Abstract
Patients with type 2 diabetes mellitus (T2DM) experience many cardiovascular complications. Several studies have demonstrated the cardioprotective effects of incretin-based therapies; however, there are few studies on the effects of long-term incretin-based therapies on cardiovascular events. Therefore, the present study conducted a systematic review and network meta-analysis to evaluate the effects of long-term incretin-based therapies on ischaemic diseases. We searched PubMed, CENTRAL, and Clinicaltrial.gov to retrieve randomised control trials reported until December 2016 and enrolled only RCTs with more than a 1-year follow-up. The network meta-analysis was performed using R Software with a GeMTC package. A total of 40 trials were included. Dipeptidyl peptidase 4 inhibitors and glucagon-like peptide-1 agonists were associated with a lower risk of myocardial infarction (MI) than were sulfonylureas (odds ratio [95% credible interval] 0.41 [0.24–0.71] and 0.48 [0.27–0.91], respectively). These results suggested that patients with T2DM receiving long-term incretin-based therapies have a lower risk of MI than do those receiving sulfonylurea-based therapy. These findings highlight the risks of cardiovascular events in patients who receive long-term incretin-based therapies, and may provide evidence for the selection of antidiabetic therapy in the future.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disorder associated with deficiency in insulin secretion and action. It is a major and growing health problem worldwide and accompanied with many complications that negatively influence the quality of life. One of the most concerned complications is cardiovascular diseases. T2DM patients are associated with two to four fold higher risk of cardiovascular diseases as compared with people under normal glycemic level1. The United Kingdom Prospective Diabetes Study (UKPDS)2 demonstrated that intensive glycemic control in patients with T2DM may reduce the risk of microvascular outcomes; however, other trials showed that lowering blood glucose intensively did not significantly prevent patients from cardiovascular events3,4.
Antidiabetic agents have also been associated with incidences of cardiovascular diseases. Previous studies showed the cardiovascular risk was increased in thiazolidinedione treatments5,6. This finding raised the attention of the cardiovascular safety of antidiabetic drugs. In 2008, the US Food and Drug Administration (FDA) revised the approval process of antidiabetic agents and the evaluation of cardiovascular events during phase II and phase III studies were required7. Since then, a number of trials have been conducted to clarify the effects of new classes of antidiabetic therapies on cardiovascular events.
Incretin-based therapies are novel medications for T2DM management. There are two types of incretin-based drugs, glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors. GLP-1 is an endogenous incretin hormone; activation of GLP-1 receptors stimulates insulin secretion and inhibits glucagon. DPP-4 inhibitors control hyperglycemia by blocking DPP-4 enzyme, which degrade incretin hormones-glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-18.
The potential cardioprotective effects of incretin-based therapies were shown in several studies9–12. Although numerous meta-analyses have been conducted to assess the cardiovascular safety of GLP-1 agonists and DPP-4 inhibitors, inconsistent results were report from different reviews, and the long-term outcomes were limited13–16. A recent meta-analysis suggest that use of exenatide and saxagliptin may increase the risk of arrhythmia and heart failure, respectively13. However, other studies did not demonstrate any differences on cardiovascular risk in comparison with other antidiabetic agents or placebo14–16. In addition, the influence of GLP-1 agonists and DPP-4 inhibitors on individual cardiovascular risk remained unclear. Furthermore, the comparisons of GLP-1 agonists versus DPP-4 inhibitors or other antidiabetic agents on cardiovascular outcomes were limited due to the lack of available long-term trial data. Therefore, in the present study we conducted a systematic review and network meta-analysis to comprehensively assess effects of the long-term use of GLP-1 agonists or DPP-4 inhibitors on ischemic heart diseases. The results of the present meta-analysis of randomized control trials may provide an evidence for a decision making of antidiabetic therapy in the future.
Methods
This systematic review and meta-analysis was conducted according to the guidance of Cochrane Handbook 17 and following the Preferred Reporting Items for Systematic reviews and Meta-Analyses Extension for Network Meta-analysis (PRISMA-NMA)18. The protocol for this systematic review was registered in PROSPERO in November 2016, the registration number is: CRD42016051259.
Search strategy and selection criteria
We searched for relevant randomize control trials (RCTs) from inception to December 2016 in PubMed and Cochrane central register of controlled trials (CENTRAL) database using medical subject heading (MeSH) terms with “ Glucagon-Like Peptide-1 Receptor/agonists”, or “Dipeptidyl-Peptidase IV Inhibitors”, (see Supplementary Tables S1 and S2). The language was limited to English. We also searched online clinical trials database (ClinicalTrials.gov) to identify additional eligible unpublished data.
Studies met the following inclusion criteria were included in this network meta-analysis: (1) randomized control trials (RCTs), (2) intervention compared DPP-4 inhibitors or GLP-1 agonist against placebo or other antidiabetic agents, (3) adults participants with type 2 diabetes, (4) at least 52 weeks follow-up, (5) Reported the events of coronary artery disease, myocardial infarction or angina in the original articles or on the ClinicalTrials.gov. The definition of these ischemic heart diseases were based on the standard medical terminology, Medical Dictionary for Regulatory Activities (MedDRA). The studies met the following criteria were excluded: (1) duplicate reports; (2) studies have not yet been terminated; (3) observational studies; (4) background treatment was the same as the one arm of studies.
The reference management software EndNote X7 was used to remove the duplicate studies by the “find duplication” function. Full texts were obtained for further review. The potentially relevant studies were identified according to pre-specified inclusion and exclusion criteria by two reviewers independently. Any discordant evaluations resolved by discussion and final consensus.
Outcome Measures and Data Extraction
Data were extracted independently by two reviewers using the standardized form including study characteristics (author name, publication year, location, sample size, mean age and percentage of male), study design (randomization, blinding, phase and interventions), and outcomes (number of participants with cardiovascular events in intervention group and control group). The primary outcome was any cardiovascular events in T2DM patients who treat with GLP-1 agonists or DPP-4 inhibitors more than one year. In addition, less trials would lead to higher heterogeneity, therefore, we analyzed the events reported in more than three trials. Discrepancies were resolved by discussion until consensus was reached. For any of the unclear information, the corresponding author of that study would be contacted for clarification.
Risk of bias assessment
The methodological quality assessment was performed by using the Cochrane Collaboration’s tool to assess risk of bias in each trials19. The evaluation items including in the present study are random sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and the potential bias. Each item was presented as “low risk”, “high risk”, or “unclear risk”. The graphs were synthesis by Review Manager version 5.3 (RevMan 5.3)20.
Statistical Analysis
The evaluation of cardiovascular outcomes was based on the synthesis of data extracted from included trials, then combine direct and indirect comparisons to estimate the overall effects of GLP-1 agonist and DPP-4 inhibitors. In this network meta-analysis we used the random-effects model and conducted in Bayesian framework. The effects of GLP-1 agonists and DPP-4 inhibitors on cardiovascular outcomes were analyzed using the odds ratios (OR) and 95% confidence intervals (CIs). The OR > 1.0 were indicated as higher risk, The CIs which did not include 1.0 was considered to be statistically significant. All the analyses were generated by R Software with GeMTC package21,22.
The consistency of network meta-analysis was assessed using the node-splitting models to detect whether the results of direct and indirect comparison were in agreement within treatment loops23. The node-splitting models cannot be performed when the outcome which lacked direct or indirect comparison. Thus, we used the analysis of heterogeneity to quantify the degree of heterogeneity by I 2 calculation. The values of I 2 > 50% was considered heterogeneity across the trials. To verify the robustness of the results, sensitivity analysis was performed to explore whether any factors might affect overall effect by excluding the heterogeneous studies one at a time then recalculated the overall effect.
Results
Study selection and characteristics
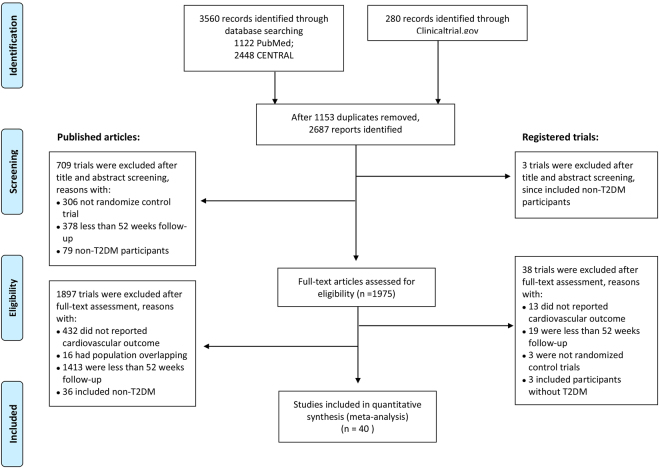
A total of 3840 references were identified using the search strategies. After removing 1153 duplicates, 2687 studies were selected through titles and abstracts screening. Full texts were obtained for further evaluation. After pre-screening, 1935 studies were excluded due to unsatisfying the inclusion criteria. Finally, a total of 40 studies which contained 35 full text publication and 5 unpublished studies, fulfilled the inclusion criteria and were reviewed in the present network meta-analysis. The range of publication year was 2007–2016. The flow diagram for results of the electronic search was described in Fig. 1 and the PRISMA NMA Checklist of Items to Include When Reporting A Systematic Review Involving a Network Meta-analysis were represented in Supplementary Table S3.
Figure 1.
PRISMA-NMA diagram of the literature search. RCTs were identified from PubMed, CENTRAL and Clinicaltrial.gov databases and the searches were done in December 2016. The medical subject heading (MeSH) terms were used in the searching of PubMed and CENTRAL. In the searching of ClinicalTrials.gov, we limited the search for completed RCTs with results. There were 3840 references identified from the databases and a total 40 studies (35 full text publication and 5 unpublished studies) were included in the present study.
The characteristics of included studies were shown in Table 1. The mean age of the included 70162 participants was 58.5 years old and the proportion of male was 55.4%. Most included trials were multicenter double-blind randomized design, whereas ten trials were open-label randomized design. There were 26 trials with active-controlled, 13 trials with placebo comparator and 1 trials both with active and placebo comparator. The trials duration ranged from 1 to 3 years.
Table 1.
Characteristics of included studies.
| Study | ClinicalTrials.gov Identifier | Location | Blind | Phase | Follow up (years) | Mean age (years) | Male (%) | Total subject | Treatment | Control |
|---|---|---|---|---|---|---|---|---|---|---|
| AWARD-2 45 | NCT01075282 | Multicenter | Open label | phase III | 1.5 | 56.66 | 51.3 | 807 | Dulaglutide 0.75–1.5 mg QW for 78 weeks (n = 545) | Insulin SC once daily for 78 weeks; dose titration based on blood glucose measures (n = 262) |
| AWARD-4 46 | NCT01191268 | Multicenter | Open label | phase III | 1 | 59.36 | 53.5 | 884 | Dulaglutide 0.75–1.5 mg QW for 52 weeks (n = 588) | Insulin SC once daily for 52 weeks; dose titration based on blood glucose measures (n = 296) |
| LEAD-3 47 | NCT00294723 | Multicenter | Double blind | phase III | 2 | 53 | 49.7 | 745 | Liraglutide 1.8 mg QD for 104 weeks (n = 497) | Glimepiride 8 mg QD for 104 weeks (n = 248) |
| Nauck, 2007 48 | NCT00082407 | Multicenter | Open-label | phase III | 1 | 58.7 | 48.7 | 501 | Exenatide 5 mcg SQ BID for 4 weeks and followed by 10 mcg for 48 weeks (n = 253) | Insulin SC twice daily; titration to target blood glucose level (n = 248) |
| LEAD-2 49 | NCT00318461 | Multicenter | Double blind | phase III | 2 | 56.7 | 58.2 | 1087 | Liraglutide 0.6–1.8 mg/day for 104 weeks (n = 724) | Glimepiride 4 mg/day for 104 weeks (n = 242)/ Metformin 1.5–2.0 g/day for 140 weeks (n = 121) |
| HARMONY-1 50 | NCT00849056 | Multicenter | Double blind | phase III | 1 | 55 | 59.8 | 301 | Albiglutide 30 mg QW (n = 150) | Placebo (n = 151) |
| Seino, 2010 51 | NCT00393718 | Japan | Double blind | phase III | 1 | 58.3 | 67.3 | 400 | Liraglutide 0.9 mg/day for 52 weeks (n = 268) | Glibenclamide 2.5 mg/day for 52 weeks (n = 132) |
| AWARD-1 2014 52 | NCT01064687 | Multicenter | Double blind | phase III | 1 | 56.5 | 58.4 | 979 | Dulaglutide 0.75–1.5 mg QW or Exenatide 5 mcg BID for 4 weeks, followed by 10 mcg BID for 48 weeks (n = 837) | Placebo (n = 142) |
| HARMONY-4 2014 53 | NCT00838916 | Multicenter | Open-label | phase III | 1 | 55.5 | 56.1 | 745 | Albiglutide 30 mg QW, n = 504 | Insulin, n = 241 |
| Seck 2010 54 | NCT00094770 | NR | Double blind | phase III | 2 | 57.3 | 60.1 | 1172 | Sitagliptin 100 mg QD (n = 588) | Glipizide 5 mg QD (n = 584) |
| Rosenstock 2013 55 | NCT00121641 | Multicenter | Double blind | phase III | 2 | 53.5 | 50.1 | 401 | Saxagliptin 2.5–10 mg QD (n = 301) | Placebo (n = 95) |
| DeFronzo 2009 56 | NCT00121667 | Multicenter | Double blind | phase III | 4 | 54.6 | 50.7 | 743 | Saxagliptin 2.5–10 mg QD (n = 564) | Placebo (n = 179) |
| Dobs 2013 57 | NCT00350779 | Multicenter | Double blind | phase III | 1 | 54.6 | 58 | 262 | Sitagliptin 100 mg QD (n = 170) | Placebo (n = 92) |
| EUREXA 2012 58 | NCT00359762 | Multicenter | Open-label | phase III | 1 | 56.4 | 53.6 | 1019 | Exenatide 10 mcg BID, n = 511 | Glimepiride 1 mg QD, n = 508 |
| Bosi 2011 59 | NCT00432276 | Multicenter | Double blind | phase III | 1 | 55.1 | 51.5 | 803 | Alogliptin 25 mg QD (n = 404) | Placebo (n = 399) |
| Corry 2013 60 | NCT00509236 | Multicenter | Double blind | phase III | 1 | 59.5 | 59.7 | 129 | Sitagliptin 25 mg QD (n = 64) | Glipizide 2.5–20 mg QD (n = 65) |
| Arjona 2013 61 | NCT00509262 | Multicenter | Double blind | phase III | 1 | 64.6 | 57.1 | 422 | Sitagliptin 25–50 mg QD (n = 210) | Glipizide 2.5–20 mg QD (n = 212) |
| Gallwitz 2012 62 | NCT00622284 | Multicenter | Double blind | phase III | 2 | 59.8 | 61 | 1551 | Linagliptin 5 mg QD (n = 776) | Glimepiride 1 mg QD (n = 775) |
| Göke 2013 63 | NCT00575588 | Multicenter | Double blind | phase III | 2 | 57.6 | 51.7 | 858 | Saxagliptin 5 mg QD (n = 428) | Glipizide 5–20 mg QD (n = 430) |
| Wilson 2013 64 | NCT00707993 | Multicenter | Double blind | phase III | 1 | 69.9 | 44.9 | 441 | Alogliptin 25 mg QD (n = 222) | Glipizide 5–10 mg QD (n = 219) |
| AWARD-5 2015 65 | NCT00734474 | Multicenter | Double blind | phase III | 2 | 54 | 46.7 | 921 | Sitagliptin 100 mg QD (n = 315) | Dulaglutide 0.75–1.5 mg QW (n = 606) |
| Barnett 2012 66 | NCT00740051 | Multicenter | Double blind | phase III | 1 | 56.6 | 39.9 | 227 | Linagliptin 5 mg QD (n = 151) | Placebo (n = 76) |
| Barnett 2013 67 | NCT00757588 | Multicenter | Double blind | phase IIIb | 1 | 57.3 | 57.8 | 455 | Saxagliptin 5 mg QD (n = 304) | Placebo (n = 151) |
| TECOS 2015 68 | NCT00790205 | Multicenter | Double blind | phase III | 3 | 65.5 | 70.3 | 14523 | Sitagliptin 100 mg QD (n = 7332) | Placebo (n = 7339) |
| HARMONY-3 2014 69 | NCT00838903 | Multicenter | Double blind | phase III | 2 | 54.5 | 47.6 | 1012 | Sitagliptin 100 mg QD (n = 302) | Glimepiride 2 mg QD (n = 307) /Albiglutide 30 mg QW (n = 302) /Placebo (n = 101) |
| Del 2014 70 | NCT00856284 | Multicenter | Double blind | phase III | 2 | 55.4 | 49.7 | 2639 | Alogliptin 12.5 mg QD (n = 1765) | Glipizide 5–20 mg QD (n = 874) |
| Ferrannini 2013 71 | NCT00881530 | Multicenter | Open- Label | phase II | 1.5 | 58.9 | 44.3 | 388 | Sitagliptin 100 mg QD (n = 56) | Empagliflozin 10–25 mg QD (n = 332) |
| Yki-Järvinen 2013 72 | NCT00954447 | Multicenter | Double blind | phase III | 1 | 60 | 52.2 | 1261 | Linagliptin 5 mg (n = 631) | Placebo (n = 630) |
| EXAMINE 2013 73 | NCT00968708 | Multicenter | Double blind | phase III | 1.5 | 61 | 67.9 | 5380 | Alogliptin 25 mg QD (n = 2701) | Placebo (n = 2679) |
| GENERATION 2015 74 | NCT01006603 | Europe,Mexico | Double blind | phase IIIb/IV | 1 | 72.6 | 61.8 | 720 | Saxagliptin 5 mg QD (n = 360) | Glimepiride 1 mg QD (n = 360) |
| Lavalle-González 2013 75 | NCT01106677 | Multicenter | Double blind | phase III | 1 | 55.4 | 46.4 | 1101 | Sitagliptin 100 mg QD (n = 366) | Canagliflozin 100–300 mg QD (n = 735) |
| SAVOR-TIMI 53 2013 76 | NCT01107886 | Multicenter | Double blind | phase IV | 2.1 | 65 | 67 | 16492 | Saxagliptin 5 mg QD (n = 8280) | Placebo (n = 8212) |
| CANTATA-D2 2013 72 | NCT01137812 | Multicenter | Double blind | phase III | 1 | 56.5 | 55.9 | 755 | Sitagliptin 100 mg QD (n = 378) | Canagliflozin 300 mg QD (n = 378) |
| ELIXA 2016 77 | NCT01147250 | Multicenter | Open-label | phase III | 2.1 | 60.3 | 69.4 | 6068 | Lixisenatide 10–20 μg QD, n = 3034 | Non-medication, n = 3034 |
| Roden 2015 45 | NCT01289990 | Multicenter | Open-Label | phase III | 1.5 | 55 | 61.3 | 899 | Sitagliptin 100 mg QD (n = 223) | Empagliflozin 10 mg/25 mg QD (n = 448) |
| NCT01098539 (139) | Multicenter | Double blind | phase III | 1 | 63.3 | 53.7 | 495 | Albiglutide 30 mg QW (n = 249) | Sitagliptin 100 mg QD (n = 246) | |
| NCT01075282(75) | Multicenter | Open-label | phase III | 1.5 | 56.7 | 51.3 | 807 | Dulaglutide 1.5 mg SC QW for 78 weeks, n = 545 | Insulin, n = 262 | |
| NCT01648582(49) | Multicenter | Open-label | phase III | 1 | 54.5 | 54.5 | 783 | Dulaglutide 0.75–1.5 mg QW for 52 weeks, n = 526 | Insulin, n = 257 | |
| NCT01087502(36) | Multicenter | Double blind | phase III | 1 | 66.6 | 63.4 | 235 | Linagliptin 5 mg (n = 118) | Placebo for 12 weeks and then switch to Glimepiride for further 40 weeks (n = 123) | |
| NCT01682759 (108) | Multicenter | Double blind | phase III | 1 | 57.7 | 55.1 | 751 | Omarigliptin 25 mg QW (n = 375) | Glimepiride 1–6 mg QD (n = 376) |
QD = once daily. BID = twice daily. QW = once per week. SC = subcutaneous. n = nu mber of participants. Registration number were identify in ClinicalTrials.gov.
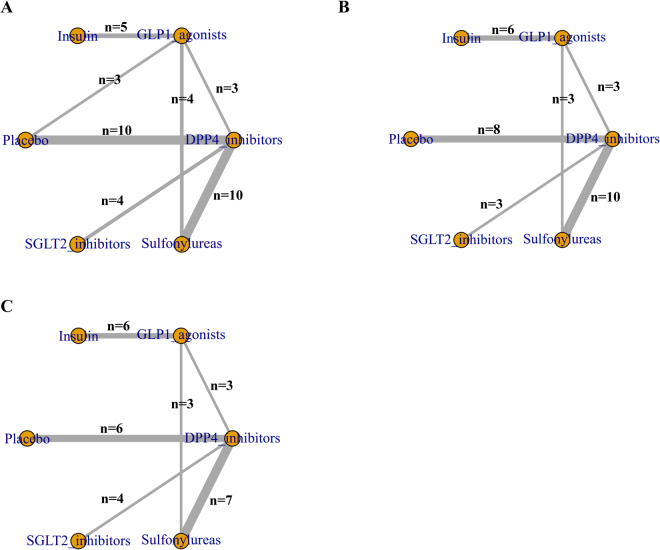
Figure 2 showed the network plots of eligible comparisons for myocardial infarction (MI), angina and coronary arterial disease (CAD). There were five classes of antidiabetic agents (DPP-4 inhibitors, GLP-1 agonists, sodium glucose transporter 2 inhibitors, sulfonylureas and insulin) have adequate trials for network- meta-analysis. Both of DPP-4 inhibitors and GLP-1 agonists were indirectly and directly compared with other antidiabetic agents.
Figure 2.
Network of eligible comparisons for (A) MI, (B) angina and (C) CAD. The size circle reflects the number of participants (sample size), and the width of the lines reflects the number of direct comparisons. n = number of trials for the direct comparisons.
Quality of included studies
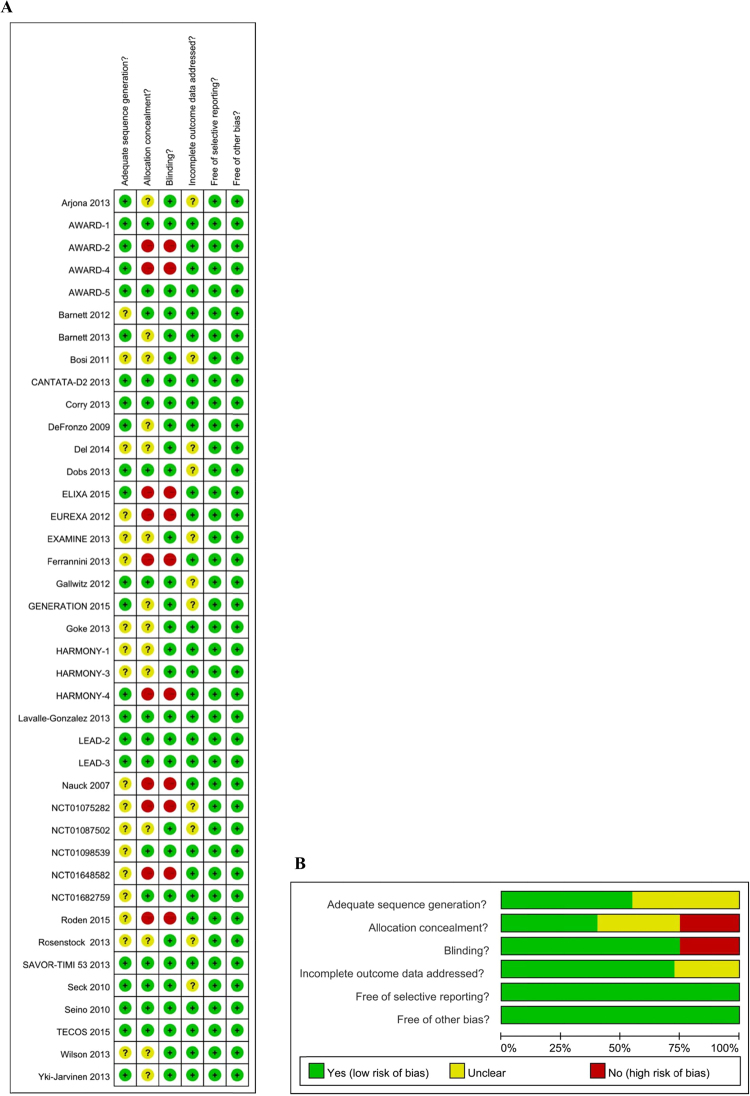
Risk of bias assessment of included trials was shown in Fig. 3. Allocation sequence generation was adequate in most of published trials except open-label trials. The unpublished trials were judged as unclear due to the insufficient information of the sequence generation process. The open random allocation schedules were used in open-label trials and were judged as high risk in the allocation concealment and blinding. The outcome measures in these open-label trials were objective parameters, such as blood glucose and cardiovascular events. These outcomes are not self-reported and blinded or not would not influence the results. Therefore, open-label RCTs were included in the present study. Eleven trials were judged as unclear risk of incomplete data, because these trials did not address the outcome analysis. Selective report biases were not identified in the included studies.
Figure 3.
Risk of bias assessment of included trials. The methodological quality assessment was performed by using the Cochrane Collaboration’s tool to assess risk of bias in for included trials. Allocation sequence generation was adequate in most of published trials except open-label trials and the unpublished trials which were judged as unclear. Open-label trials were judged as high risk in the allocation concealment and blinding. Eleven trials were judged as unclear risk of incomplete data, because these studies did not address the outcome analysis. Selective report biases were not identified in the included studies.
Effects of GLP-1 agonist and DPP-4 inhibitors on myocardial infarction events
The results of the network meta-analyses for the myocardial infarction events were presented in Fig. 4A. There was no difference effect between use of DPP-4 inhibitors and GLP-1 agonists on the risk of myocardial infarction. On the other hand, pooling data showed use of DPP-4 inhibitors favored lower risk of myocardial infarction events as compared to use of sulfonylureas (OR: 0.41, 95% CrI: 0.24–0.71), and the result of node-splitting analysis did not found any inconsistency between the direct and indirect comparisons (Table 2; p-value = 0.53125).
Figure 4.
Results of the network meta-analysis for antidiabetic agents in terms of (A) MI, (B) angina (upper right triangle) and CAD (lower left triangle). Results were presented as OR with 95% CrI, the estimations should read as column-defining treatment compared with the row-defining treatment. The OR below 1 was identified that the column-defining treatment had better effect on the cardiovascular risk. Use of DPP-4 inhibitors and GLP-1 agonists shown high probability with lower risk of myocardial infarction events as compared to use of sulfonylureas. OR = odds ratios. CrI = credible interval. * = 95% CrI did not include 1.
Table 2.
Node-splitting analysis of inconsistency within network meta-analysis.
| comparison | p-value | OR (95%CrI) |
|---|---|---|
| Myocardial infarction | ||
| DPP-4 inhibitors versus GLP-1 agonists | ||
| direct | 0.71125 | 0.37 (−0.79, 1.6) |
| indirect | 0.099 (−0.34, 0.79) | |
| network | 0.12 (−0.27, 0.75) | |
| DPP-4 inhibitors versus Placebo | ||
| direct | 0.41375 | 0.0016 (−0.35, 0.33) |
| indirect | 0.39 (−0.54, 1.4) | |
| network | 0.014 (−0.26, 0.38) | |
| DPP-4 inhibitors versus Sulfonylureas | ||
| direct | 0.53125 | 0.98 (0.38, 1.7) |
| indirect | 0.59 (−0.42, 1.7) | |
| network | 0.86 (0.30, 1.4) | |
| GLP-1 agonists versus Placebo | ||
| direct | 0.38375 | −0.037 (−0.61, 0.52) |
| indirect | −0.43 (−1.4, 0.41) | |
| network | −0.10 (−0.66, 0.26) | |
| GLP-1 agonists versus Sulfonylureas | ||
| direct | 0.5225 | 0.49 (−0.45, 1.4) |
| indirect | 0.87 (0.024, 1.7) | |
| network | 0.73 (0.072, 1.3) | |
| Angina | ||
| DPP-4 inhibitors versus GLP-1 agonists | ||
| direct | 0.43125 | −0.17 (−2., 1.7) |
| indirect | 0.77 (−0.80, 3.1) | |
| network | 0.37 (−0.83, 1.6) | |
| DPP-4 inhibitors versus Sulfonylureas | ||
| direct | 0.495 | 0.056 (−0.59, 0.80) |
| indirect | −0.83 (−3.5, 1.6) | |
| network | 0.050 (−0.65, 0.67) | |
| GLP-1 agonists versus Sulfonylureas | ||
| direct | 0.50125 | −0.68 (−2.6, 0.75) |
| indirect | 0.18 (−1.7, 2.3) | |
| network | −0.29 (−1.7, 0.83) | |
| Coronary arterial diseases | ||
| DPP-4 inhibitors versus GLP-1 agonists | ||
| direct | 0.02875 | 0.78 (−0.85, 2.8) |
| indirect | −2.2 (−4.8, −0.28) | |
| network | −0.65 (−1.9, 0.60) | |
| DPP-4 inhibitors versus Sulfonylureas | ||
| direct | 0.02 | −0.70 (−2., 0.24) |
| indirect | 2.5 (−0.036, 5.7) | |
| network | −0.34 (−1.3, 0.56) | |
| GLP-1 agonists versus Sulfonylureas | ||
| direct | 0.015 | 1.6 (−0.11, 3.9) |
| indirect | −1.5 (−3.9, 0.36) | |
| network | 0.28 (−1.0, 1.5) | |
p < 0.05: significant inconsistency between direct and indirect evidence.
In terms of GLP-1 agonists, a trend of lower risks effects on myocardial infarction risk as compared with sulfonylureas were also observed (Fig. 4A; OR: 0.48, 95% CrI: 0.27–0.91). There were no inconsistency between the direct and indirect comparisons from of node-splitting analysis (Table 2; p-value = 0.5225). In the heterogeneity analysis, global I-squared did not identified any heterogeneity across the studies (Table 3; global I 2 = 15.67).
Table 3.
Analysis of heterogeneity.
| t1 | t2 | i2.pair | i2.cons | incons.p |
|---|---|---|---|---|
| Myocardial infarction | ||||
| Per-comparison I-squared: | ||||
| DPP4_inhibitors | GLP1_agonists | 0 | 0 | 0.68 |
| DPP4_inhibitors | Placebo | 32.12 | 24.82 | 0.91 |
| DPP4_inhibitors | SGLT2_inhibitors | 44.39 | 44.38 | NA |
| DPP4_inhibitors | Sulfonylureas | 15.62 | 5.24 | 0.58 |
| GLP1_agonists | Insulin | 44.14 | 44.27 | NA |
| GLP1_agonists | Placebo | 0 | 0 | 0.86 |
| GLP1_agonists | Sulfonylureas | 0 | 0 | 0.56 |
| Global I-squared: | ||||
| 19.40 | 15.67 | |||
| Angina | ||||
| Per-comparison I-squared: | ||||
| DPP4_inhibitors | GLP1_agonists | 67.00 | 52.20 | 0.47 |
| DPP4_inhibitors | Placebo | 0 | 0 | NA |
| DPP4_inhibitors | SGLT2_inhibitors | 46.17 | 47.17 | NA |
| DPP4_inhibitors | Sulfonylureas | 6.98 | 0 | 0.76 |
| GLP1_agonists | Insulin | 33.38 | 33.05 | NA |
| GLP1_agonists | Sulfonylureas | 61.08 | 33.03 | 0.53 |
| Global I-squared: | ||||
| 30.56 | 26.56 | |||
| Coronary arterial diseases | ||||
| Per-comparison I-squared: | ||||
| DPP4_inhibitors | GLP1_agonists | 0 | 19.84 | 0.05 |
| DPP4_inhibitors | Placebo | 0 | 0 | NA |
| DPP4_inhibitors | SGLT2_inhibitors | 34.72 | 34.67 | NA |
| DPP4_inhibitors | Sulfonylureas | 32.50 | 28.73 | 0.16 |
| GLP1_agonists | Insulin | 31.44 | 30.88 | NA |
| GLP1_agonists | Sulfonylureas | 0 | 13.46 | 0.09 |
| Global I-squared: | ||||
| 19.96 | 16.26 | |||
t1: treatment 1, t2: treatment 2, i2.pair: i-square of pair-wise meta-analysis, i2.cons: i-square of network meta-analysis, incons.p: inconsistency p-values for pair-wise and network meta-analysis NA: not applicable.
Effects of GLP-1 agonist and DPP-4 inhibitors on angina events
The comparisons among five classes of antidiabetic agents, incretin-based therapies did not show significant effects on the risk of angina as compared with other antidiabetic agents or placebo (Fig. 4B upper right triangle). In addition, there was no different effect between GLP-1 agonists and DPP-4 inhibitors on angina events (OR: 1.43, 95% CrI: 0.46–4.7). The result of node-splitting analysis did not found any inconsistency between the direct and indirect comparisons (Table 2). There was no significant heterogeneity across the studies regarding angina events (Table 3; global-I² = 26.56%).
Effects of GLP-1 agonist and DPP-4 inhibitors on coronary artery disease events
Coronary artery disease risk was reported in twenty-nine RCTs. Patients with incretin-based therapies did not show superior effect on coronary artery disease risk whether compared with other antidiabetic agents or placebo (Fig. 4B lower left triangle). When further compared between GLP-1 agonists and DPP-4 inhibitors, no significant difference was detected (OR: 0.55, 95% CrI: 0.14–2.41). The results of node-splitting analysis showed inconsistency between the direct and indirect comparisons (Table 2), however, the degree of heterogeneity was low across the RCTs regarding coronary artery disease events (Table 3; global-I² = 16.26%).
Discussion
The present network meta-analysis comprehensively analysed 40 RCTs that reported the occurrence of cardiovascular events in patients receiving antidiabetic treatment for more than 1 year. The direct and indirect comparison results indicate that patients with T2DM receiving long-term incretin-based therapies are not at an increased risk of angina or coronary arterial disease. By contrast, DPP-4 inhibitors or GLP-1 agonists are associated with a lower risk of MI than are sulfonylureas.
Several systematic reviews and meta-analyses have evaluated the associations between antidiabetic treatment and cardiovascular events. Some of these studies have suggested that sulfonylurea use results in an increased risk of cardiovascular events or death24–26. Evidence has also suggested that DPP-4 inhibitors or GLP-1 agonists exert cardioprotective effects in patients with T2DM27–34. The present network meta-analysis demonstrates that patients with T2DM receiving long-term incretin-based therapies are at a low risk of MI. Additionally, in accordance with the present study, prior research has demonstrated the beneficial effects of GLP-1 agonists or DPP-4 inhibitors on MI, and has suggested that GLP-1 agonists improve myocardial blood flow and reduce regional infarction35. Furthermore, DPP-4 inhibitors are recognised as reducing the risk of MI compared with a placebo12,36.
These findings are supported by animal models and in vivo studies. In a mouse model, GLP-1 improved functional recovery after ischaemic injury by increasing cardiomyocyte viability and coronary vasodilatation10. The development of atherosclerotic lesions was also suppressed and cardiac infarct size was decreased in mice pretreated with GLP-137,38. In another study, a GLP-1 analogue exerted protective effects against cardiomyocyte hypertrophy, interstitial fibrosis, and myocardial inflammation39. These effects were associated with the reduction of inflammation and oxidative stress, which are risk factors for ischaemia. In a clinical study, lower plasma GLP-1 levels were observed in patients with coronary artery disease40. Compared with sulfonylurea use, the use of GLP-1 agonists significantly improved several cardiovascular risk factors, including body weight, waist circumference, and blood pressure, in patients with T2DM41.
Several studies have also reported controversial results regarding the cardioprotective effects of incretin-based therapies. A recent meta-analysis revealed no differences in the risk of MI between patients with T2DM receiving incretin-based therapies and those receiving a placebo (OR: 0.95, 95% CI: 0.88–1.03, P = 0.18)16. In other meta-analysis studies, no significant differences have been observed in the risk of cardiovascular events between incretin-based therapies and other antidiabetic agents14,15. However, these studies could not clarify the influence on individual cardiovascular outcomes. Furthermore, some of the included RCTs had a relatively short-term follow-up period, and therefore may have underestimated the actual benefits because the effects of a decreased risk of cardiovascular events may require long-term study (e.g., a 52-week follow-up) to be observed. In addition, there are few studies on the comparison of incretin-based therapies with each class of antidiabetic agents. One previous network meta-analysis compared the efficacy of oral antidiabetic drugs on cardiovascular events and mortality42; however, the results did not reveal differences in the effects of DPP-4 inhibitors and other antidiabetic agents or a placebo on MI in patients with T2DM. Elsewhere, patients receiving sodium glucose cotransporter-2 inhibitors were found to have a lower risk of MI than were those receiving a placebo (RR: 0.77, 95% CI: 0.63–0.93) or DPP-4 inhibitors (RR: 0.75, 95% CI: 0.60–0.94)42. The present study compared the potential cardioprotective effects of long-term incretin-based therapies and other antidiabetic drugs, namely DPP-4 inhibitors or GLP-1 agonists, or a placebo. We did not include RCTs on any other antidiabetic drugs to avoid inconsistent results.
The present results did not reveal any effects of GLP-1 agonists and DPP-4 inhibitors on the risk of angina and coronary artery disease in patients with T2DM. Coronary artery disease is caused by atherosclerosis, which can be asymptomatic. Fatty plaques accumulate on the coronary artery lumen, resulting in decreased heart blood flow, and symptoms such as chest pain, heartburn, or heart attack indicate the possible occurrence of myocardial ischaemia, angina, and MI. Therefore, this discrepancy in results might be because coronary artery disease and angina are imperceptible symptoms before MI that may have progressed to MI at diagnosis43. In addition, inconsistencies in direct and indirect comparison results regarding the risk of coronary artery disease may have been caused by the limited number of RCTs. In short, additional studies must be explored to verify the results.
The present study has several notable strengths. First, we used rigorous criteria to identify and include data from RCTs to minimise methodological bias resulting from the problematic quality of evidence, which has been observed in previous reviews. Second, the most comprehensive RCTs were included in this network meta-analysis. Apart from published data, additional unpublished data were identified from the ClinicalTrials.gov database. Obtaining data from unpublished trials can help researchers avoid publication bias, which is a major concern when attempting to establish the validity of meta-analyses. Third, the subgroup analysis of ethnic characteristics could not be performed because the results of different ethnicity were provided from the database of the sponsor and would not be able to obtain from the published studies44. We carefully identified the include data and used the random-effects model to minimize methodological bias. Fourth, we strictly ensured data authenticity by carefully evaluating the consistency of data from journal publications and trial registers, which may substantially reduce the risk of outcome-reporting bias. Nevertheless, the present study has some limitations. First, we only included RCTs published in English, and therefore may have excluded related studies published in non-English languages. However, we have included most of the major published trials as well as those from the ClinicalTrials.gov database to reduce bias. Second, some trials might not have reported all outcomes in their publications. However, we obtained the relevant information from their registration data in the ClinicalTrials.gov database. Finally, only a few trials had more than a 1-year follow-up. This limitation reflects the insufficiency of the currently available RCTs, and therefore warrants additional investigation.
In conclusion, the present systematic review and network meta-analysis comprehensively compared the risks of MI, angina, and coronary arterial disease in patients with T2DM receiving incretin-based therapies and other antidiabetic agents. This study demonstrates that more than 1 year of DPP-4 inhibitor or GLP-1 agonist use is associated with a lower risk of MI than is sulfonylurea use in patients with T2DM. Additional studies with a larger sample size, longer follow-up, and novel antidiabetic agents are recommended to derive definitive conclusions regarding the major clinical benefits and risks of these therapies.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW106-TDU-B-212–113004), National Health Research Institutes (NHRI-105A1-PDCO-1315161), China Medical University and Asia University (CMU105-ASIA-24) and China Medical University (CMU105-S-16). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This manuscript was edited by Wallace Academic Editing.
Author Contributions
C.Y.C., Y.T.C. and C.C.H. wrote the main manuscript text and J.L.Y. prepared Tables 1–3. R.Y.W., J.Y.W. and T.E.L. collected clinical data and performed statistical analyses. All authors reviewed the manuscript and approved the final submitted version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Che-Yi Chou and Ying-Tzu Chang contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16101-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Laakso M. Heart in diabetes: a microvascular disease. Diabetes care. 2011;34(Suppl 2):S145–149. doi: 10.2337/dc11-s209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ntensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet (London, England) 1998;352:837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 3.Giorgino F, Leonardini A, Laviola L. Cardiovascular disease and glycemic control in type 2 diabetes: now that the dust is settling from large clinical trials. Annals of the New York Academy of Sciences. 2013;1281:36–50. doi: 10.1111/nyas.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boussageon R, et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. Bmj. 2011;343:d4169. doi: 10.1136/bmj.d4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. The New England journal of medicine. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 6.Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Archives of internal medicine. 2010;170:1191–1201. doi: 10.1001/archinternmed.2010.207. [DOI] [PubMed] [Google Scholar]
- 7.Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes care. 2007;30:389–394. doi: 10.2337/dc06-1789. [DOI] [PubMed] [Google Scholar]
- 8.Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. International journal of clinical practice. 2006;60:1454–1470. doi: 10.1111/j.1742-1241.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- 9.Basalay MV, et al. Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovascular research. 2016;112:669–676. doi: 10.1093/cvr/cvw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ban K, et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 11.Nikolaidis LA, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 12.Monami M, Ahren B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes, obesity & metabolism. 2013;15:112–120. doi: 10.1111/dom.12000. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Wang F, Zhou J, Tang H, Giovenale S. Adverse effects of incretin-based therapies on major cardiovascular and arrhythmia events: meta-analysis of randomized trials. Diabetes/metabolism research and reviews. 2016;32:843–857. doi: 10.1002/dmrr.2804. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Yang S, Lee JI, Chang MJ. Cardiovascular Effect of Incretin-Based Therapy in Patients with Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis. PloS one. 2016;11:e0153502. doi: 10.1371/journal.pone.0153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannucci E, Monami M. Cardiovascular Safety of Incretin-Based Therapies in Type 2 Diabetes: Systematic Review of Integrated Analyses and Randomized Controlled Trials. Advances in therapy. 2017;34:1–40. doi: 10.1007/s12325-016-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoud AN, et al. Cardiovascular safety of incretin-based therapy for type 2 diabetes: A meta-analysis of randomized trials. International journal of cardiology. 2017;230:324–326. doi: 10.1016/j.ijcard.2016.12.113. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, G. S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration (2011).
- 18.Hutton B, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Annals of internal medicine. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centre, T. N. C. The Cochrane Collaboration.Review Manager (RevMan). 5.3. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration (2014).
- 21.Warn DE, Thompson SG, Spiegelhalter DJ. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Statistics in medicine. 2002;21:1601–1623. doi: 10.1002/sim.1189. [DOI] [PubMed] [Google Scholar]
- 22.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS - A Bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing. 2000;10:325–337. doi: 10.1023/A:1008929526011. [DOI] [Google Scholar]
- 23.van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Research synthesis methods. 2016;7:80–93. doi: 10.1002/jrsm.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemmingsen, B. et al. Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. The Cochrane database of systematic reviews, Cd009008, 10.1002/14651858.CD009008.pub2 (2013). [DOI] [PubMed]
- 25.Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes, obesity & metabolism. 2013;15:938–953. doi: 10.1111/dom.12116. [DOI] [PubMed] [Google Scholar]
- 26.Schramm TK, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. European heart journal. 2011;32:1900–1908. doi: 10.1093/eurheartj/ehr077. [DOI] [PubMed] [Google Scholar]
- 27.Engel SS, et al. Cardiovascular safety of sitagliptin in patients with type 2 diabetes mellitus: a pooled analysis. Cardiovascular diabetology. 2013;12:3. doi: 10.1186/1475-2840-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White WB, et al. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes, obesity & metabolism. 2013;15:668–673. doi: 10.1111/dom.12093. [DOI] [PubMed] [Google Scholar]
- 29.Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovascular diabetology. 2012;11:3. doi: 10.1186/1475-2840-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams-Herman D, et al. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC endocrine disorders. 2010;10:7. doi: 10.1186/1472-6823-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, et al. Glucagon-like peptide-1 receptor agonists and heart failure in type 2 diabetes: systematic review and meta-analysis of randomized and observational studies. BMC cardiovascular disorders. 2016;16:91. doi: 10.1186/s12872-016-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferdinand KC, Botros FT, Atisso CM, Sager PT. Cardiovascular safety for once-weekly dulaglutide in type 2 diabetes: a pre-specified meta-analysis of prospectively adjudicated cardiovascular events. Cardiovascular diabetology. 2016;15:38. doi: 10.1186/s12933-016-0355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun F, et al. Cardiovascular safety and glycemic control of glucagon-like peptide-1 receptor agonists for type 2 diabetes mellitus: a pairwise and network meta-analysis. Diabetes research and clinical practice. 2012;98:386–395. doi: 10.1016/j.diabres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Wu S, et al. The cardiovascular effects of glucagon-like peptide-1 receptor agonists: a trial sequential analysis of randomized controlled trials. Journal of clinical pharmacy and therapeutics. 2014;39:7–13. doi: 10.1111/jcpt.12102. [DOI] [PubMed] [Google Scholar]
- 35.Wroge J, Williams NT. Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists in Cardiac Disorders. The Annals of pharmacotherapy. 2016;50:1041–1050. doi: 10.1177/1060028016663218. [DOI] [PubMed] [Google Scholar]
- 36.Patil HR, et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. The American journal of cardiology. 2012;110:826–833. doi: 10.1016/j.amjcard.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 37.Nagashima M, et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia. 2011;54:2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 39.Robinson E, et al. Exendin-4 protects against post-myocardial infarction remodelling via specific actions on inflammation and the extracellular matrix. Basic research in cardiology. 2015;110:20. doi: 10.1007/s00395-015-0476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akiyama E, et al. Decreased plasma levels of active glucagon-like peptide-1 in coronary artery disease. Journal of the American College of Cardiology. 2015;65:754–755. doi: 10.1016/j.jacc.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 41.Simo R, et al. Long-term changes in cardiovascular risk markers during administration of exenatide twice daily or glimepiride: results from the European exenatide study. Cardiovascular diabetology. 2015;14:116. doi: 10.1186/s12933-015-0279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee G, et al. Comparative effectiveness of oral antidiabetic drugs in preventing cardiovascular mortality and morbidity: A network meta-analysis. PloS one. 2017;12:e0177646. doi: 10.1371/journal.pone.0177646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antman, E. M. & Loscalzo, J. In Harrison’s Principles of Internal Medicine, 19e (eds Dennis Kasper et al.) (McGraw-Hill Education, 2015).
- 44.Shomali ME, Orsted DD, Cannon AJ. Efficacy and safety of liraglutide, a once-daily human glucagon-like peptide-1 receptor agonist, in African-American people with Type 2 diabetes: a meta-analysis of sub-population data from seven phase III trials. Diabetic medicine: a journal of the British Diabetic Association. 2017;34:197–203. doi: 10.1111/dme.13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and Safety of Once-Weekly Dulaglutide Versus Insulin Glargine in Patients With Type 2 Diabetes on Metformin and Glimepiride (AWARD-2) Diabetes care. 2015;38:2241–2249. doi: 10.2337/dc14-1625. [DOI] [PubMed] [Google Scholar]
- 46.Blonde L, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. The Lancet. 2015;385:2057–2066. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 47.Garber A, et al. Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycaemic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes, obesity & metabolism. 2011;13:348–356. doi: 10.1111/j.1463-1326.2010.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nauck MA, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259–267. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 49.Nauck M, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reusch J, et al. Efficacy and safety of once-weekly glucagon-like peptide 1 receptor agonist albiglutide (HARMONY 1 trial): 52-week primary endpoint results from a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes mellitus not controlled on pioglitazone, with or without metformin. Diabetes, obesity & metabolism. 2014;16:1257–1264. doi: 10.1111/dom.12382. [DOI] [PubMed] [Google Scholar]
- 51.Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Current medical research and opinion. 2010;26:1013–1022. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 52.Wysham C, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1) Diabetes care. 2014;37:2159–2167. doi: 10.2337/dc13-2760. [DOI] [PubMed] [Google Scholar]
- 53.Weissman PN, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57:2475–2484. doi: 10.1007/s00125-014-3360-3. [DOI] [PubMed] [Google Scholar]
- 54.Seck T, et al. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2-year study. International journal of clinical practice. 2010;64:562–576. doi: 10.1111/j.1742-1241.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 55.Rosenstock J, et al. Long-term 4-year safety of saxagliptin in drug-naive and metformin-treated patients with Type 2 diabetes. Diabetic medicine: a journal of the British Diabetic Association. 2013;30:1472–1476. doi: 10.1111/dme.12267. [DOI] [PubMed] [Google Scholar]
- 56.DeFronzo RA, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes with metformin alone. Diabetes care. 2009;32:1649–1655. doi: 10.2337/dc08-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobs AS, et al. Efficacy and safety of sitagliptin added to ongoing metformin and rosiglitazone combination therapy in a randomized placebo-controlled 54-week trial in patients with type 2 diabetes. Journal of diabetes. 2013;5:68–79. doi: 10.1111/j.1753-0407.2012.00223.x. [DOI] [PubMed] [Google Scholar]
- 58.Gallwitz B, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. The Lancet. 2012;379:2270–2278. doi: 10.1016/S0140-6736(12)60479-6. [DOI] [PubMed] [Google Scholar]
- 59.Bosi E, Ellis GC, Wilson CA, Fleck PR. Alogliptin as a third oral antidiabetic drug in patients with type 2 diabetes and inadequate glycaemic control on metformin and pioglitazone: a 52-week, randomized, double-blind, active-controlled, parallel-group study. Diabetes, obesity & metabolism. 2011;13:1088–1096. doi: 10.1111/j.1463-1326.2011.01463.x. [DOI] [PubMed] [Google Scholar]
- 60.Arjona Ferreira JC, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013;61:579–587. doi: 10.1053/j.ajkd.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 61.Arjona Ferreira JC, et al. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes care. 2013;36:1067–1073. doi: 10.2337/dc12-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallwitz B, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. The Lancet. 2012;380:475–483. doi: 10.1016/S0140-6736(12)60691-6. [DOI] [PubMed] [Google Scholar]
- 63.Göke B, Gallwitz B, Eriksson JG, Hellqvist Å, Gause-Nilsson I. Saxagliptin vs. glipizide as add-on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long-term (52-week) extension of a 52-week randomised controlled trial. International journal of clinical practice. 2013;67:307–316. doi: 10.1111/ijcp.12119. [DOI] [PubMed] [Google Scholar]
- 64.Rosenstock J, Wilson C, Fleck P. Alogliptin versus glipizide monotherapy in elderly type 2 diabetes mellitus patients with mild hyperglycaemia: a prospective, double-blind, randomized, 1-year study. Diabetes, obesity & metabolism. 2013;15:906–914. doi: 10.1111/dom.12102. [DOI] [PubMed] [Google Scholar]
- 65.Weinstock RS, et al. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes, obesity & metabolism. 2015;17:849–858. doi: 10.1111/dom.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnett AH, et al. Linagliptin monotherapy in type 2 diabetes patients for whom metformin is inappropriate: an 18-week randomized, double-blind, placebo-controlled phase III trial with a 34-week active-controlled extension. Diabetes, obesity & metabolism. 2012;14:1145–1154. doi: 10.1111/dom.12011. [DOI] [PubMed] [Google Scholar]
- 67.Barnett AH, et al. Saxagliptin add-on therapy to insulin with or without metformin for type 2 diabetes mellitus: 52-week safety and efficacy. Clinical drug investigation. 2013;33:707–717. doi: 10.1007/s40261-013-0107-8. [DOI] [PubMed] [Google Scholar]
- 68.Green JB, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. The New England journal of medicine. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 69.Ahren B, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes care. 2014;37:2141–2148. doi: 10.2337/dc14-0024. [DOI] [PubMed] [Google Scholar]
- 70.Del Prato S, Camisasca R, Wilson C, Fleck P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2-year study. Diabetes, obesity & metabolism. 2014;16:1239–1246. doi: 10.1111/dom.12377. [DOI] [PubMed] [Google Scholar]
- 71.Ferrannini E, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes care. 2013;36:4015–4021. doi: 10.2337/dc13-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yki-Jarvinen H, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a ≥ 52-week randomized, double-blind study. Diabetes care. 2013;36:3875–3881. doi: 10.2337/dc12-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White WB, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. The New England journal of medicine. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 74.Schernthaner G, et al. Efficacy and tolerability of saxagliptin compared with glimepiride in elderly patients with type 2 diabetes: a randomized, controlled study (GENERATION) Diabetes, obesity & metabolism. 2015;17:630–638. doi: 10.1111/dom.12461. [DOI] [PubMed] [Google Scholar]
- 75.Lavalle-Gonzalez FJ, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scirica BM, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. The New England journal of medicine. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 77.Pfeffer MA, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. The New England journal of medicine. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.