Abstract
Cirrhosis is the terminal stage of hepatic diseases and is prone to develop into hepatocyte carcinoma. Increasing evidence suggests that the transplantation of dental pulp stem cells (DPSCs) may promote recovery from cirrhosis, but the key regulatory mechanisms involved remain to be determined. In this study, we overexpressed human hepatocyte growth factor (hHGF) in primary rat DPSCs and evaluated the effects of HGF overexpression on the biological behaviors and therapeutic efficacy of grafted DPSCs in cirrhosis. Liver cirrhosis was induced via the intraperitoneal injection of CCl4 twice weekly for 12 weeks and was verified through histopathological and serological assays. HGF was overexpressed in DPSCs via transduction with a hHGF-lentiviral vector and confirmed based on the elevated expression and secretion of HGF. The HGF-overexpressing DPSCs were transplanted into rats intravenously. The HGF-overexpressing DPSCs showed increased survival and hepatogenic differentiation in host liver tissue at 6 weeks after grafting. They also exhibited a significantly greater repair potential in relation to cirrhosis pathology and impaired liver function than did DPSCs expressing HGF at physiological levels. Our study may provide an experimental basis for the development of novel methods for the treatment of liver cirrhosis in clinical practice.
Introduction
Cirrhosis, characterized by diffuse degeneration and the death of hepatocytes, followed by nodular regeneration, extensive fibrosis and the consequent collapse of the normal liver architecture, is the terminal stage of a variety of chronic liver diseases, resulting in irreversible impaired liver function, portal hypertension and the potential to develop into hepatocellular carcinoma1,2. Epidemiological data show that liver cirrhosis is the 14th most common cause of death worldwide but the fourth in central Europe3. Because of the lack of effective anti-fibrotic methods currently available in clinical practice3–5, liver transplantation remains the only chance of a cure for patients with liver cirrhosis and is severely restricted by the source of donor organs. Hence, alternative therapeutic strategies are needed.
Mesenchymal stem cell (MSC) transplantation, which has shown notable potential for repairing hepatic architecture and function in preclinical studies, may represent a prospective therapy for liver fibrosis6. Dental pulp stem cells (DPSCs) are a unique type of dental pulp-enriched MSC that hold promise for use in tissue engineering and regenerative therapies. DPSCs are widely available, proliferate rapidly and thus easily amplified, have preserved multipotency, are able to survive during long-term cryopreservation and have minimal immune rejection when autografted7–10. Grafted DPSCs show an effective regenerative capacity in cardiac injury following ischemia-reperfusion as well as central nervous system injury, without teratoma formation11,12. Recently, DPSCs have been reported to differentiate into functional hepatocyte-like cells and express hepatic markers in vitro and in vivo 13,14. However, the determinant induction of hepatic differentiation of DPSCs and the therapeutic efficacy of DPSC grafting for cirrhosis remain poorly understood.
Hepatocyte growth factor (HGF), which is mainly secreted by mesenchymal cells, functions as a cell growth, motility, morphogenic and antiapoptotic factor by activating c-Met receptor/tyrosine kinase signaling in many cell types and plays critical roles in embryonic organogenesis, adult organ regeneration and wound healing15,16. HGF has also been verified to show anti-fibrotic activity in both the onset and progression of liver fibrosis/cirrhosis17,18. However, high exogenous HGF protein levels cannot be maintained, as the protein is extremely unstable (half-life <15 min)19. Therefore, the selection of a vehicle for the long-term transfer of therapeutic genes is important. For liver regeneration, MSCs are promising candidate. For example, transplantation of bone marrow mesenchymal stem cells (BMMSCs) and umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) overexpressing HGF in a rat model of liver fibrosis was shown to ameliorate liver injury induced by the hepatotoxin carbon tetrachloride (CCl4)20,21. However, the impact of HGF on DPSCs and the therapeutic efficacy of DPSCs in cirrhosis are still elusive.
Therefore, in the present study, we overexpressed human HGF (hHGF) in rat-derived primary DPSCs through the transduction of a hHGF-expressing lentiviral vector and evaluated the effects of elevated HGF expression on the survival, fate determination and regenerative capacity of grafted DPSCs in a rat model of cirrhosis to provide an experimental basis for the development of a novel anti-cirrhosis therapy.
Materials and Methods
Study design
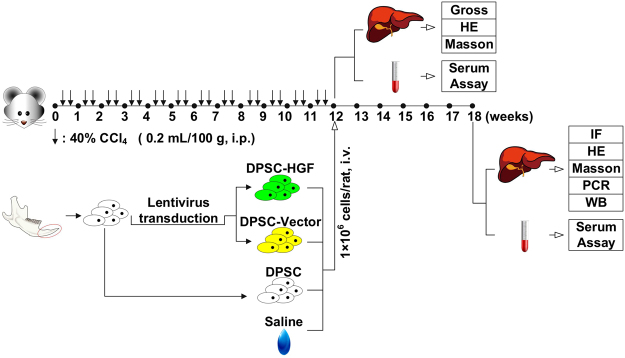
All animal experiments were conducted in strict accordance with the principles of medical ethics and were approved by the ethics committee of the Hospital of Harbin Medical University. As shown in Fig. 1, liver cirrhosis was induced in female Sprague-Dawley rats(200–250 g, purchased from the Department of the Animal Experiment Center of the Second Affiliated Hospital of Harbin Medical University) via the intraperitoneal injection of CCl4 for 12 weeks and was verified through histopathological and serological analyses. DPSCs were derived from the incisors of 4-week-old Sprague-Dawley rats. To assess their purity and multipotency, DPSCs at the 3rd passage were subjected to fluorescence-activated cell sorting (FACS) to detect surface markers or were differentiated into osteoblasts, adipocytes and hepatocytes. To investigate the impacts of elevated HGF expression on the therapeutic efficacy of DPSC grafting for liver cirrhosis, the experimental animals (50 female Sprague-Dawley rats, 200–250 g) were randomly divided into one of the following 5 groups: Control, CCl4/Saline, CCl4/DPSC, CCl4/DPSC-Vector, or CCl4/DPSC-HGF. The rats in the Control group were administered intraperitoneal injections of saline for 12 weeks. The animals in the other groups were administered intraperitoneal CCl4 injections for the same period. The rats in the CCl4/Saline, CCl4/DPSC, CCl4/DPSC-Vector and CCl4/DPSC-HGF groups underwent treatment with saline alone, wild-type DPSCs, blank lentiviral vector-transduced DPSCs or hHGF-expressing lentiviral vector-transduced DPSCs, respectively, in an equal volume via intravenous injection on Week 12. On Week 18, immunofluorescence (IF) staining was performed to determine the survival and differentiation of the grafted DPSCs in host hepatic tissue. Histopathological and molecular analyses were conducted to evaluate histological restoration, and serum assays were conducted to assess the recovery of liver function.
Figure 1.
Outline of the experimental design. Science Slides 2005 was used to crate these images (www.scienceslides.com).
Cell culture
DPSCs were isolated from rat incisors as described previously, with some modifications22. Briefly, dental pulp was extracted from the lower incisors of 4-week-old male Sprague-Dawley rats, then cut into pieces of less than 1 mm in 0.01 M phosphate-buffered saline (PBS; GIBCO-BRL, USA)23 and digested with type I collagenase (3 mg/mL; Sigma-Aldrich, USA) for 45 min (37 °C, 8 rpm). Digestion was neutralized with DPSC complete medium (Dulbecco’s modified Eagle’s medium (DMEM; GIBCO-BRL) containing 10% fetal bovine serum (FBS; GIBCO-BRL), 0.292 mg/mL glutamine (Invitrogen, USA), 100 units/mL penicillin G (GIBCO-BRL), 100 mg/mL streptomycin (GIBCO-BRL), and 2.5 mg/mL ascorbic acid (GIBCO-BRL)). After filtration, the cells were harvested and plated in 25 cm2 culture flasks (Costar, USA) in complete medium at a cell density of 1 × 106/mL. They were then grown under 5% CO2 at 37 °C. For passaging, cells were digested with 0.25% trypsin at 90% confluency.
FACS
Cultured cells at passage three (1 × 106 cells/specimen) were harvested via trypsinization and incubated with the following fluorescent molecule-conjugated antibodies: anti-rat CD29-FITC (561796, BD Biosciences, USA, 1:200), CD29 isotype control (553960, BDBiosciences, USA, 1:200), anti-ratCD34-APC (ab213058, Abcam, UK, 1:200), CD34-APC isotype control (552893, BDBiosciences, USA, 1:200), anti-rat CD45-PE-cy5 (559135, BD Biosciences, 1:200), CD45-PE-cy5 isotype control (550618, BD Biosciences, 1:200), anti-rat CD44-PE (554869, BD Biosciences, 1:200), CD44-PE isotype control (554680, BD Biosciences, 1:200), anti-rat CD90-PE (554898, BD Biosciences, 1:200), and CD90-PE isotype control (550617, BD Biosciences, 1:200). CD29 was a hamster IgM, whereas the others were mouse IgGs. The cells were treated for 30 min in the dark and subsequently suspended in PBS (5 × 105/mL) and analyzed using a FACSCanto II flow cytometer (BD Biosciences).
Multilineage differentiation
Purified DPSCs at the 3rd passage were differentiated into adipocytes and osteoblasts and hepatocytes in osteogenic and adipogenic differentiation medium, respectively, for 21 d. The osteogenic medium contained DMEM supplemented with 50 μg/mL ascorbic acid 2-phosphate, 0.1 µmol/L dexamethasone, and 10 mmol/L β-glycerol phosphate (Sigma-Aldrich) and 10% fetal bovine serum (GIBCO-BRL). The adipogenic medium contained DMEM supplemented with 0.1 µmol/L dexamethasone, 0.1 mmol/L indomethacin, 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma-Aldrich) and 10% fetal bovine serum (GIBCO-BRL). DPSCs were differentiated into hepatocytes using the OriCellTM Meschenchymal Stem Cell Hepatogenic Differetiation Medium (HUXMX-90101, Cyagen Biosciences Inc, USA) in strict accordance with Manufacturer’s instructions. Osteogenic, adipogenic and hepatogenic differentiation was verified by Alizarin Red (Sigma-Aldrich) staining, Oil Red O (Sigma-Aldrich) staining and periodic acid-Schiff (PAS; Sigma-Aldrich) staining, respectively. Hepatogenic differentiation was further confirmed by immunofluorescence staining using rabbit anti-albumin (Abcam; IgG; 1:300) according to the standard protocol.
Lentiviral vector construction and transduction
Two lentiviral vectors (a blank lentiviral vector encoding only green fluorescent protein (GFP) and a hHGF-expressing lentiviral vector encoding GFP and hHGF) were purchased from Shanghai Genechem Company (GOSL49905). The lentiviral transduction of DPSCs with a multiplicity of infection (MOI) of 20 was carried out for 72 h in 8 mg/mL polybrene (Millipore, USA). The transduction efficiency was evaluated by observation using a BX51 fluorescence microscope (Olympus, Japan).
Rat model of cirrhosis and cell transplantation
To induce liver cirrhosis, 40% CCl4 in olive oil (0.2 mL/100 g body weight) was injected intraperitoneally into female Sprague-Dawley rats (200–250 g) in the cirrhosis model groups twice weekly for 12 weeks. Rats in the Control group received an intraperitoneal injection of the same volume of saline. For transplantation, on Week 12, wild-type, Lenti-GFP-transduced or Lenti-hHGF-transduced DPSCs (1 × 106) in 0.5 mL of PBS were injected into the caudal veins of the rats in the cell transplantation groups using a Hamilton Syringe. Rats in the Vehicle group received an intravenous injection of the same volume of PBS.
Serum assays
For the rat serum assays, blood was harvested from the abdominal aorta and transferred to coagulation-promoting vacuum tubes (MDSIN, China) to collect the serum. To evaluate the serum levels of albumin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), enzyme-linked immunosorbent assays (ELISAs) were performed using ELISA kits (Invitrogen) according to the manufacturer’s instructions.
Histopathological analysis
After the rats were sacrificed, the right lobes of their livers were dissected and embedded in paraffin. Serial 4-µm sections of the right lobes of the livers were prepared. Routine hematoxylin & eosin (HE) and Masson’s trichrome staining were performed according to standard protocols. Fibrosis was evaluated using the Masson’s trichrome staining-based Laennec fibrosis scoring system24. In this system, hepatic fibrosis is scored from 0 to 6, with 0 indicating no definite fibrosis; 1, minimal fibrosis; 2, mild fibrosis; 3, moderate fibrosis; 4, mild, but definite cirrhosis; 5, moderate cirrhosis; and 6, severe cirrhosis. An immunofluorescence assay was conducted to evaluate the survival and differentiation of grafted cells. The sections were stained using mouse anti-prealbumin (transthyretin, TTR; Santa Cruz), rabbit anti-cytokeratin (CK) 18 (Abcam) or anti-albumin (Abcam) at a dilution of 1:300 (all these antibodies were IgGs), followed by incubation with Alexa 488-conjugated goat anti-rabbit IgG (R37116, Invitrogen, 1:400) and anti-mouse IgG (R28177, Invitrogen, 1:400). The stained sections were visualized, and images were captured using an IX83 confocal microscope (Olympus, Japan). The GFP+ cells in local area were counted, the precentage resident GFP+ cells of serial sections in total grafted cells were calculated and colocalization of GFP and TTR, CK18 or albumin was measured using the software Image-Pro Plus 6.0.
Quantitative PCR
Total RNA was extracted from liver tissue using TRIzol reagent (HaiGene, Harbin, China). cDNA was then synthesized using the Golden 1st cDNA Synthesis Kit (HaiGene, Harbin, China), and real-time PCR was performed using Golden HS SYBR Green qPCR Mix (HaiGene, Harbin, China) with a MiniOpticon2 system (MJ). The reaction conditions were 95 °C for 15 min, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The primers for the target genes (rat) were as follows: albumin (forward: 5′-AGACGGTGATGGGTGACTTC-3′, reverse: 5′-GCCTGAGATGGTTGTGATG TG -3′); TTR (forward: 5′-ACAGATGAGAAGTTCACGGAAGG-3′, reverse: 5′-CGATGGTGTAGTGGCGATGAC-3′); CK18 (forward: 5′-TCCGTGCCCAGTAT GAACAG-3′, reverse: 5′-AGTCCAGGTCAATCTCCAAGG-3′); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward: 5′-AACTCCCATTCTTCCACCTTT-3′, reverse: 5′-CTCTTGCTCTCAGTATCCT TG-3′). The mRNA expression of the target genes was normalized to GAPDH expression and calculated using the 2−ΔΔCt method.
Western blotting
Total protein was extracted using Super RIPA Lysis Buffer with PMSF (HaiGene, Harbin, China). Then, 30 µg of total protein was subjected to SDS-PAGE and transferred to a PVDF membrane (Millipore, USA). Target protein expression was detected using the following primary antibodies: rabbit anti-HGF (ab178359, Abcam, UK), rabbit anti-hHGF (ab24865, Abcam, UK), rabbit anti-rat CK18 (ab133263, Abcam, UK) or anti-rat albumin (ab207327, Abcam, UK), and mouse anti-TTR (SC-377517, Santa Cruz) (IgGs; 1:000). β-actin expression was used as a control using rabbit anti-β-actin (Sigma-Aldrich, USA, 1:000) The protein blots were visualized using horseradish peroxidase-conjugated goat anti-rabbit IgG (65–6120, Invitrogen, USA, 1:10,000), anti-mouse IgG (A28177, Invitrogen, USA, 1:10,000) and an enhanced chemiluminescence (ECL) system (BioRad, USA). Protein expression levels were measured using Image-Pro Plus 6.0 software. All of the antibodies used in this study were list in Table 1.
Table 1.
List of antibodies used.
| Antibody | Manufacturer | Source | Dilution (FACS) | Dilution (IF) | Dilution (WB) |
|---|---|---|---|---|---|
| Albumin | Ab207327, Abcam | Rabbit | 1:300 | 1:1000 | |
| TTR | Sc-377517 Santa Cruz | mouse | 1:300 | 1:1000 | |
| CK18 | Ab133263 Abcam | Rabbit | 1:300 | 1:1000 | |
| CD29-FITC | 561796 BD Biosciences | Hamster | 1:200 | ||
| CD29-FITC Isotype Control | 553960 BD Biosciences | Hamster | 1:200 | ||
| CD90-PE Isotype Control | 554898 BD Biosciences | Mouse | 1:200 | ||
| CD90-PE Isotype Control | 550617 BD Biosciences | Mouse | 1:200 | ||
| CD45-PE-cy5 | 559135 BD Biosciences | Mouse | 1:200 | ||
| CD45-PE-cy5 Isotype Control | 550618 BD Biosciences | Mouse | 1:200 | ||
| CD44-PE | 554869 BD Biosciences | Mouse | 1:200 | ||
| CD44-PE Isotype Control | 554680 BD Biosciences | Mouse | 1:200 | ||
| CD34-APC | Ab213058 Abcam | Mouse | 1:200 | ||
| CD34-APC Isotype Control | 552893 BD Biosciences | Mouse | 1:200 |
Statistical analysis
Statistical analyses were performed using Microsoft Excel and SPSS 23.0 software. The data are expressed as the mean ± standard deviation (SD) or the mean ± standard error of the mean (SEM). Statistical significance was determined using Student’s t test or analysis of variance (ANOVA), followed by Bonferroni’s post hoc multiple comparisons. P < 0.05 was considered to indicate statistical significance.
Results
Rat model of liver cirrhosis
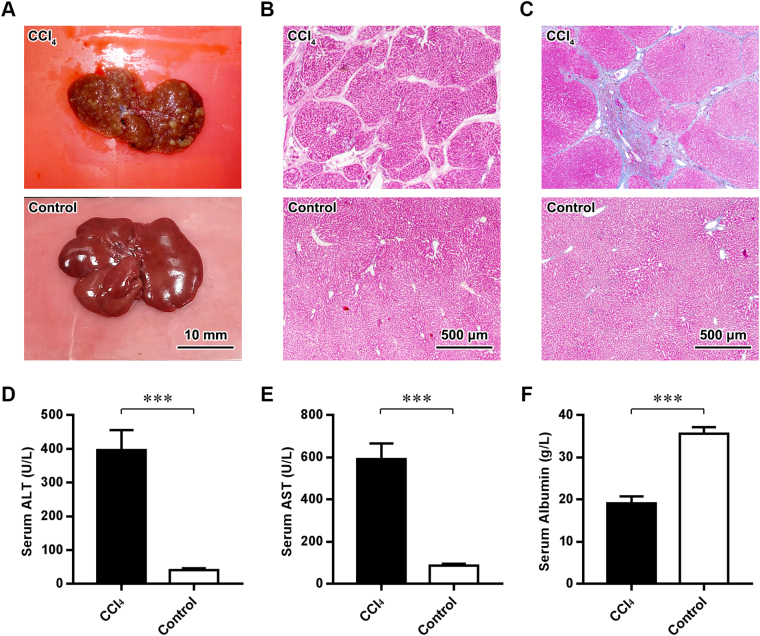
To recapitulate liver cirrhosis, rats were administered intraperitoneal injections of CCl4 for 12 weeks. The livers of CCl4-treated rats exhibited a hard texture and a rough surface with numerous nodules compared with those of control rats, which exhibited a soft texture and smooth surface (Fig. 2A). HE staining and Masson’s trichrome staining further revealed diffuse parenchymal regenerating nodules and fibrous septa in the livers of rats in the CCl4 group (Fig. 2B,C). To assess hepatic dysfunction, serum assays were performed. As shown in Fig. 2D–F, significant increases in the levels of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and a simultaneous decrease in serum albumin occurred after CCl4 treatment (all P values < 0.001). These results indicated that the major histopathological and serological changes of liver cirrhosis had emerged in CCl4-treated rats.
Figure 2.
Rat model of liver cirrhosis. (A–C) Gross observations (A), HE staining (B) and Masson’s trichrome staining of the livers of rats in the CCl4 and Control groups. Scale bars (A) = 10 mm; (B,C) = 500 μm. (D–F) ELISA of the serum ALT, AST and albumin levels of rats in the CCl4 and Control groups. ***P < 0.001.
Isolation and characterization of DPSCs
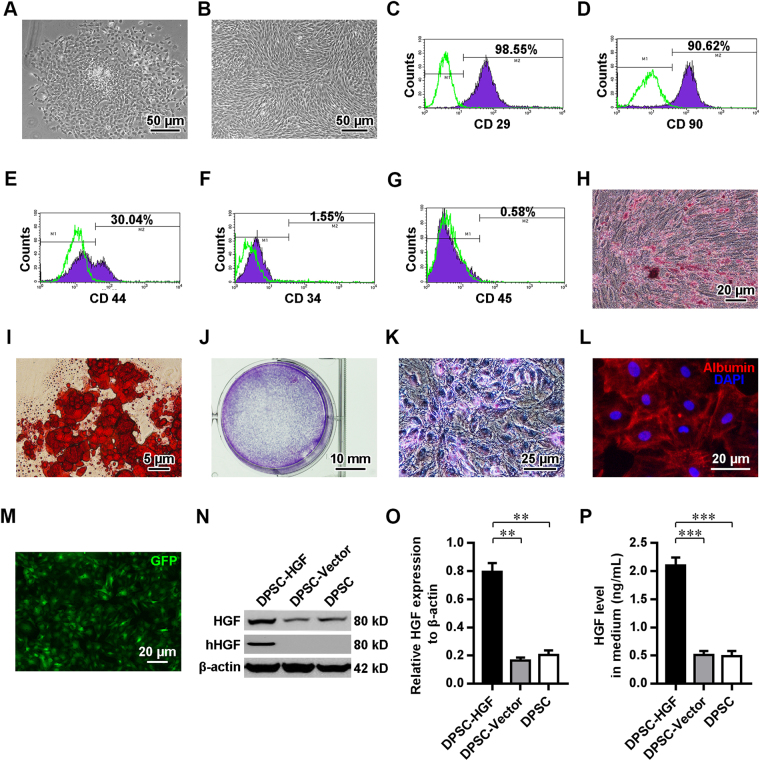
The primary dental pulp-derived cells displayed adherent growth and had formed multiple colonies 48 h after their initial plating (Fig. 3A). The cultured cells at the 3rd passage showed a relatively uniform spindle-like morphology and a vortex-like distribution (Fig. 3B). To characterize the immunophenotypes of these dental pulp-derived cells, cells at the 3rd passage were subjected to flow cytometry (Fig. 3C–G). Intriguingly, the vast majority of cultured dental pulp-derived cells expressed CD29 and CD90, which are markers of MSCs. Approximately 30% of the cells expressed CD44 (a homing cell adhesion molecule), whereas they rarely expressed CD34 (a hematopoietic progenitor marker) or CD45 (leukocyte common antigen). To evaluate the multipotency of these cultured dental pulp-derived cells, osteogenic, adipogenic and hepatogenic differentiation was induced. When grown in osteogenic and adipogenic differentiation media, the cultured cells developed into osteoblasts (Fig. 3H) or adipocytes (Fig. 3I), as shown using Alizarin Red staining for mineral deposits and Oil Red O staining for lipid vacuoles, respectively. Futhermore, when exposed to the hepatogenic differentiation media, these dental pulp-derived cells granually gained irregular polygonal and paving stone-like morphology which resembled that of primary hepatocyte (Fig. S1A–F). The hepatocyte-like glycogen accumulation (Figs 3J,K and S1G) and emerging expression of hepatocyte-produced albumin (Fig. 3L) in these dental pulp-derived cells were further revealed by PAS staining and immunostaining respectively. These data indicated that the dental pulp-derived cells with MSC-like immunophenotypes and osteogenic, adipogenic and hepatogenic multipotency were DPSCs.
Figure 3.
Isolation and characterization of DPSCs. (A,B) Phase-contrast images of primary DPSCs cultured for 48 h (A) and DPSCs at the 3rd passage (B). (C–G) FACS analyses of the expression of CD29 (C), CD90 (D), CD44 (E), CD34 (F) and CD45 (G) in DPSCs. (H,I) Images of Alizarin Red (C) and Oil Red O staining (D) in DPSCs cultured in osteogenic and adipogenic differentiation medium for 21 d. (J–K) Gross (J) and phase contrast (K) images of PAS staining in DPSCs grown in hepatogenic differentiation medium for 17 d. (L) Fluorescence microscopic image of DPSCs grown in hepatogenic differentiation medium for 17 d, immunostained with anti-albumin (red) and counterstained with DAPI (blue). (M) Fluorescence microscopic image of GFP (green)-encoding lentiviral vector-transduced DPSCs. (N,O) Images from Western blot analyses for HGF and hHGF in DPSCs (N) and related quantitative analysis (O). (P) ELISA of HGF levels in the medium of DPSCs. Scale bars (A,B) = 50 μm; (H,L,M) = 20 μm; (J) = 5 μm; (J) = 10 mm; (K) = 25 μm. **P < 0.01; ***P < 0.001.
HGF overexpression promotes the hepatocyte-like differentiation of grafted DPSCs
To investigate the effects of HGF on the determination of DPSC fate, rat DPSCs at the 3rd passage were transduced with the human HGF (hHGF)-lentiviral vector (DPSC-HGF) or a blank lentiviral vector (DPSC-Vector). Overexpression of HGF in hHGF-lentiviral vector-transduced cells was confirmed through the expression of the reporter gene (green fluorescence protein, GFP; Fig. 3M,N) as well as hHFG and the over 4-fold increase in total HGF levels in both the cells and medium (Fig. 3O,P), relative to blank lentiviral vector-transduced and wild-type (DPSC) DPSCs (both P values < 0.001).
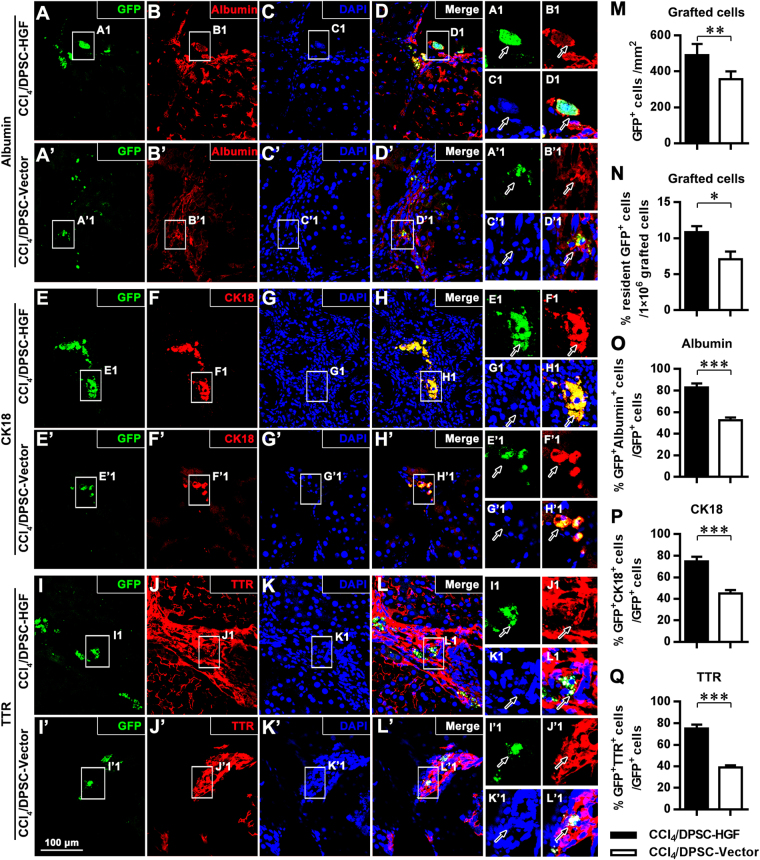
The lentiviral vector-transduced DPSCs were subsequently transplanted into rats with cirrhosis through intravenous injection. Immunofluorescence staining and confocal microscopy analysis performed 6 weeks after transplantation showed that increased amounts of GFP-positive graft-derived cells survived, migrated to and resided in the host liver tissue areas in the DPSC-HGF group compared with those in the DPSC-Vector group (P < 0.01; Fig. 4A–L,A’–L’,M). Further image analyses of serial sections verified that higher percentages of GFP-positive cells resident in host liver tissue in total grafted cells existed in DPSC-HGF group (10.84% ± 0.84%) rather than DPSC-Vector group (7.08% ± 1.07%; P < 0.05; Fig. 4N). Furthermore, the GFP fluorescence of the grafted DPSCs colocalized with the immunofluorescence of the single-layer epithelial marker CK18 as well as the hepatocyte-synthesized plasma proteins albumin and TTR, which suggested that the grafted DPSCs with hepatogenic potency confirmed in vitro (Figs S1 and 3J–L), had differentiated into hepatocyte-like cells in host liver tissue with cirrhosis pathology. Quantitative analyses further indicated that the grafted DPSCs with the elevated expression of HGF gave rise to higher frequencies of GFP+albumin+ (P < 0.001; Fig. 4O), GFP+CK18+ (P < 0.001; Fig. 4P) or GFP+TTR+ (P < 0.001; Fig. 4Q) graft-derived hepatocyte-like cells than with the blank lentiviral vector-transduced DPSCs. These results verified that the hepatocyte-like differentiation of grafted DPSCs in host liver tissue with cirrhosis could be significantly enhanced by HGF overexpression.
Figure 4.
HGF overexpression promotes the hepatocyte-like differentiation of grafted DPSCs. (A–L,A’–L’) Fluorescence microscopy images of hepatic tissue immunostained with anti-GFP (A,A’,E,E’,I,I’), anti-albumin (B,B’), anti-CK18 (F,F’), and anti-TTR (J,J’) and counterstained with DAPI (C,C’,G,G’,K,K’) and related merged fluorescence microscopy images (D,D’,H,H’,L,L’) in CCl4/DPSC-HGF (A–L) and CCl4/DPSC-HGF groups (A’–L’). A1-L1 and A’1-L’1 show magnified images of the outlined areas from the corresponding panels. (M-Q) Quantitative analyses of the number of GFP+ graft-derived cells (M), the percentage of resident GFP+ cells in total 1 × 106 grafted cells (N) and the percentages of albumin+ (O), CK18+ (P) and TTR+ (Q) cells in the GFP+ graft-derived cell population. Scale bar (A–L,A’–L’) = 100 μm. **P < 0.01; ***P < 0.001.
HGF overexpression enhances the grafted DPSC-mediated amelioration of cirrhosis in rats
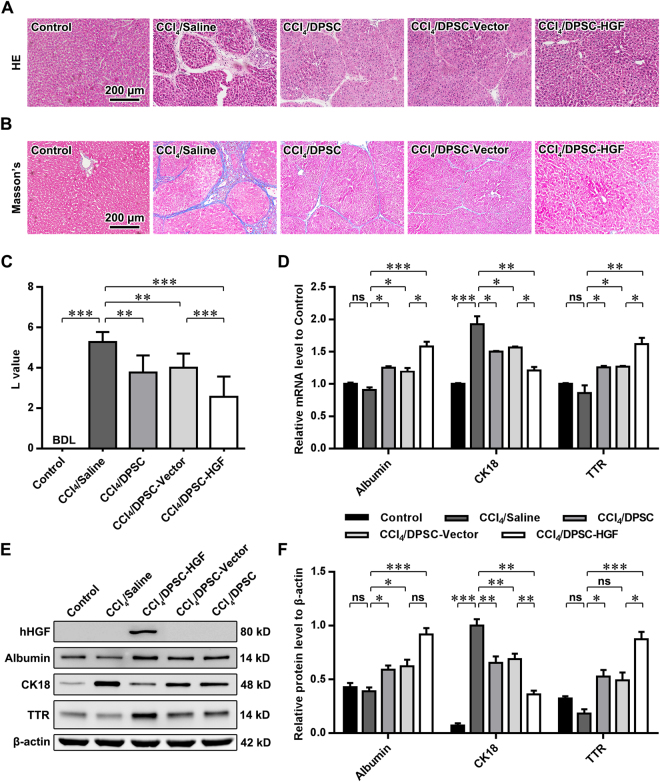
To further study the impacts of HGF on the repair potential of grafted DPSCs in cirrhosis, histopathological analyses were conducted on healthy rats (Control), sham-treated rats with cirrhosis (CCl4/Saline) and rats receiving grafts of wild-type DPSCs (DPSC), blank lentiviral vector (CCl4/DPSC-Vector)-transduced DPSCs or hHGF-lentiviral vector (CCl4/DPSC-HGF)-transduced DPSCs for 6 weeks. As shown through HE staining (Fig. 5A), the formation of regenerative nodules and fibrous septa was notably attenuated in the livers of rats with cirrhosis after DPSC grafting. Masson’s trichrome staining and subsequent image analysis (Fig. 5B,C) further revealed that cirrhosis-specific fibrosis was significantly alleviated in the livers of model rats receiving grafts of wild-type, blank lentiviral vector or hHGF-lentiviral vector-transduced DPSCs (all P values < 0.01 vs. CCl4/Saline). Compared with rats in the CCl4/DPSC-Vector group, the rats in the CCl4/DPSC-HGF group exhibited a better recovery from hepatic fibrous deposition (P < 0.001).
Figure 5.
HGF overexpression in grafted DPSCs enhances the amelioration of cirrhosis in a rat cirrhosis model. (A,B) Images of the HE staining (A) and Masson’s trichrome staining (B) of the liver tissue of rats. (C) Quantitative analysis of the Laennec fibrosis scoring for the liver tissue of rats. (D) Quantitative analyses of qPCR assays for the mRNA levels of albumin, CK18 and TTR in the liver tissue of rats. (E,F) Images of Western blot analyses for the protein expression of hHGF, albumin, CK18 and TTR in the liver tissue of rats (E) and the related quantitative analysis (F). Scale bars (A,B) = 200 μm. ns, nonsignificant; **P < 0.05; **P < 0.01; ***P < 0.001.
Molecular biological analyses were utilized to determine the levels of markers of mature and functional hepatocytes in liver tissues. Interestingly, qPCR (Fig. 5D) revealed that the sham-treated rats with cirrhosis displayed unaltered albumin and TTR mRNA levels (both P values > 0.05) and dramatically increased CK18 mRNA levels (P < 0.001) compared with the healthy Controls, whereas all the rats in the DPSC-treated groups exhibited increased albumin and TTR levels and restored CK18 transcription (all P values < 0.05). Furthermore, rats treated with hHGF-lentiviral vector-transduced DPSCs showed comparatively elevated albumin and TTR mRNA levels and recovered CK18 transcription relative to those of rats treated with blank lentiviral vector-transduced DPSCs (all P values < 0.05). Concurrent with the in vitro observations, the specific expression of human HGF in the liver tissue of rats subjected to grafting of hHGF-lentiviral vector-transduced DPSCs was detected by Western blotting (Fig. 5E,F). Consistent with the qPCR data, Western blotting (Fig. 5E) indicated no significant changes in albumin or TTR expression (both P values > 0.05) in the liver tissue of rats with cirrhosis, whereas albumin and TTR levels were increased in rats treated with DPSCs (both P values < 0.05). Additionally, TTR expression was further up-regulated in the CCl4/DPSC-HGF group (P < 0.05). Furthermore, the aberrant rise in CK18 expression in rats with cirrhosis was reversed after treatment using DPSCs expressing HGF at normal levels (both P values < 0.01) and was further restored by the grafting of HGF-overexpressing DPSCs (P < 0.01). These data indicate that the regenerative and repair capacity of grafted DPSCs in cirrhosis-related pathological injury was enhanced by HGF overexpression.
HGF overexpression in grafted DPSCs enhances the hepatic functional recovery of rats with cirrhosis
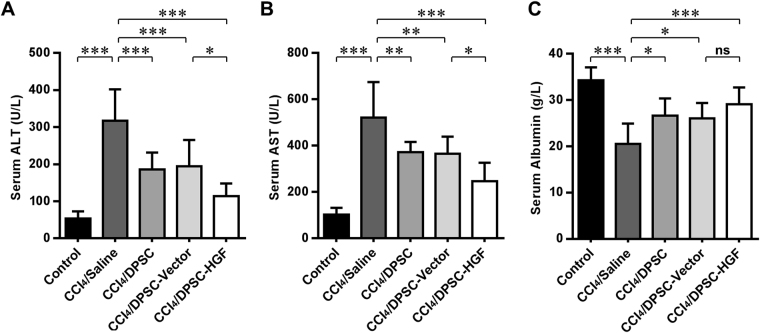
To further evaluate the effect of HGF on the protective efficacy of DPSCs against hepatic functional impairment, serum assays were performed. As shown by the ELISA results (Fig. 6), the increases in the serum levels of the enzymes ALT and AST released by injured hepatocytes in rats with cirrhosis (both P values < 0.001 vs. Control) were alleviated 6 weeks after the grafting of DPSCs with physiological HGF levels (all P values < 0.01) and recovered more significantly in rats treated with DPSCs overexpressing HGF (both P values < 0.05). Intriguingly, inconsistent with the results of Western blotting, the evident decrease in the level of serum albumin in cirrhosis model rats (P < 0.001 vs. Control) was recovered to equivalent levels via the grafting of physiological HGF-expressing and HGF-overexpressing DPSCs (all P values < 0.05 vs. CCl4/saline). These data suggest that the grafted DPSC-induced hepatic functional recovery could be further improved through elevated HGF expression.
Figure 6.
HGF overexpression in grafted DPSCs enhances the hepatic functional recovery of rats with cirrhosis. (A–C) ELISAs of the serum ALT (A), AST (B) and albumin (C) levels of the rats in each group. ns, nonsignificant; **P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In this study, we investigated the impacts of hHGF overexpression on the in vivo biological behaviors and the therapeutic efficacy of DPSCs for the treatment of liver cirrhosis. Purified multipotent rat dental pulp-derived mesenchymal stem cells were characterized for their immunophenotype, transduced with hHGF-lentiviral vectors to elevate the expression and secretion of HGF, and transplanted into rats with confirmed CCl4-induced cirrhosis. The HGF-overexpressing DPSCs showed significantly increased survival and hepatocyte-like differentiation in host liver tissue and dramatically enhanced the repair potential for cirrhosis pathology and impaired liver function compared with DPSCs expressing HGF at physiological levels.
CCl4-induced hepatic injury in rats is widely used as an induced animal model of liver fibrosis because of its high similarity to the morphological and functional alterations observed in human cirrhosis25–27. In this study, CCl4 was administered to rats twice weekly at an appropriate dosage, determined in preliminary experiments, for approximately 3 months, rather than administering a relatively high dose for a short term, to ensure the survival of the experimental animals and the emergence of identifiable pathology. As shown through histopathological and serological analyses, cirrhosis-specific formation of hepatic regenerating nodules and fibrous septa, increased serum levels of aminotransferases indicative of hepatocyte injury and a decreased level of hepatocyte-synthesized serum albumin occurred in the CCl4-treated rats, verifying that liver cirrhosis was well recapitulated in the rats.
Cirrhosis, the terminal stage of hepatic diseases, is prone to develop into hepatocyte carcinoma and hepatic failure, which is incurable in clinical practice, except through liver transplantation. Fortunately, in preclinical studies, interventions such as MSC graft-based regenerative medicine therapies, involving minimally invasive procedures and few complications, have been demonstrated to cause hepatic fibrosis to regress and to improve clinical outcomes, even in advanced stages of cirrhosis28,29. Among the various lineages of MSCs, embryonic ectomesenchyme tissue-derived uncommitted DPSCs (the target cell type in this study), which are capable of homing, self-renewal and differentiation in injured tissue, are considered one of the therapies with the most potential for use in the treatment of tissue injury. These cells display obvious advantages over other types of stem cells, including ease of accessibility for autografts, minimal immune rejection and tolerance to stress, including long-term cryopreservation12,24,30. The primary rat DPSCs isolated and purified in this study were found to express MSC surface markers (CD90 and CD29) and show some expression of the homing cell adhesion molecule CD44, but they rarely expressed hematopoietic lineage-related molecules (CD34 and CD45). These cells also exhibited osteogenic and adipogenic potency (Fig. 3). All these characteristics were in accord with the characteristics of DPSCs identified in previous studies31,32. With regard to hepatic regeneration, differentiation of DPSCs into functional hepatocyte-like cells that express hepatocyte markers, such as albumin, CK18 and TTR, and are capable of repairing liver injury in vitro and in vivo has been observed in several independent studies14,33–36 and was further confirmed by our work (Figs S1 and 3, 4 and 5). However, the key regulatory mechanisms underlying hepatocyte-like commitment and the hepatic regenerative capacity of DPSCs remain elusive.
HGF is a mesenchymal cell-secreted cytokine that extensively regulates the growth, motility and morphogenesis of a variety of cell types, especially hepatocytes15,16. Intriguingly, mesenchymal cell-derived HGF has been demonstrated to stimulate the differentiation of bone marrow-resident MSCs into hepatocyte-like cells20,21. Accordingly, our results showed that the intravenous transplantation of HGF-overexpressing DPSCs (dental pulp-derived MSCs) gave rise to higher frequencies of albumin-positive, CK18-positive and TTR-positive hepatocyte-like cells than did DPSCs expressing HGF at physiological levels (Fig. 4), which further clarified the general hepatogenic differentiation-inducing capacity of HGF in MSCs. Moreover, we found more graft-derived GFP-labeled cells in the liver tissue of animals receiving grafts of DPSCs with elevated HGF expression, suggesting possible positive modulation of the homing and survival of grafted DPSCs by HGF.
To evaluate the repair potential of HGF-overexpressing DPSCs for cirrhosis, the morphological, molecular and functional recovery of the rat livers was tested 6 weeks after transplantation, rather than within 4 weeks as in previous studies37,38. The longer observation period ensures the effective functioning of DPSCs as well as hepatic regeneration and increases the reliability and precision of the research results. As shown by histopathological tests, the DPSC grafting-facilitated reconstruction of the liver architecture and reversal of hepatic fibrosis were further reinforced by HGF overexpression (Fig. 5). The benefits of HGF-overexpressing DPSC grafting might result from both the HGF-accelerated hepatogenic differentiation of DPSCs and the specific expression and secretion of human HGF itself. The ectopic secretion of a high dose of hHGF far above intrinsic HGF secretion levels may lead to the activation of resident hepatic progenitor cells, which have been implicated in hepatic regeneration39, and/or the obvious anti-fibrogenic activities of HGF17,18. Additionally, the DPSC graft-promoted hepatocyte regeneration, enhanced by the overexpression of HGF in transplanted cells, was verified by the restoration of the mRNA and/or protein expression of albumin, TTR and CK18 (Fig. 5D–F), as reduced hepatic levels of albumin and TTR (hepatocyte-synthesized plasma proteins) and aberrantly increased expression of CK18 (single-layer epithelial Type I intermediate protein) associated with hepatogenic dedifferentiation are well-established indicators of cirrhosis-related hepatocyte injury40–43. Interestingly, as revealed by serum assays, the HGF-overexpressing DPSC-treated rats gained further recovery of the serum levels of aminotransferases, but not albumin, compared with the physiological HGF-expressing DPSC-treated animals. This result was consistent with the Western blot data for liver tissue and might suggest the heterogeneous restoration of hepatic function.
In summary, our results demonstrated that MSC-specific-marker-expressing multipotent DPSCs transduced with a hHGF-lentiviral vector showed elevated HGF expression and secretion of HGF in vitro. These cells also exhibited markedly increased survival and underwent hepatogenic differentiation in host liver tissue, significantly enhancing their therapeutic efficacy in liver cirrhosis. Our study provides a novel method for the treatment of liver cirrhosis in clinical practice.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81570951), the Special Foundation for Sino-Russian Translational Medicine Research Center of Harbin Medical University (Grant No. CR201412), the Special Foundation for Sino-Russian Translational Medicine Research Center of Harbin Medical University (Grant No. CR201504) and the Innovation Science Foundation of Harbin Medical University (Grant No.2016LCZX01).
Author Contributions
Xiao-fang Cao, Shi-zhu Jin, and Bin Zhang conceived this work. Xiao-fang Cao, Shi-zhu Jin, Liang Sun, Yuan-bo Zhan, Ying Li and Feng Lin conducted the experiments and analyzed the data. Liang Sun, Yuan-bo Zhan, Ying-lian Zhou, Xiu-mei Wang, Li Gao and Bin Zhang wrote the manuscript. All of the authors discussed the results and reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Xiao-fang Cao and Shi-zhu Jin contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14995-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz A, et al. Melatonin prevents experimental liver cirrhosis induced by thioacetamide in rats. J Pineal Res. 2005;39:143–150. doi: 10.1111/j.1600-079X.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 3.Nagano T, Mori-Kudo I, Kawamura T, Taiji M, Noguchi H. Pre- or post-treatment with hepatocyte growth factor prevents glycerol-induced acute renal failure. Renal failure. 2004;26:5–11. doi: 10.1081/JDI-120028537. [DOI] [PubMed] [Google Scholar]
- 4.Li JT, Liao ZX, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J Gastroenterol. 2008;43:419–428. doi: 10.1007/s00535-008-2180-y. [DOI] [PubMed] [Google Scholar]
- 5.Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225–231. doi: 10.1016/j.cbi.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang YO, et al. Effect of Function-Enhanced Mesenchymal Stem Cells Infected With Decorin-Expressing Adenovirus on Hepatic Fibrosis. Stem Cells Transl Med. 2016;5:1247–1256. doi: 10.5966/sctm.2015-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thesleff, I. & Tummers, M. Stem cells and tissue engineering: prospects for regenerating tissues in dental practice. Med Princ Pract 12 Suppl 1, 43–50, doi:69840 (2003). [DOI] [PubMed]
- 9.Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 10.Yu F, et al. Effect of an Experimental Direct Pulp-capping Material on the Properties and Osteogenic Differentiation of Human Dental Pulp Stem Cells. Sci Rep. 2016;6:34713. doi: 10.1038/srep34713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi S, et al. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci Rep. 2015;5:16295. doi: 10.1038/srep16295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young F, Sloan A, Song B. Dental pulp stem cells and their potential roles in central nervous system regeneration and repair. J Neurosci Res. 2013;91:1383–1393. doi: 10.1002/jnr.23250. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda E, et al. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495–505. doi: 10.1111/j.1432-0436.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 14.Cho YA, Noh K, Jue SS, Lee SY, Kim EC. Melatonin promotes hepatic differentiation of human dental pulp stem cells: clinical implications for the prevention of liver fibrosis. J Pineal Res. 2015;58:127–135. doi: 10.1111/jpi.12198. [DOI] [PubMed] [Google Scholar]
- 15.Morishita R, Aoki M, Yo Y, Ogihara T. Hepatocyte growth factor as cardiovascular hormone: role of HGF in the pathogenesis of cardiovascular disease. Endocr J. 2002;49:273–284. doi: 10.1507/endocrj.49.273. [DOI] [PubMed] [Google Scholar]
- 16.Ding BS, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda H, et al. Antifibrogenic effect of a deletion variant of hepatocyte growth factor on liver fibrosis in rats. Hepatology. 1996;24:636–642. doi: 10.1002/hep.510240328. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda Y, et al. Preventive and therapeutic effects in rats of hepatocyte growth factor infusion on liver fibrosis/cirrhosis. Hepatology. 1997;26:81–89. doi: 10.1002/hep.510260111. [DOI] [PubMed] [Google Scholar]
- 19.Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor prevents acute renal failure and accelerates renal regeneration in mice. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4357–4361. doi: 10.1073/pnas.91.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai L, et al. Transplantation of MSCs Overexpressing HGF into a Rat Model of Liver Fibrosis. Molecular Imaging and Biology. 2016;18:43–51. doi: 10.1007/s11307-015-0869-x. [DOI] [PubMed] [Google Scholar]
- 21.Seo K-W, et al. Therapeutic effects of hepatocyte growth factor-overexpressing human umbilical cord blood-derived mesenchymal stem cells on liver fibrosis in rats. Cell biology international. 2014;38:106–116. doi: 10.1002/cbin.10186. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, et al. Differentiation ability of rat postnatal dental pulp cells in vitro. Tissue Eng. 2005;11:357–368. doi: 10.1089/ten.2005.11.357. [DOI] [PubMed] [Google Scholar]
- 23.Ohshima H, et al. The eternal tooth germ is formed at the apical end of continuously growing teeth. Arch Oral Biol. 2005;50:153–157. doi: 10.1016/j.archoralbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 24.Chiang CH, et al. Enhanced antioxidant capacity of dental pulp-derived iPSC-differentiated hepatocytes and liver regeneration by injectable HGF-releasing hydrogel in fulminant hepatic failure. Cell Transplant. 2015;24:541–559. doi: 10.3727/096368915X686986. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Gil JJ, et al. Improvement in liver fibrosis, functionality and hemodynamics in CCI4-cirrhotic rats after injection of the Liver Growth Factor. J Hepatol. 1999;30:1065–1072. doi: 10.1016/S0168-8278(99)80261-X. [DOI] [PubMed] [Google Scholar]
- 26.Hou YL, Tsai YH, Lin YH, Chao JC. Ginseng extract and ginsenoside Rb1 attenuate carbon tetrachloride-induced liver fibrosis in rats. BMC Complement Altern Med. 2014;14:415. doi: 10.1186/1472-6882-14-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, et al. Protection effect of kallistatin on carbon tetrachloride-induced liver fibrosis in rats via antioxidative stress. PLoS One. 2014;9:e88498. doi: 10.1371/journal.pone.0088498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(Suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 30.Dissanayaka WL, Hargreaves KM, Jin L, Samaranayake LP, Zhang C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng Part A. 2015;21:550–563. doi: 10.1089/ten.tea.2014.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khorsand A, et al. Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J Oral Implantol. 2013;39:433–443. doi: 10.1563/AAID-JOI-D-12-00027. [DOI] [PubMed] [Google Scholar]
- 32.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishkitiev N, et al. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod. 2012;38:475–480. doi: 10.1016/j.joen.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Liang XJ, et al. Differentiation of human umbilical cord mesenchymal stem cells into hepatocyte-like cells by hTERT gene transfection in vitro. Cell Biol Int. 2012;36:215–221. doi: 10.1042/CBI20110350. [DOI] [PubMed] [Google Scholar]
- 35.Prasajak P, Leeanansaksiri W. Developing a New Two-Step Protocol to Generate Functional Hepatocytes from Wharton’s Jelly-Derived Mesenchymal Stem Cells under Hypoxic Condition. Stem Cells Int. 2013;2013:762196. doi: 10.1155/2013/762196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chien Y, et al. Synergistic effects of carboxymethyl-hexanoyl chitosan, cationic polyurethane-short branch PEI in miR122 gene delivery: accelerated differentiation of iPSCs into mature hepatocyte-like cells and improved stem cell therapy in a hepatic failure model. Acta Biomater. 2015;13:228–244. doi: 10.1016/j.actbio.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, et al. Transplantation of embryonic small hepatocytes induces regeneration of injured liver in adult rat. Transplant Proc. 2009;41:3887–3892. doi: 10.1016/j.transproceed.2009.06.205. [DOI] [PubMed] [Google Scholar]
- 38.Shams S, Mohsin S, Nasir GA, Khan M, Khan SN. Mesenchymal Stem Cells Pretreated with HGF and FGF4 Can Reduce Liver Fibrosis in Mice. Stem Cells Int. 2015;2015:747245. doi: 10.1155/2015/747245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riehle KJ, Dan YY, Campbell JS, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;26(Suppl 1):203–212. doi: 10.1111/j.1440-1746.2010.06539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Racine-Samson L, et al. The metabolic organization of the adult human liver: a comparative study of normal, fibrotic, and cirrhotic liver tissue. Hepatology. 1996;24:104–113. doi: 10.1002/hep.510240118. [DOI] [PubMed] [Google Scholar]
- 41.Kakehashi A, Inoue M, Wei M, Fukushima S, Wanibuchi H. Cytokeratin 8/18 overexpression and complex formation as an indicator of GST-P positive foci transformation into hepatocellular carcinomas. Toxicol Appl Pharmacol. 2009;238:71–79. doi: 10.1016/j.taap.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bateman AC, Hubscher SG. Cytokeratin expression as an aid to diagnosis in medical liver biopsies. Histopathology. 2010;56:415–425. doi: 10.1111/j.1365-2559.2009.03391.x. [DOI] [PubMed] [Google Scholar]
- 43.Omary MB, Ku NO, Toivola DM. Keratins: guardians of the liver. Hepatology. 2002;35:251–257. doi: 10.1053/jhep.2002.31165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.