Abstract
Resting state functional brain connectivity (rsFC) may be an important neuromarker of smoking behavior. Prior research has shown, among cigarette smokers, that nicotine administration alters rsFC within frontal and parietal cortices involved in executive control, as well as striatal regions that drive reward processing. These changes in rsFC have been associated with reductions in withdrawal symptom severity. We currently have a limited understanding of how rsFC is affected by the use of electronic cigarettes (ecigs), an increasingly popular class of products, the members of which deliver nicotine with varying effectiveness. The current study used fMRI to determine the effects of ecig use on rsFC and withdrawal symptoms. Independent component, dual regression, and permutation analyses were conducted on rsFC collected from ecig users before and after an ecig use episode (n=9) that occurred after 14 hours of nicotine abstinence. Similar to the known effects of nicotine administration, ecig use decreased rsFC of two clusters in the right frontal pole and frontal medial cortex with an attentional control salience network, and decreased rsFC of five clusters in the left thalamus, insula, and brain stem with a reward network encompassing the striatum. Ecig use increased inverse coupling between the prefrontal reward network and the right frontoparietal executive control network. Reductions in craving and difficulty with concentration were correlated with decreases in coupling strength between reward and executive control networks. These preliminary results suggest that the effects of ecig use on rsFC are similar to those seen with nicotine administration in other forms. In order to gain insight into the addictive potential of ecigs, further research is needed to understand the neural influence of ecigs across the range of nicotine delivery within this class of products.
Keywords: connectivity, electronic cigarette, fMRI, nicotine, resting state, withdrawal
1. Introduction
Electronic cigarettes (ecigs) have grown in popularity in the U.S. over the past decade, especially among youth (Department of Health and Human Services, 2016; King et al., 2014) These devices use an electrical heating element to turn a liquid that typically contains propylene glycol, glycerin, nicotine and flavorants into an aerosol (Breland et al., 2016). Their popularity has been aided by marketing campaigns portraying them as a cigarette substitute, often implying the potential to serve as a safer alternative to combustible cigarettes or a stepping stone to cessation (Grana et al., 2014). However, some non-smokers also report using ecigs, especially young adults under the age of 24 (Department of Health and Human Services, 2016). Despite concerns about ecig use leading to nicotine addiction, the prevalence of daily ecig use among 18-24 year-olds is estimated at 1.3%, which is low relative to the rates of cigarette smoking (14.7%) for this age group (Kasza et al., 2017). The FDA gained authority to regulate the manufacture and sale of ecigs in 2016, but we still know little about the safety and addictive potential of these products.
Acute and chronic nicotine use is associated with neural changes in midbrain and cortical areas involved in reward processing, motivation, learning, and executive functions (Engelmann et al., 2012; Kenny and Markou, 2006; McClernon et al., 2009). These changes can lead to the development of addiction and continued cigarette use despite physical harm (McLaughlin et al., 2015). Withdrawal symptoms, such as craving, anxiety, irritability, and negative affect, make nicotine abstinence difficult, and have neural correlates identified with functional magnetic resonance imaging (fMRI) (Buhler et al., 2010; Lerman et al., 2014; McClernon et al., 2008; McLaughlin et al., 2015; Mendrek et al., 2006). fMRI is a non-invasive brain imaging technique that measures changes in blood-oxygen-level-dependent (BOLD) neural activity over time. If ecigs produce similar effects on the brain as combustible nicotine products, this would imply that what we have learned about the neurocognitive effects of cigarette smoking will likely also apply to ecig use. However, we know little about how ecigs affect neural function, which is difficult to predict given the varying nicotine contents in the liquids and the power output (wattage) of the devices (Breland et al., 2016; Marsot and Simon, 2016).
Our prior investigation using fMRI showed that viewing images of ecigs elicits BOLD cue-reactivity in daily ecig users (Nichols et al., 2016). The effects of the ecig cue task, localized in sensorimotor cortical regions, were similar to cue-induced reactivity seen among daily smokers when viewing pictures of cigarettes (Engelmann et al., 2012; Nichols et al., 2016). fMRI is also used to measure associations between spontaneous fluctuations in BOLD signal throughout the brain when a person is not engaged in a goal-directed task, called resting state functional connectivity (rsFC) (Laird et al., 2011). Whole-brain networks of correlated rsFC have been linked to behavior relevant to addictive processes (Laird et al., 2011; Sutherland et al., 2012). Common networks include those that drive attention to external and internal stimuli to perform executive functions (e.g., frontoparietal, salience, and default mode networks), the visuospatial processing of complex numerical and emotional stimuli, and networks involved in evaluating and responding to reward (e.g, striatal and prefrontal networks) (Laird et al., 2011). RsFC has been shown to be altered among dependent smokers and predictive of relapse during quit attempts (Addicott et al., 2015; Fedota and Stein, 2015; Lerman et al., 2014; Sutherland et al., 2013; Sweitzer et al., 2016; Wang et al., 2016; Wetherill et al., 2015). When compared to non-smokers, daily smokers who are nicotine satiated show reduced rsFC of limbic, insular, and prefrontal cortical circuitry involved in reward processing, increased coupling strength between prefrontal and parietal cortical circuitry, and decreased coupling strength between insular and frontal cortical circuitry involved in executive functions (Bi et al., 2016; Fedota et al., 2016; Janes et al., 2012; Shen et al., 2016; Yuan et al., 2016).
After a period of nicotine abstinence, rsFC changes in response to nicotine administration via smoking, patch, and lozenge (Cole et al., 2010; Ding and Lee, 2013; Hong et al., 2009). For daily smokers, smoking after a 12-hour period of abstinence was related to reductions in coupling strength between the default mode and salience networks, as well as enhanced coupling strength between the salience network with the combined frontoparietal and default mode networks (Ding and Lee, 2013). Enhanced rsFC between cingulate-cortical regions has also been noted with nicotine patch administration among daily smokers (Hong et al., 2009). In a separate study, daily smokers who had abstained from nicotine for 8 hours showed reductions in rsFC in the salience network (i.e, insula, orbitofrontal cortex) and the default mode network (i.e., precuneus and cuneus) after being administered two doses of a 4mg nicotine lozenge as compared to when they were administered a placebo lozenge (Cole et al., 2010). Changes in rsFC were associated with changes in withdrawal symptoms (i.e., craving, concentration, restlessness, and hunger): Specifically, reductions in total withdrawal symptoms were related to nicotine-induced increases in rsFC within the default mode (prefrontal, occipital, parietal, and hippocampal regions) and salience networks (prefrontal cortex), as well as decreases in temporal and occipital regions of the salience network. Nicotine-induced reductions in coupling strength between the salience and default mode networks were associated with fewer difficulties with concentration after satiety (Cole et al., 2010). These results suggest that while nicotine may produce overall reductions in rsFC, withdrawal symptoms may be driven by more complicated network dynamics that include increases and decreases in network rsFC, as well as changes in connectivity strength between networks.
If nicotine is the mechanism driving these changes, one could expect to see similar changes in rsFC connectivity with nicotine administration via ecig use; however, given the lack of testing and regulations regarding nicotine delivery with these devices, their effects on neural processes remain unclear. The current study aimed to use a similar data-driven, whole-network approach as Cole and colleagues (2010) to identify anatomical locations of significant within-subject changes in rsFC induced by using an ecig after a 14-hour period of nicotine abstinence among regular ecig users. We also assessed for associations between changes in rsFC with changes in withdrawal symptoms and blood nicotine levels measured during the ecig use episode. We expected to observe changes in rsFC similar those reported with other forms of nicotine administration, including decreased rsFC and reduced between-network coupling among executive and reward networks after ecig use.
2. Materials and methods
2.1 Participants
Participants were recruited from an online anonymous survey posted on websites and ecig forums that asked about smoking history, ecig use, and device preference. The survey was described previously in (Foulds et al., 2015) and (Yingst et al., 2015). Participants interested in research participation provided their contact information and those who lived locally were contacted and screened via phone for eligibility for the fMRI study. Eligible participants were between the ages of 18 and 60, used an ecig for at least 20 days out of the last 28 with a nicotine concentration in their ecig liquid of at least 12 mg/mL. Participants were excluded if they reported a chronic health condition (e.g., diabetes, hypertension, cancer), or cardiovascular or respiratory illness, current psychopathology or prescribed psychiatric medication, current drug or alcohol abuse, current pregnancy, difficulty donating blood in the past, or other safety MRI contraindications (e.g., metal fragments or implants). Eleven eligible participants completed MRI scans: One participant was excluded for incomplete data collection and another for excessive head motion during scanning. Data were collected across two research labs on Penn State University campuses (Hershey and University Park, PA) from May, 2014 to April, 2015. All participants provided informed consent prior to participation.
2.2 Procedures
Ecig users completed resting state fMRI scans before and after a use episode with their own ecig device. This strategy allowed for a comparison of rsFC while nicotine abstinent and after ecig use. Participants were asked to abstain from combustible cigarettes 4-days prior to the study visit (verified by carbon monoxide sample <8 parts per million) and all nicotine and caffeine products 14 hours prior to the study visit. Participants first completed a series of questionnaires on demographic, smoking history, and the 10-item Penn State Electronic Cigarette Dependence Index questionnaire (PSECDI) (Foulds et al., 2015). Directly after the first scan, participants responded to 24 computerized visual analogue scale (VAS) items regarding their withdrawal symptoms (e.g., “Please respond to each word or phrase with how you feel RIGHT NOW”) by clicking on a horizontal line ranging from “0-Not at all” to “100-Very much” for each withdrawal symptom (e.g., “difficulty concentrating”). Participants were then instructed to take one puff from their own ecig every 20 seconds for 10 minutes for a total of 30 puffs. Nursing staff sampled blood periodically during and after the use episode from a catheter inserted prior to the session. After removing the catheter, participants again completed VAS withdrawal items and returned to the scanner for an identical MRI scan.
2.3 MRI Data Acquisition
Scanning was conducted on 3-T Siemens Trio magnets on the University Park (n=2) and Hershey (n=7) campuses. After completing cue-reactivity and n-back working memory tasks, participants were asked to keep their eyes closed, but remain awake for the resting state scan. Images were acquired using 34-slice oblique-axial series (3 × 3 × 3 mm voxels) for 120 volumes using an EPI pulse sequence with the following parameters at the Hershey campus: repetition time=2000ms; echo time=25ms; field of view=24cm; flip angle=80°; in-plane matrix size 80×80; and the following parameters at the University Park campus: repetition time=2000ms; echo time=25ms; field of view=19.2cm; flip angle=80°; in-plane matrix size 64×64. Functional sequences were followed by a high-resolution structural volume (1 × 1 × 1 mm voxels) acquired using an MPRAGE sequence.
2.4 Data analysis
2.4.1 Blood and self-report measures
SPSS 24 (IBM Corp., 2016) was used to conduct descriptive statistics on sample characteristics, blood nicotine concentrations, and self-report measures. Difference scores of self-reported withdrawal from the VAS were calculated by subtracting pre-episode ratings from post-episode ratings (negative values indicate symptom reductions). A mean total withdrawal score, based on DSM-V criteria (American Psychological Association, 2013), was calculated by averaging the following VAS symptom items: irritability, anxiety, difficulty with concentration, restlessness, hunger, and depression. Blood nicotine boost, an estimate of the amount of nicotine absorbed into the blood during ecig use, was calculated by subtracting the baseline nicotine concentration level from the peak recorded value during or after the ecig use episode. Paired t-tests compared pre- to post-episode withdrawal symptoms. Significance was determined by p-values ≤.05.
2.4.2 Standard fMRI preprocessing
Image preprocessing and analyses were performed with FSL 5.0.1 (Smith et al., 2004). Fsl's standard preprocessing was completed, including motion correction with MCFLIRT, skull-stripping using the Brain Extraction Tool (Smith, 2002), slice timing correction, spatial smoothing (FWHM of 6 mm), intensity normalization (mean of 1000 for each 3D volume), registration to high resolution anatomical images (full search with 12 degrees of freedom [dof]), and non-linear registration to standard MNI-152 space (normal search with 12 dof) with FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001). The preprocessed functional data was further motion-corrected with ICA-based automatic removal of motion artifacts (ICA- AROMA), which employs an algorithm to identify signal associated with artifacts, while preserving signal of interest (Pruim et al., 2015). ICA-AROMA involves three steps: 1) probabilistic independent components analysis with FSL's MELODIC (Beckmann and Smith, 2004); 2) identification of components comprised of artifact signal based on a pre-determined classifier; 3) ordinary least squares regression to regress out the components identified as artifact. As the final preprocessing step, a high-pass temporal filter of 100 seconds was applied and the filtered, denoised data was registered to MNI standard space so that all images were in the same voxel and image dimensions.
2.4.3 Network identification
To identify networks, functional runs from all sessions and all participants were subjected to probabilistic independent components analysis (PICA) using MELODIC with multi-session temporal concatenation and component estimation constrained to 30 components (Beckmann and Smith, 2004). Pre-processing in MELODIC included masking to exclude non-brain voxels, voxel-wise de-meaning of the data, and normalization of the voxel-wise variance. The group ICA approach decomposes the 4D data set into sets of vectors that describe signal variation across the temporal domain (time-courses), the session/subject domain, and the spatial domain (maps). Component maps were thresholded at p ≤ 0.05.
2.4.4 Network labeling
Previously described networks of interest were chosen a priori, and templates provided by Laird and colleagues (2011) were used to run spatial cross-correlations between the templates and the 30 sample components derived with MELODIC. The sample components with the strongest correlations to each template of interest were used in all subsequent analyses.
2.4.5 Dual regression
Dual regression was conducted to estimate a version of each group-level spatial map and time-course for each participant's 4D data (Beckmann et al., 2009; Filippini et al., 2009). In stage one, the group spatial maps were regressed onto each participant's 4D dataset to give a set of time-courses. In stage two, those time-courses were then regressed onto the same 4D dataset to get a subject-specific set of spatial maps. This analysis resulted in a component time-course and spatial map for each subject and each component.
2.4.6 Changes in within-network rsFC
To identify anatomical regions within the eight sample component spatial maps that had significant changes in rsFC across sessions, we conducted one-sample t-tests on subject-specific difference scores using voxelwise permutation testing with FSL's randomise (512 permutations) (Winkler et al., 2014). To identify anatomical regions where changes in rsFC were correlated with changes in withdrawal symptoms and nicotine boost, correlations between change scores of each were conducted with randomise permutation testing (500 permutations).
2.4.7 Between-network coupling
We used the time-courses from the first stage of the dual regression analyses to conduct cross-correlations on the components for each subject. The time- courses consisted of component connectivity estimates (volume-wise slope in the regression) for each of the 120 volumes for each component. This resulted in a correlation matrix in which each matrix element was the pairwise association between each network. The resulting partial correlation coefficients of rsFC between the resting state networks of interest were then transformed to Fisher's Z statistics to normalize the distributions. Between-network connectivity strength was compared across MRI sessions with a series of paired t-tests using SPSS Version 24 (IBM Corp., 2016). SPSS was used to conduct correlations between change scores of between-network rsFC with self-reported withdrawal symptoms and nicotine boost.
3. Results
3.1 Participant and device characteristics
The final sample included 5 women and 4 men, all non-Hispanic white, ages 25 to 58 years (M=35.11, SD=12.30) with educational attainment ranging from high school to graduate degrees. One participant was a current daily cigarette smoker, two occasional smokers, and six past smokers. Five of the six past smokers quit in the prior year after beginning to use their ecig and one participant reported beginning to use their ecig 6 months after quitting smoking. Participants reported using their ecig devices 3 to 36 times per day (M=14.00; SD=11.70) for the past 1 to 24 months (M=9.56; SD=8.34). All participants reported having a preferred device, but only 5 had a preferred liquid flavor; 8 out of 9 participants used a device larger than a combustible cigarette that included a manual button to initiate heating of the coil prior to puffing. Devices cost between $15 and $160 dollars (M=52.33; SD=43.10) and participants reported spending between $3 and $15 dollars per week to maintain their device. The strength of nicotine concentration in liquids ranged from 12 to 24 mg/mL (M=16.44; SD=4.22).
3.2 Blood and self-report measures
The sample's average self-reported dependence on ecigs was low based on PSECDI total scores, which ranged from 3 to 8 (M=6.33; SD=1.80) out of a possible score range from 0 to 20. Baseline nicotine ranged from 0 to 3.6 ng/mL (M=0.83; SD=1.14), suggesting good compliance with the required 14-hour nicotine abstinence prior to the visit. Nicotine boost ranged from 2.02 to 35.45 mg/mL (M=10.08; SD=10.67) and the time to reach peak nicotine levels from the beginning of the ecig use episode ranged from 8 to 15 minutes (M=11.78; SD=2.28). On average, all withdrawal ratings decreased (negative change scores), albeit not significantly, after the ecig use episode with the only exception being hunger, which increased slightly: craving M=-16.67, SD=42.39, (t(8)=1.18, p=0.272); difficulty concentrating M=-5.56, SD=20.56, (t(8)=2.27, p=0.053); restlessness M=-3.22, SD=6.28, (t(8)=1.54, p=0.162); anxiety M=-11.22, SD=19.49, (t(8)=1.73, p=0.122); hunger M=5.00, SD=24.11, (t(8)=-0.62, p=0.551); MNWS total score, M=-6.57, SD=13.28, (t(8)=1.49, p=0.18). Changes in depression and irritability were not analyzed independently because over half of the sample reported no change after the ecig use episode.
3.3 Independent component analysis
Group probabilistic independent component analysis identified eight independent components that corresponded closely to previously described resting state networks of interest for the current study (Figure 1): 1) prefrontal reward, including the anterior cingulate and orbitofrontal cortex; 2) striatal reward, including the basal ganglia and thalamus; 3) salience, including the anterior insula and frontal operculum; 4) visuospatial, including the middle frontal gyri and superior parietal cortices; 5) default mode, including the medial prefrontal cortex, posterior cingulate, and precuneus; 6) emotion, including middle and inferior temporal gyri; 7) right frontoparietal, including the right-lateralized frontal and parietal cortices; and 8) left frontoparietal, including left-lateralized frontal and parietal cortices.
Figure 1.
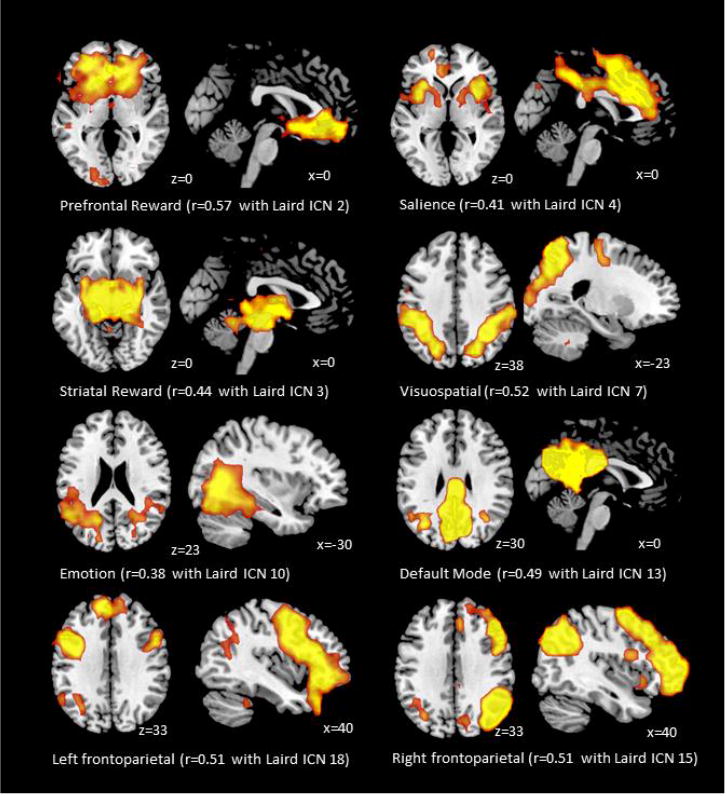
Whole-brain networks identified with probabilistic independent component analysis and cross-correlations with templates provided by Laird and colleagues (2011).
3.4 Voxel-wises changes in rsFC after ecig use episode
RsFC between the salience network and two clusters in the right frontal medial cortex and right frontal pole significantly decreased in strength after ecig use (Table 1, Figure 2). Similarly, connectivity between the striatal reward network and five clusters in the left thalamus, insula, and brain stem significantly decreased in strength after ecig use.
Table 1.
Anatomical regions where resting state functional connectivity significantly decreased after electronic cigarette use.
| Network | Anatomical location | Voxels(mm3) | Max p-value | mni coordinates |
|---|---|---|---|---|
| Salience network | ||||
| R juxtapositional lobule cortex | 1192 | 0.004 | 14, 6, 44 | |
| R frontal pole | 400 | 0.023 | 34, 38, 32 | |
| Striatal reward network | ||||
| L thalamus | 360 | 0.014 | -2, 2, 0 | |
| R brain stem | 264 | 0.012 | 2, -22, -12 | |
| L brain stem | 216 | 0.029 | -6, -30, -4 | |
| R insular cortex | 104 | 0.031 | -34, 2, 4 | |
| L thalamus | 24 | 0.049 | -2, -6, 8 | |
Note: L=Left, R=Right
Figure 2.
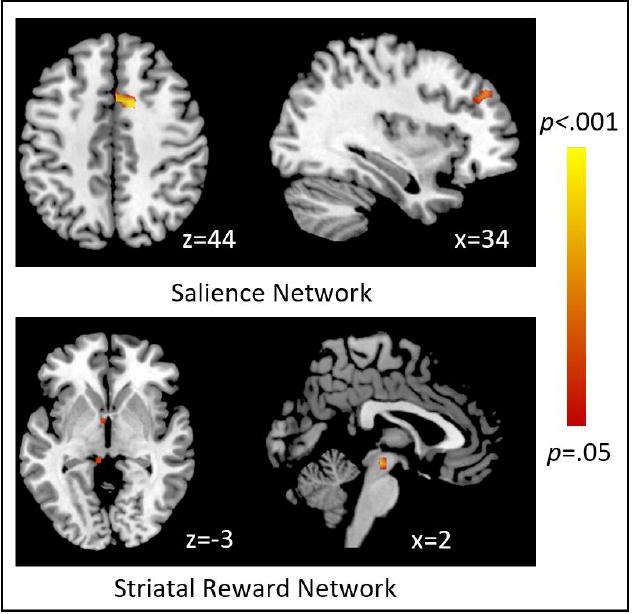
Anatomical regions identified with voxel-wise permutation testing where resting state functional connectivity strength decreased after electronic cigarette use
3.5 Voxel-wise correlations between changes in rsFC and withdrawal symptoms (Table 2)
Table 2. Anatomical regions where changes in resting state functional connectivity were significantly correlated with changes in self-reported withdrawal symptoms.
| Anatomical location | Voxels (mm3) | Max p-value | MNI Coordinates |
|---|---|---|---|
| Reductions in emotion network rsFC with reductions in craving | |||
| L precuneus | 1888 | 0.002 | -26, -58, 12 |
| L lateral occipital cortex | 112 | 0.042 | -26, -74, 16 |
| L occipital fusiform gyrus | 56 | 0.042 | -26, -78, -8 |
| Increases in visuospatial rsFC with reductions in anxiety | |||
| L lateral occipital cortex | 240 | 0.032 | -42, -58, 56 |
| Increases in right frontoparietal network rsFC with reductions in craving | |||
| R frontal pole | 48 | 0.046 | 50, 46, -8 |
| Reductions in salience network rsFC with reductions in concentration difficulty | |||
| R superior frontal gyrus | 8 | 0.036 | 14, 10, 68 |
Note: L=Left, R=right, rsFC=resting state functional connectivity
Reductions in craving were correlated with decreased rsFC in three clusters encompassing the left precuneus, lateral occipital cortex, and occipital fusiform gyrus within the emotion network (Figure 3). Reductions in craving were also correlated with increased rsFC in a cluster encompassing the right frontal pole within the right frontoparietal network. Reductions in anxiety were correlated with increased rsFC in a cluster encompassing the left lateral occipital cortex in the visuospatial network. Reductions in difficulty with concentration were associated with decreased rsFC in a small cluster in the right superior frontal gyrus within the salience network.
Figure 3.
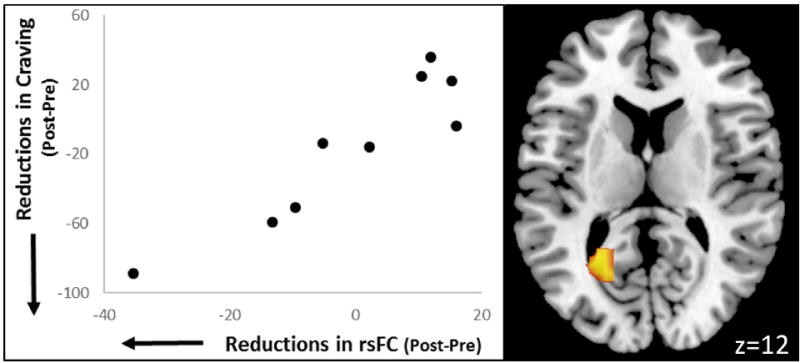
Reductions in resting state functional connectivity strength between a cluster in the precuneus with the emotion network correlated with reductions in craving identified via voxel-wise permutation testing.
3.6 Changes in between-network coupling from before and after ecig use
There was a significant change in mean coupling strength between the prefrontal reward and right frontoparietal networks, which was weakly positive prior to the use episode (r=.01) and moderately negative after the use episode (r=-0.141) (t(8)=2.90, p=0.20). There were no other significant changes in between-network coupling.
3.7 Correlations between changes in between-network coupling and withdrawal symptoms
Scatter plots of significant correlations between changes in coupling strength and withdrawal symptoms are displayed in Figure 4. Reductions in craving were correlated with reductions in coupling strength between the prefrontal reward and visuospatial networks (r=.74). Reductions in difficulty with concentration were correlated with reductions in coupling strength of the striatal reward network with the default mode network (r=.72) and the left frontoparietal network (r=.77). Other significant correlations with withdrawal symptoms and nicotine boost emerged and were determined through visual inspection to be largely driven by one outlier and thus are not reported.
Figure 4.
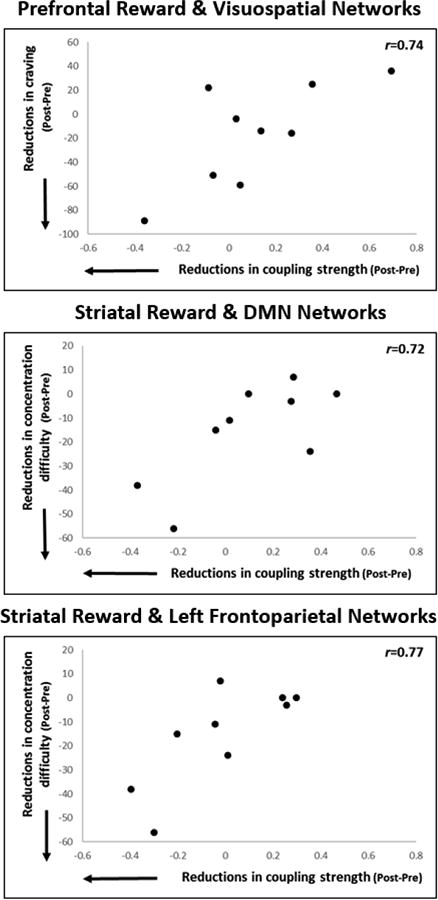
Significant correlations between changes in coupling strength with changes in withdrawal symptoms.
4. Discussion
The current study examined the hypothesis that ecig use would be associated with changes in resting state functional connectivity and nicotine withdrawal symptoms, as has been described in prior studies using other forms of nicotine administration (Cole et al., 2010). In support of this hypothesis, the current study found that an ecig use episode was associated with nicotine delivery and reductions in rsFC in the salience and striatal reward networks. The rsFC reductions we identified in the right insula and prefrontal cortex are consistent with those found by Cole and colleagues (2010) after nicotine lozenge administration among smokers. The insula is increasingly recognized as an important brain structure that is linked to cognitive control and craving among smokers (Fedota et al., 2016; Naqvi and Bechara, 2010; Naqvi et al., 2007; Zelle et al., 2016). Our cluster fits most consistently with the far posterior aspect of the dorsal anterior insula (Addicott et al., 2015). Prior studies have found that smokers show reduced activity in the right anterior insula in response to increasing cognitive demands and weaker connectivity of the anterior insula with prefrontal regions involved in executive control when compared to nonsmokers (Fedota et al., 2016). Further, smokers who relapsed during a quit attempt also demonstrated weaker connectivity between the anterior insula and prefrontal regions involved in executive control compared to smokers who did not relapse (Addicott et al., 2015). Our clusters of reduced rsFC encompassing the left thalamus and brain stem were similar to a cluster showing significantly stronger visual BOLD cue-reactivity after the ecig use episode in our prior analysis (Nichols et al., 2016). Thus, the anatomical regions identified in the current study were expected based on prior research and associated with structures highly involved in craving and executive functions relevant to the development and maintenance of addiction to nicotine and other substances.
Despite overall reductions in rsFC from nicotine abstinence to satiety via ecig use, associations with withdrawal symptoms revealed a more complicated picture, as has been seen in prior studies among smokers (Cole et al., 2010). For example, we found reductions in craving to be associated with decreases in rsFC in the precuneus and visual cortices within a network typically associated with emotion regulation. Alternatively, reductions in anxiety were associated with increases in rsFC in the visuospatial network. The anatomical regions identified in these analyses are strikingly similar to a meta-analysis on smoking cue-reactivity, which found that the precuneus and regions in the visual cortex were consistently associated with cue-reactivity among dependent smokers (Engelmann et al., 2012). The current results highlight the importance of the precuneus, a key region of the default mode network, for understanding craving and its modulation via ecig nicotine administration.
Decreases in network coupling were also associated with reductions in withdrawal symptoms. More specifically, when coupling between networks became weaker (closer to zero) or negative after ecig use, this was associated with relief from withdrawal symptoms. Cole (2010) found reductions in default mode and salience network coupling to be linked to reductions in difficulty concentrating. We likewise found that decreased coupling strength was associated with less difficulty concentrating; however, our associations were between the striatal reward network with the default mode and the left frontoparietal networks, two networks involved in executive function and task completion. There is growing evidence that drug-seeking behavior may be a consequence of an imbalance in the dynamic relationship between dopaminergic reward circuitry and cortical circuitry that directs inhibition, emotional control, and decision-making (Volkow et al., 2011). Our results suggest that ecig use reduces connectivity strength between these circuits, which relieves withdrawal symptoms. During a state of nicotine abstinence, these networks appeared to be more strongly connected than during satiety, which may contribute to craving and difficulty concentrating. Future research could provide insight into how the chronic use of ecigs changes connections between this circuitry.
The study had several limitations. This was a preliminary analysis designed to identify statistically large, within-subject effects of ecig use and was underpowered to detect small effects. Additionally, the current study did not include a placebo control condition to account for changes across sessions that may have been due to confounding factors such as practice effects and measurement variation, or to rule out that changes were not related to inhalation, rather than the inhalation of nicotine specifically. The differences in scanning parameters across campuses may have further contributed to measurement error. However, the pattern of results reported here is similar to that reported by Cole et al (2010) using a placebo-controlled design. We expected changes in rsFC to be associated with changes in nicotine absorption during the ecig use episode, which was not supported by the results. However, the current analysis may have been underpowered to detect effects of nicotine absorption. This variable may be more accurately examined as a moderator of the effects of ecig use on rsFC, such that higher levels or faster rates of nicotine absorption are associated with more pronounced effects of ecig use on rsFC or more lasting neural changes in reward and cognitive control circuitry with chronic use. Additionally, the current study was not designed or powered to examine for other moderating variables like levels of nicotine dependence, length of smoking history, etc. Future studies using larger samples with more selective criteria for the inclusion of participants, device heating element strengths, and liquid nicotine concentrations could provide more insight into importance of these factors for understanding the neural effects of ecigs. Finally, research on ecig use has been influenced by rapid changes in the characteristics of marketed ecig devices and liquids, and the current results, which were collected in 2014-2015, may not generalize to future device/liquid combinations.
Overall, these preliminary findings suggest that ecigs, although a potentially less harmful alternative to combustible cigarettes, may have neural effects similar to those seen among combustible cigarettes and other forms of nicotine administration, despite varying levels of nicotine delivery and absorption. Previous research suggests that rsFC is altered among smokers, which may be related to craving and withdrawal symptoms and predictive of smoking relapse during quit attempts. The results of the current study, showing that ecig use has similar effects on rsFC as nicotine administration via other forms, has implications for understanding the addictive potential of these products as well as their potential as cessation aid. Considering the increasing rates of ecig use among youth and young adults who may not be using ecigs as a quit aid, further research on their impact on brain circuitry involved in nicotine addiction is warranted.
Acknowledgments
This project and the data collection tools for survey responses were supported by the Penn State Clinical & Translational Research Institute, Pennsylvania State University CTSA (NIH/NCATS Grant Number UL1 TR000127). Additional support was provided by the Penn State Hershey Cancer Institute and the Penn State Social Science Research Institute. JF, JR, SV, JY, & SH are primarily funded by the National Institute on Drug Abuse of the National Institutes of Health (NIH-NIDA) and the Center for Tobacco Products of the U.S. Food and Drug Administration (under Award Numbers P50DA036107, P50DA036105). ALH is supported by an NIH-NIDA fellowship (F32DA038519). TE is supported by FDA/NIH grant P50DA036105. We would like to thank the staff at the Penn State University Clinical Research Center at the Hershey and University Park campuses for blood collection, Neil Trushin for completing the blood assays, and David Cole for providing information to assist with replicating his work.The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH, FDA, or any other funding agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ. Increased Functional Connectivity in an Insula-Based Network is Associated with Improved Smoking Cessation Outcomes. Neuropsychopharmacology. 2015;40:2648–2656. doi: 10.1038/npp.2015.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and statistical manual of mental disorders. 5th. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM. Group comparison of resting-state fMRI data using multi-subject ICA and dual regression. OHBM. 2009 [Google Scholar]
- Beckmann CF, Smith SM. FMRIB Technical Report. Oxford Centre for Functional Magnetic Resonance Imaging of the Brain; Oxford, UK: 2004. Probabilistic independent component analysis for functional magnetic resonance imaging. [DOI] [PubMed] [Google Scholar]
- Bi Y, Yuan K, Guan Y, Cheng J, Zhang Y, Li Y, Yu D, Qin W, Tian J. Altered resting state functional connectivity of anterior insula in young smokers. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9511-z. [DOI] [PubMed] [Google Scholar]
- Breland A, Soule E, Lopez A, Ramoa C, El-Hellani A, Eissenberg T. Electronic cigarettes:what are they and what do they do? Ann N Y Acad Sci. 2016:1–26. doi: 10.1111/nyas.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Buchel C, Smolka MN. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. Centers for Disease Control. Rockville, MD: 2016. E-cigarete use among youth and young adults. [Google Scholar]
- Ding X, Lee SW. Changes of functional and effective connectivity in smoking replenishment on deprived heavy smokers: a resting-state FMRI study. PLoS One. 2013;8:e59331. doi: 10.1371/journal.pone.0059331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Matous AL, Salmeron BJ, Gu H, Ross TJ, Stein EA. Insula Demonstrates a Non-Linear Response to Varying Demand for Cognitive Control and Weaker Resting Connectivity With the Executive Control Network in Smokers. Neuropsychopharmacology. 2016;41:2557–2565. doi: 10.1038/npp.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, Macintosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine Tob Res. 2015;17:186–192. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine's actioins with separate cingulate cortex functional circuits. Archives General Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statitistics for Windows. IBM Corp.; Armonk, NY: 2016. [Google Scholar]
- Janes AC, Nickerson LD, Frederick Bde B, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, Cummings KM, Sharma E, Pearson JL, Green VR, et al. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N Engl J Med. 2017;376:342–353. doi: 10.1056/NEJMsa1607538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- King BA, Patel R, Nguyen K, Dube SR. Trends in awareness and use of electronic cigarettes among U.S. adults, 2010-2013. Nicotine & Tobacco Research. 2014 doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of instinsic connectivity networks. Journal of Cognitive Neuroscience. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71:523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsot A, Simon N. Nicotine and Cotinine Levels With Electronic Cigarette: A Review. Int J Toxicol. 2016;35:179–185. doi: 10.1177/1091581815618935. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI- BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- McLaughlin I, Dani JA, De Biasi M. Nicotine withdrawal. Curr Top Behav Neurosci. 2015;24:99–123. doi: 10.1007/978-3-319-13482-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 214(2010):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TT, Foulds J, Yingst JM, Veldheer S, Hrabovsky S, Richie J, Eissenberg T, Wilson SJ. Cue-reactivity in experienced electronic cigarette users: Novel stimulus videos and a pilot fMRI study. Brain Res Bull. 2016;123:23–32. doi: 10.1016/j.brainresbull.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Shen Z, Huang P, Qian W, Wang C, Yu H, Yang Y, Zhang M. Severity of dependence modulates smokers' functional connectivity in the reward circuit: a preliminary study. Psychopharmacology (Berl) 2016;233:2129–2137. doi: 10.1007/s00213-016-4262-5. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 17(2002):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down- regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74:538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Addicott MA, Denlinger R, Raiff BR, Dallery J, McClernon FJ, Donny EC. Smoking Abstinence-Induced Changes in Resting State Functional Connectivity with Ventral Striatum Predict Lapse During a Quit Attempt. Neuropsychopharmacology. 2016;41:2521–2529. doi: 10.1038/npp.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shen Z, Huang P, Qian W, Yu X, Sun J, Yu H, Yang Y, Zhang M. Altered spontaneous activity of posterior cingulate cortex and superior temporal gyrus are associated with a smoking cessation treatment outcome using varenicline revealed by regional homogeneity. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9538-1. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Fang Z, Jagannathan K, Childress AR, Rao H, Franklin TR. Cannabis, cigarettes, and their co-occurring use: Disentangling differences in default mode network functional connectivity. Drug Alcohol Depend. 2015;153:116–123. doi: 10.1016/j.drugalcdep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, Foulds J. Factors Associated With Electronic Cigarette Users' Device Preferences and Transition From First Generation to Advanced Generation Devices. Nicotine Tob Res. 2015;17:1242–1246. doi: 10.1093/ntr/ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Yu D, Bi Y, Li Y, Guan Y, Liu J, Zhang Y, Qin W, Lu X, Tian J. The implication of frontostriatal circuits in young smokers: A resting-state study. Hum Brain Mapp. 2016;37:2013–2026. doi: 10.1002/hbm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelle SL, Gates KM, Fiez JA, Sayette MA, Wilson SJ. The first day is always the hardest: Functional connectivity during cue exposure and the ability to resist smoking in the initial hours of a quit attempt. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


