Abstract
Dogs may be beneficial in reducing cardiovascular risk in their owners by providing social support and motivation for physical activity. We aimed to investigate the association of dog ownership with incident cardiovascular disease (CVD) and death in a register-based prospective nation-wide cohort (n = 3,432,153) with up to 12 years of follow-up. Self-reported health and lifestyle habits were available for 34,202 participants in the Swedish Twin Register. Time-to-event analyses with time-updated covariates were used to calculate hazard ratios (HR) with 95% confidence intervals (CI). In single- and multiple-person households, dog ownership (13.1%) was associated with lower risk of death, HR 0.67 (95% CI, 0.65–0.69) and 0.89 (0.87–0.91), respectively; and CVD death, HR 0.64 (0.59–0.70), and 0.85 (0.81–0.90), respectively. In single-person households, dog ownership was inversely associated with cardiovascular outcomes (HR composite CVD 0.92, 95% CI, 0.89–0.94). Ownership of hunting breed dogs was associated with lowest risk of CVD. Further analysis in the Twin Register could not replicate the reduced risk of CVD or death but also gave no indication of confounding by disability, comorbidities or lifestyle factors. In conclusion, dog ownership appears to be associated with lower risk of CVD in single-person households and lower mortality in the general population.
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, accounting for 45% of all deaths (>4 million) in Europe in 20161,2. Dogs may be beneficial in reducing cardiovascular risk by providing a non-human form of social support and increasing physical activity. Dog ownership has been reported to be associated with alleviation of social isolation and improved perception of wellbeing, particularly in single persons and the elderly3–5. A meta-analysis of eleven observational studies found that dog owners walked more and were more physically active than non-owners6. Two studies assessing changes in physical activity after acquisition of a dog or other pet found increased self-reported recreational walking7,8. A recent study showed that dog ownership also supports the maintenance of physical activity during poor weather9.
Previous findings on dog ownership and the risk of CVD are conflicting. In individuals without CVD, dog ownership has been reported as inversely associated with the prevalence of cardiovascular risk factors, such as dyslipidemia, hypertension and diabetes10,11, but other studies found absent or inconclusive associations12,13. In patients with coronary artery disease, dog ownership is reportedly associated with improved survival14–16.
Although the American Heart Association issued a Scientific Statement in 2013 concluding that “[…] dog ownership is probably associated with decreased CVD risk […]” and that this effect “[…] may be causal […]”17, earlier studies have had several limitations, including lack of power, low responder rates and incomplete adjustment for potential confounders.
We aimed to clarify the association of dog ownership with CVD and mortality by studying over 3.4 million Swedish adults followed through nationwide register linkage over a 12-year period.
Methods
Study population – National Cohort
All Swedish residents aged 40 to 80 years on January 1, 2001 (n = 3,987,937) were eligible for this study. The age range was chosen to exclude younger individuals at low risk of CVD and the elderly at low odds of owning a dog. Participants were identified using the Register of the Total Population, which contains information on birth, migration, changes of citizenship, civil status and death on all Swedish citizens and residents18. By using the unique 10-digit personal identity number, records were linked to national registers for socioeconomic variables and health outcomes (see Supplementary Material).
Study population - Swedish Twin Register
To verify findings from the national cohort in a subgroup with more available information on additional potential confounders, we repeated our analysis in the “Screening Across the Lifespan Twin study” (SALT) sub-study of the Swedish Twin Register (STR). The STR, initiated in 1958, is a longitudinal study of the vast majority of twins born in Sweden after 188619 and SALT is a telephone-based interview conducted between March 1998 and March 2002 that collected information on twins born in 1958 or earlier. Of the 44,821 (74%) responders, 41,039 were aged 42–80 years in 2001 and hence eligible for this study20.
Exclusions
Individuals (n = 163,156) were excluded from the National Cohort if they had not resided continuously in Sweden since 1987 to ensure sufficient information on dog ownership registration and complete medical history. Those with re-used or unconfirmed personal identity numbers (n = 5,057) were also excluded.
All Swedish residents are covered by the public health care system, and all hospital visits are registered in the National Patient Register21. Individuals with inpatient visits (n = 387,571 in national cohort and n = 3,163 in twin cohort) between January 1, 1987 to December 31, 2000 for CVD (ICD-9 codes 390–459 or ICD-10 I00-I99; main or secondary diagnosis) and/or had a coronary artery bypass grafting or percutaneous coronary artery intervention (Nordic surgical procedure codes FNA, FNC and FNG) were excluded from the study The final data set included 3,432,153 participants in the national cohort and 34,202 in the twin cohort (Supplementary Figure 1).
Exposure
Since January 1, 2001, it has been a statutory requirement that every dog in Sweden has a unique identifier (ear tattoo or subcutaneous chip) registered at the Swedish Board of Agriculture. In addition, the Swedish Kennel Club has registered all dogs with a certified pedigree with complete information on owner’s personal identity number since 200122. In 2012, an estimated 83% (95% confidence interval, CI, 78–87%) dogs were registered in at least one of these two registers23.
Dog ownership was defined as periods registered or having a partner registered as a dog owner in either of the two dog registers (Supplementary Figure 2). The linkage to each respective partner (defined as a married couple or a cohabiting couple with common children) was possible through annual extracts from the Register of the Total Population. If information on dog death was missing, we assumed a maximum lifespan of ten years. If dates were discrepant between the two registers, we randomly selected one of the two. We also used the Swedish Kennel Club’s definition of breed groups to categorize the 339 breeds into ten groups (Supplementary Table 1). All non-purebred dogs and those of unknown breeds were grouped as mixed breed.
In the twin data, we did not have access to information on partners’ dog ownership and only each person’s own dog registrations were used.
Outcomes
We obtained death data from the Cause of Death Register24 and incident disease data from the National Patient Register from January 1, 2001 to December 31, 2012 in the national cohort, and up to December 31, 2014 in the twin cohort. In addition to all-cause mortality, the main diagnosis in inpatient and outpatient care and underlying cause of death were used to define four incident disease outcomes: (1) acute myocardial infarction (ICD-10 I21); (2) heart failure (ICD-10 I50); (3) ischemic stroke (ICD-10 I63) and (4) hemorrhagic stroke (ICD-10 I60-I62). Any occurrence of these diagnoses was additionally considered as a composite CVD outcome. CVD mortality was defined as death with the underlying cause being any of the CVD outcomes defined above. In the twin register only the composite CVD outcome and all-cause mortality were assessed.
Covariates
Theoretical structured ordering of factors was undertaken by use of directed acyclic graphs to identify potential confounders (Supplementary Figure 3). Socioeconomic variables were accessed from annual excerpts of the Register of the Total Population and the Longitudinal Integration Database for Health Insurance and Labor Market Studies. Variables assessed only at baseline included sex, year of birth, country of birth (Sweden, other Nordic countries and non-Nordic countries) and educational attainment (compulsory, ≤9 years; secondary, 10–11 years; or tertiary education, ≥12 years; not available for those aged >75 years). Time-updated covariates included marital status (single, married/cohabiting, divorced or widowed: definition in Supplementary Methods), presence of children in the home (yes/no), area of residence (Norrland, Götaland and Svealand), population density in municipality of residence (continuous variable), and annual household income (birth year-standardized quintiles). We further accounted for a north-south gradient by including the latitude of the municipality of residence. We further added a time-updated variable for two variants of the socioeconomic index, the international socio-economic index (ISEI)25 and the European Socioeconomic index (ESeC)26 in those with sufficient information (Supplementary Methods).
To avoid reverse effects of outcomes on covariates, we used covariate data from the preceding year to time-update information on January 1 in every year. A stratification variable for household was created with individuals assigned to ‘single-person household’ if the participant lived alone, or to ‘multiple-person household’ if they were married, living with a partner and/or a child. Non-married partners are only traceable in the registers if the couple has children together. A second stratification variable was created for age group in decades.
In the twin register, no register data on socioeconomic and demographic covariates were available. Instead, the following self-reported variables at baseline were used (Supplementary Table 2): body mass index (BMI), physical activity (less than average, average, more than average), employment status (employed, retired, long-term sick leave/early retirement, unemployed), type of accommodation (independent housing, assisted living, other), smoking status (never, former, current), any physical impairment, requirement of assistance for routine daily activities and socioeconomic index (unskilled employees, lower skilled non-manual employees, self-employed excluding independent occupations, intermediate non-manual workers and higher non-manual workers). Data from the National Patient Register from January 1 1996 to December 31 2000 were used to calculate Charlson’s Comorbidity Index27,28 (Supplementary Table 3).
Statistical Analysis
Cox proportional hazards regression with attained age as time-scale was used to calculate hazard ratios (HR) and 95% confidence intervals (CI) for each of the seven outcomes separately. The proportional hazards assumption was verified using log-log plots and Schoenfield residuals plots. Participants were censored at emigration (available in national cohort only), death or at the end of the study; whichever came first. For each outcome, we estimated one sex-adjusted model and one multivariable adjusted model.
National cohort
The multivariable-adjusted model included sex, country of birth, marital status, presence of children in the home, area of residence, population density, age-adjusted income and latitude. All analyses were implemented in the full study sample and stratified on household-type (single/multiple person), age group in decades and sex. In sensitivity analysis, we added adjustment for education in those <75 years at baseline and adjustment for ISEI and ESeC in those with sufficient information. Additionally, we tested for associations between different dog breed groups and outcomes.
Swedish Twin Register
The first multivariable model was similar to the national cohort model with socioeconomic index replacing age-adjusted income. All analyses were implemented in the full study sample and stratified on household type. In additional models, further adjustments for body mass index, smoking, Charlson Comorbidity Index, employment status and level of exercise was done. We repeated analyses after excluding those residing in assisted living accommodation, those with any physical impairments and those requiring assistance for routine daily activities. Standard errors were adjusted with the robust sandwich estimator for dependent observations.
Analyses were conducted in Stata/ MP 14.1 (StataCorp LP, TX, US). The study was approved by the Regional Ethical Review Board in Stockholm, Sweden (2012/1114-31/2 with amendments 2013-1687-32 and 2016/1392-31/1) and allowed the researchers to waive the requirement for obtaining informed consent in the national register part of the study. Participants in the SALT (Twin register) had given informed consent. The study was performed in accordance with relevant guidelines and regulations.
Data availability statement
The register data that support the findings of this study were made available by record-linkage with data from Statistics Sweden, the National Board of Health and Welfare, the Swedish Kennel Club, Swedish Board of Agriculture and the Swedish Twin Register. Restrictions apply to the availability of these data, which were used under license and ethical approval for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Regional Ethical Review Board in Stockholm, Sweden.
Results
National cohort
The final sample included 3,432,153 individuals (48% men) with a mean age of 57 years. We identified 13.1% as dog owners at any time during the 12-year study period. Baseline characteristics on January 1 2001 are shown in Table 1. Dog owners were younger than non-owners at baseline (mean age, 52 vs. 58 years) and more likely to reside in areas of lower population density (median 57 inhabitants per km2 vs. 74 inhabitants per km2).
Table 1.
Baseline characteristics of Swedish adults aged 40–80 years without cardiovascular disease on 1 January 2001 from the Register of the Total Population (national cohort, n = 3,432,153) and the Swedish Twin Register (twin cohort, n = 34,202).
| National cohort | Twin cohort | |||||
|---|---|---|---|---|---|---|
| Dog owners4 n = 448,298 (13.1%) | Non-dog owners n = 2,983,855 (86.9%) | All n = 3,432,153 (100%) | Dog owners n = 2,909 (8.5%) | Non-dog owners n = 31,293 (91.5%) | All n = 34,202 (100%) | |
| Age - mean ± SD | 51.7 ± 8.2 | 57.9 ± 11.1 | 57.1 ± 11.0 | 53.3 ± 7.7 | 57.8 ± 9.8 | 57.4 ± 9.7 |
| Male | 219,105 (48.9%) | 1,419,396 (47.6%) | 1,638,501 (47.7%) | 1,149 (39.5%) | 14,179 (45.3%) | 15,328 (44.8%) |
| Marital status | ||||||
| Married or cohabiting | 355,882 (79.4%) | 1,863,429 (62.5%) | 2,219,311 (64.7%) | 2,301 (79.1%) | 23,608 (75.4%) | 25,909 (75.8%) |
| Never married | 33,470 (7.5%) | 417,700 (14.0%) | 451,170 (13.1%) | 250 (8.6%) | 3,016 (9.6%) | 3,266 (9.5%) |
| Divorced | 49,766 (11.1%) | 463,259 (15.5%) | 513,025 (14.9%) | 274 (9.4%) | 3,006 (9.6%) | 3,280 (9.6%) |
| Widowed | 9,180 (2.0%) | 239,467 (8.0%) | 248,647 (7.2%) | 84 (2.9%) | 1,663 (5.3%) | 1,747 (5.1%) |
| Type of family | ||||||
| Children at home | 244,875 (54.6%) | 983,221 (33.0%) | 1,228,096 (35.8%) | 909 (31.2%) | 6,234 (19.9%) | 7,143 (20.9%) |
| No children at home | 203,423 (45.4%) | 2,000,634 (67.0%) | 2,204,057 (64.2%) | 2,000 (68.8%) | 25,059 (80.1%) | 27,059 (79.1%) |
| Education 1 | ||||||
| Compulsory | 114,638 (25.8%) | 870,007 (32.3%) | 984,645 (31.4%) | 1,149 (39.5%) | 13,899 (44.4%) | 15,048 (44.0%) |
| Secondary | 208,856 (47.0%) | 1,138,523 (42.3%) | 1,347,379 (43.0%) | 920 (31.6%) | 9,111 (29.1%) | 10,031 (29.3%) |
| University | 120,575 (27.2%) | 684,072 (25.4%) | 804,647 (25.7%) | 840 (28.9%) | 8,283 (26.5%) | 9,123 (26.7%) |
| Income quintile 2 | ||||||
| 1 (lowest quintile) | 97,492 (21.8%) | 590,760 (19.8%) | 688,252 (20.0%) | — | — | — |
| 2 | 93,336 (20.8%) | 593,387 (19.9%) | 686,723 (20.0%) | — | — | — |
| 3 | 90,760 (20.2%) | 594,863 (19.9%) | 685,623 (20.0%) | — | — | — |
| 4 | 85,743 (19.1%) | 600,078 (20.1%) | 685,821 (20.0%) | — | — | — |
| 5 (highest quintile) | 80,967 (18.1%) | 604,767 (20.3%) | 685,734 (20.0%) | — | — | — |
| Country of birth | ||||||
| Sweden | 418,344 (93.3%) | 2,666,384 (89.4%) | 3,084,728 (89.9%) | 2,909 (100%) | 31,293 (100%) | 34,202 (100%) |
| Other Nordic countries3 | 18,421 (4.1%) | 150,397 (5.0%) | 168,818 (4.9%) | 0 | 0 | 0 |
| Non-Nordic countries | 11,533 (2.6%) | 167,074 (5.6%) | 178,607 (5.2%) | 0 | 0 | 0 |
| Population density - median (interquartile range) inhabitant per square kilometre | ||||||
| 56.5 (20.9–123.1) | 74.2 (26.5–341.9) | 72.5 (26.4–248.2) | 45.0 (19.6–107.7) | 68.1 (26.3–153.9) | 64.2 (25.6–149.9) | |
| Region of Residence | ||||||
| Svealand | 158,532 (35.4%) | 1,146,703 (38.4) | 1,305,235 (38.0) | 957 (32.9) | 10,949 (35.0) | 11,906 (34.8) |
| Götaland | 216,373 (48.2%) | 1,429,956 (47.9) | 1,646,329 (48.0) | 1,396 (47.8) | 15,808 (50.5) | 17,198 (50.3) |
| Norrland | 73,393 (16.4%) | 407,196 (13.7) | 480,589 (14.0) | 556 (19.1) | 4,542 (14.5) | 5,098 (14.9) |
Numbers and % of the respective cohort are reported unless stated otherwise. 1Characteristics of n = 295,482 individuals who had missing values were: 98.6% non-dog owners; 91.3% Swedish-born; median population density 69.3 per km2 (interquartile range, 25.5–341.9); median age 77.5 years (interquartile range, 76.5–79.5); 13.6% resident in Norrland, 49.3% resident in Götaland, 37.1% resident in Svealand; 2Information on income not available for the SALT sub-study in the Swedish Twin Register; 3Other Nordic countries: Norway, Denmark, Iceland, Finland, the territories of the Åland Islands and the Faroe Islands. 4For descriptive purposes, dog owners in this table are individuals who had a registered dog at any time point during the study period.
In age and sex-adjusted analysis, dog ownership was inversely associated with risk of acute myocardial infarction, ischemic stroke, heart failure, and composite CVD (HRs 0.93–0.94; Table 2). After multivariable adjustment, associations with CVD outcomes were attenuated (HRs 0.97–1.01), but remained significant in acute myocardial infarction, HR 0.97 (95% CI 0.95–0.99). Dog ownership was inversely associated with cardiovascular mortality (HR 0.77, 95% CI, 0.73–0.80), and all-cause mortality (adjusted HR 0.80, 95% CI, 0.79–0.82; Table 2). Additional adjustment for education and socioeconomic index in those with enough information available (n = 3,136,671 and n = 1,660,140, respectively) did not affect the results compared to non-adjusted models (Supplementary Tables 4 and 5).
Table 2.
Hazard ratios (HR) and confidence intervals (CI) examining associations between dog ownership and CVD outcomes in the National Cohort (n = 3,432,153) using Cox proportional hazards regression with attained age as time-scale.
| Cardiovascular disease | Number of events | Person-years at risk | Crude1 HR (95% CI) | Adjusted2 HR (95% CI) |
|---|---|---|---|---|
| Acute Myocardial Infarction | 172,999 | 37,773,460 | 0.93 (0.92–0.96) | 0.97 (0.95–0.99) |
| Ischemic Stroke | 136,305 | 37,866,672 | 0.94 (0.92–0.96) | 0.98 (0.96–1.01) |
| Hemorrhagic Stroke | 41,286 | 38,274,834 | 0.97 (0.93–1.01) | 1.02 (0.98–1.07) |
| Heart failure | 107,843 | 38,089,903 | 0.93 (0.90–0.96) | 1.01 (0.98–1.05) |
| Composite CVD3 | 399,600 | 36,910,720 | 0.94 (0.93–0.96) | 0.99 (0.98–1.01) |
| CVD mortality4 | 76,106 | 38,408,267 | 0.68 (0.65–0.71) | 0.77 (0.73–0.80) |
| All–Cause mortality | 502,896 | 38,408,267 | 0.72 (0.71–0.73) | 0.80 (0.79–0.82) |
1Adjusted for sex. 2Adjusted for sex, marital status, presence of children in the home, population density, area of residence, region of birth, income and latitude. 3Composite CVD comprises all incident (fatal and non-fatal) ischemic stroke, myocardial infarction, hemorrhagic stroke and heart failure cases. 4Representing death from ischemic strokes, myocardial infarction, hemorrhagic stroke and heart failure.
Stratified analysis showed effect measure modification of household type for all outcomes except haemorrhagic stroke, with lower multivariable adjusted HRs found in single households (Fig. 1, upper panel). There seemed to be no effect measure modification by sex (Fig. 1, middle panel). Age seemed to modify the association between dog ownership and myocardial infarction, with lower HRs in older age groups (Fig. 1, lower panel).
Figure 1.
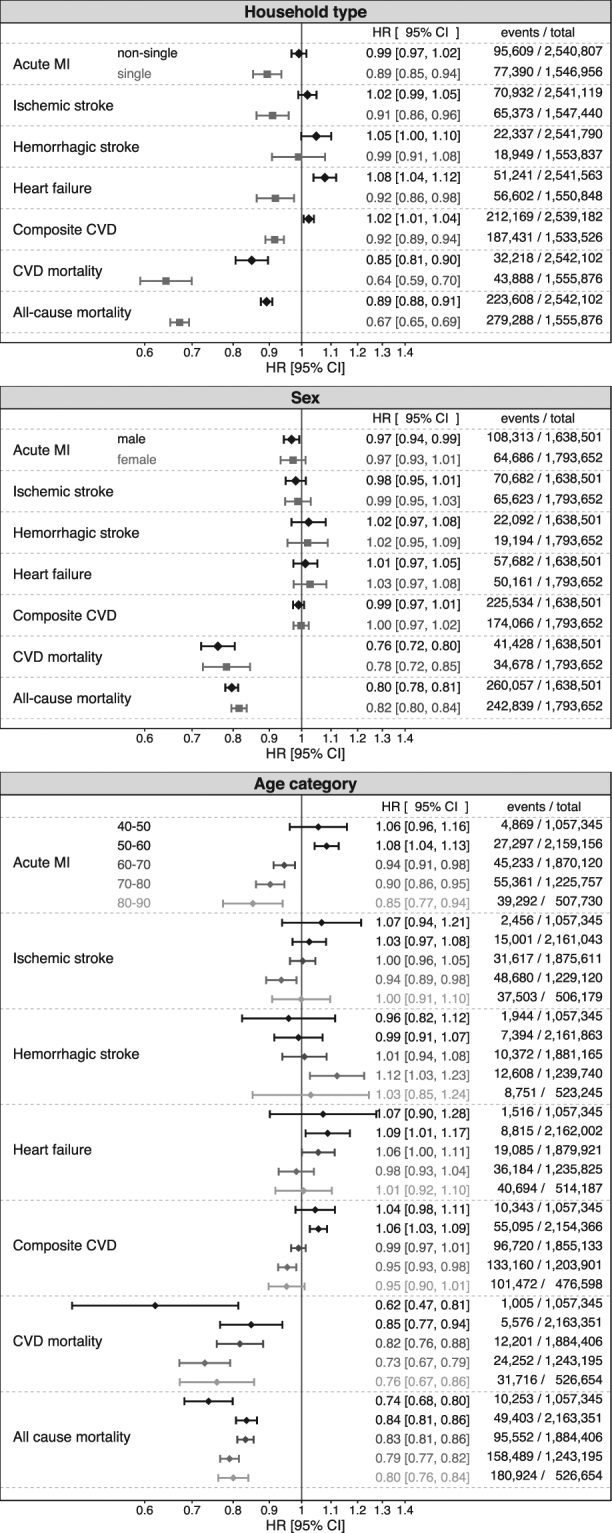
Hazard ratios (HR) and confidence intervals (CI) of the associations between dog ownership and CVD outcomes in the National cohort stratified by household type, sex, and age using Cox proportional hazards regression with attained age as time-scale.
In the breed group analysis, we found that ownership of a dog from breeds originally bred for hunting (including terriers, retrievers, scent hounds and related dogs) was associated with a lower risk of CVD (Table 3). Ownership of a mixed-breed dog was associated with higher risk of CVD, HR 1.13 (95% CI, 1.09–1.17). The HR for all-cause mortality was <1 for all breed groups, with pointing dogs associated with the lowest estimate (HR 0.60, 95% CI, 0.53–0.68) and mixed-breed dogs with estimates closest to one (HR 0.98, 95% CI, 0.94–1.01).
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) for associations between dog breed groups and CVD outcomes in the National cohort using Cox proportional hazards regression with attained age as time-scale.
| Breed Groups | Composite Cardiovascular Disease1 | All-Cause Mortality | ||
|---|---|---|---|---|
| Crude HR2 | Adjusted HR3 | Crude HR2 | Adjusted HR3 | |
| Sheep and cattle dogs | 0.99 (0.95–1.03) | 1.02 (0.98–1.07) | 0.76 (0.73–0.80) | 0.84 (0.80–0.88) |
| Pinscher and schnauzer dogs | 0.91 (0.87–0.95) | 0.97 (0.93–1.02) | 0.68 (0.64–0.72) | 0.78 (0.74–0.82) |
| Terriers | 0.88 (0.84–0.92) | 0.95 (0.91–0.99) | 0.70 (0.67–0.74) | 0.81 (0.78–0.86) |
| Dachshunds | 0.88 (0.83–0.93) | 0.94 (0.89–1.00) | 0.67 (0.63–0.71) | 0.76 (0.72–0.81) |
| Spitz and primitive types | 0.97 (0.92–1.02) | 0.98 (0.94–1.03) | 0.68 (0.65–0.72) | 0.72 (0.68–0.76) |
| Scent hounds and related dogs | 0.90 (0.86–0.95) | 0.93 (0.88–0.97) | 0.58 (0.55–0.62) | 0.63 (0.60–0.67) |
| Pointing dogs | 0.84 (0.77–0.93) | 0.90 (0.82–1.00) | 0.52 (0.46–0.59) | 0.60 (0.53–0.68) |
| Retrievers | 0.83 (0.80–0.86) | 0.90 (0.87–0.94) | 0.63 (0.60–0.66) | 0.74 (0.71–0.77) |
| Companion and toy dogs | 1.00 (0.96–1.03) | 1.04 (1.01–1.08) | 0.77 (0.74–0.80) | 0.85 (0.82–0.89) |
| Sight hounds | 0.97 (0.83–1.14) | 1.02 (0.87–1.18) | 0.76 (0.64–0.90) | 0.83 (0.70–0.99) |
| Mixed Pedigree4 | 1.12 (1.08–1.16) | 1.13 (1.09–1.17) | 0.93 (0.90–0.97) | 0.98 (0.94–1.01) |
1Composite CVD comprises all ischemic stroke, myocardial infarction, hemorrhagic stroke and heart failure. 2Adjusted for sex. 3Adjusted for sex, marital status, presence of children in the home, population density, area of residence, region of birth, income and latitude. 4Group comprising all non-pure pedigree dogs.
Swedish Twin Register
The final dataset included 34,202 individuals (45% men) with a mean age of 57 years. We identified 8.5% as registered dog owners at any time during the 14-year follow-up period. The cohort had similar characteristics as the national cohort, except that fewer individuals lived in single households, probably due to differences in accuracy of measurement (register not complete for individuals cohabiting without common children vs phone survey in which partner information was recorded) (Supplementary Table 6).
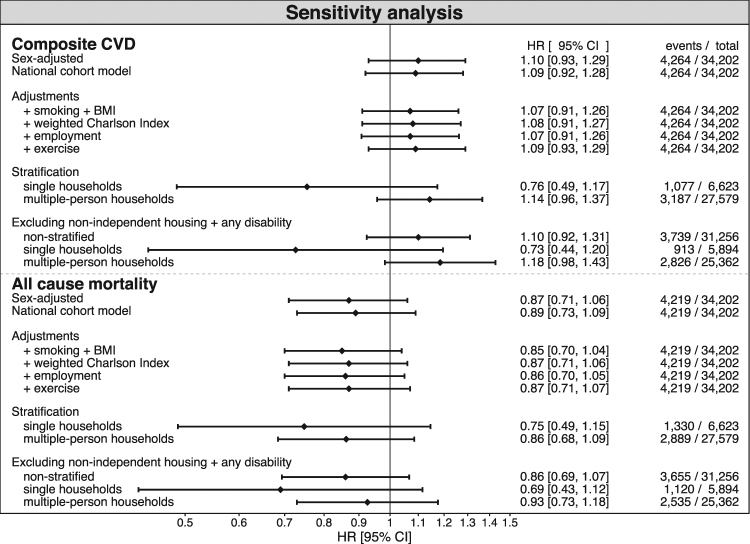
Multivariable-adjusted estimates for composite CVD, HR 1.09 (95% CI, 0.92–1.28), and all-cause mortality, HR 0.89 (95% CI 0.73–1.09) overlapped with estimates from the national cohort, but the estimate for CVD was in the opposite direction. The serial addition of the different lifestyle covariates to the model only slightly altered the estimates (Fig. 2). Overall, the results from the Twin Register did not demonstrate any difference in risk of CVD or mortality between dog owners and non-owners but remained robust to a range of different modifications explored in sensitivity analyses, including the exclusion of individuals with disabilities or unable to care for themselves (Fig. 2).
Figure 2.
Association of dog ownership with cardiovascular disease and all-cause mortality in the Swedish Twin Register following adjustments and exclusions in sensitivity analysis using Cox proportional hazards regression with attained age as time-scale. The “National cohort model” was adjusted for sex, marital status, type of family, area of residence, education level, population density and occupation level.
Discussion
In this register-based nationwide prospective study including 3,432,153 individuals, dog ownership was associated with a lower risk of incident cardiovascular disease in single-person households and with lower cardiovascular and all-cause mortality in the general population. Ownership of hunting dog breeds was associated with a decreased risk of CVD, and ownership of all purebred breeds were associated with a lower risk of all-cause mortality. Although further investigation in the Twin cohort did not show any association between dog ownership and CVD and mortality likely due to the smaller sample size, additional adjustment for detailed lifestyle and socioeconomic factors only marginally altered these estimates.
A recent Norwegian prospective cohort study within a subset of the HUNT study (n = 28,746 in adjusted analysis, 4,233 events) showed no difference in all-cause mortality between dog owners and non-owners (adjusted HR 1.00, 95% CI 0.91–1.09)29. Adjustments were similar as in our study, but stratification by family type was not investigated, which would have been interesting to compare to our results, as the single household stratum was where the largest protective effect sizes were observed. The differences in results from our study could be due to differences in the population studied (largely rural in the HUNT study), difference in the study design (cohort vs register-based), measurement of dog-ownership (questionnaire vs register) and time of exposure definition (baseline vs time-updated) or pure chance. Three other previous studies conducted within the Second and Third National Health and Nutrition Examination Survey (NHANES II-III) of 4,435 (44% dog owners), 5,903 (11% dog owners) and 3,964 (22% dog owners) individuals, respectively, reported no effect of dog ownership on all-cause mortality (adjusted HRs 1.00, 95% CI, 0.85–1.20; 1.17, 95% CI, 0.94–1.46; and 0.82, 95% CI, 0.51–1.34, respectively)30–32. Whilst statistical methods were similar to our study, these studies suffered from a high degree of incomplete follow-up (~50%) and small sample sizes, which do not apply to the present study based on comprehensive registers of the total population. Further, three smaller unrelated studies in the United States support an association of dog ownership with lower mortality after cardiovascular events14,16,33.
Our sample size is hundredfold larger than the largest previously reported study29 and allows for more precise effect estimates and analyses of different CVD outcomes and in different subgroups. Using updated exposure information on dog ownership, we reduce the influence and analyses of misclassification. Cumulative dog ownership in the national cohort was similar to the 12.9% estimate in a cross-sectional 2012 survey and somewhat lower in the Swedish Twin Registry cohort since we were not able to take partners’ registration of dog ownership into account.
Our observational study cannot provide evidence for a causal effect of dog ownership on cardiovascular disease or mortality. Although careful attention was paid to adjusting for potential confounders in a set of sensitivity analyses, it is still possible that personal characteristics that we did not have information about affect the choice of not only acquiring a dog, but also the breed and the risk of CVD. Such residual confounding in the present study may be indicated by the opposite direction of the association with CVD in mixed-breed dogs.
There might be direct effects of dog ownership on health outcomes. One mechanism by which dog ownership could reduce CVD risk and mortality is by alleviating psychosocial stress factors, such as social isolation, depression and loneliness - all reportedly lower in dog owners3–5,34. These factors have been linked to increased risk of coronary heart disease, cardiovascular death and all-cause mortality35,36. Dog ownership has also been associated with elevated parasympathetic and diminished sympathetic nervous system activity37, lower reactivity to stress, and faster recovery of blood pressure following stressful activity38. Apart from the social support, it has consistently been shown that dog owners achieve more physical activity and spend more time engaged in outdoor activities4. We found that individuals in single households benefitted most from dog ownership regarding protection from CVD. Here, we defined a single household based on both marital status and the presence of children in the household. A study on the psychological effects of dog ownership suggested that ownership benefits single persons more than married individuals3 Moreover, single dog-owners were shown to walk their dog more often than individuals in multiple-person households39, and in general, it is plausible that not all members of a multiple-person household interact with the dog as much as a single owner. In both multiple- and single-person household strata, we found lower hazard ratios for dog ownership on all-cause mortality and cardiovascular mortality than on incident CVD. This discrepancy may be explained by less severe disease at hospital presentation in dog-owners, similar to the effect described in physically active persons40. Alternatively, owning a dog may improve rehabilitation after an incident disease event by acting as motivation and support to mobilize for walks again.
To our knowledge, our prospective nationwide study is by far the largest investigation of associations of dog ownership with human health reported to date. The comprehensiveness of the Swedish population register system gave us access to a rich set of possible confounders and outcomes, allowed for accurate censoring and low numbers of missing data or lost participants. Our results are based on the Swedish population and likely generalizable to other European populations with similar demographics and cultural practices regarding dog ownership. We found statistically robust estimates that were consistent, although with lower precision, after adjustment in the smaller twin cohort for lifestyle factors such as smoking, obesity, physical impediment, assisted living and comorbidity.
Some limitations apply to our study. As earlier mentioned, there is a risk of unmeasured confounding, especially from personal characteristics affecting the choice of not only acquiring a dog, but also lifestyle and the risk of CVD and death. Further, although we excluded individuals with a history of CVD at baseline and adjusted for comorbidities in sensitivity analyses, there remains a risk of reverse causation, in that individuals with disabilities or comorbidity may be less likely to own a dog, and may be at higher risk for CVD and death. Although registration of dog ownership is mandatory in Sweden, not all dogs are registered, and we noted that the number of dogs in the Board of Agriculture Register increased during the study period. Some dog owners might be misclassified as non-owners in the beginning of the study period, which could have introduced bias away from the null if misclassification was associated with cardio-protective factors. Likelihood of dog registration could also be linked to factors like personality that we were not able to control for.
Taken together, we believe our longitudinal population-wide design provides the most robust evidence so far of a link between dog ownership and health outcomes, although bias from reverse causation, misclassification and confounding cannot be excluded.
Conclusion
In conclusion, in a nationwide population based study with 12 years of follow-up, we show that dog ownership is associated with a lower risk of cardiovascular disease in single households and with a reduced risk of cardiovascular and all-cause death in the general population.
Infrastructure support
Support by NBIS (National Bioinformatics Infrastructure Sweden) for the use of the Mosler system for sensitive data is gratefully acknowledged. There was no compensation received for this assistance.
We would also like to acknowledge the Swedish Kennel Club and the National Board of Agriculture for granting access to the dog registers. They were not involved in any part of the study design, analysis, data interpretation, manuscript preparation or approval.
Scientific support
We acknowledge the assistance provided by Malin Ericsson of the Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Sweden in defining the socioeconomic variables from the Swedish Twin Registry.
We acknowledge the assistance provided by Dr Erik Bihagen of the Swedish Institute for Social Research in defining the socioeconomic variables in the Register of the Total Population.
We acknowledge Dr Mark S. Clements of the Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Sweden for assistance with the statistical analysis.
Electronic supplementary material
Author Contributions
T.F., A.E., E.I. and L.B. designed the study. T.F. acquired the national data and P.M. is responsible for the Swedish Twin Register data. M.M. performed data cleaning. M.M. and T.F. ran statistical analyses. C.N. prepared Figures. M.M. drafted the manuscript and all authors reviewed the manuscript.
Competing Interests
T.F. received funding from the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS), grant number 2013-1673 and from the Agria and SKK Research Foundation and the Göran Gustafsson foundation. The funders were not involved in any part of the study design, data collection, analysis manuscript preparation or approval. E.I. is a scientific advisor for Precision Wellness and Olink Proteomics for work unrelated to the present project. The authors report that no other competing interests exist.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16118-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Townsend N, et al. Cardiovascular disease in Europe: epidemiological update 2016. European heart journal. 2016;37:3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 3.Cline KM. Psychological effects of dog ownership: role strain, role enhancement, and depression. The Journal of social psychology. 2010;150:117–131. doi: 10.1080/00224540903368533. [DOI] [PubMed] [Google Scholar]
- 4.Mullersdorf M, Granstrom F, Sahlqvist L, Tillgren P. Aspects of health, physical/leisure activities, work and socio-demographics associated with pet ownership in Sweden. Scandinavian journal of public health. 2010;38:53–63. doi: 10.1177/1403494809344358. [DOI] [PubMed] [Google Scholar]
- 5.Wood L, et al. The pet factor–companion animals as a conduit for getting to know people, friendship formation and social support. PloS one. 2015;10:e0122085. doi: 10.1371/journal.pone.0122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christian HE, et al. Dog ownership and physical activity: a review of the evidence. Journal of physical activity & health. 2013;10:750–759. doi: 10.1123/jpah.10.5.750. [DOI] [PubMed] [Google Scholar]
- 7.Cutt H, Giles-Corti B, Knuiman M, Timperio A, Bull F. Understanding dog owners’ increased levels of physical activity: results from RESIDE. American journal of public health. 2008;98:66–69. doi: 10.2105/AJPH.2006.103499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serpell J. Beneficial effects of pet ownership on some aspects of human health and behaviour. Journal of the Royal Society of Medicine. 1991;84:717–720. doi: 10.1177/014107689108401208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu, Y. T., Luben, R. & Jones, A. Dog ownership supports the maintenance of physical activity during poor weather in older English adults: cross-sectional results from the EPIC Norfolk cohort. J Epidemiol Community Health, 10.1136/jech-2017-208987 (2017). [DOI] [PMC free article] [PubMed]
- 10.Lentino C, Visek AJ, McDonnell K, DiPietro L. Dog walking is associated with a favorable risk profile independent of moderate to high volume of physical activity. Journal of physical activity & health. 2012;9:414–420. doi: 10.1123/jpah.9.3.414. [DOI] [PubMed] [Google Scholar]
- 11.Anderson WP, Reid CM, Jennings GL. Pet ownership and risk factors for cardiovascular disease. The Medical journal of Australia. 1992;157:298–301. [PubMed] [Google Scholar]
- 12.Parslow RA, Jorm AF. Pet ownership and risk factors for cardiovascular disease: another look. The Medical journal of Australia. 2003;179:466–468. doi: 10.5694/j.1326-5377.2003.tb05649.x. [DOI] [PubMed] [Google Scholar]
- 13.Wright JD, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Pet ownership and blood pressure in old age. Epidemiology. 2007;18:613–618. doi: 10.1097/EDE.0b013e3181271398. [DOI] [PubMed] [Google Scholar]
- 14.Friedmann E, Thomas SA. Pet ownership, social support, and one-year survival after acute myocardial infarction in the Cardiac Arrhythmia Suppression Trial (CAST) Am J Cardiol. 1995;76:1213–1217. doi: 10.1016/S0002-9149(99)80343-9. [DOI] [PubMed] [Google Scholar]
- 15.Friedmann E, Thomas SA, Son H. Pets, depression and long term survival in community living patients following myocardial infarction. Anthrozoos. 2011;24:273–285. doi: 10.2752/175303711X13045914865268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedmann E, Katcher AH, Lynch JJ, Thomas SA. Animal companions and one-year survival of patients after discharge from a coronary care unit. Public Health Rep. 1980;95:307–312. [PMC free article] [PubMed] [Google Scholar]
- 17.Levine GN, et al. Pet ownership and cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2013;127:2353–2363. doi: 10.1161/CIR.0b013e31829201e1. [DOI] [PubMed] [Google Scholar]
- 18.Ludvigsson JF, et al. Registers of the Swedish total population and their use in medical research. European journal of epidemiology. 2016;31:125–136. doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein P, et al. The Swedish Twin Registry in the third millennium: an update. Twin Res Hum Genet. 2006;9:875–882. doi: 10.1375/twin.9.6.875. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein P, et al. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 21.Ludvigsson JF, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansson M, Laikre L. Recent breeding history of dog breeds in Sweden: modest rates of inbreeding, extensive loss of genetic diversity and lack of correlation between inbreeding and health. Journal of Animal Breeding and Genetics. 2014;131:153–162. doi: 10.1111/jbg.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Statistics Sweden, Survey Report, Hundar, katter och andra sällskapsdjur 2012. Swedish.
- 24.Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. International journal of epidemiology. 2000;29:495–502. doi: 10.1093/intjepid/29.3.495. [DOI] [PubMed] [Google Scholar]
- 25.Ganzeboom HB, De Graaf PM, Treiman DJ. A standard international socio-economic index of occupational status. Social science research. 1992;21:1–56. doi: 10.1016/0049-089X(92)90017-B. [DOI] [Google Scholar]
- 26.Rose D, Harrison E. The European socio-economic classification: a new social class schema for comparative European research. European Societies. 2007;9:459–490. doi: 10.1080/14616690701336518. [DOI] [Google Scholar]
- 27.Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American journal of epidemiology. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Torske MO, Krokstad S, Stamatakis E, Bauman A. Dog ownership and all-cause mortality in a population cohort in Norway: The HUNT study. PLoS One. 2017;12:e0179832. doi: 10.1371/journal.pone.0179832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gillum RF, Obisesan TO. Living with companion animals, physical activity and mortality in a U.S. national cohort. Int J Environ Res Public Health. 2010;7:2452–2459. doi: 10.3390/ijerph7062452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qureshi AI, Memon MZ, Vazquez G, Suri MF. Cat ownership and the Risk of Fatal Cardiovascular Diseases. Results from the Second National Health and Nutrition Examination Study Mortality Follow-up Study. Journal of vascular and interventional neurology. 2009;2:132–135. [PMC free article] [PubMed] [Google Scholar]
- 32.Ogechi, I. et al. Pet Ownership and the Risk of Dying from Cardiovascular Disease Among Adults Without Major Chronic Medical Conditions. High blood pressure & cardiovascular prevention: the official journal of the Italian Society of Hypertension, 10.1007/s40292-016-0156-1 (2016). [DOI] [PubMed]
- 33.Friedmann E, Thomas SA, Son H. Pets, depression and long-term survival in community living patients following myocardial infarction. Anthrozoös. 2011;24:273. doi: 10.2752/175303711X13045914865268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegel JM. Companion animals: In sickness and in health. Journal of Social Issues. 1993;49:157–167. doi: 10.1111/j.1540-4560.1993.tb00915.x. [DOI] [Google Scholar]
- 35.Curtis BM, O’Keefe JH., Jr. Autonomic tone as a cardiovascular risk factor: the dangers of chronic fight or flight. Mayo Clinic proceedings. 2002;77:45–54. doi: 10.4065/77.1.45. [DOI] [PubMed] [Google Scholar]
- 36.Das S, O’Keefe JH. Behavioral cardiology: recognizing and addressing the profound impact of psychosocial stress on cardiovascular health. Current hypertension reports. 2008;10:374–381. doi: 10.1007/s11906-008-0070-6. [DOI] [PubMed] [Google Scholar]
- 37.Aiba N, et al. Usefulness of pet ownership as a modulator of cardiac autonomic imbalance in patients with diabetes mellitus, hypertension, and/or hyperlipidemia. The American journal of cardiology. 2012;109:1164–1170. doi: 10.1016/j.amjcard.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 38.Allen K, Blascovich J, Mendes WB. Cardiovascular reactivity and the presence of pets, friends, and spouses: the truth about cats and dogs. Psychosomatic medicine. 2002;64:727–739. doi: 10.1097/01.psy.0000024236.11538.41. [DOI] [PubMed] [Google Scholar]
- 39.Westgarth C, Christian HE, Christley RM. Factors associated with daily walking of dogs. BMC veterinary research. 2015;11:116. doi: 10.1186/s12917-015-0434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitsavos C, et al. Physical activity status and acute coronary syndromes survival The GREECS (Greek Study of Acute Coronary Syndromes) study. Journal of the American College of Cardiology. 2008;51:2034–2039. doi: 10.1016/j.jacc.2008.01.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The register data that support the findings of this study were made available by record-linkage with data from Statistics Sweden, the National Board of Health and Welfare, the Swedish Kennel Club, Swedish Board of Agriculture and the Swedish Twin Register. Restrictions apply to the availability of these data, which were used under license and ethical approval for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Regional Ethical Review Board in Stockholm, Sweden.