Abstract
microRNA (miRNA) play critical roles in the pathological processes of diabetic retinopathy, including inflammatory responses, insulin signaling, and angiogenesis. In addition to their regulatory functions on gene expression, miRNA is considered as a potential therapeutic target, as well as a diagnostic marker for many diseases. Our understanding on the pathological mechanisms underlying diabetic retinopathy is still incomplete and additional investigations are required to develop novel therapeutic strategies. The aim of this study was to investigate our hypothesis that miR-146a plays a role in suppressing pro-inflammatory pathways, involving STAT3 and VEGF, through regulating IL-6 signaling to reduce apoptosis of human retinal endothelial cells (REC) in high glucose conditions. Human REC were cultured in normal (5mM) glucose or high glucose medium (25 mM) for 3 days. We performed transfections on REC with miRNA mimics (hsa-miR-146a-5p). Overexpression of miR-146a reduced IL-6 levels, STAT3 phosphorylation, and VEGF levels in REC cultured in high glucose. Cellular apoptosis was decreased in REC overexpressing miR-146a, as demonstrated by the inhibition of DNA fragmentation. More importantly, we demonstrated that the regulatory role of miR-146a on STAT3/VEGF and apoptosis was mediated by IL-6 receptor signaling in REC.
Overall, we report that miR-146a suppressed IL-6 signaling, leading to reduced levels of STAT3 and VEGF in REC in high glucose conditions, leading to decreased apoptosis. The outcome suggests that miR-146a is a potential molecular target for inhibiting inflammation and apoptosis in the diabetic retina through the suppression of the IL-6-mediated STAT3/VEGF pathway.
Keywords: miR-146a, REC, IL-6, STAT3, VEGF
1. Introduction
In 2012, 9.3% of Americans or 29 million people had diabetes (National Diabetes Statistics Report, 2014). The prevalence of diabetes will increase by 366 million people worldwide by 2030; with the population at risk of blindness escalating with increased diabetes incidence, according to World Health Organization (WHO). The molecular mechanisms of diabetic retinopathy remain poorly understood with extensive investigations ongoing focusing on the cellular pathways underlying hyperglycemic-induced retinal damage and diabetic retinopathy, with the goal of development of novel strategies for therapeutics. miRNA regulate target genes by binding to 3′ UTR of mRNA, inducing post-transcriptional reduction in gene expression (2). miRNA can be detected in serum, plasma, and a variety of body fluids, including saliva and urine (3,4). Thus, miRNA are novel and powerful candidates for diagnostic biomarkers and for assisting in the development of therapeutic strategies (5). Altered expressions of miRNA have been reported in a variety of pathological conditions, including diabetic retinopathy (6,7) and inflammatory responses (8,9).
There is increasing evidence of the functions of miRNA in many diseases, we still have a limited understanding on how specific miRNA may influence the molecular mechanisms in the pathology of diabetic retinopathy. miR-146a is one of many candidates, in addition to miR-15b-, -16, -18b, -29, -195, and -200b, -221, that have been studied for a regulatory role in diabetic retinopathy and/or hyperglycemia (10–17). In addition to diabetic retinopathy, miR-146a has been studied in different types of ocular disorders, such as corneal disease (18), age-related macular degeneration (19), uveal melanomas (20), autoimmune uveo-retinitis (21), and Graves’ ophthalmopathy (22). In diabetic retinopathy, reduced levels of miR-146a were found in the serum of T2D patients, which was associated with chronic inflammation (23). Also, modified rhythmic expression of miR-146a was shown in human retinal endothelial cells from diabetic donors (24). Our previous study demonstrated inhibitory roles of miR-146a on high glucose-induced TLR4 signaling leading to altered NF-κ and TNFα in REC (25).
Elevated IL-6 levels, a key pro-inflammatory cytokine, have been reported in diabetic retinopathy (26–29). IL-6 was reported as a potential target of miR-146a, as shown by the homology between the 3′-UTR of human IL-6 mRNA and miR-146a (30). Negative regulatory effects of miR-146a on IL-6 were reported in patients with T2DM (31) and in human aortic endothelial cells (30). Classic IL-6 signaling works through membrane bound IL-6 receptors, which is found on only limited types of cells, including subsets of leukocytes, epithelial cells, and hepatocytes (32). It is suggested that IL-6 trans-signaling, acting through soluble IL-6 receptor (sIL-6R), plays a role in the pathophysiology of diabetic retinopathy. Elevated levels of IL-6 and sIL-6R were shown in the serum and aqueous humor of subjects with diabetic retinopathy (33). In addition, higher levels of sIL-6R were found in the serum and vitreous fluid of patients with proliferative DR. Furthermore, the levels of sIL-6R had a significant correlation with VEGF levels in the vitreous fluid (34).
IL-6 can stimulate Jak/STAT3 signaling in the eye (35–37). Additionally, high glucose can stimulate STAT3 phosphorylation in retinal endothelial and RPE cells (38–40). The activation of STAT3 plays a role in inflammatory pathways and can initiate ER stress under high glucose conditions in TR-iBRB cells (39). Moreover, IL-6-induced STAT3-mediated nitric oxide resulted in apoptosis (41). Phosphorylation of STAT3 played a mediatory role in retinal neuronal apoptosis in streptozotocin (STZ)-diabetic rats (42). Additionally, STAT3-induced apoptosis was mediated through the downregulation of PI3K-Akt pathway in mammary epithelial cells (43) and through Bcl-2/Fas signaling in cerebral ischemia/reperfusion (44).
Vascular endothelial growth factor (VEGF), one downstream effector of the STAT3 pathway, is a crucial factor in diabetic retinopathy, mediating increased vascular permeability and pathological angiogenesis (45). Upregulation of VEGF mediated by STAT3 has been shown in bovine retinal capillary endothelial cells under high glucose conditions (38). In addition, the proliferation of endothelial cells induced by VEGF was inhibited when STAT3 was reduced in HUVEC and human retinal microvascular endothelial cells (24). A stimulatory role of VEGF on cell death was shown in mouse cerebral endothelial cells after oxygen-glucose deprivation (46).
Understanding cell type- and disease-specific functions of miR-146a is crucial for development of potential therapeutics for clinical application in diabetic retinopathy. In the present study, we tested the hypothesis that elevated levels of miR-146a suppress IL-6 signaling to inhibit pro-inflammatory pathways of STAT3 and VEGF, leading to the prevention of REC apoptosis in high glucose conditions.
2. Materials and Methods
2.1. Cell culture
Human REC were cultured as described in our previous study (16). Briefly, REC were purchased from Cell Systems Corporation (CSC, Kirkland, WA). Cells are grown in Cell Systems Medium. Microvascular growth supplement (MVGS, Invitrogen), 10 μg/mL gentamycin, and 0.25 μg/mL amphotericin B are added to all cell cultures. Cells are maintained in normal (5mM) glucose or transferred to high glucose medium (25 mM) (Cell Systems) for 3 days. Only primary cells within passage 5 were used. Cells were quiesced by incubating in the appropriate medium without growth supplementation for 20 hours before the experiments.
2.2. Cell transfection with microRNA-mimics and IL-6 siRNA
REC transfection was performed using the miRNA mimic (hsa-miR-146a-5p) (Invitrogen, Carlsbad, CA) or IL-6 siRNA (Dharmacon, Lafayette, CO) using oligofectamine (Invitrogen), following manufacturer instructions. We transfected cells for 48 hours before the cell harvest. A final concentration of 50 nM was used for the mimic, with a negative control for all studies with equal concentration of the mimic negative control (Invitrogen) in REC grown in high glucose. For studies with human IL-6 siRNA, a final concentration of 20 nM was used and a negative control group was used for all studies using an equal concentration of a scrambled siRNA (Dharmacon, Lafayette, CO) in REC grown in high glucose. Mock-treated controls, normal glucose (NG) and high glucose (HG), were treated with oligofectamine only.
2.3. Real-time quantitative PCR
Total RNA was isolated and purified using the Trizol method. Purity and quantity of RNA was measured using a Synergy HTX multi-mode reader (BioTek; Winooski, VT). For polyA tail reverse-transcriptase PCR, 5 μg of total RNA was treated with DNase I for 15 min at room temperature (Promega; Madison, WI) followed by the addition of polyA using (polyA) polymerase (NEB; Ipswich, MA) at 37°C for 1 h. The final reaction mixtures were extracted with phenol/chloroform, precipitated with isopropanol, and re-dissolved in 25 μl diethylpyrocarbonate (DEPC)-treated water. PolyA-tailed RNA (6 μl) was reverse-transcribed into first-strand cDNA using Superscript II reverse transcriptase (Invitrogen) with the oligo-dT adapter primer 5′GCGAGCACAGAATTAATACGACTCACTATAGGTTTTTTTTTTTTVN3′. For PCR, 1 μl of RT product was diluted three times and used as a template in each reaction. Sequences of primer pairs used to detect human IL-6R (Life Technologies, Carlsbad, CA) were as follows (47): 1) mIL-6R, fwd: 5′-CTCCTCTGCATTGCCATTGT-3′, rev: 5′-TGTGGCTCGAGGTATTGTCA-3′, and 2) s-IL6R, fwd: 5′-CGACAAGCCTCCCAGGTTCA-3′, rev: 5′-CGGTTGTGGCTCGAGGTATT-3′. GAPDH (OriGene, Rockville, MD) was used as the internal control. SYBR-Green-based real-time PCR was performed using a CFX Connect PCR system (BioRad; Hercules, CA). The relative expression of miRNA was calculated based on the formula: 2 (−Delta Delta Ct). Delta-Delta Ct values are Delta Ct exp.− Delta Ct cont..
2.4. Western Blot Analysis
As described in our previous study (16), REC were rinsed with cold PBS, collected in lysis buffer containing protease and phosphatase inhibitors, and scraped into tubes. Equal amounts of protein were separated on precast tris-glycine gels (Invitrogen, Carlsbad, CA), and then blotted onto a nitrocellulose membrane. After blocking in TBST (10mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA, the membrane was treated with appropriate primary antibodies followed by incubation with secondary antibodies labeled with horseradish peroxidase. Antigen-antibody complexes were detected by chemilluminescence reagent kit (Thermo Scientific, Pittsburgh, PA). Primary antibodies used were phosphorylated STAT3 (Tyr705) and total STAT3 (purchased from Cell Signaling, Danvers, MA), VEGF (Abcam, Cambridge, MA), ADAM10, ADAM17 (Santa Cruz Biotechnology, Dallas, TX), and beta actin (Santa Cruz, Santa Cruz, CA).
2.5. ELISA Analysis
A human IL-6 ELISA (Thermo Fisher scientific, Grand Island, NY) was used to measure levels of the pro-inflammatory cytokine, IL-6, in cell lysates. Equal protein was loaded (100 μg) into all wells to allow for comparisons based on O.D. To quantify the levels of cytoplasmic histone-associated DNA fragments, cell death detection ELISA (Roche, Indianapolis, IN) was used. Equal protein was loaded (20 μg) into all wells to allow for comparisons based on O.D. In addition, a human sIL-6R ELISA (Abcam, Cambridge, MA) was used to assess the levels of soluble IL-6 receptors in culture supernatant. Equal volume of samples was loaded (100 μl) into all wells to allow for comparisons based on O.D. Manufacturer’s instructions were followed for all ELISA, except extended incubation of samples and antibody reagents overnight at 4°C.
2.6. Statistics
Data were statistically analyzed using Prism software (GraphPad, La Jolla, CA). Analyses were done using unpaired Student t test with two-tailed p value. p<0.05 was considered significant. Data are presented as mean±SEM. For Western blots, a representative blot is presented.
3. Results
3.1. miR-146a reduced IL-6 levels in high glucose conditions
Our previous study demonstrated that exposure to high glucose decreased miR-146a expression in REC, and we confirmed the elevated levels of miR-146a after transfection using miRNA mimic by qRT-PCR (25). As a negative correlation of miR-146a and IL-6 was shown in patients with T2DM (31) and in human aortic endothelial cells (30), thus, we aimed to determine whether miRNA-146a affected IL-6 levels in REC under high glucose conditions. We performed transfections with miR-146a mimics to increase miRNA expression to study downstream signaling pathways. REC were transfected with mimic, miR-146a-5p at a final concentration of 50 nM for 48 hours. We demonstrated that miR-146a overexpression resulted in a significant decrease of IL-6 levels in high glucose conditions (Fig 1). That suggests that miR-146a may directly or indirectly target IL-6, and that IL-6 is a target pathway affected by miR-146a in REC.
Figure 1.
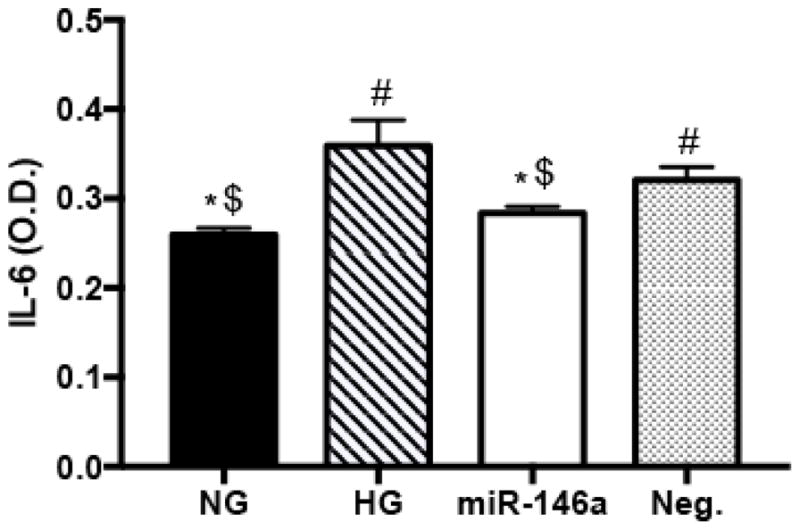
Changes in IL-6 levels in REC. ELISA results for IL-6 on REC in normal glucose (NG, 5 mM) or high glucose (HG, 25 mM) and transfected groups. Cells were transfected with miR146a mimic (50 nM of final concentration) to elevate the level of expression in high glucose conditions. miR-146a decreased IL-6 levels significantly, compared to control HG condition. #p < 0.05 versus NG, *p < 0.05 versus HG, $p < 0.05 versus Neg., N=5; Data are mean±S.E.M.
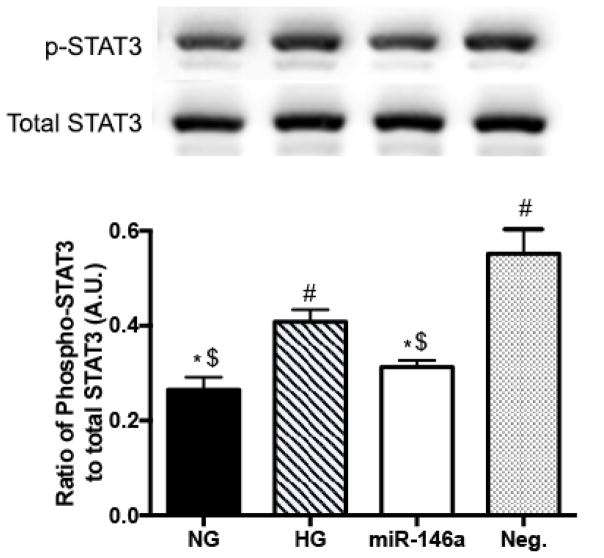
3.2. miR-146a inhibited STAT3 activation in high glucose conditions
It has been reported that the IL-6 family stimulates Jak/STAT3 signaling in the eye (35–37). Also, high glucose can stimulate STAT3 phosphorylation in retinal endothelial and RPE cells (38–40). Thus, we investigated whether miR-146a regulated levels of STAT3 phosphorylation in REC under high glucose conditions. Our results showed that high glucose significantly increased the phosphorylation of STAT3. However, the elevated levels of STAT3 activation were reduced by miR-146a overexpression under high glucose conditions (Fig 2). We, therefore, demonstrated that miR-146a plays a regulatory role in suppressing STAT3 activation in REC in high glucose.
Figure 2.
Changes of STAT3 phosphorylation levels. Western blot results for STAT3 phosphorylation (Tyr705) on REC in normal glucose (5 mM, NG) or high glucose (25 mM, HG) and transfected groups. The levels of STAT3 phosphorylation (Tyr705) were significantly decreased with overexpression of miR-146a in high glucose. A representative blot is shown. #p < 0.05 versus NG, *p < 0.05 versus HG, $p < 0.05 versus Neg., N=3; Data are mean±S.E.M.
3.3. miR-146a decreased the levels of VEGF expression
As STAT3 plays a role as an upstream effector to VEGF in retinal endothelial cells (24,38), we examined whether VEGF levels could be altered in response to the increase of miR-146a and decreased STAT3. Our results demonstrated that high glucose culturing conditions significantly increased VEGF levels compared to cells in the control NG group. However, miR-146a overexpression resulted in the suppression of VEGF levels in HG (Fig 3). Therefore, this suggests that elevated levels of miR-146a induced a significant decrease of VEGF levels, likely mediated through the reduction of STAT3 phosphorylation.
Figure 3.
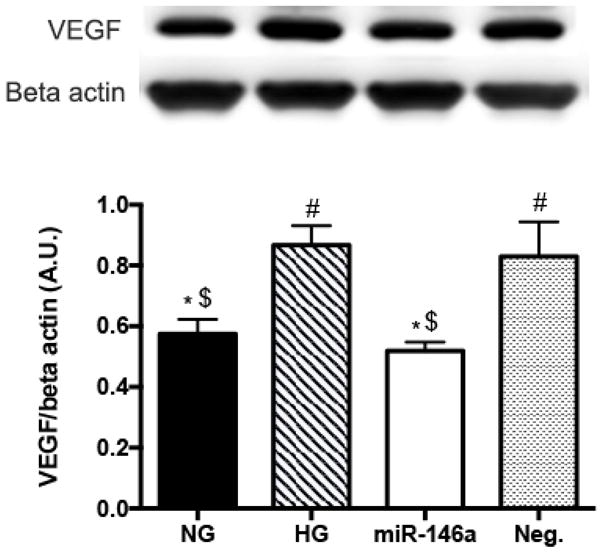
Effects of miR-146a on VEGF levels in high glucose. REC were cultured in normal glucose (5 mM, NG) or high glucose (25 mM, HG). HG-induced increase of VEGF levels was significantly decreased in REC use of miR-146a mimics. A representative blot is shown. #p < 0.05 versus NG, *p < 0.05 versus HG, $p < 0.05 versus Neg., N=4; Data are mean±S.E.M.
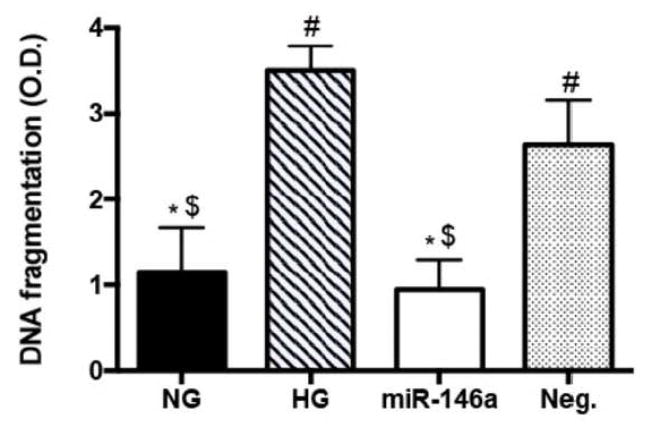
3.4. miR-146a reduced apoptosis in high glucose conditions
Next, we examined whether miR-146a can protect REC from apoptosis, which may be caused by high glucose-induced pro-inflammatory signaling. Previous studies have shown mediatory role of STAT3 signaling (41–44) and VEGF (46) in inducing cellular apoptosis, and our results demonstrated inhibitory role of miR-146a on the phosphorylation of STAT3 and VEGF levels in REC. To examine the effects of increased levels of miR-146a on apoptosis, we examined DNA fragmentation of REC under high glucose conditions. Our results demonstrated that overexpression of miR-146a significantly reduced DNA fragmentation levels in REC cultured in high glucose (Fig 4). These results suggest that miR-146a can protect REC from high glucose-induced apoptosis, potentially through inhibition of pro-inflammatory pathways.
Figure 4.
Effects of miR-146a on REC apoptosis in high gluocse. REC were cultured in normal glucose (5 mM, NG) or high glucose (25 mM, HG). ELISA results demonstrated that miR-146a significantly reduced the levels of DNA fragmentation in high glucose conditions. #p < 0.05 versus NG, *p < 0.05 versus HG, $p < 0.05 versus Neg., N=3; Data are mean±S.E.M.
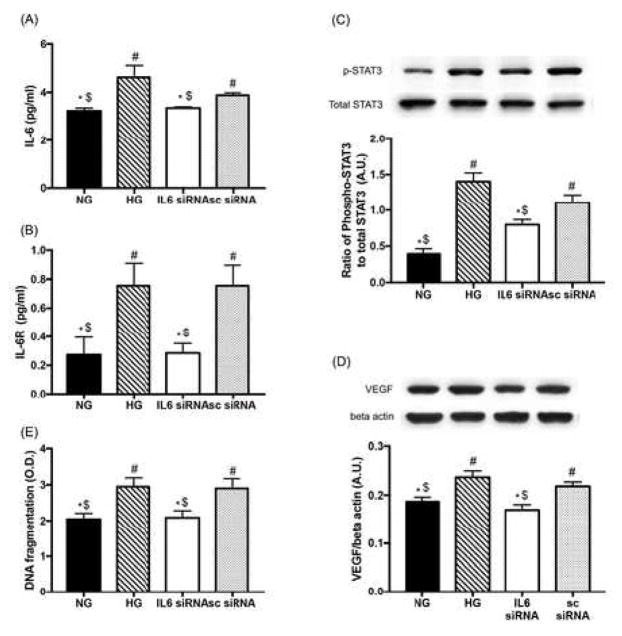
3.5. The regulatory role of miR-146a was mediated by IL-6 signaling in REC
Then, we examined whether IL-6 acts as a mediator in the regulatory process of miR-146a on pro-inflammatory signaling in REC. REC were transfected with human IL-6 siRNA at a final concentration of 20 nM for 48 hours. We demonstrated that both IL-6 and sIL-6R levels were increased in REC under high glucose conditions and the expression was suppressed following IL-6 siRNA treatment (Fig 5A and B). That indicates that IL-6 signaling plays a role in REC as a molecular mechanism underlying high glucose conditions. We also demonstrated that STAT3 phosphorylation and VEGF levels were significantly reduced following inhibition of IL-6 signaling (Fig 5C and D). The suppression of IL-6 signaling prevented REC apoptosis, as shown with decreased levels of DNA fragmentation (Fig 5E). The results suggest that IL-6 plays a role in the upregulation of STAT3/VEGF pathway and apoptosis, via binding to sIL-6R in REC under high glucose conditions.
Figure 5.
The role of IL-6 signaling on STAT3/VEGF pathway and apoptosis. REC were cultured in normal glucose (5 mM, NG) or high glucose (25 mM, HG) and transfected with human IL-6 siRNA (20 nM of final concentration). ELISA results showed that the levels of both IL-6 (A) and sIL-6R (B) in REC were suppressed by IL-6 siRNA in high glucose conditions. (C and D) STAT3 phosphorylation and VEGF levels were significantly reduced by inhibited IL-6 signaling. A representative blot is shown. (E) ELISA data showing decreased levels of DNA fragmentation in REC. #p < 0.05 versus NG, *p < 0.05 versus HG, $p < 0.05 versus Neg.; N=4 (A, B, and D), N=7 (C and E); Data are mean±S.E.M.
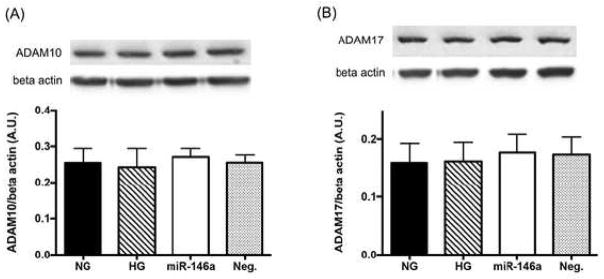
Human s-IL-6R protein can be generated by either alternative splicing or proteolytic cleavage of membrane-bound IL-6R (47,48). To demonstrate IL-6 signaling in a direct approach, we examined the levels of membrane-bound IL-6R (mIL-6R) and soluble IL-6R (sIL-6R) using RNA isolated from REC by real-time quantitative PCR (Table 1). We demonstrated that the expression of sIL-6R was significantly increased in HG when compared to NG. REC treated with IL-6 siRNA showed significantly reduced levels of sIL-6R than high glucose only (Table 1). The overexpression of miR-146a did not change sIL-6R expression compared to high glucose only (Table 1). Additionally, we assessed the levels of ADAM10 and ADAM17, the sheddases of IL-6R, which can generate sIL-6R proteolytically from the membrane-bound form in humans (49). We showed that the levels of ADAM10/and 17 were not changed in different culturing conditions (Fig 6A and 6B). The results suggest that sIL-6R was produced in human REC via alternative splicing of mIL-6R under high glucose conditions.
Table 1.
Fold changes of mIL-6R and sIL-6R expression in REC.
| Experimental groups | |||||
|---|---|---|---|---|---|
|
| |||||
| Primer | NG vs. | HG vs. | |||
| HG | miR-146a | IL6 siRNA | Neg.mimic | sc siRNA | |
| mIL-6R | 2.63 (0.48) | 1.08 (0.3) | 0.48* (0.12) | 1.03 (0.31) | 0.83 (0.33) |
| sIL-6R | 4.9# (0.87) | 1.3 (0.7) | 0.2* (0) | 0.9 (0.31) | 1.07 (0.37) |
p < 0.05 versus NG,
p < 0.05 versus HG, N≥3; Values are Mean (S.E.M.).
Figure 6.
ADAM10 and ADAM17 levels in REC treated with miR146a. The levels of ADAM10 and ADAM17 were not changed between different culture groups. A representative blot is shown. N≥3; Data are mean±S.E.M.
4. Discussion
Our knowledge of the molecular and cellular mechanisms by which miRNA function in diabetic retinopathy is still limited and additional studies are required to develop novel therapeutics for diabetic retinopathy. So far, only a small number of studies have been performed to reveal the relation of miR-146a in pathological processes of diabetic retinopathy. Predominantly, miR-146a has been implicated as an epigenetic regulator of inflammatory responses. The regulatory roles of miR-146a that affects inflammatory pathways have been shown in various cell types, including human RPE cells (50), trabecular meshwork cells (51), brain endothelial cells (52), brain microglical cells (53), and cells from intervertebral discs (54). Much work has demonstrated that REC are an important cell type substantially affected in diabetic retinopathy (55–57). Since the expression and functions of miRNA are specific to the type of cell, tissue, and disease, condition-specific examinations are critical to get clear understanding on the regulatory networks between miRNA and their associated molecular pathways. Some studies have reported that miR-146a plays a pro-inflammatory role in the retina and diabetic retinopathy. In human RPE cells, miR-146a expression was IL-1β-dependent and the miRNA expression was significantly increased by the addition of the mixture of IFN-γ, TNFα, and IL-1β in culture (50). Additionally, a negative regulatory role of miR-146a on the levels of IRAK1 and ICAM-1 was shown in human REC (24). Our recent study demonstrated that miR-146a inhibited TLR4, NF-κB, and TNFα in REC grown in high glucose (25). However, it is highly likely that miR-146a regulates a number of pro-inflammatory pathways key to diabetic retinopathy. In this study, we examined the effects of miR-146a regulation on pro-inflammatory and survival/apoptotic pathways in REC cultured in high glucose. Our results provide novel clues to the inhibitory role of miR-146a on STAT3/VEGF pathway through IL-6 signaling in high glucose-induced diabetic retinopathy.
We previously showed that high glucose induced a significant decrease in miR-146a expression in REC (25). Thus, in this study we induced the overexpression of miR-146a using miR146a mimics in REC under high glucose conditions to examine the roles in IL-6/STAT3/VEGF signaling. Our data demonstrated that overexpression of miR-146a reduced IL-6 levels in high glucose conditions, suggesting that miR-146a may inhibit IL-6 in REC in high glucose. Consistent with our results, a negative correlation of miR-146a expression with IL-6 levels was demonstrated in human aortic endothelial cells in vitro (30) and patients with T2DM (31).
IL-6 can stimulate Jak/STAT3 signaling in the eye (35–37). Activation of STAT3 pathway plays a role in high glucose-induced endoplasmic reticulum stress and contributes to endothelial inflammation in the retina of Type 1 diabetes (39). Our results demonstrated that increased levels of STAT3 phosphorylation were reduced by miR-146a overexpression in high glucose conditions. This suggests that increased levels of STAT3 phosphorylation in high glucose conditions are decreased by miR-146a-driven suppression of IL-6.
De novo synthesis of sIL-6R has been shown in human B cells (58). Our qPCR results showed a significant elevation of sIL-6R expression under HG conditions with no changes in ADAM10 or ADAM17 levels was found between culture conditions, suggesting that de novo synthesis of sIL-6R occurred in REC through alternative splicing of mIL-6R to induce IL-6 signaling. miR-146a overexpression in REC did not result in decreased expression of mIL-6R and sIL-6R in HG. It is possible that miR-146a regulated other unknown signaling pathways and that, in turn, could counterbalance the inhibitory effects of miR-146a on IL-6 signaling. We will explore these other pathways in future studies.
It has been reported that inhibition of the STAT3 pathway decreases VEGF expression (38,40,59). Our results demonstrated that miR-146a overexpression decreased the levels of VEGF protein, in addition to STAT3 phosphorylation. Our findings of reduced VEGF by miR-146a are consistent with other studies as reported in HUVECs (60) and in a nude mouse model (61). Therefore, the reduction of STAT3 and VEGF by miR-146a may have a therapeutic potential as a molecular target and genetic regulatory element for treating angiogenic disorders.
Previous studies have shown STAT3-induced apoptosis in the retina of diabetic rats (42), IL-6-treated beta cells (41), focal cerebral ischemia/reperfusion rats (44), and mammary gland involution (43). VEGF also played a role on inducing endothelial cell death after oxygen-glucose deprivation (46). Our previous studies (16,62–65) and many others (66–68) have demonstrated that high glucose increased the levels of apoptosis in REC. We showed that miR-146a played a role in reducing REC apoptosis under high glucose conditions by decreasing the levels of DNA fragmentation. Finally, we demonstrated that the regulatory role of miR-146a on pro-inflammatory pathway and apoptosis was mediated by IL-6 signaling in REC under high glucose conditions. That suggests that miR-146a can protect REC from high glucose-induced apoptosis, potentially through the suppression of the STAT3/VEGF pathway via IL-6 signaling.
5. Conclusions
Taken together, our study demonstrated that elevated expression of miR-146a resulted in inhibition of STAT3 and VEGF signaling through IL-6 signaling in REC under high glucose conditions. Therefore, we present a potential regulatory mechanism whereby miR-146a can downregulate IL-6-mediated STAT3/VEGF signaling, resulting in reduced apoptosis in REC. The outcome suggests that miR-146a is a potential therapeutic target for rescuing diabetic retina through the inhibition of pro-inflammatory pathways of IL-6/STAT3/VEGF.
Acknowledgments
This work was supported by R01EY022330 (JJS), P30EY04068 (PI:Hazlett) and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute).
Abbreviations
- miR
microRNA
- REC
retinal endothelial cells
- PCR
polymerase chain reaction
- ELISA
enzyme-linked immunosorbent assay
- IL-6
interleukin-6
- sIL-6R
soluble interleukin-6 receptor
- mIL-6R
membrane-bound interleukin-6 receptor
- STAT3
signal transducer and activator of transcription 3
- VEGF
vascular endothelial growth factor
- ADAM-10 and -17
A disintegrin and metalloproteinase domain -10, and -17
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Authors’ contributions
EY completed the studies and wrote the manuscript. JS guided designing the study. JS and EY edited the manuscript. All authors have read and approved of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bai Y, Bai X, Wang Z, Zhang X, Ruan C, Miao J. MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Experimental and molecular pathology. 2011;91:471–477. doi: 10.1016/j.yexmp.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Olena AF, Patton JG. Genomic organization of microRNAs. J Cell Physiol. 2010;222:540–545. doi: 10.1002/jcp.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert opinion on biological therapy. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- 4.Igaz I, Igaz P. Diagnostic Relevance of microRNAs in Other Body Fluids Including Urine, Feces, and Saliva. Exs. 2015;106:245–252. doi: 10.1007/978-3-0348-0955-9_11. [DOI] [PubMed] [Google Scholar]
- 5.McClelland AD, Kantharidis P. microRNA in the development of diabetic complications. Clinical science. 2014;126:95–110. doi: 10.1042/CS20130079. [DOI] [PubMed] [Google Scholar]
- 6.Sun CY, She XM, Qin Y, Chu ZB, Chen L, Ai LS, Zhang L, Hu Y. miR-15a and miR-16 affect the angiogenesis of multiple myeloma by targeting VEGF. Carcinogenesis. 2013;34:426–435. doi: 10.1093/carcin/bgs333. [DOI] [PubMed] [Google Scholar]
- 7.Xiong F, Du X, Hu J, Li T, Du S, Wu Q. Altered retinal microRNA expression profiles in early diabetic retinopathy: an in silico analysis. Current eye research. 2014;39:720–729. doi: 10.3109/02713683.2013.872280. [DOI] [PubMed] [Google Scholar]
- 8.Ichii O, Otsuka S, Sasaki N, Namiki Y, Hashimoto Y, Kon Y. Altered expression of microRNA miR-146a correlates with the development of chronic renal inflammation. Kidney international. 2012;81:280–292. doi: 10.1038/ki.2011.345. [DOI] [PubMed] [Google Scholar]
- 9.Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Seminars in cancer biology. 2008;18:131–140. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Feng B, Chen S, Chu Y, Chakrabarti S. Mechanisms of endothelial to mesenchymal transition in the retina in diabetes. Investigative ophthalmology & visual science. 2014;55:7321–7331. doi: 10.1167/iovs.14-15167. [DOI] [PubMed] [Google Scholar]
- 11.Cowan C, Muraleedharan CK, O’Donnell JJ, 3rd, Singh PK, Lum H, Kumar A, Xu S. MicroRNA-146 inhibits thrombin-induced NF-kappaB activation and subsequent inflammatory responses in human retinal endothelial cells. Investigative ophthalmology & visual science. 2014;55:4944–4951. doi: 10.1167/iovs.13-13631. [DOI] [PubMed] [Google Scholar]
- 12.Fulzele S, El-Sherbini A, Ahmad S, Sangani R, Matragoon S, El-Remessy A, Radhakrishnan R, Liou GI. MicroRNA-146b-3p regulates retinal inflammation by suppressing adenosine deaminase-2 in diabetes. BioMed research international. 2015;2015:846501. doi: 10.1155/2015/846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin X, Zhou X, Liu D, Yun L, Zhang L, Chen X, Chai Q, Li L. MicroRNA-29 regulates high-glucose-induced apoptosis in human retinal pigment epithelial cells through PTEN. In vitro cellular & developmental biology. Animal. 2016 doi: 10.1007/s11626-015-9990-z. [DOI] [PubMed] [Google Scholar]
- 14.Mortuza R, Feng B, Chakrabarti S. miR-195 regulates SIRT1-mediated changes in diabetic retinopathy. Diabetologia. 2014;57:1037–1046. doi: 10.1007/s00125-014-3197-9. [DOI] [PubMed] [Google Scholar]
- 15.Wu JH, Wang YH, Wang W, Shen W, Sang YZ, Liu L, Chen CM. MiR-18b suppresses high-glucose-induced proliferation in HRECs by targeting IGF-1/IGF1R signaling pathways. The international journal of biochemistry & cell biology. 2016;73:41–52. doi: 10.1016/j.biocel.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Ye EA, Steinle JJ. miR-15b/16 protects primary human retinal microvascular endothelial cells against hyperglycemia-induced increases in tumor necrosis factor alpha and suppressor of cytokine signaling 3. Journal of neuroinflammation. 2015;12:44. doi: 10.1186/s12974-015-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Song YH, Li F, Yang T, Lu YW, Geng YJ. MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochemical and biophysical research communications. 2009;381:81–83. doi: 10.1016/j.bbrc.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funari VA, Winkler M, Brown J, Dimitrijevich SD, Ljubimov AV, Saghizadeh M. Differentially expressed wound healing-related microRNAs in the human diabetic cornea. PloS one. 2013;8:e84425. doi: 10.1371/journal.pone.0084425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menard C, Rezende FA, Miloudi K, Wilson A, Tetreault N, Hardy P, SanGiovanni JP, De Guire V, Sapieha P. MicroRNA signatures in vitreous humour and plasma of patients with exudative AMD. Oncotarget. 2016 doi: 10.18632/oncotarget.8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragusa M, Caltabiano R, Russo A, Puzzo L, Avitabile T, Longo A, Toro MD, Di Pietro C, Purrello M, Reibaldi M. MicroRNAs in vitreus humor from patients with ocular diseases. Molecular vision. 2013;19:430–440. [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe T, Keino H, Kudo A, Sato Y, Okada AA. MicroRNAs in retina during development of experimental autoimmune uveoretinitis in rats. The British journal of ophthalmology. 2016;100:425–431. doi: 10.1136/bjophthalmol-2015-306924. [DOI] [PubMed] [Google Scholar]
- 22.Li K, Du Y, Jiang BL, He JF. Increased microRNA-155 and decreased microRNA-146a may promote ocular inflammation and proliferation in Graves’ ophthalmopathy. Medical science monitor: international medical journal of experimental and clinical research. 2014;20:639–643. doi: 10.12659/MSM.890686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldeon RL, Weigelt K, de Wit H, Ozcan B, van Oudenaren A, Sempertegui F, Sijbrands E, Grosse L, Freire W, Drexhage HA, Leenen PJ. Decreased serum level of miR-146a as sign of chronic inflammation in type 2 diabetic patients. PloS one. 2014;9:e115209. doi: 10.1371/journal.pone.0115209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Bozack SN, Yan Y, Boulton ME, Grant MB, Busik JV. Regulation of retinal inflammation by rhythmic expression of MiR-146a in diabetic retina. Investigative ophthalmology & visual science. 2014;55:3986–3994. doi: 10.1167/iovs.13-13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye EA, Steinle JJ. miR-146a Attenuates Inflammatory Pathways Mediated by TLR4/NF-kappaB and TNFalpha to Protect Primary Human Retinal Microvascular Endothelial Cells Grown in High Glucose. Mediators of inflammation. 2016;2016:3958453. doi: 10.1155/2016/3958453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canataroglu H, Varinli I, Ozcan AA, Canataroglu A, Doran F, Varinli S. Interleukin (IL)-6, interleukin (IL)-8 levels and cellular composition of the vitreous humor in proliferative diabetic retinopathy, proliferative vitreoretinopathy, and traumatic proliferative vitreoretinopathy. Ocular immunology and inflammation. 2005;13:375–381. doi: 10.1080/09273940490518900. [DOI] [PubMed] [Google Scholar]
- 27.Kawashima M, Shoji J, Nakajima M, Kamura Y, Sato Y. Soluble IL-6 receptor in vitreous fluid of patients with proliferative diabetic retinopathy. Japanese journal of ophthalmology. 2007;51:100–104. doi: 10.1007/s10384-006-0411-4. [DOI] [PubMed] [Google Scholar]
- 28.Koskela UE, Kuusisto SM, Nissinen AE, Savolainen MJ, Liinamaa MJ. High vitreous concentration of IL-6 and IL-8, but not of adhesion molecules in relation to plasma concentrations in proliferative diabetic retinopathy. Ophthalmic research. 2013;49:108–114. doi: 10.1159/000342977. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Kubo Y, Kobayashi Y, Zhou Y, Nakama T, Yamaguchi M, Tachibana T, Ishikawa K, Arita R, Nakao S, Sassa Y, Oshima Y, Kono T, Ishibashi T. Increased vitreous concentrations of MCP-1 and IL-6 after vitrectomy in patients with proliferative diabetic retinopathy: possible association with postoperative macular oedema. The British journal of ophthalmology. 2015;99:960–966. doi: 10.1136/bjophthalmol-2014-306366. [DOI] [PubMed] [Google Scholar]
- 30.Wu S, He L, Li Y, Wang T, Feng L, Jiang L, Zhang P, Huang X. miR-146a facilitates replication of dengue virus by dampening interferon induction by targeting TRAF6. The Journal of infection. 2013;67:329–341. doi: 10.1016/j.jinf.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Balasubramanyam M, Aravind S, Gokulakrishnan K, Prabu P, Sathishkumar C, Ranjani H, Mohan V. Impaired miR-146a expression links subclinical inflammation and insulin resistance in Type 2 diabetes. Molecular and cellular biochemistry. 2011;351:197–205. doi: 10.1007/s11010-011-0727-3. [DOI] [PubMed] [Google Scholar]
- 32.Rose-John S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int J Biol Sci. 2012;8:1237–1247. doi: 10.7150/ijbs.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Zhang XZ, Liao NY, Wen F. Increased levels of IL-6, sIL-6R, and sgp130 in the aqueous humor and serum of patients with diabetic retinopathy. Molecular vision. 2016;22:1005–1014. [PMC free article] [PubMed] [Google Scholar]
- 34.Kawashima M, Shoji J, Nakajima M, Kamura Y, Sato Y. Soluble IL-6 receptor in vitreous fluid of patients with proliferative diabetic retinopathy. Jpn J Ophthalmol. 2007;51:100–104. doi: 10.1007/s10384-006-0411-4. [DOI] [PubMed] [Google Scholar]
- 35.Elsaeidi F, Bemben MA, Zhao XF, Goldman D. Jak/Stat signaling stimulates zebrafish optic nerve regeneration and overcomes the inhibitory actions of Socs3 and Sfpq. J Neurosci. 2014;34:2632–2644. doi: 10.1523/JNEUROSCI.3898-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fasler-Kan E, Barteneva NS, Ketterer S, Wunderlich K, Reschner A, Nurzhanova A, Flammer J, Huwyler J, Meyer P. Human cytokines activate JAK-STAT signaling pathway in porcine ocular tissue. Xenotransplantation. 2013;20:469–480. doi: 10.1111/xen.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samardzija M, Wenzel A, Aufenberg S, Thiersch M, Reme C, Grimm C. Differential role of Jak-STAT signaling in retinal degenerations. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:2411–2413. doi: 10.1096/fj.06-5895fje. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Z, Chen H, Zhao H, Liu K, Luo D, Chen Y, Chen Y, Yang X, Gu Q, Xu X. Inhibition of JAK2/STAT3-mediated VEGF upregulation under high glucose conditions by PEDF through a mitochondrial ROS pathway in vitro. Investigative ophthalmology & visual science. 2010;51:64–71. doi: 10.1167/iovs.09-3511. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Wang JJ, Li J, Hosoya KI, Ratan R, Townes T, Zhang SX. Activating transcription factor 4 mediates hyperglycaemia-induced endothelial inflammation and retinal vascular leakage through activation of STAT3 in a mouse model of type 1 diabetes. Diabetologia. 2012;55:2533–2545. doi: 10.1007/s00125-012-2594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Cai Y, Wang YS, Shi YY, Hou W, Xu CS, Wang HY, Ye Z, Yao LB, Zhang J. Hyperglycaemia exacerbates choroidal neovascularisation in mice via the oxidative stress-induced activation of STAT3 signalling in RPE cells. PloS one. 2012;7:e47600. doi: 10.1371/journal.pone.0047600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh YS, Lee YJ, Park EY, Jun HS. Interleukin-6 treatment induces beta-cell apoptosis via STAT-3-mediated nitric oxide production. Diabetes-Metab Res. 2011;27:813–819. doi: 10.1002/dmrr.1233. [DOI] [PubMed] [Google Scholar]
- 42.Li PY, Xu X, Zheng Z, Zhu BJ, Shi YH, Liu K. Protective Effects of Rosiglitazone on Retinal Neuronal Damage in Diabetic Rats. Current eye research. 2011;36:673–679. doi: 10.3109/02713683.2011.572220. [DOI] [PubMed] [Google Scholar]
- 43.Abell K, Bilancio A, Clarkson RWE, Tiffen PG, Altaparmakov AI, Burdon TG, Asano T, Vanhaesebroeck B, Watson CJ. Stat3-induced apoptosis requires a molecular switch in PI(3)K subunit composition. Nat Cell Biol. 2005;7:392–398. doi: 10.1038/ncb1242. [DOI] [PubMed] [Google Scholar]
- 44.Guo K, Yin G, Zi XH, Yan WG. Activation of STAT3 is involved in neuronal apoptosis in focal cerebral ischemia/reperfusion rats via Bcl 2/Fas pathway. Int J Clin Exp Patho. 2016;9:2660–2669. [Google Scholar]
- 45.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Progress in retinal and eye research. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 46.Narasimhan P, Liu J, Song YS, Massengale JL, Chan PH. VEGF Stimulates the ERK 1/2 Signaling Pathway and Apoptosis in Cerebral Endothelial Cells After Ischemic Conditions. Stroke. 2009;40:1467–1473. doi: 10.1161/STROKEAHA.108.534644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Rokavec M, Hermeking H. Soluble IL6R represents a miR-34a target: potential implications for the recently identified IL-6R/STAT3/miR-34a feed-back loop. Oncotarget. 2015;6:14026–14032. doi: 10.18632/oncotarget.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Briso EM, Dienz O, Rincon M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. Journal of immunology. 2008;180:7102–7106. doi: 10.4049/jimmunol.180.11.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garbers C, Janner N, Chalaris A, Moss ML, Floss DM, Meyer D, Koch-Nolte F, Rose-John S, Scheller J. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. The Journal of biological chemistry. 2011;286:14804–14811. doi: 10.1074/jbc.M111.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kutty RK, Nagineni CN, Samuel W, Vijayasarathy C, Jaworski C, Duncan T, Cameron JE, Flemington EK, Hooks JJ, Redmond TM. Differential regulation of microRNA-146a and microRNA-146b-5p in human retinal pigment epithelial cells by interleukin-1beta, tumor necrosis factor-alpha, and interferon-gamma. Molecular vision. 2013;19:737–750. [PMC free article] [PubMed] [Google Scholar]
- 51.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells. Investigative ophthalmology & visual science. 2010;51:2976–2985. doi: 10.1167/iovs.09-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu D, Cerutti C, Lopez-Ramirez MA, Pryce G, King-Robson J, Simpson JE, van der Pol SM, Hirst MC, de Vries HE, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA. Brain endothelial miR-146a negatively modulates T-cell adhesion through repressing multiple targets to inhibit NF-kappaB activation. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:412–423. doi: 10.1038/jcbfm.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma N, Verma R, Kumawat KL, Basu A, Singh SK. miR-146a suppresses cellular immune response during Japanese encephalitis virus JaOArS982 strain infection in human microglial cells. Journal of neuroinflammation. 2015;12:30. doi: 10.1186/s12974-015-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu SX, Li X, Hamilton JL, Chee A, Kc R, Chen D, An HS, Kim JS, Oh CD, Ma YZ, van Wijnen AJ, Im HJ. MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc. Gene. 2015;555:80–87. doi: 10.1016/j.gene.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kowluru RA, Kowluru A, Kanwar M. Small molecular weight G-protein, H-Ras, and retinal endothelial cell apoptosis in diabetes. Mol Cell Biochem. 2007;296:69–76. doi: 10.1007/s11010-006-9299-z. [DOI] [PubMed] [Google Scholar]
- 56.Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002;80:771–779. doi: 10.1046/j.0022-3042.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 57.Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003;35:1491–1499. doi: 10.1016/j.freeradbiomed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 58.Korholz D, Nussbaum P, Pafferath B, Mauz-Korholz C, Hempel L, Burdach S. Activation of protein kinase C induces de novo synthesis of the soluble interleukin-6 receptor in human B cells. Scandinavian journal of immunology. 1994;40:515–520. doi: 10.1111/j.1365-3083.1994.tb03498.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Li G, Wang Z, Zhang X, Yao L, Wang F, Liu S, Yin J, Ling EA, Wang L, Hao A. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience. 2012;202:58–68. doi: 10.1016/j.neuroscience.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 60.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. The Journal of clinical investigation. 2013;123:2143–2154. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME, Liechty KW. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes. 2012;61:2906–2912. doi: 10.2337/db12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams KP, Steinle JJ. Maintenance of beta-adrenergic receptor signaling can reduce Fas signaling in human retinal endothelial cells. Experimental eye research. 2009;89:448–455. doi: 10.1016/j.exer.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 63.Jiang YD, Zhang QH, Soderland C, Steinle JJ. TNF alpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cell Signal. 2012;24:1086–1092. doi: 10.1016/j.cellsig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panjala SR, Steinle JJ. Insulin and beta-adrenergic receptors inhibit retinal endothelial cell apoptosis through independent pathways. Neurochemical research. 2011;36:604–612. doi: 10.1007/s11064-010-0303-3. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Q, Steinle JJ. DNA-PK phosphorylation of IGFBP-3 is required to prevent apoptosis in retinal endothelial cells cultured in high glucose. Investigative ophthalmology & visual science. 2013;54:3052–3057. doi: 10.1167/iovs.12-11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nahomi RB, Palmer A, Green KM, Fort PE, Nagaraj RH. Pro-inflammatory cytokines downregulate Hsp27 and cause apoptosis of human retinal capillary endothelial cells. Biochimica et biophysica acta. 2014;1842:164–174. doi: 10.1016/j.bbadis.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang L, Zhang ZK, Liang S. Epigallocatechin-3-gallate protects retinal vascular endothelial cells from high glucose stress in vitro via the MAPK/ERK-VEGF pathway. Genetics and molecular research: GMR. 2016:15. doi: 10.4238/gmr.15027874. [DOI] [PubMed] [Google Scholar]
- 68.Safi SZ, Qvist R, Yan GO, Ismail IS. Differential expression and role of hyperglycemia induced oxidative stress in epigenetic regulation of beta1, beta2 and beta3-adrenergic receptors in retinal endothelial cells. BMC medical genomics. 2014;7:29. doi: 10.1186/1755-8794-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]