Abstract
Breast cancer is composed of several well-recognized subtypes including estrogen receptor, progesterone receptor and HER2 triple-negative breast cancer (TNBC). Without available targeted therapy options, standard of care for TNBC remains chemotherapy. It is of interest to note that TNBC tumors generally have better responses to chemotherapy compared with other subtypes. However, patients without complete response account for approximately 80% of TNBC. Mounting evidence suggests significant heterogeneity within the TNBC subtype, and studies have focused on genetic targets with high rates of altered expression. Recent studies suggest clear possibilities for benefits from targeted therapy in TNBC. In this review, we summarize studies of targeted therapy, including within mouse models, and discuss their applications in the development of combinatorial treatments to treat TNBC.
Keywords: : chemotherapy, clinical trials, combinatorial treatments, mouse models, omics, targeted therapy, triple-negative breast cancer
The lifetime breast cancer incidence in women is one in eight and it is estimated that 30% of the newly diagnosed women's cancer cases will be breast cancer in 2017. Consistent with mortality trends in all cancers, overall survival rate of breast cancer has increased over the past decade. However, approximately 24% of breast cancer patients are first diagnosed while under 40 years of age and breast cancer is the leading cause of cancer mortality in this age group. In addition, cancer diagnosis prior to 40 is commonly made in African–Americans and BRCA1/2 mutation carriers [1].
Breast cancer heterogeneity is well known and is commonly observed using routine markers. Clinically, breast cancer is routinely categorized into four subtypes based on the presence of estrogen receptor (ER), progesterone receptor (PR), HER2 and ki67 [2]. With the addition of transcriptomics, many of these ER- and PR-positive tumors have been classified into the commonly observed luminal A and luminal B subtypes [3–5]. Tumors with HER2 amplification and/or overexpression are classified into the HER2-positive subtype. Triple-negative breast cancer (TNBC) represents tumors without the expression of ER, PR and HER2 and largely overlaps with the basal subtype. Although TNBC only represents 15–20% of breast cancer, distant recurrence and mortality in the TNBC is significantly worse than the other subtypes [6]. Considering the malignancy of TNBC and the death rate of metastatic breast cancer, further studies are required to improve the outcome of this subtype of breast cancer.
Molecular subtypes of breast cancer
With the advent of gene expression microarray technology, seminal manuscripts initially stratified breast cancer into five intrinsic subtypes [3]. These subtypes, including luminal A, luminal B, HER2, basal-like (BL) and normal-like, are differentiated based on gene expression. The BL tumors have high expression of keratin 5/6 and 17; in contrast, the luminal subtype expresses luminal keratin 8/18 [5]. Linked back to clinical markers, 79% of TNBC is defined as BL subtype [7]. With increased samples, inclusion of additional genes and a comparison to mouse models, Perou and colleagues uncovered an additional subtype called claudin-low [8]. Claudin-low tumors are histologically triple negative with increased expression of those genes involved in epithelial-to-mesenchymal transition [9]. Although these six subtypes have been useful, there is additional complexity within each subtype. Indeed, even within subtypes there is variation in survival as shown when the basal group was subdivided to three unique pathway expression patterns [10]. Lehmann et al. categorized TNBC tumors into six subtypes on the basis of the gene expression and gene ontology [11]. The representative gene ontologies in BL1 and BL2 subtypes include cell cycle, EGF and MET pathways. The immunomodulatory subtype was enriched for multiple immune signaling pathways. The mesenchymal-like (M) and mesenchymal stem like (MSL) subtypes have high expression of genes associated with cell differentiation. The luminal androgen receptor (LAR) subtype was marked with increased androgen expression and enriched androgen and estrogen metabolism. Recently, Burstein et al. split TNBC patients into four subtypes, LAR, mesenchymal, BL immunosuppressed and BL immune-activated based on mRNA expression and various clustering methods [12]. In an alternative approach using immune metagene information, TNBC tumors were clustered into three subtypes, including LAR, BL with a low-immune response and BL with high-immune response [13]. Given these various classifications based on gene expression, genomic events regulating development of basal breast cancer have been examined, resulting in the identification of deletion of chromosome 5q in many basal tumors [14].
In addition to the deletion event, other heterogeneous characteristics of breast cancer have been noted at the epigenetic and protein expression level [15]. Importantly, these gene expression differences among the subtypes of breast cancer are strongly correlated with the therapeutic responses.
Molecular characteristics in current treatment & identification of targets
In the past, decisions regarding therapeutic options for patients with different subtypes of breast cancer were based on the histological properties. For instance, hormone therapy is the standard of care for ER- and/or PR-positive breast cancer patients, and HER2-targeted therapy is standard for HER2-positive tumors. Due to the lack of those three receptors in TNBC, chemotherapy is still the primary option for these patients. Although TNBC is the subtype with the most complete response to chemotherapy (22%), the recurrence and metastasis rate of TNBC patients was also higher than non-TNBC tumors [16]. The urgent need to improve the therapeutic outcomes of TNBC patients is the focus of numerous studies on the association of molecular characteristics with this subtype, therapeutic responses and identification of new targets.
Molecular characteristics in response to chemotherapy
In addition to histological subtypes directing therapeutic options, a number of studies have worked on linking the correlation between molecular characteristics of subtypes of breast cancer and their therapeutic outcomes. For example, a recent report outlined that BL and HER2+ve patients have a better response to taxane and anthracycline treatment. Interestingly, the gene expression patterns associated with therapeutic responses in these two populations were distinct [17]. Among the basal patients, half of BL1 patients had pathological complete responses while BL2 patients had the least treatment benefits from chemotherapy [18]. These studies clearly demonstrate the impact of molecular and genomic heterogeneity in patients receiving the same treatment.
Molecular characteristics of TNBC & potential targets
To explore the potential targets for TNBC treatment, the genomic impacts inherent within TNBC patients’ response to treatment have been examined. Silver et al. demonstrated that alteration of BRCA1 expression due to promoter methylation and p53 mutations was correlated with good response to cisplatin treatment [19]. Likewise, high expression of CD73 was related to resistance to doxorubicin in TNBC patients [20]. Another study focusing on the genomic spectrum of BL tumors showed that the genomic alterations in BL tumors include mutations of p53 and PIK3CA, loss of PTEN and RB1, amplification of cyclin E1 and increased expression of Myc and HIF1-α [15]. In addition to the primary tumor, Balko et al. analyzed the residual breast cancer after neoadjuvant chemotherapy, and identified additional amplification of several genes (such as CDK4, MCL1, JAK2, AKT1 and EGFR) and loss of mutations in BRCA1/2, ATM and CDKN2A [21]. These findings provide additional information that may well impact development of targeted therapies for TNBC.
Based on the gene expression studies of TNBC and the common characteristics of cancers, several potential targets have been identified in studies from in vitro, in vivo and clinical trials. A representative summary of current trials of targeted therapy, the combination of different target therapeutic reagents and the combination of target therapy and chemotherapy is listed in Table 1. Here we describe these targets and treatments based on their known functions, including DNA repair and damage, growth and angiogenesis, survival and proliferation for the PI3K pathway and others (Table 2 & Figure 1).
Table 1. . Ongoing clinical trials in triple-negative breast cancer.
| Target | Drug | Phase | NCT identifiers | Status |
|---|---|---|---|---|
| BRCA1/2 | ||||
| PARP | Veliparib | Phase I | NCT00892736 | Active, not recruiting |
| Veliparib + chemotherapy | Phase I, II or III | NCT02595905; NCT01251874; NCT01145430; NCT00576654; NCT01818063; NCT02032277 | Recruiting or active, not recruiting | |
| Veliparib + lapatinib (EGFR/HER2i) | Pilot study | NCT02158507 | Recruiting | |
| BGB290 + temozolomide | Phase I – Phase II | NCT03150810 | Recruiting | |
| BMN-673 (talazoparib) | Phase II | NCT02401347 | Recruiting | |
| BMN-673 + carboplatin + paclitaxel | Phase I | NCT02358200 | Active, not recruiting | |
| Rucaparib + cisplatin | Phase II | NCT01074970 | Active, not recruiting | |
| Olaparib | Phase II | NCT02681562; NCT00679783 | Active, not recruiting | |
| Olaparib ± carboplatin ± paclitaxel | Phase I | NCT00516724; NCT01445418; NCT00707707 | Active, not recruiting | |
| Phase II; Phase II – Phase III | NCT02789332; NCT03150576 | Recruiting | ||
| Olaparib ± AZD2014 (mTORi) ± AZD5363 (AKTi) | Phase I – Phase II | NCT02208375 | Recruiting | |
| Olaparib + onalespib (HSP90i) | Phase I | NCT02898207 | Recruiting | |
| Olaparib ± BKM120 (PI3Ki) ± BYL719 (PI3Ki) | Phase I | NCT01623349 | Active, not recruiting | |
| Olaparib + cediranib (VGEFi) | Phase I – Phase II | NCT01116648 | Active, not recruiting | |
| Phase II | NCT02498613 | Recruiting | ||
| Cyclin-dependent kinases | ||||
| CDKs | Dinaciclib + epirubicin | Phase I | NCT01624441 | Active, not recruiting |
| Abemaciclib | Phase II | NCT03130439 | Recruiting | |
| Trilaciclib | Phase II | NCT02978716 | Recruiting | |
| Ribociclib + bicalutamide (ARi) | Phase I – Phase II | NCT03090165 | Recruiting | |
| CHK1 and WEE1 | ||||
| CHK1 | LY2880070 + gemcitabine | Phase I – Phase II | NCT02632448 | Recruiting |
| CHK1/2 | LY2606368 | Phase II | NCT02203513 | Recruiting |
| Wee1 | AZD1775 + cisplatin | Phase II | NCT03012477 | Recruiting |
| AZD1775 | Phase I | NCT02482311 | Active, not recruiting | |
| Growth factors and angiogenesis | ||||
| EGFR | Aftatinib + paclitaxel | Phase II | NCT02511847 | Recruiting |
| Cetuximab + ixabepilone | Phase II | NCT01097642 | Active, not recruiting | |
| Erlotinib + chemotherapy | Phase II | NCT00491816 | Active, not recruiting | |
| Icotinib | Phase II | NCT02362230 | Recruiting | |
| Panitumumab ± cisplatin ± paclitaxel ± carboplatin | Phase II | NCT02546934; NCT02593175; NCT02876107 | Recruiting | |
| HER2 | Trastuzumab + paclitaxel + cyclophosphamide | Phase II | NCT01750073 | Recruiting |
| VEGF | Bevacizumab + paclitaxel | Phase II | NCT01898117 | Recruiting |
| Bevacizumab + paclitaxel + erlotinib (EGFRi) | Phase II | NCT00733408 | Active, not recruiting | |
| VEGFR2 | Apatinib + vinorelbine | Phase II | NCT03254654 | Recruiting |
| Apatinib + fluzoparib (PARPi) | Phase I | NCT03075462 | Recruiting | |
| VEGFR/FGFR | Lucitanib | Phase II | NCT02202746 | Active, not recruiting |
| Androgen receptor | ||||
| Androgen | Enzalutamide | Phase II | NCT02750358 | Recruting |
| NCT01889238 | Active, not recruiting | |||
| Enzalutamide + paclitaxel | Phase IIb | NCT02689427 | Recruting | |
| Bicalutamide | Phase III | NCT03055312 | Recruting | |
| GTx-024 | Phase II | NCT02368691 | Active, not recruiting | |
| CYP17 | VT-464 | Phase II | NCT02130700 | Recruiting |
| PI3K/AKT/mTOR pathway | ||||
| PIK3CA | Taselisib + enzalutamide (ARi) | Phase I – Phase II | NCT02457910 | Recruiting |
| PI3K | BKM120 | Phase II | NCT01790932 | Active, not recruiting |
| BKM120 + MEK162 (MEKi) | Phase I | NCT01363232 | Active, not recruiting | |
| BKM120 + capecitabine + trastuzumab (HER2i) | Phase II | NCT02000882 | Recruiting | |
| AZD8186 | Phase I | NCT01884285 | Recruiting | |
| Gedatolisib + docetaxel + cisplatin + dacomitinib (EGFRi) | Phase I | NCT01920061 | Recruiting | |
| PI3K/mTOR | PQR309 + eribulin | Phase I – Phase II | NCT02723877 | Recruiting |
| Tak-228 (mTORi) + Tak-117 (PI3Ki) + cisplatin + paclitaxel | Phase II | NCT03193853 | Recruiting | |
| Akt | Ipatasertib | Phase II | NCT02162719; NCT02301988 | Active, not recruiting |
| ARQ092 + carboplatin + paclitaxel | Phase Ib | NCT02476955 | Recruiting | |
| GSK2141795 + trametinib (MEKi) | Phase II | NCT01964924 | Active, not recruiting | |
| mTOR | Everolimus | Phase II | NCT01931163 | Recruiting |
| Everolimus + eribulin | Phase I | NCT02616848 | Recruiting | |
| Everolimus + cisplatin + paclitaxel | Phase I – Phase II | NCT01031446 | Active, not recruiting | |
| Phase I | NCT02120469 | Recruiting | ||
| Everolimus + doxorubicin + bevacizumab (VEGFi) | Phase II | NCT02456857 | Recruiting | |
| Everolimus + cisplatin + paclitaxel | Phase II | NCT02531932 | Recruiting | |
| Everolimus + 5-fluorouracil + epirubicin + cyclophosphamide | Phase II | NCT00499603 | Active, not recruiting | |
| Src and WNT signaling | ||||
| Src | Dasatinib | Phase II | NCT02720185 | Recruiting |
| WNT | PTK7-ADC (WNTi) + gedatolisib (PI3Ki) | Phase I | NCT03243331 | Not yet recruiting |
The representative ongoing clinical trials of targeted therapy, including recruiting and active, not recruiting ones, are summarized based on the data from ClinicalTrials.gov as of 24 August 2017. The trials of evaluating the effect of the combination of targeted therapy and immunotherapy are excluded. The letter i represents inhibitor.
Table 2. . Examples of potential targets and drugs for triple-negative breast cancer.
| Targets | Drugs | Mechanisms/actions | Ref. |
|---|---|---|---|
| Androgen receptor | Bicalutamide | AR antagonist | [11,46] |
| Enzalutamide | AR antagonist | [45] | |
| 17-DMAG | HSP90 inhibitor; regulate the stabiltiy of AR | [44] | |
| VT-464 | CYP17 lyase; involved in the synthesis of androgen | [44] | |
| BRCA1/2 | Olaparib | Targeting PARP in BRCA1/2 mutant cells | [24] |
| CDKs | Dinaciclib | Pan-CDK inhibitor; G1/S and G2/M arrest; synthetic lethal in Myc-dependent tumor cells | [28] |
| Purvalanol A | CDK1 inhibitor; G2/M arrest; synthetic lethal in Myc-dependent tumor cells | [28] | |
| RO-3306 | CDK1 inhibitor; G2/M arrest; synthetic lethal in Myc-dependent tumor cells | [29] | |
| CGP74514A | CDK1 inhibitor; G2/M arrest; synthetic lethal in Myc-dependent tumor cells | [29] | |
| Palbociclib (PD0332991) | CDK4/6 inhibitor; G1/S arrest | [29,30] | |
| Ribociclib | CDK4/6 inhibitor; G1/S arrest | [27] | |
| CHK1 | V158411 | Inhibitors sensitize cells to DNA-damaging agents (gemcitabine and cisplatin) | [33] |
| DNMT1 | Decitabine | Inhibit DNA methyltransferase | [58] |
| EGR | Cetuximab | EGFR monoclonal antibody | [38] |
| Erlotinib | EGFR monoclonal antibody | [39] | |
| FGFR | PD173074 | ATP-competitive inhibitor of FGFR | [37] |
| p53 | PRMIA-1 | p53 activator; rescue the function of p53 in p53-deficient cells | [36] |
| APR-246 | p53 activator; rescue the function of p53 in p53-deficient cells | [36] | |
| PI3K/AKT/mTOR | GDC-0941 | PI3K inhibitor | [47] |
| NVP-BKM120 (BKM-120) | PI3K inhibitor | [47] | |
| Everolimus | mTOR inhibitor | [48,53] | |
| Temsirolimus | mTOR inhibitor | [53] | |
| GDC-0980 | PI3K/mTOR dual inhibitor | [47] | |
| BEZ235 (NVP-BEZ235) | PI3K/mTOR dual inhibitor | [49] | |
| VEGF | Bevacizumab | VEGF monoclonal antibody | [42] |
| SRC | Dasatinib | SRC inhibitor | [11,55] |
| Wee1 | MK1775 | Inhibit Wee1 kinase; G2/M arrest; sensitize cells to cisplatin | [35] |
| WNT | LGK974 | Inhibit a WNT-specific acyltransferase (porcupine) to block the secretion of WNT ligands | [57] |
Targeted therapeutic agents and their reported mechanisms and actions are listed.
AR: Androgen receptor.
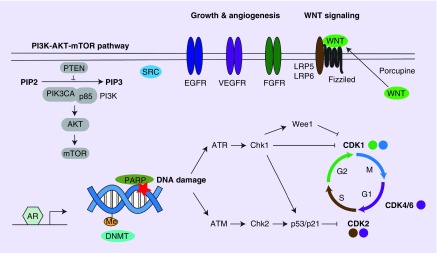
Figure 1. . Potential targets for triple-negative breast cancer.
A simplified schematic figure of all potential targets discussed is depicted. The colored circles of CDKs represent the corresponding phases of the cell cycle.
AR: Androgen receptor; ATM: Ataxia-telangiectasia mutated; ATR: Ataxia telangiectasia and Rad3 related; DNMT: DNA methyltransferase; Me: Methyl group.
DNA damage & repair
DNA damage responses are important in both normal and cancer cells. It is known that the cytotoxic effects of chemotherapeutic reagents are through various mechanisms. Thus, it is plausible that the alterations of genes involved in the responses to DNA damage could be targets for treating TNBC. In response to DNA damage, CDKs and Chk1 act as checkpoints regulating cell cycle, and BRCA1 is involved in repairing DNA damage.
BRCA1 & BRCA2
A total of 19.5% of TNBC patients have been shown to carry BRCA1/2 mutations. BRCA1/2 functions in repairing double-strand breaks through homologous recombination, and PARP mediates nonhomologous end joining and base-excision repair. Inhibition of PARP blocked the alternative DNA repair mechanism mediated by BRCA1/2 [22]. Mutations of BRCA1/2 have been shown to sensitize tumor cells to PARP inhibitors, and the combination of a PARP inhibitor (olaparib/AZD2281) and cisplatin or carboplatin prolonged the overall survival of mice implanted with BL mouse tumors [23]. Olaparib has also been tested in clinical trials although resistance has been observed in patients. Patients receiving high doses of olaparib (400 mg twice daily) had a better objective response (41%) with acceptable toxicity compared with those patients treated with low doses of olaparib (100 mg twice daily) [24]. Interestingly, a second mutation of BRCA1 can restore the normal functions of BRCA1 in TNBC patients with acquired resistance to chemotherapy [25].
Cyclin-dependent kinases
Altered expression of CDKs, including CDK4 and CDK6, has been observed in residual TNBC [21]. Cell cycle is tightly regulated by the formation of various cyclin–CDK complexes in normal cells; in contrast, the hyperactive CDK4 and CDK6 expedite G1-S transition through phosphorylation of Rb and the release of Rb-bound E2Fs in many cancers. In addition to the G1 arrest caused by CDK4/6 inhibitors, CDK1/2 inhibition results in cell cycle arrest and apoptosis [26]. Recently, an aromatase inhibitor plus ribociclib (a CDK4/6 inhibitor) has been approved for hormone-receptor-positive/HER2-negative metastatic breast cancer after successful clinical trials [27]. Preliminary in vitro and in vivo studies illustrated the potential of targeting CDKs in TNBC. Inhibition of CDKs using a pan-CDK inhibitor (dinaciclib) or a CDK1 inhibitor (purvalanol A) had a synthetic lethal effect in TNBC cells with elevated Myc pathway activity compared with receptor-positive breast cancer cell lines [28]. Later, another study concluded that Myc-dependent breast cancer cells are more sensitive to CDK1 inhibitors compared with other CDK inhibitors [29]. In addition, inhibition of CDK4 decreased the percentage of chemotherapy-resistant cells and breast cancer stem cells in TNBC [30]. However, the subtype-specific benefit of CDK inhibitors has not yet been elucidated in TNBC patients.
P53 & CHK1
Mutations in p53 have been noted in more than 80% of BL breast cancers [15]. This is a significant opportunity for therapy in TNBC. In response to double-strand DNA breaks, the ATM-Chk2 pathway is activated, followed by the activation of p53. The ATR kinases bind to the complex at the single-stranded DNA sites, and subsequently activate Chk1 [31]. Thus, Chk1 serves as a druggable target in p53-deficient tumor cells. Indeed, inhibiting Chk1 sensitized p53-mutant TNBC cells to DNA damaging agents [32]. However, Chk1 inhibition in patients remains an open area of investigation due to the toxicity observed in clinical trials of other cancers [33]. Instead of targeting Chk1 directly, an emerging approach is to inhibit the downstream of Cdk1, WEE1. The activated Wee1 phosphorylates CDK1 and causes G2/M arrest [34]. The combination of MK1775 (a Wee1 inhibitor) and cisplatin resulted in enhanced tumor growth inhibition in a TNBC xenograft model (MDA-MB-231) [35]. Furthermore, in a promising line of investigation, Synnott et al. have shown that two compounds (PRMIA-1 and APR-246) can rescue p53 functions in p53-deficient TNBC cells and had synergistic therapeutic effects when combined with chemotherapy [36].
Growth factors & angiogenesis
EGFR, FGFRs and VEGFRs are major growth factors consistently found overexpressed or amplified in TNBC patients [15,21,37]. Upon ligand binding, these growth factors activate the downstream signaling promoting tumor growth and angiogenesis. The EGFR inhibitors (antibodies and tyrosine kinase inhibitors) have been routinely used in the clinic to treat non-small-cell lung cancer. Addition of cetuximab (an anti-EGFR antibody) to cisplatin significantly increased the response rate (overall survival: 20 vs 10%, and progression-free survival: 3.7 vs 1.5 months) in metastatic TNBC patients [38]. However, in another Phase I clinical trial of erlotinib (an EGFR inhibitor) plus bendamustine (a nitrogen mustard derivative alkylating agent) in metastatic TNBC patients, severe lymphopenia was noted and only one out of 11 patients had partial responses [39] (ClinicalTrials.gov number NCT00834678). No selective FGF inhibitors have been approved by US FDA in cancer treatment, and no clinical trials of the FGF inhibitors have been completed in TNBC. Preliminary studies with PD173074 (an FGFR inhibitor) inhibited the growth of TNBC cells known with amplified FGFR2 and CAL-51 (a BL breast cancer cell line) xenografts [37,40]. The indications of bevacizumab (a VEGFR inhibitor) include treatments in non-small-cell lung cancer, metastatic colorectal cancer and other cancers. However, the combination of bevacizumab and chemotherapy in HER2-ve metastatic breast cancer did not improve overall survival significantly [41]. Another completed Phase III clinical trial reported that the addition of bevacizumab to chemotherapy did not prolong the overall survival or invasive disease-free survival, indicating that further studies are required to define the benefits of the combination of bevacizumab and chemotherapy in TNBC patients [42].
Androgen receptor
The expression of androgen receptor (AR) has been noted in 60.5% of breast cancer patients, correlating with increased overall survival and disease-free survival [43]. However, in the LAR subtype of TNBC, elevated AR expression was associated with decreased relapse-free survival [11]. In addition to androgen receptor, examples of known alternative targets are HSP90 which regulates the function and the stability of ARs and CYP17 lyase which is required in the synthesis of androgen [44]. Four human breast cancer cell lines sharing gene expression patterns with LAR TNBC had higher sensitivity to bicalutamide (AR antagonist) or 17-DMAG (a HSP90 inhibitor) than those ones shared with BL TNBC [11]. Cochrane et al. concluded that enzalutamide was effective in treating AR-positive TNBC cells or ER+ cells in vitro and in xenografts [45]. A Phase II clinical trial of bicalutamide (150 mg daily) in AR+ve/PR-ve/ER-ve metastatic breast cancer patients showed that 19% of patients have a therapeutic response to this agent for at least 6 months, and the toxicity of this treatment is moderate [46]. In addition to those AR inhibitors, a clinical trial will evaluate the benefit of a CYP17 lyase inhibitor in AR+ve TNBC patients (ClinicalTrials.gov number NCT02130700).
PI3K/AKT/mTOR pathway
PIK3CA mutation frequency was noted to be elevated in both luminal and HER2 subtype tumors, ranging from 32 to 49%. In contrast, basal subtype tumors are enriched for loss of PTEN and amplification of AKT3 [15]. In TNBC, the PIK3CA mutation rate was significantly increased in the residual TNBC and AR-positive TNBC [11,21]. Given these findings, it is interesting to note that a PI3K inhibitor plus bicalutamide synergistically inhibited the growth of AR-positive TNBC cells [47]. Everolimus (an mTOR inhibitor) plus exemestane (an aromatase inhibitor) extended the progression-free survival of metastatic hormone-receptor-positive breast cancer [48], and has been approved for clinical use. Translating this to TNBC, activation of mTOR was observed in TNBC, and BEZ235 (a PI3K/mTOR inhibitor) enhanced the tumor inhibition effects of chemotherapy reagents (carboplatin and docetaxel) in mice orthotopically injected with MDA-MB231 cells in a proof of concept experiment [49]. In addition, clinical trials have evaluated the clinical benefits of combinations of chemotherapy and PI3K or mTOR inhibitors in TNBC. A Phase II trial of GDC-0941 (a PI3K inhibitor) plus cisplatin in metastatic TNBC was terminated (ClinicalTrials.gov number NCT01918306). Another Phase II trial showed that 36% of metastatic TNBC patients benefited from the combination of everolimus and carboplatin [50] (ClinicalTrials.gov number NCT01127763). Compared with the arm receiving chemotherapy, the combined everolimus and chemotherapy did not improve the complete response. Of note, the mutations in PI3K signaling were not related to the response to the treatment [51] (ClinicalTrials.gov NCT00930930).
Additionally, several feedback loops involved in the resistance to the inhibitors targeting PI3K/mTOR pathway have been identified [52]. Some trials have focused on the combination of different targeted therapeutic agents. For instance, a Phase I clinical trial has evaluated the combination of BEZ235 and MEK162 (a MEK1/2 inhibitor) in TNBC and other cancers (completed but results not yet available; ClinicalTrials.gov number NCT01337765). The combination of doxorubicin, bevacizumab and mTOR inhibitors (temsirolimus or everolimus) has been used in a Phase I clinical trial in metaplastic TNBC. Interestingly, this study found that patients with abnormal PI3K activation have a better objective response rate [53]. Taken together, these studies suggest that further analysis is required for determining the subpopulation of TNBC which will benefit from PI3K/mTOR inhibitors treatment.
SRC, WNT signaling & DNMT1
Compared with non-TNBC tumors, detection of Src at the membrane was significantly increased in TNBC tumors [54]. Considered with this finding, it is not surprising that TNBC cell lines are more sensitive to the Src inhibitor (dasatinib), especially in the M and MSL subtypes [11]. However, only 4.7% of TNBC patients had complete or partial response to dasatinib treatment in a Phase II study [55]. In addition to Src, WNT signaling was enhanced in BL2, M and MSL subtypes of TNBC [11], and was correlated with development of lung and brain metastases [56]. LGK974 (a WNT-specific O-acyltransferase porcupine inhibitor) inhibited WNT signaling in a mouse model of breast cancer [57], and has been proposed in a clinical trial in TNBC and other cancers (ClinicalTrials.gov number, NCT01351103). An additional gene elevated in TNBC is DNMT1, playing a key role in maintenance of methylation. At the expression level, DNMT1 was elevated in TNBC [58]. The combination of the DNMT inhibitor (decitabine) and PARP inhibitors resulted in additional tumor inhibition in wild-type BRCA1 TNBC cells [59]. Currently, 17 early-stage clinical trials targeting WNT signaling in cancers other than breast cancer are underway or are recruiting patients (based on data from ClinicalTrials.gov as of 22 August 2017). Conversely, both DNMT inhibitors and Src inhibitors have been approved by FDA for treating other cancers, such as myeloid malignancies.
Development of new targeted therapies & beyond
With the recent advances in ‘omics’, the number of novel targets has continued to rise for potential pathways that could be used to treat TNBC. This includes several new approaches described below to identify therapy for previously undruggable targets. Given the heterogeneity of TNBC, the combination of these varied methods will be refined to treat specific patients.
Exploiting ‘omics’ to identify new therapeutic targets & treatments
In recent years, genome- and transcriptome-wide studies have been the mainstay of identifying genes associated with the sensitivity and resistance to chemotherapy in TNBC as well as the BL subtype. For instance, tumors with genetic aberrations may be vulnerable to specific treatments based on specific expression or pathway effects. Likewise, more recent studies in epigenomic and proteomic analyses of TNBC have delineated therapeutic potential of various targets. With the development of these various sources of ‘omic’ data in hand, drug repurposing is now feasible. This essentially integrates the expression of various genetic programs with drug response data through approaches such as Connectivity Map [60]. However, to date relatively few drugs have been identified this way for breast cancer treatment [61,62]. With additional data and stratification of tumor types, there is obvious potential in this approach. The various means to use ‘omic’ data are reviewed below.
Sensitivity & resistance
The relevance of several novel targets to chemotherapy resistance has recently been investigated. For example, CD73 is highly expressed in TNBC patients and is related to the resistance to anthracycline treatment. Mechanistically, CD73 activates A2A adenosine receptor to inhibit CD8+ cells. The combination of the A2A antagonist and doxorubicin inhibited tumor growth in mice more efficiently than doxorubicin alone [20]. A2A antagonists also decreased the number of lung metastasis colonies in mice injected with CD73+ mouse mammary tumor cells significantly [63]. Likewise, overexpression of IRAK1 in TNBC patients was associated with resistance to paclitaxel, and IRAK-inh (the IRAK1 inhibitor) rescued the sensitivity to paclitaxel in paclitaxel-resistant cells. Increased expression of IRAK1 was found both in metastatic TNBC cells and patients with poorly differentiated breast tumors. IRAK-inh also reduced the lung metastasis in mice engrafted with metastatic breast cancer cells [64]. In addition to the role of resensitizing cells to chemotherapy, these two emerging targets also target metastasis.
Vulnerability
In addition to the studies of sensitivity and resistance caused by the alteration of single genes, recent omic studies highlight two functional dependencies within TNBC. First, BL TNBC cells with increased expression of genes involved in proteasome degradation had a markedly better response to proteasome inhibitors relative to other subtypes [65]. In a good example of drug repurposing targeting the protein translation pathway, a recent study identified that compared with Rb1-mutated cells, TNBC cells with deficient Rb1 and p53 were more sensitive to the mitochondrial protein translation pathway inhibitor tigecycline, an FDA-approved antibiotic [61]. In a second line of investigation, elevated synthesis of pyrimidines was observed in TNBC cells after chemotherapy. After blocking de novo pyrimidine synthesis, the sensitivity of TNBC cells to chemotherapeutic agents was restored [66]. These approaches demonstrate the utility of targeting broad functional pathways instead of individual genes to restore vulnerability to chemotherapy in TNBC.
Undruggable targets
There are several genetic pathways elevated in numerous cancers, including both Myc and Ras, that lack targeted therapy and these molecules have been termed ‘undruggable’. Despite the difficulty in directly inhibiting these pathways, their expression and importance in cancer have ensured the continued exploration of methods to target these pathways indirectly. Myc is of particular interest since a large number of TNBC cases have Myc overexpression. Interestingly, a recent metabolomic study identified a synthetic lethal approach for TNBC, including those with Myc overexpression. In this study, using an unbiased screening approach in TNBC, LDHB was found to contribute to the glycolysis dependency of TNBC cells, and knockdown of LHDB-inhibited tumor growth in xenograft mice. Considering that the expression of LHDB in TNBC was higher than that in luminal subtypes and high expression of LDHB was correlated with survival, LDHB was noted as potentially having a role in the treatment of TNBC [67]. Another study examined the depletion of essential genes in the fatty acid oxidation pathway which blocked the growth of TNBC cells with high Myc expression in vitro and in vivo [68]. In addition to these broad pathways, patients with elevated Myc expression were also noted to have elevated SHP2 and ZEB1, correlating with poor prognosis. Moreover, TNBC expression of ERK1, ERK2 and ZEB1 was activated by SHP2 [69]. Thus, when SHP2 was inhibited in TNBC cells, this resulted in decreased activity of multiple signaling pathways, including EGFR, Ras and Akt [70]. Together these examples demonstrate the opportunities to use alternative approaches to target undruggable pathways.
Combination of therapeutic targets to combat TNBC
The concept of combining different therapeutic agents for synergistic benefits in treatment has been utilized for decades. Moving forward, this may be especially valuable in TNBC where personalized medicine targeting signaling molecules is difficult. In TNBC, as the number of synthetic lethal treatments, identification of novel targets and methods for combatting drug resistance increase and are better understood, it will be critical to assess potential for combinatorial treatment. Driving this forward are studies reviewed below with transgenic mice, human TNBC cell lines, xenografts with these cell lines and patient-derived xenografts (PDXs). These various tools provide a key opportunity to elucidate the response of heterogeneous TNBC to various combination therapies in different experimental models prior to clinical trials. The results of this work can be compared with addition of targeted therapy to chemotherapy in TNBC patients, with several studies reporting remarkable improvements in the response rate in clinical trials.
Targets associated with response to treatment
A major strategy in improving TNBC treatment outcomes is to combine targets that mediate the responses to current chemotherapy, including those genes identified from the omic studies mentioned previously. One method that has added significantly to this data is high-throughput screening in cell lines to a collection of drugs, revealing both synthetic lethal interactions and identifying genes conferring resistance to targeted therapy. Indeed, in addition to confirming established interactions, this approach revealed that NOTCH1 pathway activation resulted in a surprising resistance to PI3K inhibitors [71]. Given the importance of PI3K inhibitors in breast cancer, and the frequent resistance to PI3K inhibitors, this work is highly significant. Recent work by Castel et al. was significant in identifying a new mechanism of PI3K resistance, mediated by PDK1 and SGK1 [72]. Indeed, with PI3K inhibition and development of resistance, they noted SGK1-mediated mTORC1 activity. Addition of a PDK1 inhibitor then rescued the sensitivity of the PI3K inhibitor in these cell lines [72]. Another example of target upregulation with inhibitor treatment was observed with MEK inhibitors. In TNBC cells treated with an MEK inhibitor, elevated activation of several receptor tyrosine kinases, such as AXL, VEGFR2 and PDGFR-β, has been noted. The combination of AZD6244 (an MEK inhibitor) and sorafenib (a multiple receptor kinase inhibitor) led to a synergistic inhibition of tumor growth [73].
Targeting cancers with shared mutations
In TNBC and ovarian cancer, BRCA1/2 mutations are commonly observed. However, the development of a second mutation in BRCA1 is associated with resistance to chemotherapy in both ovarian and breast cancers [25,74]. Moreover, mutations of CDK12 often co-occur with BRCA1/2 mutations. In two studies, the depletion of CDK12 expression rescued the sensitivity to PARP inhibitors in ovarian cancer and in TNBC cell lines [75,76]. Following this, a preclinical study of the combination of PI3K and PARP inhibitors demonstrated the synergistic effects in these two cancers. A recent Phase I clinical trial strongly suggests that this inhibitor combination is practical in treating both cancers [77]. Together, these studies demonstrate the utility of targeting mutations that co-occur in TNBC.
Signaling pathway & network-based approaches
Gene expression data from breast cancer can be analyzed through the previously described intrinsic approach to stratify into various subtypes. However, this does not allow for the identification of signaling pathway activity. By using gene set enrichment analysis [78] or a Bayesian approach to predict cell signaling pathway activation, considerable knowledge as to the active signaling components within a tumor can be gained [79–81]. Indeed, by combining these predictions with known inhibitors there is significant potential to predict the optimal treatment. For instance, it has been demonstrated that each subtype of breast cancer is enriched for specific pathways while maintaining significant heterogeneity for these pathways [10]. This may well explain the variability in response when individual tumors within a subtype have differential responses to the same drugs. Moving forward in therapeutic development using this type of data, initial studies have focused on overcoming the resistance to one targeted therapeutic agent by adding another one. For instance, the resistance to PI3K/Akt/mTOR pathway in TNBC was addressed by combining these pathway inhibitors with an inhibitor of JAK2 (NVP-BSK805) [82]. However, our recent publication has combined the strategies of developing treatments based on computational analysis of pathways with a combination therapy protocol. In this report we described the development of tailored combinatorial treatments for heterogeneous subtypes of mammary tumors after analyzing the pathway activation patterns, and observed a significant inhibition of tumor growth [83]. Importantly, while the initial proof of principle work with this study was completed in mouse tumors, we then went on to make and test predictions for human TNBC, demonstrating that a three-drug combination targeting specific cell signaling pathways was able to significantly reduce tumor volume.
Experimental models
Different models, ranging from cell culture assays to mouse models and PDX samples, have unique advantages in breast cancer research. For instance, cell lines are routinely used to understand the characteristics and mechanisms inherent in breast cancer. Advantage of cell line research is that it permits high-throughput drug or RNA interference screening to rapidly develop information about drug resistance or susceptibility. However, given the additional mutations accumulated over time in culture, there may be limitations for the ability to extend tissue culture findings to breast cancer. To address this, one additional avenue of research has been the use of mouse models. Various models, ranging from transgenics to knockouts and chemical mutagens have been used to study many facets of breast cancer, including tumorigenesis, tumor microenvironment and metastasis. Of note, decoding the genomic information from tumors in transgenic mice has demonstrated the similarity between transgenic mouse models of breast cancer and human breast cancer. For instance, significant gene-expression heterogeneity has been observed in both MMTV-Myc and MMTV-Met transgenic mice, with noted similarity to human breast cancer [84–86]. Moving past gene expression, the same data have been used to predict copy number alterations across numerous transgenic mouse models, revealing potential driver events shared between mouse and human breast cancer [87]. In addition to the basic characterization of the mouse models, recent studies have shown a promising role for transgenic mice in development of treatment. Drost et al. found that the transgenic mice carrying different BRCA1 mutations have disparate responses to targeted therapy, and went on to identify a domain of the BRCA1 protein essential for the drug resistance [88]. For those targets without FDA-approved drugs, ablation of targets in transgenic mice is another method to examine the widespread effects of targets in a certain oncogene-driven background, such as knockout Stat3 in Myc mice [89]. A more recent advance has been the development of PDX models, particularly in TNBC research. These PDXs recapitulate the molecular characteristics of both primary and metastatic breast cancer [90]. In one study of BRCA1-associated drug resistance, one PDX sample had intrinsic resistance to PARP inhibitors, similar to the original breast cancer patient [76]. More importantly, a platform with collections of PDX samples at multiple institutes will be highly influential in examining the response to drug treatments [91].
Future perspective
The focus of understanding TNBC within the past several years has led to significant progress in the development of targeted therapy and combinatorial treatments. This is exemplified in one of the newest findings in the I-SPY2 trial where the promising benefit of using PARP inhibitor contained treatment in unselected TNBC patients was observed [92]. In this review, we have described some of the major approaches used to address the unmet needs for TNBC treatments. Here, we discuss a couple of challenges that remain in TNBC treatment.
First, a major challenge that remains is both inter- and intratumor heterogeneity. To address this, TNBC patients should be stratified into subtypes based on the integrated omics data. Moreover, the examination of intratumor heterogeneity is required for the development of treatments. As one tumor consists of multiple colonies, these colonies may well have diverse functions and orchestrate the various properties of tumors [93]. In addition, if one dominant clone is targeted, other metastatic clones or other minor clones within the tumor may continue to grow and metastasize.
Indeed, the advances in single-cell sequencing have enabled the interpretation of the differential gene expression among the populations of cells in a tumor, and the ability to trace the evolution of TNBC [94]. This indicates the requirement of a deeper and broader understanding of targets and targeted therapeutic agents, especially for rare populations, including cancer stem cells and metastatic cells.
A second major hurdle is to uncover additional drivers, modifying genes and potential targets for development of effective therapy for TNBC. With each new screen, it is clear that only a portion of the targets involved in key networks have been uncovered. In order to maximize effectiveness of combinatorial therapy, these events and their mechanistic details need to be elucidated.
A third challenge is the integration of omic technologies with the various model systems. Although many investigators are expert in mouse models, PDX, drug screening or omic technologies, it is still rare for these disciplines to be combined. Given that the NCI's Molecular Analysis for Therapy Choice (MATCH) trial pan-cancer Phase II trial integrates alterations in gene expression in tumors with determination for treatment modalities, it is clear that this type of approach is required [95]. It is anticipated that the MATCH trial will accelerate approval of targeted therapeutic agents in different cancers with the same gene alterations. Extending this approach to mouse models, PDX samples with mechanistic understanding will open up new treatment regimens, especially for patients with resistant tumors.
Executive summary.
Molecular subtypes of breast cancer
Different classifications of subtypes of breast cancer.
Triple-negative breast cancer (TNBC) has been stratified into at least three subtypes.
Molecular characteristics in current treatment & identification of targets
Subtypes of breast cancer or TNBC have differential responses to the same treatment.
Highlight targets chosen mainly based on the prevalence of aberrations.
Most of these targets have been widely studied in vitro, in vivo and clinical trials.
BRCA1/2-deficient patients are sensitive to PARP inhibitors.
CDK4/6 inhibitors have been approved in the hormone-receptor-positive metastatic breast cancer treatment.
Targets used in treating other cancers, such as SRC, androgen receptor and DNMT1.
Examples of targets associated with the malignant characteristics.
Other targets include PI3K/mTOR, p53, CHK1 and WNT signaling.
Development of new targeted therapy & beyond
A list of targets has been identified based on the omics approaches.
Targeting genes associated with sensitivity and resistance to chemotherapy or targeted therapy.
Genetic alterations make tumors vulnerable to targeted therapy.
Some of the novel targets in cancer treatment were identified from studies of metabolomics and epigenetics.
Overcome the resistances to current treatments through combining targeted therapy agents.
Extend the application of the same combinatorial treatments for other types of cancer to TNBC based on the shared alterations.
Signaling pathway based combinatorial treatments led to the inhibition of subtypes of breast cancer individually.
Recent advances in mouse models offer opportunities to assess the treatments in preclinical stages.
Future perspective
Inter- and intratumor heterogeneity are still challenges.
Uncovering the additional drivers to treat TNBC systematically and efficiently.
Integrative approaches are critical in the development of TNBC treatment.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH R01CA160514 to ERA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Savci-Heijink CD, Halfwerk H, Hooijer GK, Horlings HM, Wesseling J, Van De Vijver MJ. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res. Treat. 2015;150(3):547–557. doi: 10.1007/s10549-015-3352-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 7.Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist. 2013;18(2):123–133. doi: 10.1634/theoncologist.2012-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8(5):R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prat A, Parker JS, Karginova O, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12(5):R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatza ML, Lucas JE, Barry WT, et al. A pathway-based classification of human breast cancer. Proc. Natl Acad. Sci. USA. 2010;107(15):6994–6999. doi: 10.1073/pnas.0912708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015;21(7):1688–1698. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jezequel P, Loussouarn D, Guerin-Charbonnel C, et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res. 2015;17:43. doi: 10.1186/s13058-015-0550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Network TCGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 17.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin. Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 18.Masuda H, Baggerly KA, Wang Y, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin. Cancer Res. 2013;19(19):5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J. Clin. Oncol. 2010;28(7):1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loi S, Pommey S, Haibe-Kains B, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl Acad. Sci. USA. 2013;110(27):11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balko JM, Giltnane JM, Wang K, et al. Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discovery. 2014;4(2):232–245. doi: 10.1158/2159-8290.CD-13-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farmer H, Mccabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 23.Rottenberg S, Jaspers JE, Kersbergen A, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl Acad. Sci. USA. 2008;105(44):17079–17084. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 25.Afghahi A, Timms KM, Vinayak S, et al. Tumor BRCA1 reversion mutation arising during neoadjuvant platinum-based chemotherapy in triple-negative breast cancer is associated with therapy resistance. Clin. Cancer Res. 2017;23(13):3365–3370. doi: 10.1158/1078-0432.CCR-16-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. 2006;24(11):1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 27.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl. J. Med. 2016;375(18):1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 28.Horiuchi D, Kusdra L, Huskey NE, et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J. Exp Med. 2012;209(4):679–696. doi: 10.1084/jem.20111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang J, Sergio CM, Sutherland RL, Musgrove EA. Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells. BMC Cancer. 2014;14:32. doi: 10.1186/1471-2407-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai M, Zhang C, Ali A, et al. CDK4 regulates cancer stemness and is a novel therapeutic target for triple-negative breast cancer. Sci. Rep. 2016;6:35383. doi: 10.1038/srep35383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 32.Bryant C, Rawlinson R, Massey AJ. Chk1 inhibition as a novel therapeutic strategy for treating triple-negative breast and ovarian cancers. BMC Cancer. 2014;14:570. doi: 10.1186/1471-2407-14-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakurikar N, Eastman A. Will targeting Chk1 have a role in the future of cancer therapy? J. Clin. Oncol. 2015;33(9):1075–1077. doi: 10.1200/JCO.2014.60.0767. [DOI] [PubMed] [Google Scholar]
- 34.Matheson CJ, Backos DS, Reigan P. Targeting WEE1 kinase in cancer. Trends Pharmacol. Sci. 2016;37(10):872–881. doi: 10.1016/j.tips.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Zheng H, Shao F, Martin S, Xu X, Deng CX. WEE1 inhibition targets cell cycle checkpoints for triple negative breast cancers to overcome cisplatin resistance. Sci. Rep. 2017;7:43517. doi: 10.1038/srep43517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Synnott NC, Murray A, Mcgowan PM, et al. Mutant p53: a novel target for the treatment of patients with triple-negative breast cancer? Int. J. Cancer. 2017;140(1):234–246. doi: 10.1002/ijc.30425. [DOI] [PubMed] [Google Scholar]
- 37.Turner N, Lambros MB, Horlings HM, et al. Integrative molecular profiling of triple negative breast cancers identifies amplicon drivers and potential therapeutic targets. Oncogene. 2010;29(14):2013–2023. doi: 10.1038/onc.2009.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baselga J, Gomez P, Greil R, et al. Randomized Phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 2013;31(20):2586–2592. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Layman RM, Ruppert AS, Lynn M, et al. Severe and prolonged lymphopenia observed in patients treated with bendamustine and erlotinib for metastatic triple negative breast cancer. Cancer Chemother. Pharmacol. 2013;71(5):1183–1190. doi: 10.1007/s00280-013-2112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharpe R, Pearson A, Herrera-Abreu MT, et al. FGFR signaling promotes the growth of triple-negative and basal-like breast cancer cell lines both in vitro and in vivo . Clin. Cancer Res. 2011;17(16):5275–5286. doi: 10.1158/1078-0432.CCR-10-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brufsky AM, Hurvitz S, Perez E, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2011;29(32):4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 42.Cameron D, Brown J, Dent R, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, Phase III trial. Lancet Oncol. 2013;14(10):933–942. doi: 10.1016/S1470-2045(13)70335-8. [DOI] [PubMed] [Google Scholar]
- 43.Vera-Badillo FE, Templeton AJ, De Gouveia P, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J. Natl Cancer Inst. 2014;106(1) doi: 10.1093/jnci/djt319. djt319. [DOI] [PubMed] [Google Scholar]
- 44.Proverbs-Singh T, Feldman JL, Morris MJ, Autio KA, Traina TA. Targeting the androgen receptor in prostate and breast cancer: several new agents in development. Endocr. Relat. Cancer. 2015;22(3):R87–R106. doi: 10.1530/ERC-14-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cochrane DR, Bernales S, Jacobsen BM, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16(1):R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin. Cancer Res. 2013;19(19):5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehmann BD, Bauer JA, Schafer JM, et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014;16(4):406. doi: 10.1186/s13058-014-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montero JC, Esparis-Ogando A, Re-Louhau MF, et al. Active kinase profiling, genetic and pharmacological data define mTOR as an important common target in triple-negative breast cancer. Oncogene. 2014;33(2):148–156. doi: 10.1038/onc.2012.572. [DOI] [PubMed] [Google Scholar]
- 50.Singh J, Novik Y, Stein S, et al. Phase II trial of everolimus and carboplatin combination in patients with triple negative metastatic breast cancer. Breast Cancer Res. 2014;16(2):R32. doi: 10.1186/bcr3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jovanovic B, Mayer IA, Mayer EL, et al. A randomized Phase II neoadjuvant study of cisplatin, paclitaxel with or without everolimus in patients with stage II/III triple-negative breast cancer (TNBC): responses and long-term outcome correlated with increased frequency of DNA damage response gene mutations, TNBC subtype, AR status, and Ki67. Clin. Cancer Res. 2017;23(15):4035–4045. doi: 10.1158/1078-0432.CCR-16-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozengurt E, Soares HP, Sinnet-Smith J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: an unintended consequence leading to drug resistance. Mol. Cancer Ther. 2014;13(11):2477–2488. doi: 10.1158/1535-7163.MCT-14-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basho RK, Gilcrease M, Murthy RK, et al. Targeting the PI3K/AKT/mTOR pathway for the treatment of mesenchymal triple-negative breast cancer: evidence from a Phase I trial of mTOR inhibition in combination with liposomal doxorubicin and bevacizumab. JAMA Oncol. 2017;3(4):509–515. doi: 10.1001/jamaoncol.2016.5281. [DOI] [PubMed] [Google Scholar]
- 54.Tryfonopoulos D, Walsh S, Collins DM, et al. Src: a potential target for the treatment of triple-negative breast cancer. Ann. Oncol. 2011;22(10):2234–2240. doi: 10.1093/annonc/mdq757. [DOI] [PubMed] [Google Scholar]
- 55.Finn RS, Bengala C, Ibrahim N, et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label Phase II study. Clin. Cancer Res. 2011;17(21):6905–6913. doi: 10.1158/1078-0432.CCR-11-0288. [DOI] [PubMed] [Google Scholar]
- 56.Dey N, Barwick BG, Moreno CS, et al. Wnt signaling in triple negative breast cancer is associated with metastasis. BMC Cancer. 2013;13:537. doi: 10.1186/1471-2407-13-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Pan S, Hsieh MH, et al. Targeting Wnt-driven cancer through the inhibition of porcupine by LGK974. Proc. Natl Acad. Sci. USA. 2013;110(50):20224–20229. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin E, Lee Y, Koo JS. Differential expression of the epigenetic methylation-related protein DNMT1 by breast cancer molecular subtype and stromal histology. J. Transl. Med. 2016;14:87. doi: 10.1186/s12967-016-0840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muvarak NE, Chowdhury K, Xia L, et al. Enhancing the cytotoxic effects of PARP inhibitors with DNA demethylating agents – a potential therapy for cancer. Cancer Cell. 2016;30(4):637–650. doi: 10.1016/j.ccell.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lamb J, Crawford ED, Peck D, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. doi: 10.1126/science.1132939. [DOI] [PubMed] [Google Scholar]
- 61.Jones RA, Robinson TJ, Liu JC, et al. RB1 deficiency in triple-negative breast cancer induces mitochondrial protein translation. J. Clin. Invest. 2016;126(10):3739–3757. doi: 10.1172/JCI81568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takai K, Le A, Weaver VM, Werb Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7(50):82889–82901. doi: 10.18632/oncotarget.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beavis PA, Divisekera U, Paget C, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc. Natl Acad. Sci. USA. 2013;110(36):14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wee ZN, Yatim SM, Kohlbauer VK, et al. IRAK1 is a therapeutic target that drives breast cancer metastasis and resistance to paclitaxel. Nature Comm. 2015;6:8746. doi: 10.1038/ncomms9746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrocca F, Altschuler G, Tan SM, et al. A genome-wide siRNA screen identifies proteasome addiction as a vulnerability of basal-like triple-negative breast cancer cells. Cancer Cell. 2013;24(2):182–196. doi: 10.1016/j.ccr.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown KK, Spinelli JB, Asara JM, Toker A. Adaptive reprogramming of de novo pyrimidine synthesis is a metabolic vulnerability in triple-negative breast cancer. Cancer Discovery. 2017;7(4):391–399. doi: 10.1158/2159-8290.CD-16-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mccleland ML, Adler AS, Shang Y, et al. An integrated genomic screen identifies LDHB as an essential gene for triple-negative breast cancer. Cancer Res. 2012;72(22):5812–5823. doi: 10.1158/0008-5472.CAN-12-1098. [DOI] [PubMed] [Google Scholar]
- 68.Camarda R, Zhou AY, Kohnz RA, et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat. Med. 2016;22(4):427–432. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aceto N, Sausgruber N, Brinkhaus H, et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat. Med. 2012;18(4):529–537. doi: 10.1038/nm.2645. [DOI] [PubMed] [Google Scholar]
- 70.Matalkah F, Martin E, Zhao H, Agazie YM. SHP2 acts both upstream and downstream of multiple receptor tyrosine kinases to promote basal-like and triple-negative breast cancer. Breast Cancer Res. 2016;18(1):2. doi: 10.1186/s13058-015-0659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Muellner MK, Uras IZ, Gapp BV, et al. A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat. Chem. Biol. 2011;7(11):787–793. doi: 10.1038/nchembio.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castel P, Ellis H, Bago R, et al. PDK1-SGK1 signaling sustains AKT-independent mTORC1 activation and confers resistance to PI3Kalpha inhibition. Cancer Cell. 2016;30(2):229–242. doi: 10.1016/j.ccell.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duncan JS, Whittle MC, Nakamura K, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149(2):307–321. doi: 10.1016/j.cell.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68(8):2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bajrami I, Frankum JR, Konde A, et al. Genome-wide profiling of genetic synthetic lethality identifies CDK12 as a novel determinant of PARP1/2 inhibitor sensitivity. Cancer Res. 2014;74(1):287–297. doi: 10.1158/0008-5472.CAN-13-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson SF, Cruz C, Greifenberg AK, et al. CDK12 inhibition reverses de novo and acquired PARP inhibitor resistance in BRCA wild-type and mutated models of triple-negative breast cancer. Cell Rep. 2016;17(9):2367–2381. doi: 10.1016/j.celrep.2016.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matulonis UA, Wulf GM, Barry WT, et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann Oncol. 2017;28(3):512–518. doi: 10.1093/annonc/mdw672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bild AH, Yao G, Chang JT, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439(7074):353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 80.Andrechek ER. HER2/Neu tumorigenesis and metastasis is regulated by E2F activator transcription factors. Oncogene. 2015;34(2):217–225. doi: 10.1038/onc.2013.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hollern DP, Honeysett J, Cardiff RD, Andrechek ER. The E2F transcription factors regulate tumor development and metastasis in a mouse model of metastatic breast cancer. Mol. Cell Biol. 2014;34(17):3229–3243. doi: 10.1128/MCB.00737-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Britschgi A, Andraos R, Brinkhaus H, et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012;22(6):796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 83.Jhan JR, Andrechek ER. Effective personalized therapy for breast cancer based on predictions of cell signaling pathway activation from gene expression analysis. Oncogene. 2017;23(25):3553–3561. doi: 10.1038/onc.2016.503. [DOI] [PubMed] [Google Scholar]
- 84.Hollern DP, Andrechek E. A genomic analysis of mouse models of breast cancer reveals molecular features of mouse models and relationships to human breast cancer. Breast Cancer Res. 2014;16:R59. doi: 10.1186/bcr3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andrechek ER, Cardiff RD, Chang JT, et al. Genetic heterogeneity of Myc-induced mammary tumors reflecting diverse phenotypes including metastatic potential. Proc. Natl Acad. Sci. USA. 2009;106(38):16387–16392. doi: 10.1073/pnas.0901250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ponzo MG, Lesurf R, Petkiewicz S, et al. Met induces mammary tumors with diverse histologies and is associated with poor outcome and human basal breast cancer. Proc. Natl Acad. Sci. USA. 2009;106(31):12903–12908. doi: 10.1073/pnas.0810402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rennhack J, To B, Wermuth H, Andrechek ER. Mouse models of breast cancer share amplification and deletion events with human breast cancer. J. Mammary Gland Biol. Neoplasia. 2017;22(1):71–84. doi: 10.1007/s10911-017-9374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Drost R, Dhillon KK, Van Der Gulden H, et al. BRCA1185delAG tumors may acquire therapy resistance through expression of RING-less BRCA1. J. Clin. Invest. 2016;126(8):2903–2918. doi: 10.1172/JCI70196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jhan JR, Andrechek ER. Stat3 accelerates Myc induced tumor formation while reducing growth rate in a mouse model of breast cancer. Oncotarget. 2016;7(40):65797–65807. doi: 10.18632/oncotarget.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Derose YS, Wang G, Lin YC, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 2011;17(11):1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bruna A, Rueda OM, Greenwood W, et al. A biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anticancer compounds. Cell. 2016;167(1):260–274.e22. doi: 10.1016/j.cell.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rugo HS, Olopade OI, Demichele A, et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N. Engl. J. Med. 2016;375(1):23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tabassum DP, Polyak K. Tumorigenesis: it takes a village. Nat. Rev. Cancer. 2015;15(8):473–483. doi: 10.1038/nrc3971. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Waters J, Leung ML, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature. 2014;512(7513):155–160. doi: 10.1038/nature13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McNeil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J. Natl Cancer Inst. 2015;107(7) doi: 10.1093/jnci/djv193. pii: djv193. [DOI] [PubMed] [Google Scholar]