Abstract
Pharmacotherapy for traumatic brain injury (TBI) is focused on resuscitation, prevention of secondary injury, rehabilitation and recovery. Pharmacogenomics may play a role in TBI for predicting therapies for sedation, analgesia, seizure prevention, intracranial pressure-directed therapy and neurobehavioral/psychiatric symptoms. Research into genetic predictors of outcomes and susceptibility to complications may also help clinicians to tailor therapeutics for high-risk individuals. Additionally, the expanding use of genomics in the drug development pipeline has provided insight to novel investigational and repurposed medications that may be useful in the treatment of TBI and its complications. Genomics in the context of treatment and prognostication for patients with TBI is a promising area for clinical progress of pharmacogenomics.
Keywords: : biomarkers, drug development, neurocritical care, pharmacogenomics, pharmacokinetics, prognostication, therapy, transporters, traumatic brain injury
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide [1]. Despite substantial research efforts, major knowledge gaps remain. This may be due to individual variability in clinical presentation and incomplete understanding of the molecular mechanisms driving neuronal damage after TBI [2]. TBI pathology is divided into acute primary injury (initial trauma) and the subsequent response referred to as secondary injury. Secondary injury can be driven by disturbances in cerebral blood flow, loss of neurovascular autoregulation, cerebral hypoxia, metabolic dysfunction and axonal injury. These effects are augmented by an inflammatory response, generation of reactive oxygen species and excitotoxicity, among other mechanisms. These processes create an environment of evolving axonal fiber tract damage, synaptic dysfunction, cerebral edema (CE), ischemia and neuronal death that can persist after the primary injury [3,4]. Despite growing knowledge of the molecular mechanisms associated with secondary injury, there remains no pharmacologic therapy that uniformly mitigates the damage from secondary injury and improves clinical outcomes. The use of preclinical in vivo and in vitro models for TBI has also elevated our understanding of TBI pathology, but pharmacologic interventions in preclinical models have not translated well into humans [5]. This is complicated by the fact that TBI is a chronic and sometimes terminal disease state with pathology influenced by individual characteristics [6]. Discovery of therapeutics for TBI has been increasingly led by transcriptomics, metabolomics, proteomics and genomics, which may inform tailored treatment approaches effective for specific subpopulations of patients with TBI [7,8].
Therapeutic response after TBI may be influenced by injury phenotype, patient age, co-morbidities, concurrent extracerebral insults, genetics and other factors. Efforts to identify genetic predictors of outcomes following TBI have identified novel pathways that support the effort to discover treatments for TBI [7,9]. Many studies in the past decade have investigated the role of genetics in prognosticating post TBI, with over 20 genetic variations identified to date that have associations with TBI outcomes and/or complications [7]. Despite the lack of US FDA-approved therapies, many medications are used in TBI for acute care, rehabilitation and neuropsychiatric care. Pharmacogenomics is one approach to tailor treatments for individuals with TBI through precision critical care medicine and/or prophylactic pharmacotherapy to reduce TBI complications [10].
The objective of this review is to discuss the current treatments used for individuals with TBI and how they may be impacted clinically by pharmacogenomics in severe TBI. Of note, an extended definition of pharmacogenomics that encompasses the role of genomics in prognostication as it relates to pharmacotherapy decisions will be used. How genomics may improve prognostication and treatment decisions in the future is also discussed. To build consistency into variant descriptions, all genetic variants will be first identified by GRCh38 position/allele change and subsequently by reference single nucleotide polymorphism identification number (RSID) or other common identifier when available [11]. HUGO Gene Nomenclature Committee names for proteins and genes will be used in lieu of common names [12].
Pharmacogenomics of the blood–brain barrier
The blood–brain barrier (BBB) controls the influx of nutrients, efflux of waste products and protection from xenobiotics and neurotoxic compounds present in the blood. Transporters mediate this selective permeability, and transporter genes are major considerations in the pharmacogenomics of TBI and other neurologic conditions [13,14]. Transporters are generally categorized into ATP-binding cassette (ABC) and solute carrier (SLC) transporters, which are exclusively efflux or mixed efflux/influx, respectively. Independent of drug use evaluation, Cousar et al. investigated genetic variations in BBB transporters (ABCB1, ABCC1 and ABCC2) in 305 adult patients with severe TBI [15]. They found that ABCB1 rs1045642 (NC_000007.14:g.87509329A>G; AA) and ABCC1 rs4148382 (NC_000016.10:g.16144637G>A; GG) genotypes were associated with lower odds of unfavorable 6-month Glasgow Outcome Scale (GOS) scores, defined as GOS of 1–3 (odds ratio: 0.71 and 0.73, respectively) [15]. The finding with ABCB1 rs1045642 was reversed in a study by Wang et al. who studied 182 patients with TBI [16]. They defined favorable outcomes as GOS of 3–5 and found that patients with the (AG, GG) genotypes were more likely to have favorable GOS scores at 6 months post TBI (odds ratio: 2.71) [16]. These contradictory results may be due to different definitions of outcomes and/or racial makeup (i.e., Caucasian vs Chinese). Focusing on another ABC transporter highly expressed on the human BBB, Adams et al. studied variations in the ABCG2 gene for association with GOS scores evaluated at 3, 6, 12 and 24 months following injury. They found that ABCG2 rs2231142 (NC_000004.12:g.88131171G>T; TG, TT) genotypes were associated with better outcomes (higher GOS scores) following TBI [17]. These findings suggest that the function of these genes impacts recovery from TBI, which may relate to changes in drug disposition or other mechanisms. It also exemplifies the differences in magnitude and direction that can be found in different population and/or with different evaluations of the same outcome measure. Transporters are the gatekeepers for the penetrance and/or removal of medications into/from the brain, and variations that impact transporter expression and/or function may have implications in therapeutics with brain targets.
Pharmacotherapy of TBI & associated pharmacogenomics
Treatments for TBI are time sensitive and start with medications used for resuscitation and critical care management. Early pharmacotherapy typically includes sedatives, analgesia and neuromuscular blocking agents. Individuals with TBI may have a high risk for post-traumatic seizures (PTS; both clinical and subclinical), require intubation and sedation, need frequent neurologic assessment while sedated and require constant monitoring of cerebrovascular parameters (e.g., intracranial pressure [ICP], cerebral perfusion pressure) [18]. The role of pharmacogenomics in the intensive care unit is not a new concept, but implementation entails practical challenges due to the complexity of cases and the presence of comorbidities [10,19]. In patients with TBI, pharmacogenomics may inform pharmacotherapy for seizures, pain and sedation [19–21]. Drug–gene pairs relevant to TBI are summarized in Table 1.
Table 1. . Traumatic brain injury drug–gene pairs.
| Medication | Use(s) | Gene | Variant | Association | Clinical recommendation |
|---|---|---|---|---|---|
| Phenytoin | Post-traumatic seizures, post-traumatic epilepsy | HLA-B | *15:02 | Increased risk for SCAR | Avoid |
| CYP2C9 | Intermediate/poor metabolizers | Decreased systemic clearance | Use lower dose | ||
| Ketamine | Sedation, analgesia | CYP2B6 | *6/*6 | Decreased systemic clearance | N/A† |
| Midazolam | Sedation | CYP3A5 | *3 | Decreased systemic clearance | N/A† |
| Fentanyl | Sedation, analgesia | CYP3A5 | *3 | Decreased systemic clearance | N/A† |
| OPRM1 | rs1799971 (GG) | Decreased sensitivity | N/A† | ||
| ABCB1 | rs1045642 (TT) | Increased brain/CSF concentrations | N/A† | ||
| Morphine | Sedation, analgesia | ABCB1 | rs1045642 (TT) | Increased brain/CSF concentrations | N/A† |
| OPRM1 | rs1799971 (GG) | Decreased sensitivity | N/A† | ||
| Citalopram, escitalopram, sertraline | Post-traumatic depression | CYP2C19 | Poor metabolizers | Decreased systemic clearance | Decrease dose |
| Ultra-rapid metabolizers | Increased systemic clearance | Use alternative agent‡ | |||
| Paroxetine | CYP2D6 | Ultra-rapid metabolizers | Increased systemic clearance | Use alternative agent | |
| Poor metabolizers | Decreased systemic clearance | Use alternative agent | |||
| Fluvoxamine | Decrease dose | ||||
†No specific clinical guidance available.
‡Does not apply to sertraline.
CSF: Cerebrospinal fluid; N/A: Not available or defined; SCAR: Severe cutaneous adverse reaction.
Seizure treatment/prophylaxis in the acute period following TBI
PTS can be clinical or subclinical in nature, and both types can contribute to secondary injury [22]. PTS occur within 7 days in as many as 22% of patients with moderate and severe TBI [23]. Current treatment guidelines recommend the use of seizure prophylaxis in the first 7 days post injury to prevent PTS. Based on work by Temkin et al. in 1990, the drug of choice for PTS prophylaxis has been phenytoin [24]. However, a growing body of literature supports levetiracetam as potentially equivalent or superior to phenytoin [25], and potentially with neuroprotective benefits [26] – particularly when treatment is continued daily into the postacute period [27]. Pharmacogenomics considerations for agents regarding PTS prophylaxis and treatment can be divided into genetic predictors of drug response (i.e., traditional pharmacogenomics) and in PTS risk assessment (i.e., guided prophylaxis).
Pharmacogenomic measures for phenytoin pharmacokinetics and response include CYP2C9 and HLA-B, respectively. The Clinical Pharmacogenomics Implementation Consortium and Dutch Pharmacogenomics Working Group guidelines for phenytoin suggest that patients with decreased CYP2C9 activity (e.g., CYP2C9 intermediate/poor metabolizers) receive a 25–50% lower maintenance dose and patients carrying the HLA-B*15:02 haplotype should avoid phenytoin due to over four-fold increased risk for severe cutaneous adverse reactions [20,21]. Siddiqui et al. also reported that ABCB1 rs1045642 (GG) was associated with higher incidence of drug-resistant epilepsy (odds ratio: 2.66), which suggests that some anti-epileptic drugs’ ability to cross the BBB may be impacted by ABCB1 function [28]. However, this finding has not been directly linked to phenytoin, and additional studies have not supported a significant contribution of variations in the ABCB1 gene and phenytoin pharmacokinetics/pharmacodynamics [29,30]. These pharmacogenomic markers may be useful in TBI to identify patients who may require lower maintenance doses or an alternative agent, such as levetiracetam, during PTS prophylaxis and treatment.
The use of genetic markers to predict risk for PTS may help guide therapeutic decision making for individual patients. The variant ADORA1 rs3766553 (NC_000001.11:g.203163914A>G; AA) was associated with over five-fold increased risk for PTS in a study with 206 subjects with severe TBI, although the mechanistic basis was not established by this association [31]. Kochanek et al. evaluated the impact of Adora1 knockout in a murine model for experimental TBI and found that knockout mice develop lethal status epilepticus following TBI, suggesting a role for ADORA1 in preventing PTS [32]. Activation of ADORA1 seems to inhibit the microglial response following TBI, which may indicate that ADORA1 and its associated pathways represent a therapeutic option for TBI [33]. These findings not only suggest new treatment modalities for PTS, but may help identify patients more likely to benefit from PTS prophylaxis. Darrah et al. investigated GAD1 and GAD2, which catalyze the conversion of glutamate to GABA. In a study of 257 adults with severe TBI, they found that GAD1 rs3828275 (NC_000002.12:g.170826230C>T; CT, TT) was associated with higher risk for PTS (odds ratio: 5.6) [34]. APOE has been studied for its role in PTS, and some investigations have suggested an association between APOE ε4 homozygotes and PTS, but this finding has not been consistent [35,36]. Variability in outcome measures, statistical power, patient characteristics and methodologies seems to be the driver of this variability; however, a systematic review by Lawrence et al. found that over 63% of studies investigating APOE ε4 were detrimental to outcomes post TBI [37].
Sedation & analgesia
In the intensive care setting, sedation is used to minimize patient discomfort and control ICP in patients with TBI [23]. Preferred agents for sedation in adults with TBI include propofol and midazolam due to their favorable effects on cerebral metabolism, ICP and cerebral perfusion pressure [18,38,39]. Midazolam is metabolized by the CYP3A4/5 enzymes. The CYP3A5*3 haplotype is associated with decreased midazolam metabolism, but its effect may only be clinically relevant in the presence of concomitant moderate/strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin) [40–42]. Ketamine and dexmedetomidine may be used in some patients with TBI as adjunctive or solo agents due to decreased accumulation, lower risk for dependence and/or ease with which patients can be awakened for neurologic assessments. Nevertheless, their use in routine clinical practice for TBI is less well established due to decreased time on the market, but their use has gained popularity [18]. Some studies assessing ketamine for ICP control among individuals with severe TBI have suggested a very favorable profile, although this remains controversial [43]. Ketamine is primarily metabolized by CYP3A4, which is not associated with common functional pharmacogenomic variants, but at higher concentrations the metabolic contribution of CYP2B6 is more pronounced [44]. Li and colleagues found that CYP2B6 (*6/*6) was associated with 59% and 40% decreased steady state clearance of ketamine compared with CYP2B6 (*1/*1, *1/*6), respectively, in a group of 49 adult patients [45]. Decreased clearance of ketamine may increase risk for adverse events including adverse psychomimetic and cognitive reactions, and hepatic and/or renal toxicity [45,46]. Variability in dexmedetomidine pharmacokinetics/pharmacodynamics may be explained by variations in its metabolic pathway through CYP2A6 and UGT1A4, though no link has been found at this point. However, dexmedetomidine pharmacodynamics may be affected by variations in its target, ADRA2A [47]. Yağar et al. found that ADRA2A rs1800544 (NC_000010.11:g.111076745G>C; GG, GC) was associated with slightly decreased efficacy at some time points as measured by sedation scores, though the clinical relevance of this finding is not clear [48]. Barbiturates, particularly pentobarbital, are also used as sedatives for TBI patients with refractory intracranial hypertension [23].
TBI patients may also receive therapy with opioid analgesics/sedatives like fentanyl, remifentanil or morphine [18]. Variants in ABCB1, CYP3A5 and OPRM1 may provide insight into the variable dosing associated with opioid medications commonly used for TBI [49,50]. ABCB1 rs1045642 (TT) is associated with decreased expression of ABCB1, which may increase penetration of ABCB1 substrates across the intestinal epithelium and the BBB. Fentanyl and morphine are substrates for ABCB1, and individuals carrying ABCB1 rs1045642 (TT) may require lower doses. Lötsch et al. measured oral morphine equivalent dosing in subjects treated with ABCB1 substrates (e.g., morphine, fentanyl) [49]. They found that subjects with ABCB1 rs1045642 (TT) required 135.4 vs (CT) 194.9 vs (CC) 274.5 (ANOVA; p = 0.014) [9,51]. Similarly, Horvat et al. found in a diverse pediatric population (n = 61) that patients with ABCB1 rs1045642 (TT) required 18.6 mcg/kg/day less fentanyl than (CT, CC) [52]. Patients with CYP3A5*3 may also require a reduction in dose of fentanyl due to decreased hepatic clearance [51]. The OPRM1 variation rs1799971 (NC_000006.12:g.154039662A>G; GG) is associated with decreased efficiency of endogenous opioid signaling [53]. OPRM1 rs1799971 (GG) may predict increased dosing requirements for analgesics, and individuals with AG or AA genotypes may experience higher rates of adverse reactions [19,50].
Role of pharmacogenomics during TBI rehabilitation
Chronic neurologic and psychiatric care is necessary for many individuals post-TBI and pharmacotherapy may be guided by pharmacogenomics. The use of pharmacogenomic concepts to guide treatments for TBI rehabilitation in light of genetic risk factors and expected response to medications complements the rehabilomics framework introduced by Wagner in 2010 [54]. Rehabilomics in the context of TBI refers to systematic use of biomarker, genetic, phenotypic and other patient-specific factors that impact rehabilitation and long-term recovery [54,55]. It focuses on TBI as a chronic disease state that requires long-term care with patient-specific approaches to rehabilitation. An example of this is found in the approach by Myrga et al. in evaluating the sex-stratified risk for post-TBI cognitive decline in association with dopamine pathways. They found an important sex*gene interaction in patient outcomes, suggesting sex-associated stratification of genetic risk [56]. While rehabilomics is all-encompassing, pharmacogenomics is most applicable in the psychiatric and neurologic pathologies requiring pharmacotherapy such as post-traumatic depression (PTD), cognitive decline and post-traumatic epilepsy (PTE) [55,57]. PTD and changes in cognitive capacity are multifactorial, but incidence, nature and onset, as well as severity and duration may be predictable through genetic risk factors associated with monoamine pathways, specifically the dopamine (DA) pathways in the prefrontal cortex and by serotonin pathways [56]. PTE may share some risk factors with PTS, but is thought to have unique pathophysiology [58]. Genetic influences on risk for these complications are summarized in Table 2.
Table 2. . Genetic modifiers of traumatic brain injury rehabilitation.
| Gene | GRCh38/hg38 or common name | rsID | Associated genotype(s) | Outcome or complication |
|---|---|---|---|---|
| ABCB1 | NC_000007.14:g.87509329A>G | rs1045642 | AA | Unclear† |
| ABCC1 | NC_000016.10:g.16144637G>A | rs4148382 | GG | Improved 6-month outcomes (GOS scores) |
| ABCC8 | NC_000011.10:g.17440757A>C | rs2283261 | CC | Higher risk for cerebral edema |
| NC_000011.10:g.17465190C>T | rs3819521 | TT | Higher risk for cerebral edema | |
| NC_000011.10:g.17451890C>T | rs2283258 | TT | Higher risk for cerebral edema | |
| ABCG2 | NC_000004.12:g.88131171G>T | rs2231142 | TG, TT | Improved outcomes (GOS scores) |
| ADK | NC_000010.11:g.74683339A>G | rs11001109 | GG | Increased seizure duration, shorter time to first seizure |
| ADORA1 | NC_000001.11:g.203163914A>G | rs3766553 | AA | Higher risk for post-traumatic seizures |
| NC_000001.11:g.203139380T>C | rs10920573 | CT | Higher risk for post-traumatic epilepsy | |
| NC_000001.11:g.203163914A>G | rs3766553 | AA | Higher risk for post-traumatic epilepsy | |
| ANKK1 | NC_000011.10:g.113400106G>A | rs1800497 | GA | Higher composite cognitive score |
| APOE | Epsilon 4 | ‡ | ε4/ε4 | Higher risk for post-traumatic seizures |
| AQP4 | NC_000018.10:g.26855854C>T | rs3763043 | TT | Unfavorable 6-month outcomes (GOS score) |
| NC_000018.10:g.26865469T>C | rs3875089 | CT, CC | Poor 6-month outcomes (GOS score) | |
| COMT | NC_000022.11:g.19963748G>A | rs4680 | GA, GG | Higher risk for cognitive decline |
| DRD2 | NC_000011.10:g.113410351G>C | rs6279 | GG, GC | Higher composite cognitive score |
| GAD1 | NC_000002.12:g.170826230C>T | rs3828275 | TT | Higher risk for post-traumatic seizures |
| NC_000002.12:g.170852920A>G | rs769391 | AA | Higher risk for post-traumatic epilepsy | |
| NC_000002.12:g.170815681G>T | rs3791878 | GG | Higher risk for post-traumatic epilepsy | |
| IL-1β | NC_000002.12:g.112832813G>A | rs1143634 | AG | Higher risk for post-traumatic epilepsy |
| MTHFR | NC_000001.11:g.11796321G>A | rs18001133 | TC, TT | Higher risk for post-traumatic epilepsy |
| NT5E | NC_000006.12:g.85465856G>A | rs9444348 | GA | Increased seizure duration, shorter time to first seizure |
| SLC1A1 | NC_000009.12:g.4557296C>G | rs10974620 | GG | Higher risk for post-traumatic epilepsy |
| SLC6A4 | 5-HTTLPR | ‡ | Long/long | Higher risk for post-traumatic depression |
| VMAT2 | NC_000010.11:g.117265701G>C | rs363226 | GC, GG | Higher risk for cognitive decline |
†Conflicting studies.
‡No assigned rsID or multiple IDs associated with haplotype.
GOS: Glasgow outcome scale.
Post-traumatic depression
Post-traumatic depression occurs in up to 50% of individuals in the first year after TBI [59]. Selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants are frequently prescribed antidepressants, yet require up to 6 weeks to have an effect and have a poor response rate (the first choice only works in 50% of individuals) [60]. This necessitates a trial-and-error approach to antidepressant selection, which can result in patients waiting several months before finding an optimal therapy. Understanding if and how genetics moderates premorbid psychiatric disease relationships to PTD risk is also an important consideration. The 5-HTTLPR variation is a tandem repeat in the promoter region for SLC6A4, which is defined as either long (L) or short (S). L-homozygotes for this variation were found by Failla et al. to be at nearly a threefold higher risk for PTD, which may help to select patients who should be treated with antidepressants (e.g., SSRIs) [61].
Genetic predictors of risk for depression may be augmented by published pharmacogenomics guidelines for selection and dosing of SSRIs when genetic data are available. Decreased doses of citalopram, escitalopram and sertraline are recommended in CYP2C19 poor metabolizers. Citalopram and escitalopram are not recommended in CYP2C19 ultra-rapid metabolizers. Paroxetine and fluvoxamine are metabolized by CYP2D6. Paroxetine is not recommended in ultra-rapid metabolizers or poor metabolizers at CYP2D6, and a 25–50% dose reduction for fluvoxamine is recommended in poor metabolizers at CYP2D6 [62]. Information about depression risk and drug metabolism may help clinicians appropriately monitor patients and to select appropriate pharmacotherapy early post TBI.
Post-traumatic cognitive decline
Post-traumatic cognitive decline is common in patients with severe TBI and is thought to be driven by white matter loss (i.e., progressive damage to axonal tracts) and hippocampal atrophy. While some patients’ cognitive function improves in the immediate year following TBI, many suffer from life-long progressive cognitive decline [63]. Genetic predictors may provide insight into the heterogeneity for risk and severity of cognitive decline [7]. They may also provide insight into what patients will benefit from early treatment. Pharmacotherapy for cognitive decline is not currently well defined and presents unique challenges. This is evident by a Cochrane review by Dougall et al., which concluded that there is insufficient evidence for the effectiveness of pharmacotherapy for cognitive decline post TBI, however; their inclusion only evaluated modafinil, atomoxetine, rivastigmine and an investigative monoamine modulator [64]. Additional study into other agents for cognitive decline is warranted.
Failla and colleagues evaluated the role of the DRD2 and its genomic neighbor; ANKK1 in 99 Caucasians with severe TBI [65]. They found that DRD2 rs6279 (NC_000011.10:g.113410351G>C; GG, GC) was associated with higher (improved) composite cognitive score at 6 months and ANKK1 rs1800497 (NC_000011.10:g.113400106G>A; GA) was associated with higher composite cognitive score at 6 and 12 months post TBI [65]. Wagner et al. investigated the role of WWC1 in 129 patients with severe TBI for its impact on memory. They found that WWC1 rs17070145 NC_000005.10:g.168418786C>T (CC) was associated with improved performance on episodic memory tests [66]. The rs363226 (NC_000010.11:g.117265701G>C; GC, GG) genotypes in VMAT2 were identified by Markos et al. to be associated with increased risk for cognitive decline as measured by cognitive composite T scores [67]. VMAT2 takes up monoamine neurotransmitters from the cytosol to vesicles, where they are stored for later release into the synapse [67]. The COMT rs4680 (NC_000022.11:g.19963748G>A; GA, GG) genotypes are associated with numerous cognitive impairments after TBI, including worse nonverbal cognitive performance, post-traumatic stress disorder (PTSD), worse self-reported behavior among survivors with PTD and worse executive functioning in pediatric TBI [68–71].
Beyond its utility as a predictor of outcomes, variations in dopamine pathways are associated with response to stimulants, antipsychotics and others [72]. Pharmacotherapy with methylphenidate has shown benefits for post-TBI cognitive impairment, and increased knowledge of how genetics influences various elements of cognitive performance that are amenable to improvement with a particular pharmacological intervention may guide cognitive testing, medication selection and follow-up and care post-injury [73]. This may help to answer the controversy surrounding appropriate treatment for post-traumatic cognitive decline.
Post-traumatic epilepsy
Post-traumatic epilepsy is defined by the occurrence of unprovoked seizures that occur 7 days or more after TBI. The 5-year risk for PTE is 11.5% for people with severe TBI and 1.6% for those with moderate TBI [58]. Genetic markers may predict risk for PTE and guide therapeutic decisions. Diamond et al. evaluated both IL-1β levels and associated genetic variations in the IL-1β gene with risk for PTE development [74]. They found that the IL-1β rs1143634 (NC_000002.12:g.112832813G>A; AG) genotype was associated with nearly threefold increased risk or PTE and higher cerebrospinal fluid (CSF)/serum ratios of IL-1β [74]. The MTHFR gene has been investigated for its role in epilepsy, including PTE, as MTHFR dysfunction can lead to elevated homocysteine and lowered seizure threshold [75,76]. Scher et al. investigated the role of MTHFR in a population of the armed forces with evidence of epilepsy diagnosis and history of TBI [76]. They found that MTHFR rs18001133 (NC_000001.11:g.11796321G>A; TC, TT) genotypes were associated with higher risk for PTE when they limited their population to subjects with two or more encounters for epilepsy (adjusted odds ratio: 2.55) [76]. Similar to the associations of the adenosine pathway and PTS, ADORA1 rs10920573 (NC_000001.11:g.203139380T>C; CT) and rs3766553 (NC_000001.11:g.203163914A>G; GG) are associated with increased risk for PTE [31]. Also in that pathway; ADK rs11001109 (NC_000010.11:g.74683339A>G; GG) and NT5E rs9444348 (NC_000006.12:g.85465856G>A; GA) are associated with increased seizure duration and shorter time to initial seizure in patients who develop PTE [77]. In addition to their work in PTS, Darrah et al. found that GAD1 rs769391 (NC_000002.12:g.170852920AG; AA) and rs3791878 (NC_000002.12:g.170815681GT; GG) were associated with PTE risk between 1–6 months post-TBI [34]. Ritter et al. found that SLC1A1 rs10974620 (NC_000009.12:g.4557296C>G; GG) and rs7858819 (NC_000009.12:g.4559892C>T; TT) were associated with higher risk for PTE [78].
The use of genetic markers to predict incidence of PTE may help clinicians monitor and treat patients with PTE more effectively. Importantly, prophylactic treatment with anti-epileptic drugs does not reduce the incidence of PTE [79]. Genetic variations previously unaccounted for may have contributed to failure of randomized controlled trials and may have a role in supporting future drug development. Pharmacogenomics may also have similar application to the long-term use of phenytoin in relation to CYP2C9 metabolizer status, or the decision to not use it based on presence of HLA-B*15:02 [20,21].
Pipeline: new & repurposed medications for TBI
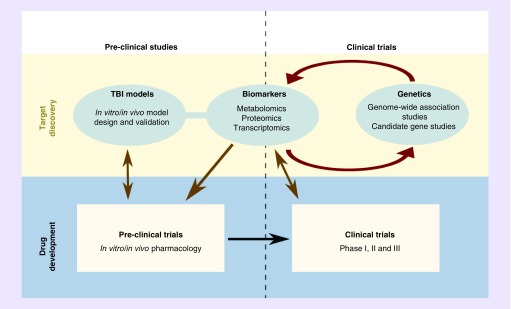
TBI is a highly active area for drug development, with many promising leads for future pharmacotherapy [2]. An exhaustive review of all areas for drug development for TBI is beyond the scope for this review, however; some proposed therapeutics has pharmacogenomic considerations with respect to their development, efficacy and appropriateness. The landscape of TBI drug development has been driven by a cycle of research translation that focuses on novel discovery and repurposing existing agents. This often begins with biomarker discovery in humans, which drives follow-up candidate gene studies in humans, mechanistic studies in animals and begins the preclinical to clinical translation of new and/or existing pharmacologic agents (Figure 1). The two leading targets in the drug development pipeline are antioxidant therapy in patients with TBI and medications targeting the ABC transporter: ABCC8 [80–83].
Figure 1. . Drug development landscape surrounding traumatic brain injury.
TBI: Traumatic brain injury.
CNS antioxidants
Oxidative stress drives the secondary injury early post-TBI, and brain antioxidant reserve is depleted after severe TBI [84,85]. CNS antioxidant use has been under investigation as a treatment strategy for TBI. N-acetylcysteine is an FDA-approved medication that is under investigation as a therapy for TBI to increase the brain concentration of glutathione (GSH), a potent CNS antioxidant [2]. Due to its hydrophilicity and poor penetrance through the BBB, it is also being investigated in combination therapy with the SLC22A6 and SLC22A8 inhibitor, probenecid. This strategy has been shown to increase brain concentrations of N-acetylcysteine in preclinical studies, and might increase its efficacy [86]. Another target in this area are the ABC transporters, ABCC1 and ABCG2. The previously mentioned association between rs4148382 and ABCG2 rs2231142 with clinical outcomes following TBI may be due to their influence on the endogenous substrates such as glutathione and uric acid [15,17]. These variations may therefore have use in predicting patients who are less likely to require additional agents supporting antioxidant reserve [87].
Sulfonylurea medications
Development of CE occurs in nearly half of patients with severe TBI, with preponderance to those with a computerized tomography (CT) evidence of mass lesion [88]. Prevention and treatment strategies include decreasing ICP through CSF drainage, hyperosmolar therapy with mannitol or hypertonic saline and/or decompressive craniectomy [23]. ABCC8 has received attention recently due to its association with TRPM4, specifically in the traumatically injured brain. TRPM4 permits the transcellular flux of Na+, which leads to ionic edema (i.e., driven by fluid high in sodium and low in protein). ABCC8 regulates TRPM4 by closing the channel when intracellular ATP is high [81]. The ABCC8 antagonist, glyburide (glibenclamide), is thought to prevent the opening of the ABCC8-TRPM4 channel post-TBI. It is under investigation as a treatment for CE post-TBI, has been successful in preclinical studies, has shown promise in clinical trials in stroke and is currently in Phase II clinical trials for prevention of CE in moderate/severe TBI (www.clinicaltrials.gov/show/NCT01454154) [2,89].
Jha et al. studied ABCC8 with a candidate gene approach for risk of developing CE post severe TBI. They found significant associations with rs2283261 (NC_000011.10:g.17440757A>C; CC), rs3819521 (NC_000011.10:g.17465190C>T; TT) and rs2283258 (NC_000011.10:g.17451890C>T; TT) in the ABCC8 gene with increased risk of CE following severe TBI (odds ratio: 2.45, 2.95 and 3.00, respectively) [90]. This finding suggests a genomic role wherein ABCC8 pharmacogenomics may help predict the occurrence of CE, and may help guide the appropriate use of glyburide in the intensive care unit. An additional contributor to risk for CE post-TBI is AQP4 [91]. Dardiotis et al. investigated the role of AQP4 with clinical outcomes following TBI and found that AQP4 rs3763043 (NC_000018.10:g.26855854C>T; TT) and rs3875089 (NC_000018.10:g.26865469T>C; C) were associated with a higher odds of having an unfavorable and favorable 6-month GOS scores, respectively, which suggests that a molecular moderator of CE risk (e.g., AQP4) impacts TBI outcomes [92]. These findings support the important role of pharmacological protection from CE in TBI patients. Glyburide, among other sulfonylurea medications, is also metabolized by the polymorphic CYP2C9 [20]. While there are no current clinical recommendations regarding dosing when used as an antidiabetic agent, glyburide is given at a subtherapeutic dose in TBI relative to its FDA-approved dosing for diabetes. Hypoglycemia is undesirable post TBI, and it is possible that CYP2C9 poor/intermediate metabolizers may be more likely to develop hypoglycemia [20,81].
Brain-specific metabolism
Cytochrome P450 enzymes (CYPs) are also present in the brain and in the cerebrovascular endothelium [93]. Little has been investigated regarding the role of genetics of brain-specific CYPs, but Donnelly et al. investigated CYP polymorphisms in patients with subarachnoid hemorrhage [94]. They found that CYP4A11 rs9332978 (NC_000001.11:g.46942278T>C; CT, CC) was associated with decreased CSF of the cerebral vasoconstrictor and 20-hydroxyeicosatetraenoic acid. Results also showed that CYP4F2 rs3093089 (NC_000019.10:g.15898482A>G; GG) was associated with decreased risk for clinical neurologic deterioration. Finally, they found that CYP4A11 rs3890011 (NC_000001.11:g.46933071G>C; GC, CC), CYP4F2 rs3093156 (NC_000019.10:g.15889799T>A; TA, TT) and CYP4F2 rs3093168 (NC_000019.10:g.15885435A>G; AA) were associated with higher odds of having a favorable outcome [94]. Further investigations are needed to delineate how brain-specific CYPs affect drug disposition in the context of TBI.
Conclusion
Pharmacotherapy for TBI is a growing area of research and active drug development. With the application of existing therapies and the discovery of novel treatment targets, the potential of precision medicine using pharmacogenomics is high [10]. Pharmacogenomics for any CNS pathology may utilize genetic variations in genes that function in the brain, on the BBB or involved in systemic clearance. Variations in CYP enzymes and ABC transporters may play a role in understanding how drug disposition changes following injury. Many of these genetic variations may help clinicians to tailor therapeutics associated with acute and/or chronic care. Genetic markers for neurotransmission may better explain pathophysiology of post-TBI complications like seizures, depression, and may help guide therapeutic choices and doses.
Future perspective
As the field of pharmacogenomics progresses, genetic studies investigating outcomes and biomarkers for specific diseases, such as TBI, will become prevalent. As with many initiatives geared to improve our understanding of neuropathologies, the integration of neuroscience with genomics will provide unique opportunities for personalized medicine in numerous disease states. These are likely to lead to new approaches to monitor patients with TBI and novel targets for treatment. Treatment for TBI does not currently involve routine genetic testing, but instead utilizes evidence and practice-based guidelines to maximize patient survival and recovery [38,95]. The addition of patient-specific factors and pharmacogenomics is an excellent opportunity to further precision medicine and decrease the heterogeneity of TBI outcomes. With the growing preponderance of evidence for the ability of genetics to augment TBI care, it is likely that clinicians will routinely use genetic data to select the best pharmacotherapy to treat and prevent TBI.
Executive summary.
Traumatic brain injury pathophysiology
Patients suffer from postimpact progressive neuronal damage after traumatic brain injury (TBI) called secondary injury. This contributes to inflammatory response, oxidative stress and excitotoxicity.
Secondary injury is an active area of drug development and genetic factors have been investigated to help identify treatment targets.
Pharmacogenomics of the blood–brain barrier
The blood–brain barrier protects the brain from undesired solutes in the blood, allows passage of nutrients and provides mechanisms to remove waste from the brain compartment.
Transporters (e.g., ABCB1) provide active mechanisms to selectively allow entry and exit of solutes from the brain.
Genetic variations in ABCB1, ABCC2 and ABCG2 are associated with clinical outcomes after TBI, possibly due to changes in xenobiotics and endogenous solutes.
Pharmacotherapy of TBI and associated pharmacogenomics
Post-traumatic seizures are seizures that occur within 7 days of TBI and phenytoin is the preferred therapy.
Phenytoin should be avoided with HLA-B*15:02 and dose adjusted for CYP2C9 intermediate/poor metabolizers.
Propofol and midazolam are preferred sedatives for TBI in adults. Ketamine, dexmedetomidine or barbiturates may be used in some patients.
Midazolam is metabolized by CYP3A4/5 and may have higher levels in patients with CYP3A5*3. Ketamine clearance is decreased in patients with CYP2B6*6/*6.
Fentanyl and morphine are used for adjunctive sedation and/or analgesia in patients with TBI, and may be impacted by variations in CYP3A5, ABCB1 and/or OPRM1.
Role of pharmacogenomics for TBI rehabilitation
Post-traumatic depression and cognitive changes following injury may be predicted by variations in monoamine pathways (e.g., COMT, VMAT2, SLC6A4, DRD2).
Selective serotonin reuptake inhibitors are commonly used for post-traumatic depression. Citalopram, escitalopram and sertraline have clinical guidance for use with CYP2C19 intermediate, poor and ultra-rapid metabolizers. Paroxetine and fluvoxamine have clinical guidance associated with CYP2D6 poor and ultra-rapid metabolizers.
Post-traumatic epilepsy refers to seizures that occur beyond 7 days post TBI.
Variations in IL-1β, MTHFR and genes in the adenosine neurotransmission pathway may predict patients at risk for post-traumatic epilepsy.
Genetic drivers of TBI treatments in the pipeline
N-Acetylcysteine is under investigation for TBI due to its ability to raise brain glutathione (GSH) levels.
ABCC1 and ABCG2 variations influence TBI outcomes, possibly by impacting the brain’s antioxidant reserve.
Glyburide is in clinical trials for cerebral edema TBI, and genetic variations in its target, ABCC8, are independently associated with cerebral edema occurrence post TBI.
Use of glyburide and other sulfonylurea medications may be impacted by CYP2C9 intermediate/poor metabolizer status.
Conclusion & future perspective
TBI is an highly active area for drug development and investigation.
Pharmacogenomics may help discover novel drug targets for TBI, predict patient risk for complications that require pharmacotherapy and aid clinicians in selecting medications for patients with TBI.
More studies are needed to fully determine the role of pharmacogenomics post-TBI.
Footnotes
Financial & competing interests disclosure
Support for this research from grants from the NIH 1TL1 TR001858–01 (SMA). SM Adams is a paid consultant for Ariel Precision Medicine. This role is unrelated to the subject matter of this article. SM Poloyac has served as a consultant for CSL Behring. PM Kochanek supported by Department of Defense grant WH81XWH-14–12–0018 NIH/NINDS NS087978. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Coronado V, Xu L, Basavaraju S, et al. Surveillance for traumatic brain injury-related deaths – United States, 1997–2007. MMWR Surveill. Summ. 2011;60(5):1–32. [PubMed] [Google Scholar]
- 2.Diaz-Arrastia R, Kochanek P, Bergold P, et al. Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma. 2014;31(2):135–158. doi: 10.1089/neu.2013.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearn M, Niesman I, Egawa J, et al. Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell. Mol. Neurobiol. 2016;37(4):571–585. doi: 10.1007/s10571-016-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 5.Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: where do we go from here? Br. J. Pharmacol. 2011;164(4):1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner A, Zitelli K. A rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology. 2013;20(1):39–48. doi: 10.1016/j.pathophys.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Bennett E, Reuter-Rice K, Laskowitz D. Genetic influences in traumatic brain injury. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. CRC Press/Taylor and Francis Group; FL, USA: 2016. [PubMed] [Google Scholar]
- 8.Orešič M, Posti J, Kamstrup-Nielsen M, et al. Human serum metabolites associate with severity and patient outcomes in traumatic brain injury. EBioMedicine. 2016;12:118–126. doi: 10.1016/j.ebiom.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson J, Cusimano M, Bendena W. Post-traumatic brain injury: genetic susceptibility to outcome. Neuroscientist. 2015;21(4):424–441. doi: 10.1177/1073858414543150. [DOI] [PubMed] [Google Scholar]; • Recent review of the relationship of genetic variation with traumatic brain injury (TBI) recovery.
- 10.Empey P. Genetic predisposition to adverse drug reactions in the intensive care unit. Crit. Care Med. 2010;38(6 Suppl.):S106–S116. doi: 10.1097/CCM.0b013e3181de09f8. [DOI] [PubMed] [Google Scholar]
- 11.den Dunnen J, Dalgleish R, Maglott D, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum. Mutat. 2016;37(6):564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 12.Gray K, Yates B, Seal R, Wright M, Bruford E. Genenames.org: the HGNC resources in 2015. Nucleic Acids Res. 2015;43(Database issue):D1079–D1085. doi: 10.1093/nar/gku1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Yee S, Chen L, Giacomini K. Pharmacogenomics of membrane transporters: past, present and future. Pharmacogenomics. 2010;11(4):475–479. doi: 10.2217/pgs.10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cousar J, Conley Y, Willyerd F, et al. Influence of ATP-binding cassette polymorphisms on neurological outcome after traumatic brain injury. Neurocrit. Care. 2013;19(2):192–198. doi: 10.1007/s12028-013-9881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Xu D, Wang Y, Qin H, Geng D. Effect of single nucleotide polymorphisms in the ATP-binding cassette B1 gene on the clinical outcome of traumatic brain injury. Genet. Mol. Res. 2015;14(3):10948–10953. doi: 10.4238/2015.September.21.6. [DOI] [PubMed] [Google Scholar]
- 17.Adams S, Conley Y, Ren D, et al. ABCG2 C.421C>A is associated with outcomes following severe traumatic brain injury. J. Neurotrauma. 2017 doi: 10.1089/neu.2017.5000. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oddo M, Crippa I, Mehta S, et al. Optimizing sedation in patients with acute brain injury. Crit. Care. 2016;20(1):128. doi: 10.1186/s13054-016-1294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Comprehensive description of sedation in patients with TBI, with comparisons of different pharmacotherapy with respect to patient characteristics.
- 19.MacKenzie M, Hall R. Pharmacogenomics and pharmacogenetics for the intensive care unit: a narrative review. Can. J. Anesth. 2016;17:1–20. doi: 10.1007/s12630-016-0748-1. [DOI] [PubMed] [Google Scholar]
- 20.Swen J, Nijenhuis M, Boer de A, et al. Pharmacogenetics: from bench to byte – an update of guidelines. Clin. Pharmacol. Ther. 2011;89(5):662–673. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 21.Caudle K, Rettie A, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin. Pharmacol. Ther. 2014;96(5):542–548. doi: 10.1038/clpt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vespa P, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit. Care Med. 2007;35(12):2830–2836. [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy G, Gopinath S, Robertson C. Critical care management of the patient with traumatic brain injury. Semin. Neurol. 2016;36(6):570–576. doi: 10.1055/s-0036-1592169. [DOI] [PubMed] [Google Scholar]
- 24.Temkin N, Dikmen S, Wilensky A, Keihm J, Chabal S, Winn H. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N. Engl. J. Med. 1990;323(8):497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- 25.Khan N, Vanlandingham M, Fierst T, et al. Should levetiracetam or phenytoin be used for posttraumatic seizure prophylaxis? A systematic review of the literature and meta-analysis. Neurosurgery. 2016;79(6):775–781. doi: 10.1227/NEU.0000000000001445. [DOI] [PubMed] [Google Scholar]
- 26.Zou H, Brayer S, Hurwitz M, Niyonkuru C, Fowler L, Wagner A. Neuroprotective, neuroplastic, and neurobehavioral effects of daily treatment with levetiracetam in experimental traumatic brain injury. Neurorehabil. Neural Repair. 2013;27(9):878–888. doi: 10.1177/1545968313491007. [DOI] [PubMed] [Google Scholar]
- 27.Zou H, Hurwitz M, Fowler L, Wagner A. Abbreviated levetiracetam treatment effects on behavioural and histological outcomes after experimental TBI. Brain Inj. 2015;29(1):78–85. doi: 10.3109/02699052.2014.955528. [DOI] [PubMed] [Google Scholar]
- 28.Siddiqui A, Kerb R, Weale M, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1 . N. Engl. J. Med. 2003;348(15):1442–1448. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- 29.Allabi A, Gala J, Horsmans Y. CYP2C9, CYP2C19, ABCB1 (MDR1) genetic polymorphisms and phenytoin metabolism in a Black Beninese population. Pharmacogenet. Genomics. 2005;15(11):779–786. doi: 10.1097/01.fpc.0000174787.92861.91. [DOI] [PubMed] [Google Scholar]
- 30.Tate S, Depondt C, Sisodiya S, et al. Genetic predictors of the maximum doses patients receive during clinical use of the anti-epileptic drugs carbamazepine and phenytoin. Proc. Natl Acad. Sci. USA. 2005;102(15):5507–5512. doi: 10.1073/pnas.0407346102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner A, Miller M, Scanlon J, Ren D, Kochanek P, Conley Y. Adenosine A1 receptor gene variants associated with post-traumatic seizures after severe TBI. Gene. 2011;90(3):259–272. doi: 10.1016/j.eplepsyres.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochanek P, Vagni V, Janesko K, et al. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2006;26(4):565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- 33.Haselkorn M, Shellington D, Jackson E, et al. Adenosine A1 receptor activation as a brake on the microglial response after experimental traumatic brain injury in mice. J. Neurotrauma. 2010;27(5):901–910. doi: 10.1089/neu.2009.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darrah S, Miller M, Ren D, et al. Genetic variability in glutamic acid decarboxylase genes: associations with post-traumatic seizures after severe TBI. Epilepsy Res. 2013;103(2–3):180–194. doi: 10.1016/j.eplepsyres.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson G, Temkin N, Dikmen S, et al. Haptoglobin phenotype and apolipoprotein E polymorphism: relationship to posttraumatic seizures and neuropsychological functioning after traumatic brain injury. Epilepsy Behav. 2009;16(3):501–506. doi: 10.1016/j.yebeh.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller M, Conley Y, Scanlon J, et al. APOE genetic associations with seizure development after severe traumatic brain injury. Brain Inj. 2010;24(12):1468–1477. doi: 10.3109/02699052.2010.520299. [DOI] [PubMed] [Google Scholar]
- 37.Lawrence D, Comper P, Hutchison M, Sharma B. The role of apolipoprotein E episilon (ϵ)-4 allele on outcome following traumatic brain injury: a systematic review. Brain Inj. 2015;29(9):1018–1031. doi: 10.3109/02699052.2015.1005131. [DOI] [PubMed] [Google Scholar]
- 38.Carney N, Totten A, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2016;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 39.Bratton S, Chestnut R, Ghajar J, et al. Guidelines for the Management of Severe Traumatic Brain Injury. XI. Anesthetics, analgesics, and sedatives. J. Neurotrauma. 2007;24(Suppl. 1):S71–S76. doi: 10.1089/neu.2007.9985. [DOI] [PubMed] [Google Scholar]
- 40.Yu K, Cho J, Jang I, et al. Effect of the CYP3A5 genotype on the pharmacokinetics of intravenous midazolam during inhibited and induced metabolic states. Clin. Pharmacol. Ther. 2004;76(2):104–112. doi: 10.1016/j.clpt.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Khojasteh S, Prabhu S, Kenny J, Halladay J, Lu A. Chemical inhibitors of cytochrome P450 isoforms in human liver microsomes: a re-evaluation of P450 isoform selectivity. Eur. J. Drug Metab. Pharmacokinet. 2011;36(1):1–16. doi: 10.1007/s13318-011-0024-2. [DOI] [PubMed] [Google Scholar]
- 42.Skrobik Y, Leger C, Cossette M, Michaud V, Turgeon J. Factors predisposing to coma and delirium: fentanyl and midazolam exposure; CYP3A5, ABCB1, and ABCG2 genetic polymorphisms; and inflammatory factors. Crit. Care Med. 2013;41(4):999–1008. doi: 10.1097/CCM.0b013e318275d014. [DOI] [PubMed] [Google Scholar]
- 43.Bar-Joseph G, Guilburd Y, Tamir A, Guilburd J. Effectiveness of ketamine in decreasing intracranial pressure in children with intracranial hypertension. J. Neurosurg. Pediatr. 2009;4(1):40–46. doi: 10.3171/2009.1.PEDS08319. [DOI] [PubMed] [Google Scholar]; • Refute of the notion that ketamine raises intracranial pressure in TBI patients, opening the way to more research into the potential benefits of ketamine post TBI.
- 44.Hijazi Y, Boulieu R. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab. Dispos. 2002;30(7):853–858. doi: 10.1124/dmd.30.7.853. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Jackson K, Slon B, et al. CYP2B6*6 allele and age substantially reduce steady-state ketamine clearance in chronic pain patients: impact on adverse effects. Br. J. Clin. Pharmacol. 2015;80(2):276–284. doi: 10.1111/bcp.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peltoniemi M, Hagelberg N, Olkkola K, Saari T. Ketamine: a review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin. Pharmacokinet. 2016;55(9):1059–1077. doi: 10.1007/s40262-016-0383-6. [DOI] [PubMed] [Google Scholar]
- 47.Holliday SF, Kane-Gill SL, Empey PE, Buckley MS, Smithburger PL. Interpatient variability in dexmedetomidine response: a survey of the literature. Sci. World J. 2014;2014:805013. doi: 10.1155/2014/805013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yağar S, Yavaş S, Karahalil B. The role of the ADRA2A C1291G genetic polymorphism in response to dexmedetomidine on patients undergoing coronary artery surgery. Mol. Biol. Rep. 2011;38(5):3383–3389. doi: 10.1007/s11033-010-0446-y. [DOI] [PubMed] [Google Scholar]
- 49.Lötsch J, von Hentig N, Freynhagen R, et al. Cross-sectional analysis of the influence of currently known pharmacogenetic modulators on opioid therapy in outpatient pain centers. Pharmacogenet. Genomics. 2009;19:429–436. doi: 10.1097/fpc.0b013e32832b89da. [DOI] [PubMed] [Google Scholar]
- 50.Ting S, Schug S. The pharmacogenomics of pain management: prospects for personalized medicine. J. Pain Res. 2016;9:49–56. doi: 10.2147/JPR.S55595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takashina Y, Naito T, Mino Y, Yagi T, Ohnishi K, Kawakami J. Impact of CYP3A5 and ABCB1 gene polymorphisms on fentanyl pharmacokinetics and clinical responses in cancer patients undergoing conversion to a transdermal system. Drug Metab. Pharmacokinet. 2012;27(4):414–421. doi: 10.2133/dmpk.dmpk-11-rg-134. [DOI] [PubMed] [Google Scholar]
- 52.Horvat C, Au A, Conley Y, et al. ABCB1 genotype is associated with fentanyl requirements in critically ill children. Pediatr. Res. 2017;82(1):29–35. doi: 10.1038/pr.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walter C, Doehring A, Oertel B, Lötsch J. μ-opioid receptor gene variant OPRM1 118 A>G: a summary of its molecular and clinical consequences for pain. Pharmacogenomics. 2013;14(15):1915–1925. doi: 10.2217/pgs.13.187. [DOI] [PubMed] [Google Scholar]
- 54.Wagner A. TBI translational rehabilitation research in the 21st century: exploring a rehabilomics research model. Eur. J. Phys. Rehabil. Med. 2010;46(4):549–555. [PubMed] [Google Scholar]; • Introduction of the concept of rehabilomics as it relates to TBI.
- 55.Wagner AK, Sowa G. Rehabilomics research: a model for translational rehabilitation and comparative effectiveness rehabilitation research. Am. J. Phys. Med. Rehabil. 2014;93(10):913–916. doi: 10.1097/PHM.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 56.Myrga J, Failla M, Ricker J, et al. A dopamine pathway gene risk score for cognitive recovery following traumatic brain injury: methodological considerations, preliminary findings, and interactions with sex. J. Head Trauma Rehabil. 2016;31(5):E15–E29. doi: 10.1097/HTR.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 57.Zaninotto A, Vicentini J, Fregni F, et al. Updates and current perspectives of psychiatric assessments after traumatic brain injury: a systematic review. Front. Psychiatry. 2016;7:95. doi: 10.3389/fpsyt.2016.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Szaflarski J, Nazzal Y, Dreer L. Post-traumatic epilepsy: current and emerging treatment options. Neuropsychiatr. Dis. Treat. 2014;10:1469–1477. doi: 10.2147/NDT.S50421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bombardier C, Fann J, Temkin N, Esselman P, Barber J, Dikmen S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA. 2010;303(19):1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinyor M, Schaffer A, Levitt A. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial: a review. Can. J. Psychiatry. 2010;55(3):126–135. doi: 10.1177/070674371005500303. [DOI] [PubMed] [Google Scholar]
- 61.Failla M, Burkhardt J, Miller M, et al. Variants of SLC6A4 in depression risk following severe TBI. Brain Inj. 2013;27(6):696–706. doi: 10.3109/02699052.2013.775481. [DOI] [PubMed] [Google Scholar]
- 62.Hicks J, Bishop J, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin. Pharmacol. Ther. 2015;98(2):127–134. doi: 10.1002/cpt.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker K, Tesco G. Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. Aging Neurosci. 2013;5 doi: 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dougall D, Poole N, Agrawal N. Pharmacotherapy for chronic cognitive impairment in traumatic brain injury. Cochrane Database Syst. Rev. 2015;(12) doi: 10.1002/14651858.CD009221.pub2. CD009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Failla M, Myrga J, Ricker J, Dixon C, Conley Y, Wagner A. Posttraumatic brain injury cognitive performance is moderated by variation within ANKK1 and DRD2 genes. J. Head Trauma Rehabil. 2015;30(6):E54–E66. doi: 10.1097/HTR.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wagner A, Hatz L, Scanlon J, et al. Association of KIBRA rs17070145 polymorphism and episodic memory in individuals with severe TBI. Brain Inj. 2012;26(13–14):1658–1669. doi: 10.3109/02699052.2012.700089. [DOI] [PubMed] [Google Scholar]
- 67.Markos S, Failla M, Ritter A, et al. Genetic variation in the vesicular monoamine transporter. J. Head Trauma Rehabil. 2017;32(2):E24–E34. doi: 10.1097/HTR.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kurowski B, Backeljauw B, Zang H, et al. Influence of catechol-O-methyltransferase on executive functioning longitudinally after early childhood traumatic brain injury: preliminary findings. J. Head Trauma Rehabil. 2016;31(3):E1–E9. doi: 10.1097/HTR.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myrga J, Juengst S, Failla M, et al. COMT and ANKK1 genetics interact with depression to influence behavior following severe TBI: an initial assessment. Neurorehabil. Neural Repair. 2016;30(10):920–930. doi: 10.1177/1545968316648409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winkler E, Yue J, McAllister T, et al. COMT Val 158 Met polymorphism is associated with nonverbal cognition following mild traumatic brain injury. Neurogenetics. 2016;17(1):31–41. doi: 10.1007/s10048-015-0467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winkler E, Yue J, Ferguson A, et al. COMT Val(158)Met polymorphism is associated with post-traumatic stress disorder and functional outcome following mild traumatic brain injury. J. Clin. Neurosci. 2017;35:109–116. doi: 10.1016/j.jocn.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mi H, Thomas P, Ring H, et al. PharmGKB summary: dopamine receptor D2. Pharmacogenet. Genomics. 2011;21(6):350–356. doi: 10.1097/FPC.0b013e32833ee605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McDonald B, Flashman L, Arciniegas D, et al. Methylphenidate and memory and attention adaptation training for persistent cognitive symptoms after traumatic brain injury: a randomized, placebo-controlled trial. Neuropsychopharmacology. 2017:1766–1775. doi: 10.1038/npp.2016.261. August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diamond M, Ritter A, Failla M, et al. IL-1beta associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia. 2015;56(7):991–1001. doi: 10.1111/epi.13100. [DOI] [PubMed] [Google Scholar]
- 75.Diaz-Arrastia R. Homocysteine and neurologic disease. Arch. Neurol. 2000;57(10):1422–1427. doi: 10.1001/archneur.57.10.1422. [DOI] [PubMed] [Google Scholar]
- 76.Scher A, Wu H, Tsao J, et al. MTHFR C677T genotype as a risk factor for epilepsy including post-traumatic epilepsy in a representative military cohort. J. Neurotrauma. 2011;28(9):1739–1745. doi: 10.1089/neu.2011.1982. [DOI] [PubMed] [Google Scholar]
- 77.Diamond M, Ritter A, Jackson E, et al. Genetic variation in the adenosine regulatory cycle is associated with posttraumatic epilepsy development. Epilepsia. 2015;56(8):1198–1206. doi: 10.1111/epi.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ritter A, Kammerer C, Brooks M, Conley Y, Wagner A. Genetic variation in neuronal glutamate transport genes and associations with posttraumatic seizure. Epilepsia. 2016;57(6):984–993. doi: 10.1111/epi.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thompson K, Pohlmann-Eden B, Campbell L, Abel H. Pharmacological treatments for preventing epilepsy following traumatic head injury. Cochrane Database Syst. Rev. 2015;(8) doi: 10.1002/14651858.CD009900.pub2. CD009900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simard J, Kilbourne M, Tsymbalyuk O, et al. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma. 2009;26(12):2257–2267. doi: 10.1089/neu.2009.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Initial discovery of the role for ABCC8 in the brain post-TBI, with initial studies into the potential use of sulfonylurea medications for TBI.
- 81.Simard J, Kahle K, Gerzanich V. Molecular mechanisms of microvascular failure in central nervous system injury–synergistic roles of NKCC1 and SUR1/TRPM4. J. Neurosurg. 2010;113(3):622–629. doi: 10.3171/2009.11.JNS081052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mendes Arent A, de Souza L, Walz R, Dafre A. Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. Biomed. Res. Int. 2014;2014:1–18. doi: 10.1155/2014/723060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martínez-Valverde T, Vidal-Jorge M, Martínez-Saez E, et al. Sulfonylurea receptor 1 in humans with post-traumatic brain contusions. J. Neurotrauma. 2015;32(19):1478–1487. doi: 10.1089/neu.2014.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bayir H, Kagan V, Tyurina Y, et al. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr. Res. 2002;51(5):571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 85.Cornelius C, Crupi R, Calabrese V, et al. Traumatic brain injury: oxidative stress and neuroprotection. Antioxid. Redox Signal. 2013;19(8):836–853. doi: 10.1089/ars.2012.4981. [DOI] [PubMed] [Google Scholar]
- 86.Hagos F, Daood M, Ocque J, et al. Probenecid, an organic anion transporter 1 and 3 inhibitor, increases plasma and brain exposure of N -acetylcysteine. Xenobiotica. 2017;47(4):346–353. doi: 10.1080/00498254.2016.1187777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chamorro Á, Amaro S, Castellanos M, et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind Phase 2b/3 trial. Lancet Neurol. 2014;13(5):453–460. doi: 10.1016/S1474-4422(14)70054-7. [DOI] [PubMed] [Google Scholar]
- 88.Narayan R, Kishore P, Becker D, et al. Intracranial pressure: to monitor or not to monitor? A review of our experience with severe head injury. J. Neurosurg. 1982;56(5):650–659. doi: 10.3171/jns.1982.56.5.0650. [DOI] [PubMed] [Google Scholar]
- 89.Sheth K, Elm J, Molyneaux B, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled Phase 2 trial. Lancet Neurol. 2016;15(11):1160–1169. doi: 10.1016/S1474-4422(16)30196-X. [DOI] [PubMed] [Google Scholar]
- 90.Jha R, Puccio A, Okonkwo D, et al. ABCC8 single nucleotide polymorphisms are associated with cerebral edema in severe TBI. Neurocrit. Care. 2017;26(2):213–224. doi: 10.1007/s12028-016-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang G, Yang G. Aquaporin-4: a potential therapeutic target for cerebral edema. Int. J. Mol. Sci. 2016;17(10):1–11. doi: 10.3390/ijms17101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dardiotis E, Paterakis K, Tsivgoulis G, et al. AQP4 tag single nucleotide polymorphisms in patients with traumatic brain injury. J. Neurotrauma. 2014;31(23):1920–1926. doi: 10.1089/neu.2014.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agúndez J, Jiménez-Jiménez F, Alonso-Navarro H, García-Martín E. Drug and xenobiotic biotransformation in the blood–brain barrier: a neglected issue. Front. Cell. Neurosci. 2014;8:335. doi: 10.3389/fncel.2014.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Donnelly M, Crago E, Conley Y, et al. 20-HETE is associated with unfavorable outcomes in subarachnoid hemorrhage patients. J. Cereb. Blood Flow Metab. 2015;35(9):1515–1522. doi: 10.1038/jcbfm.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kochanek P, Carney N, Adelson P, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents – second edition. Pediatr. Crit. Care Med. 2012;13(Suppl. 1):S1–S82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]