Abstract
Introduction
The aim of this study was to determine treatment delivery patterns for patients with neovascular age-related macular degeneration (nAMD) across the UK through an ophthalmology trainee research network delivered observational study.
Methods
Data were collected via an online tool by potential research collaborators identified by the Ophthalmology Trainee Clinical Trial Network (OCTN). Collaborators were asked to comment on periprocedural practices of treatment of nAMD in their eye unit including treatment location and injectors, clinical assessment and routine observation in patients undergoing intravitreal treatment.
Results
Data were available from 26 units around the United Kingdom. Survey methodology refinement was approximately 3 months, and the average response time was 4.9 ± 2.4 days. The majority of responders confirmed that treatment was undertaken as a “one-stop” service (n = 15, 58%), delivered in a clean room (n = 23, 88%). In the majority of units, doctors administered injections (n = 24, 92%), but significant treatment was also given by nurse injectors (n = 21, 81%). All collaborators reported that patients underwent visual acuity testing and optical coherence tomography imaging at all visits, but other imaging including fundus fluorescein angiography (FFA) did not take place in all cases (n = 17, 65%) and only at baseline visit.
Conclusions
These results demonstrate the feasibility of conducting ophthalmology trainee led and delivered observational studies. Our results show that FFA is not routinely used in the diagnosis of nAMD in the units sampled; most injections are carried out in a clean room, and ophthalmic nurses delivering injections is a highly prevalent model of care in the UK.
Keywords: Age-related macular degeneration, Anti-angiogenic, Intravitreal
Introduction
The development of intravitreal anti-vascular endothelial growth factor (anti-VEGF) agents has revolutionised the treatment of neovascular age-related macular degeneration (nAMD). Whilst guidelines, local protocols, and expert opinion do exist and inform treatment practice, it is not clear whether variation exists in local patterns of treatment delivery in the UK [1, 2]. The evolution of treatment paradigms and imaging techniques are a likely contributory cause of variation in practice, as is the requirement for clinicians to manage the needs of their own patient population within the local constraints of funding and resources. The increasing burden of nAMD within an ageing population poses a challenge to clinical services and has led to an extended role for non-consultant staff and use of virtual clinical assessment for stable nAMD cases [3]. Recent research suggests variation in treatment outcomes for nAMD exists across the UK [4]. Variation in periprocedural practice may directly or indirectly impact on treatment outcomes and could in part underlie the variability in treatment outcomes reported elsewhere [4].
Despite the need to understand better how care is delivered for patients with nAMD across the UK, there are no previous reports relating to practice patterns in care delivery for patients with nAMD in the UK. Our aim was to engage ophthalmology trainees in the design and delivery of an observational study describing periprocedural practice in treatment delivery for nAMD across the UK. Recently, several surgical specialties, including orthopaedics and neurosurgery, have developed collaborative trainee research networks supported by consultant mentors that have delivered several projects including clinical trials [5, 6]. Involvement of multiple trainee research collaborators supported by experienced research design services with consultant expertise may offer an efficient and robust way of carrying out clinical research.
The purpose of this study was to evaluate variation in current treatment practice in treatment of nAMD with anti-VEGF (including location of treatment, injector profession, and clinical assessment of nAMD activity at baseline and monitoring). In order to help define treatment methodology, acquire data from multiple sites efficiently, and analyse subsequent results, we used the Ophthalmology Trainee Clinical Trial Network (OCTN) to rapidly deliver this observational study.
Methods
Study Population
Invitation for involvement as a collaborator in this trainee-led study was disseminated by email from the OCTN to trainees and consultants around the United Kingdom (UK). Those wishing to be involved were designated as OCTN research collaborators for the project and were sent by email a link to an electronic data reporting form (SurveyMonkey®). This article does not contain any new studies with human or animal subjects performed by any of the authors. This study was not a clinical trial and did not therefore require clinical trial number registration.
Survey Questionnaire
The data reporting form consisted of ten questions regarding treatment location (including number of assessment and treatment sites) and pattern of delivery (“one-stop” versus “two-stop”) and type of injector (doctor including training grade if appropriate, nurse, optometrist, etc.). In addition to the theatre operating room, possible treatment sites included an outpatient clean room (as defined in the Royal College of Ophthalmologists AMD guidelines 2013) [7]. A clean room would consist of an enclosed room, free of interruptions with a washable floor and handwashing facilities. The room would also provide an appropriate patient chair or table to enable the patient to lie supine with adequate space for injectors to move easily around the patient. Collaborators also indicated which assessments were included in evaluation of nAMD disease activity in their unit at baseline (prior to commencement of treatment), monitoring and injection visits including visual acuity and imaging (optical coherence tomography, fundus fluorescein angiography, and colour fundus photography). Collaborators were also asked if measurement of intraocular pressure and baseline systemic observation were routinely assessed.
Results
Data Reporting Response
Data were available from 26 units across the UK, including nine units in London (34% of total response), a further 10 in the rest of England, and two in Wales and one in Scotland. Six trainees were involved as part of the working group, responsible for methodology refinement, data acquisition and analysis and consisted of a range in ophthalmology specialty training (OST) years and one fellow. There were 26 trainee research collaborators who responded with data collection from their units. The design of the survey took place over approximately 3 months and the mean (±SD) response time of collaborators from survey dissemination was 4.9 ± 2.4 days.
Intravitreal Treatment Location
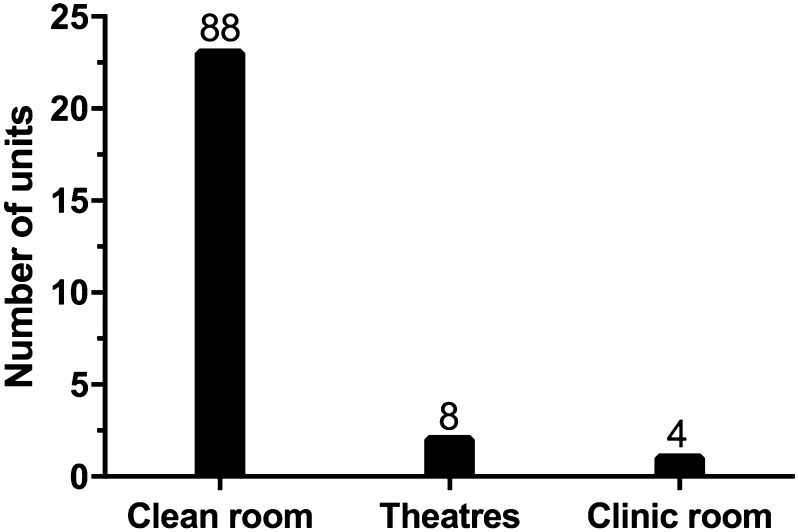
The majority of respondents (n = 15, 58%) reported that their unit followed a “one-stop” treatment delivery pattern where both assessment and intravitreal treatment occurred on the same day. Fourteen (54%) of collaborators responded that the unit had a single assessment and injection site whilst 12 (46%) reported more than one assessment and injection site. The majority of injections took place in a clean room (n = 23, 88%) with very few being undertaken in a theatre (Fig. 1).
Fig. 1.
Location of intravitreal injection treatment within hospital. 23/26 units (88%) use a clean room, 2 units (8%) use theatres and 1 unit (4%) uses a clinic room
Injector Profession
Our study showed that injections are typically administered by staff grade or specialty doctors (n = 24, 92%) in addition to nurse injectors (n = 21, 81%), OST year 3 and above doctors (n = 21, 81%), consultants (n = 19, 73%), OST years 1-2 doctors (n = 13, 50%) and rarely performed by orthoptist/optometrist injectors (n = 1, 4%).
Clinical Assessments
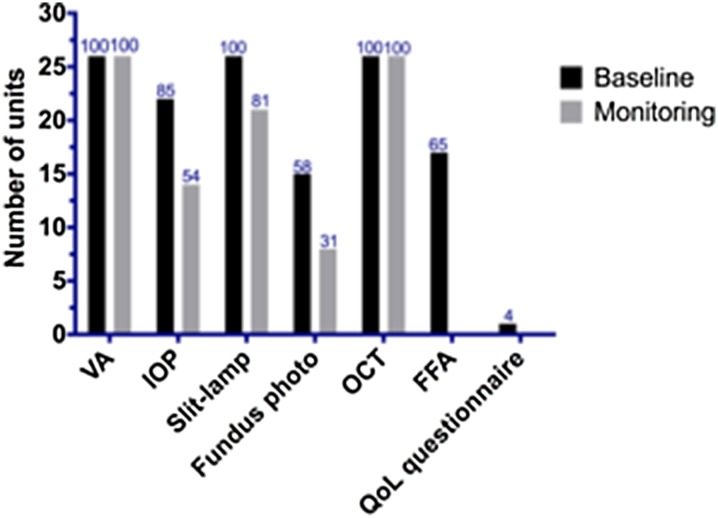
At baseline and monitoring visits, all units appeared to carry out both visual acuity testing and optical coherence tomography (OCT) imaging (Fig. 2). The majority of patients undergo intraocular pressure (IOP) measurement, slit lamp examination, and colour fundus photography at baseline visits [22 (85%), 26 (100%), 15 (58%) respectively], but reduced at monitoring visits [14 (54%), 21 (81%), eight (31%) respectively]. Interestingly, only 17 (65%) respondents replied that their unit carried out regular fundus fluorescein angiography (FFA) at baseline visit and only one1 unit carried out a quality of life assessment in the form of a formal quality of life questionnaire.
Fig. 2.
Clinical assessments performed at units on the day of intravitreal injection treatment. VA visual acuity, IOP intraocular pressure before (pre) or after (post) intravitreal anti-VEGF injection, BM blood glucose measurement, INR international normalised ratio, for patients taking warfarin, HR heart rate. Percentage of respondents shown above each bar
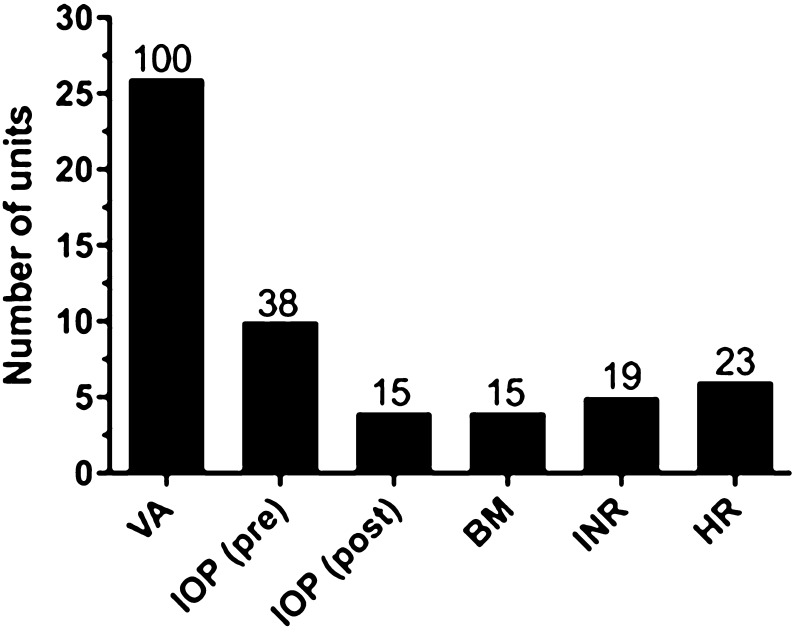
On the day of injection visits (whether as a one or two stop), whilst all patients appeared to have a visual acuity assessment, 10 (38%) had IOP measurement prior, and four (15%) had post-injection IOP measurement (Fig. 3). Systemic observations were made in a minority including blood sugar (n = 4, 15%), INR (international normalised ratio) if on warfarin (n = 5, 19%) and heart rate (n = 6, 23%).
Fig. 3.
Clinical assessments performed at units at baseline and monitoring visits. Visual acuity (VA), n = 26 (100%) at baseline and monitoring. Intraocular pressure (IOP), n = 22 (25%) at baseline and n = 14 (54%) at monitoring. Slit-lamp biomicroscopy, n = 26 (100%) at baseline and n = 21 (77%) at monitoring. Fundus photography n = 15 (58%) at baseline and n = 8 (31%) at monitoring. Optical coherence tomography (OCT), n = 26 (100%) at baseline and monitoring. Fundus fluorescein angiography (FFA), n = 17 (65%) at baseline only. Quality of life (qol) questionnaire, n = 1 (4%) at baseline only. Percentage of respondents shown above each bar
Discussion
We report the results of the first OCTN trainee-led and delivered observational study reporting patterns of nAMD treatment delivery in the UK. Our results show that variation exists in the treatment setting, injector profession and method of clinical assessment (both ophthalmic and systemic) across the sites reviewed in the study.
The increasing reliance on nurse practitioners as nurse injectors is a reflection of the expanded role of non-consultant staff due to the increasing demand for injection capacity in the treatment of nAMD. Indeed, nurse-delivered injections have received favourable feedback from patients [8]. OCT appeared to be used in all centres at baseline assessment and monitoring visits. This is unsurprising as current treatment decisions with anti-VEGF are generally guided by morphological features of nAMD shown on OCT imaging. FFA was not completed at all baseline assessments and in none of the monitoring visits. This was an interesting finding and suggests that OCT may be replacing FFA as the imaging modality of choice in diagnosis of nAMD in some centres in the UK; in part this may be due to an effort in avoiding delays between patient presentation and treatment if organising and carrying out an FFA is likely to lead to delay in commencement of anti-VEGF therapy. As NICE guidelines involve measurement of lesion size, it would suggest that units could be using alternative methods to FFA to determine this such as measurements of lesion size from en face OCT.
The issue of IOP measurement still appears to be variable. Relatively few centres appear to measure IOP with even less performing regular IOP measurement. Suggestion of an increase in IOP after chronic anti-VEGF treatment, both in clinical and experimental studies, may indicate that screening of patients for raised pressure is a sensible consideration [9, 10]. Post-injection measurement is carried out in some centres due to the possibility of acute IOP rise, although most studies suggest these changes are likely transient in the vast majority of cases [11]. Reported systemic adverse events are low with-anti-VEGF agents [12]. A minority of the units appeared to check systemic observations (INR, blood sugar, heart rate) prior to injection. Interesting, a recent study found increase in systemic blood pressure after treatment with anti-VEGF agents only in a small proportion (seven cases, 0.59%). The need to monitor these observations in those undergoing treatment with anti-VEGF agents needs further consideration.
Our study has shown that a collaborative research network in ophthalmology has the ability to deliver trainee-led and trainee-delivered multi-centre observational studies. Trainees were involved in different aspects of the project (including project design and conduct, collection and management of data, analysis and interpretation and presentation). We suggest that this project delivers proof-of-concept of an ophthalmology trainee research network and we hope that this model may be useful in further ophthalmology trainee-led research projects in the UK (including observational and prospective studies).
Strengths of this study include the development of an ophthalmology trainee research collaborative, the use of multi-centre data over short collection time frame. Our study could be further improved by the use of a multicentre audit to investigate about adherence to local protocols and it would be interesting to investigate variation in treatment regimens. It would be helpful for future research to assess the treatment protocol utilized in the UK, in particular characterizing the use of pro re nata, treat and extend, and fixed dosed regimes. A multicenter audit could capture this information accurately. It would also be helpful to assess the percentage of injections in each unit given by different healthcare professionals.
Conclusions
In summary, we report the results of a trainee research collaborative network project into periprocedural practice in the treatment of nAMD. We have shown that there is significant variation in the clinical assessments (systemic and ophthalmic) made at monitoring and baseline visits. There is an increasing reliance on extended roles for nurse practitioners as nurse injectors.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. We would like to thank Mr Barny Foot for critically advising on our methodology. Mr PJ Patel, has received a proportion of their funding from the Department of Health’s NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of Ophthalmology. Daren Hanumunthadu and Victoria A. Nowak contributed equally to this work. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. Collaborators: Mihai Teodor Bica, Miranda Buckle, Tomas R. Burke, Argyrios Chronopoulos, Matthew Gillam, Li Yen Goh, Jan O. Huelle, Simerdip Kaur, Alasdair Kennedy, Soyang Ella Kim, Varo Kirthi, Lyudmila Kishikova, Lauren Van Lancker, Graeme Loh, Gemma S. L. Manasseh, Kareem Mahgoub, Laura Maubon, Muhammad Ali Memon, Symeon Nicolaou, Tina Parmar, Jonathan C. P. Roos, Kamran Saha, Christopher Schulz, Christina Soare, Siddarth Subramani, Damien Chia Ming Yeo, Georgios Vakros, Siegfried Karl Wagner, Haoyu Wang.
Disclosures
Mr. PJ Patel is a consultant for Novartis UK and Bayer UK and has received travel grants from them and research funding from Bayer UK. Ms S George has received sponsorship for international conferences from Novartis, Allergan and Bayer. She has been a speaker for Novartis and Roche. Daren Hanumunthadu, Victoria A. Nowak, Farida Hassan, Ibtesham Hossain, Darshak S. Patel, Lamia Hamidovic, Dalia Abdulhussein, Isra Hausien, Esther Papamichael, Meena Arunakirinathan and Claudia Quijano have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/9FEBF0607973688D.
The members of Ophthalmology Trainee Clinical Trial Network are listed in “Acknowledgements”.
Contributor Information
Praveen J. Patel, Email: praveen.patel@moorfields.nhs.uk
on behalf of the Ophthalmology Trainee Clinical Trial Network:
Mihai Teodor Bica, Miranda Buckle, Tomas R. Burke, Argyrios Chronopoulos, Matthew Gillam, Li Yen Goh, Jan O. Huelle, Simerdip Kaur, Alasdair Kennedy, Soyang Ella Kim, Varo Kirthi, Lyudmila Kishikova, Lauren Van Lancker, Graeme Loh, Gemma S. L. Manasseh, Kareem Mahgoub, Laura Maubon, Muhammad Ali Memon, Symeon Nicolaou, Tina Parmar, Jonathan C. P. Roos, Kamran Saha, Christopher Schulz, Christina Soare, Siddarth Subramani, Damien Chia Ming Yeo, Georgios Vakros, Siegfried Karl Wagner, and Haoyu Wang
References
- 1.Schmidt-Erfurth U, Chong V, Loewenstein A, Larsen M, Souied E, Schlingemann R, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA) Br J Ophthalmol. 2014;98(9):1144–1167. doi: 10.1136/bjophthalmol-2014-305702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bruin DM, Burnes DL, Loewenstein J, Chen Y, Chang S, Chen TC, et al. In vivo three-dimensional imaging of neovascular age-related macular degeneration using optical frequency domain imaging at 1050 nm. Invest Ophthalmol Vis Sci. 2008;49(10):4545–4552. doi: 10.1167/iovs.07-1553. [DOI] [PubMed] [Google Scholar]
- 3.Wilde C, Poostchi A, Mehta RL, MacNab HK, Hillman JG, et al. Prevalence of age-related macular degeneration in an elderly UK Caucasian population-The Bridlington Eye Assessment Project: a cross-sectional study. Eye (Lond) 2017;31(7):1042–1050. doi: 10.1038/eye.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liew G, Lee AY, Zarranz-Ventura J, Stratton I, Bunce C, Chakravarthy U, et al. The UK Neovascular AMD Database Report 3: inter-centre variation in visual acuity outcomes and establishing real-world measures of care. Eye (Lond) 2016;30(11):1462–1468. doi: 10.1038/eye.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan PM, Kolias AG, Joannides AJ, Shapey J, Marcus HJ, Gregson BA et al. The management and outcome for patients with chronic subdural hematoma: a prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg. 2017;1–8. doi:10.3171/2016.8.JNS16134.test. [DOI] [PubMed]
- 6.Collaborative Orthopaedic Research Network The provision of total hip replacement for displaced intracapsular hip fractures. Ann R Coll Surg Engl. 2016;98(2):96–101. doi: 10.1308/rcsann.2016.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Royal College of Ophthalmologists. Age related macular degeneration: guidelines for management. 2013. Sect. 9.3, p 76.
- 8.Gregg E. Nurse-led ranibizumab intravitreal injections in wet age-related macular degeneration: a literature review. Nurs Stand (Royal College of Nursing (Great Britain): 1987). 2017;31(33):44–52. [DOI] [PubMed]
- 9.Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011;95(8):1111–1114. doi: 10.1136/bjo.2010.180729. [DOI] [PubMed] [Google Scholar]
- 10.Wen JC, Reina-Torres E, Sherwood JM, Challa P, Liu KC, Li G, et al. Intravitreal Anti-VEGF injections reduce aqueous outflow facility in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(3):1893–1898. doi: 10.1167/iovs.16-20786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol. 2008;146(6):930–934.e931. [DOI] [PubMed]
- 12.Wu L, Martinez-Castellanos MA, Quiroz-Mercado H, Arevalo JF, Berrocal MH, Farah ME et al. Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008;246(1):81–87. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.