiovascular autonomic dysfunction that leads to low BP levels in HIV‐infected persons irrespective of level of immunosuppression.38, 39 Furthermore, HIV‐infected patients in our study population tended to have advanced HIV disease at entry into HIV care and might have had comorbid opportunistic infections that could lower their BP levels directly or through weight loss.40 Conversely, other studies have found that HIV infection with ART is associated with higher BP levels compared with HIV‐uninfected persons with a similar sex and age distribution.7, 17 The discrepancy between these results and our observation could be caused by the rare (<2%) use of PIs in our study population. These agents are known to increase BP levels7, 41 and induce metabolic complications.17
We found that HIV infection was associated with lower PWV (arterial stiffness) compared with age‐, sex‐, and neighborhood‐matched HIV‐uninfected controls.42 This finding is in contrast to a few previous reports that found that HIV‐infected patients taking ART had higher PWV values compared with HIV‐infected persons not taking ART or HIV‐uninfected matched controls.43, 44 Most of our HIV‐infected participants were not taking PIs that have been shown to be proatherogenic and had suppressed viral replication at the time of the study. The combined effect of low use of PIs and suppressed viral replication may have resulted in lower systemic inflammation and endothelial dysfunction leading to reduced arterial stiffness in HIV‐infected participants.20 Furthermore, in our study, cardiovascular risk factors such as obesity, physical inactivity, and dyslipidemia were more prevalent in the HIV‐uninfected controls than the HIV‐infected participants. This suggests that traditional cardiovascular risk factors other than HIV‐related characteristics are responsible for atherosclerosis and hypertension in HIV.10, 45
We found that BMI mediated a quarter of the association between HIV and SBP. This confirms our expectation that lower body weight in HIV‐infected patients is associated with lower BP and is also consistent with previous reports of increase in BP and body weight after initiation of ART.40, 45, 46 We did not find any prior studies that investigated the role of BMI in mediating the effects of HIV on BP to compare with ours.
As expected, we found that in both sexes, HIV‐infected participants with hypertension were more aware of their hypertension status and were more likely to be taking treatment as compared with HIV‐uninfected participants. However, among women, control rates were lower for HIV‐infected participants. Although these differences are not statistically significant, they point to better rates of diagnosis and treatment for hypertension among HIV‐infected participants but the overall rates are still suboptimal. The differences in prevalence rates of awareness, treatment, and control between HIV‐infected and ‐uninfected participants might be partly attributable to the differences in health‐seeking behaviors of HIV‐infected and HIV‐uninfected persons47 as well as differences in resources (medical staff, facilities, and supplies) available in general health facilities compared with HIV care facilities48 Strategies to integrate HIV care with other health programs have focused primarily on tuberculosis, sexually transmitted infections, malaria, and reproductive health49, 50 and there is only limited experience on integrating cardiovascular disease prevention into HIV care. A recent report from South Africa found that integrating noncommunicable diseases care into HIV care is cost‐effective and leads to improved functional ability and health status in patients infected with HIV.51
5. STUDY STRENGTHS AND LIMITATIONS
Our study has several strengths. HIV‐infected participants were sampled from comprehensive databases of all patients receiving treatment in two of the three HIV clinics in Mbarara, covering most of the diagnosed patients in the area. HIV‐uninfected participants were selected from the same neighborhood, which greatly reduces the potential for confounding by location of residence and socioeconomic status. In addition to BP, we measured PWV as an indicator of early atherosclerosis, which has not been investigated with respect to HIV status in sub‐Saharan Africa. We used a rigorous and standard BP measurement protocol and collected information on many lifestyle and socioeconomic factors using standard and validated questionnaires. However, our results should be interpreted with some limitations in mind. Although we collected data on many potential confounders, there is always a potential for unmeasured confounding and residual confounding caused by measurement error in the selected confounders. As most of the participants were taking ART, we were not able to examine the impact of ART on BP and hypertension as a mediator of the effect of HIV infection. In addition, there was little variability in ART regimens in our study population, precluding analysis of the impact of different ART regimens on BP.
6. CONCLUSIONS
HIV infection was associated with lower SBP, PWV, and odds of hypertension. Although HIV infection was associated with a higher rate of diagnosis and treatment of hypertension, and better control rates among men, it was associated with worse control rates among women. To improve cardiovascular disease care among patients with HIV, health‐centered services should be combined with community‐level approaches to improve the reach and cost‐effectiveness of preventive interventions. Such healthcare models remain largely untapped in HIV‐endemic countries such as Uganda.
CONFLICT OF INTEREST
The authors have no financial or other potential conflict of interest with regard to this manuscript.
AUTHOR CONTRIBUTIONS
S.O, P.U, A.K, E.B, W.R.M, and G.D conceptualized and designed the study; S.O and P.U analyzed the data; S.O wrote the first draft of the manuscript; and S.O, P.U, A.K, E.B, A.K, G.A, W.R.M, and G.D contributed to the write‐up and edited the manuscript. All authors approved the manuscript.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the staff at the Immune Suppression Syndrome (ISS) clinic and Mbarara Municipal Council Health Center IV, Medexpert Ltd Budapest (Hungary) for providing arteriogram devices. Study staff: Abia Begumisa, Agnes Alupo, Boaz Niwamanya, Chris Mugume, Christine Kazungu, Daniel Nabimanya, Daphine K. Atwine, Elizabeth Ninsiima, Emily Ashaba, Emmanuel Ndyabahika, Gertrude Kyarimpa, Harriet Akidi, Hope Namanya, Jonath Tumusiime, Jovita Kyosiimire, Martin Kiwe, Mary Tumweshengyereze, Milliam Korukiiko, Opherous Oshabahebwa, Patrick Oroma, Phionah Ampaire, Resty Nalugya, Ronald Mwesigye, Thelema B. Kateeba, and Tony Engwau.
Okello S, Ueda P, Kanyesigye M, et al. Association between HIV and blood pressure in adults and role of body weight as a mediator: Cross‐sectional study in Uganda. J Clin Hypertens. 2017;19:1181–1191. 10.1111/jch.13092
Funding information
This study was supported by the Bernard Lown Scholars in Cardiovascular Health Program and a pilot grant through the Center for the Global Demography of Aging (National Institute of Health: AG024409) at Harvard T. H. Chan School of Public Health. The funders had no role in study design, conduct, data analysis, or production of this article
REFERENCES
- 1. UNAIDS . Global Report 2012: UNAIDS Report on the Global AIDS Epidemic. ebookpartnership. com; 2013.
- 2. Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low‐income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209‐216. [DOI] [PubMed] [Google Scholar]
- 3. Floyd S, Molesworth A, Dube A, et al. Population‐level reduction in adult mortality after extension of free anti‐retroviral therapy provision into rural areas in northern Malawi. PLoS ONE. 2010;5:e13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bor J, Herbst AJ, Newell M‐L, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale‐up of HIV treatment. Science. 2013;339:961‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nduka CU, Stranges S, Bloomfield GS, et al. A plausible causal link between antiretroviral therapy and increased blood pressure in a sub‐Saharan African setting: a propensity score‐matched analysis. Int J Cardiol. 2016;220:400‐407. [DOI] [PubMed] [Google Scholar]
- 7. Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953‐960. [DOI] [PubMed] [Google Scholar]
- 8. Baekken M, Os I, Sandvik L, Oektedalen O. Hypertension in an urban HIV‐positive population compared with the general population: influence of combination antiretroviral therapy. J Hypertens. 2008;26:2126‐2133. [DOI] [PubMed] [Google Scholar]
- 9. Durand M, Sheehy O, Baril J‐G, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case–control study using Quebec's public health insurance database. JAIDS J Acquir Immune Defic Syndr. 2011;57:245‐253. [DOI] [PubMed] [Google Scholar]
- 10. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506‐2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schutte AE, Schutte R, Huisman HW, et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5‐year prospective study. Int J Epidemiol. 2012;41:1114‐1123. [DOI] [PubMed] [Google Scholar]
- 12. Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub‐Saharan Africa: a systematic review and meta‐analysis. Int J Epidemiol. 2013;42:1754‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scholten F, Mugisha J, Seeley J, et al. Health and functional status among older people with HIV/AIDS in Uganda. BMC Public Health. 2011;11:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bergersen B, Sandvik L, Dunlop O, Birkeland K, Bruun J. Prevalence of hypertension in HIV‐positive patients on highly active retroviral therapy (HAART) compared with HAART‐naive and HIV‐negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22:731‐736. [DOI] [PubMed] [Google Scholar]
- 15. Khalsa A, Karim R, Mack WJ, et al. Correlates of prevalent hypertension in a large cohort of HIV‐infected women: Women's Interagency HIV Study. AIDS. 2007;21:2539‐2541. [DOI] [PubMed] [Google Scholar]
- 16. Jericó C, Knobel H, Montero M, et al. Hypertension in HIV‐infected patients: prevalence and related factors. Am J Hypertens. 2005;18:1396‐1401. [DOI] [PubMed] [Google Scholar]
- 17. Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. J Hypertens. 2003;21:1377‐1382. [DOI] [PubMed] [Google Scholar]
- 18. Schouten J, Wit FW, Stolte IG, et al. Cross‐sectional comparison of the prevalence of age‐associated comorbidities and their risk factors between HIV‐infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787‐1797. [DOI] [PubMed] [Google Scholar]
- 19. Ikonomidis I, Lekakis J, Papadopoulos C, et al. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never‐treated patients with essential hypertension. Am J Hypertens. 2008;21:806‐813. [DOI] [PubMed] [Google Scholar]
- 20. Echeverría P, Bonjoch A, Moltó J, et al. Pulse wave velocity as index of arterial stiffness in HIV‐infected patients compared with a healthy population. JAIDS J Acquir Immune Defic Syndr. 2014;65:50‐56. [DOI] [PubMed] [Google Scholar]
- 21. Maia‐Leite LH, Catez E, Boyd A, et al. Aortic stiffness aging is influenced by past profound immunodeficiency in HIV‐infected individuals: results from the EVAS‐HIV (EValuation of Aortic Stiffness in HIV‐infected individuals). J Hypertens. 2016;34:1338‐1346. [DOI] [PubMed] [Google Scholar]
- 22. Althoff K, Gange S. A critical epidemiological review of cardiovascular disease risk in HIV‐infected adults: the importance of the HIV‐uninfected comparison group, confounding, and competing risks. HIV Med. 2013;14:191‐192. [DOI] [PubMed] [Google Scholar]
- 23. Grotto I, Huerta M, Sharabi Y. Hypertension and socioeconomic status. Curr Opin Cardiol. 2008;23:335‐339. [DOI] [PubMed] [Google Scholar]
- 24. Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta‐analysis. J Hypertens. 2015;33:221‐229. [DOI] [PubMed] [Google Scholar]
- 25. Sliwa K, Acquah L, Gersh BJ, Mocumbi AO. Impact of socioeconomic status, ethnicity, and urbanization on risk factor profiles of cardiovascular disease in Africa. Circulation. 2016;133:1199‐1208. [DOI] [PubMed] [Google Scholar]
- 26. Le KT, Brick JM, Diop A, Alemadi D. Within‐household sampling conditioning on household size. Int J Public Opin Res. 2013;25:108‐118. [Google Scholar]
- 27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385‐396. [PubMed] [Google Scholar]
- 28. Sobngwi E, Mbanya JC, Unwin NC, Aspray TJ, Alberti KG. Development and validation of a questionnaire for the assessment of physical activity in epidemiological studies in Sub‐Saharan Africa. Int J Epidemiol. 2001;30:1361‐1368. [DOI] [PubMed] [Google Scholar]
- 29. Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT‐C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208‐1217. [DOI] [PubMed] [Google Scholar]
- 30. Consultation WE. Waist circumference and waist‐hip ratio. Report of a WHO Expert Consultation Geneva: World Health Organization; 2008:8‐11. [Google Scholar]
- 31. Mente A, O'Donnell MJ, Dagenais G, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24‐h measures in 11 countries. J Hypertens. 2014;32:1005‐1015. [DOI] [PubMed] [Google Scholar]
- 32. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560‐2571. [DOI] [PubMed] [Google Scholar]
- 33. TensioMed . ArterioGraph User's Manual. TensioMed Arteriograph. Vol 5‐02. Budapest, Hungary: TensioMed; 2011. [Google Scholar]
- 34. Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38:115‐132. [DOI] [PubMed] [Google Scholar]
- 35. Himelein K. Interviewer effects in subjective survey questions: evidence from Timor‐Leste. Int J Public Opin Res. 2015;28:511‐533. [Google Scholar]
- 36. MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Emsley R, Liu H. PARAMED: Stata module to perform causal mediation analysis using parametric regression models. Statistical Software Components. 2013. [Google Scholar]
- 38. Nzuobontane D, Ngu BK, Christopher K. Cardiovascular autonomic dysfunction in Africans infected with human immunodeficiency virus. J R Soc Med. 2002;95:445‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mattana J, Siegal FP, Sankaran RT, Singhal PC. Absence of age‐related increase in systolic blood pressure in ambulatory patients with HIV infection. Am J Med Sci. 1999;317:232‐237. [DOI] [PubMed] [Google Scholar]
- 40. Feigl AB, Bloom DE, Danaei G, et al. The effect of HIV and the modifying effect of anti‐retroviral therapy (ART) on body mass index (BMI) and blood pressure levels in rural South Africa. PLoS ONE. 2016;11:e0158264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chow DC, Souza SA, Chen R, Richmond‐Crum SM, Grandinetti A, Shikuma C. Elevated blood pressure in HIV‐infected individuals receiving highly active antiretroviral therapy. HIV Clin Trials. 2003;4:411‐416. [DOI] [PubMed] [Google Scholar]
- 42. Papita AM, Albu A, Fodor D, Bondor C, Itu C, Cârstina D. Markers of preclinical vascular disease and left ventricular diastolic dysfunction in patients with HIV infection. Med Ultrason. 2012;14:10. [PubMed] [Google Scholar]
- 43. Lekakis J, Ikonomidis I, Palios J, et al. Association of highly active antiretroviral therapy with increased arterial stiffness in patients infected with human immunodeficiency virus. Am J Hypertens. 2009;22:828‐834. [DOI] [PubMed] [Google Scholar]
- 44. Schillaci G, De Socio GV, Pucci G, et al. Aortic stiffness in untreated adult patients with human immunodeficiency virus infection. Hypertension. 2008;52:308‐313. [DOI] [PubMed] [Google Scholar]
- 45. Okello S, Kanyesigye M, Muyindike WR, et al. Incidence and predictors of hypertension in adults with HIV initiating antiretroviral therapy in Southwestern Uganda. J Hypertens. 2015;33:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palacios R, Santos J, Garcia A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV‐infected patients. A prospective study in a cohort of naive patients. HIV Med. 2006;7:10‐15. [DOI] [PubMed] [Google Scholar]
- 47. Crawford TN, Sanderson WT, Breheny P, Fleming ST, Thornton A. Impact of non‐HIV related comorbidities on retention in HIV medical care: does retention improve over time? AIDS Behav. 2014;18:617‐624. [DOI] [PubMed] [Google Scholar]
- 48. Mayosi BM, Benatar SR. Health and health care in South Africa—20 years after Mandela. N Engl J Med. 2014;371:1344‐1353. [DOI] [PubMed] [Google Scholar]
- 49. Hermans SM, Castelnuovo B, Katabira C, et al. Integration of HIV and TB services results in improved TB treatment outcomes and earlier, prioritized ART initiation in a large urban HIV clinic in Uganda. J Acquir Immune Defic Syndr. 2012;60:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hope R, Kendall T, Langer A, Bärnighausen T. Health systems integration of sexual and reproductive health and HIV services in sub‐Saharan Africa: a scoping study. J Acquir Immune Defic Syndr. 2014;67:S259‐S270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Levitt NS, Steyn K, Dave J, Bradshaw D. Chronic noncommunicable diseases and HIV‐AIDS on a collision course: relevance for health care delivery, particularly in low‐resource settings—insights from South Africa. Am J Clin Nutr. 2011;94:1690S‐1696S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


