Abstract
Background
The aim of this study was to create a screening system for diabetic cardiovascular autonomic neuropathy (DCAN) in diabetic patients.
Material/Methods
A Chinese cohort of 455 diabetic participants was recruited between 2011 and 2013. Short-term heart rate variability testing was used to evaluate cardiovascular autonomic function. A simple model was developed using multiple variable regression to include only significant risk factors that were simple and easily assessed. A DCAN score was determined based on the coefficients of the multiple variable model. This score was tested on the entire cohort of 455 diabetic patients and another independent, external cohort of 115 diabetic patients.
Results
The screening system consisted of age, body mass index, duration of diabetes mellitus, and resting heart rate, and these factors were significantly (P<0.05) associated with DCAN. Receiver operating characteristic (ROC) curve analysis was done. The areas under the ROC curve were 0.798, 0.756, and 0.729 for the total sample, validation cohort, and external set, respectively. A cutoff DCAN score of 12 out of 25 produced optimal results for sensitivity (80.36%), specificity (58.27%), and percentage of patients that needed subsequent testing (43.55%) for the validation set.
Conclusions
The study concludes that a simple and practical DCAN screening can be applied for early intervention to delay or prevent the disease in the Chinese population.
MeSH Keywords: China, Diabetic Neuropathies, Multiphasic Screening, Risk Assessment
Background
The incidence of diabetic cardiovascular autonomic neuropathy (DCAN) as a diabetic microvascular complication [1,2] is rising rapidly throughout the world [3]. The prevalence of DCAN in diabetic patients is estimated at 30–60% [4]. The damage due to DCAN involves autonomic nerve fibers that innervate the heart and blood vessels and consequentially contributes to abnormalities in heart rate control and vascular dynamics [5–8]. This disease carries an increased risk of cardiovascular morbidity and mortality in diabetic patients [9,10]. Short-term heart rate variability (HRV) testing is sensitive and non-invasive, and it can easily be applied as a test of cardiovascular autonomic function test on a large scale [4,6,11]. Our previous study provided evidence that a short-term HRV test had high sensitivity (Sen) and specificity (Spe) for DCAN diagnosis and that the HRV test was not inferior to the traditional Ewing’s test for DCAN [12,13].
Early detection of patients with DCAN is important toward decreasing cardiovascular morbidity and mortality. An accurate and simple screening tool that identifies those at high risk for DCAN would be of great value in clinical and public health practice. During the past decade, several risk score systems have been developed for diabetes, primarily in white populations [14–19]. However, few diabetes risk scores have been based on Asian populations [20–22]. In our previous studies, we developed a screening system to predict people with a high risk of cardiovascular autonomic dysfunction neuropathy (CAN) in the Chinese population but not in diabetic patients [23,24].
Recently, we conducted a risk association analysis of metabolic indices involved in blood pressure profiles, lipid profiles, and blood glucose profiles for DCAN, and our results suggest that these profiles are significantly and independently associated with DCAN [25–27]. Moreover, the combination of these risk factors has predictive value for DCAN. However, to the best of our knowledge, there are no risk score systems for DCAN screening in diabetic patients worldwide. Based on our previous studies [25–28], we hypothesized that risk factors related to DCAN can be used to create a simple risk score system for DCAN screening.
Material and Methods
The aim, design, and setting of the study
In this study, our aim was to create a screening system for identifying and predicting DCAN in Chinese diabetic patients. Like our previous studies [25–29], this study was based on data from a community-based cohort in China.
The characteristics of participants
Association analysis was conducted using diabetic patient data from the cross-sectional study sample toward development of a screening system for DCAN. Of these subjects, 455 diabetic participants with complete clinical baseline data were available for risk analysis of DCAN. To create an external dataset, 115 diabetic patients with the same inclusion and exclusion criteria were recruited as an independent, external cohort from healthy examination centers and other sources. All participants freely signed informed consent forms before the study. The Ethics Committee of Huashan Hospital approved this work. The methods were carried out in accordance with the approved guidelines.
Measurement
Demographic information and assessments of glucose profiles, lipid profiles, renal profiles, and medical history were detailed in earlier studies [25–29]. The definitions of hypertension (HTN), body mass index (BMI), diabetes mellitus (DM), and metabolic syndrome (MetS) were also detailed in earlier studies [25–29]. As we discussed in an earlier study [25,26,29], short-term HRV tests can be used for the evaluation of cardiovascular autonomic function, and we used short-term HRV tests in this study. The protocol for the short-term HRV test was detailed earlier [25–27]. The definition of DCAN was based on at least 2 abnormal results from cardiovascular autonomic function testing [4,25–28].
Development and validation of the screening system
The methodology used for the development and validation of our screening system was similar to that used in our previous work [28]. Firstly, the association analysis for DCAN was performed. Significant association factors for DCAN were estimated using univariate logistic regression (ULR) analysis. Secondly, the parameters of the screening system were validated. The β-coefficients for the associated factors of DCAN were estimated using multiple variable logistic regression analysis (MLR). The MLR model was developed using the stepwise backward elimination method and included significant simple variables (alpha level=5%). Thirdly, the screening system was created. A simple scoring system was developed based on a sum score that was derived for each participant by adding the score of each variable in the MLR model. Finally, the performance validation for the screening system was conducted. Similar to our earlier study [28], the area under the curve (AUC) of the receiver operating characteristic (ROC) curve was used to estimate the performance of the screening system for DCAN. The Youden Index, which is the maximum sum of the Sen and Spe, was computed. In addition, the cutoff scores for low and high risk of DCAN were evaluated to calculate the optimal positive predictive value (PPV) and the negative predictive value (NPV). The parameters involved in the ROC curve analysis included Sen, Spe, Youden Index, PPV, and NPV. The proportion of individuals who needed subsequent testing was also evaluated as a parameter (%Need). These parameters were evaluated for the validation set, total sample, and external set. The bootstrapping technique was employed to compute confidence intervals (CIs) for these parameters [30].
Results
Characteristics of subjects
As was detailed earlier, and as shown in Table 1 [25,26], the characteristics of the 455 diabetic patients were reported. The average age of the total sample and the external set was 62.8 years and 58.34 years, respectively. The mean heart rate (HR) was 75 bpm for the total sample. The prevalence of DCAN was 29.01% and 39.39% in the total sample and external set, respectively. The total sample was randomly divided into the exploratory set (n=237) and the validation set (n=218). There were no significant differences in the baseline characteristics between these 2 sets (P<0.05 for all).
Table 1.
The clinical baseline characteristics of individuals.
| Variable | Modeling dataset | External dataset | |||
|---|---|---|---|---|---|
| Total sample | Exploratory set | Validation set | P value | ||
| Demographic information | |||||
| N | 455 | 237 | 218 | – | 115 |
| Age years | 62.8±8.61 | 62.94±8.98 | 62.64±8.19 | 0.606 | 58.34±9.9 |
| Gender male, % | 208 (45.71%) | 105 (43.88%) | 103 (47.71%) | 0.247 | 62 (53.91%) |
| Height cm | 162.12±8.15 | 161.93±8.06 | 162.32±8.26 | 0.471 | 163.31±8.84 |
| Weight kg | 66.63±11.65 | 66.19±10.95 | 67.16±12.3 | 0.081 | 65.35±12.59 |
| SBP mmHg | 134.3±20.3 | 134.57±20.4 | 134.01±20.2 | 0.678 | 131.88±20.1 |
| DBP mmHg | 81.08±10.12 | 81.18±10.44 | 80.97±9.78 | 0.764 | 76.72±11.08 |
| Laboratory assays | |||||
| FPG mmol/L | 7.34±2.69 | 7.41±2.62 | 7.26±2.77 | 0.420 | 9.23±5.08 |
| 6PBG mmol/L | 11.98±4.42 | 11.93±4.37 | 12.03±4.49 | 0.744 | 13.07±5.44 |
| FINS uml/L | 10.45±24.39 | 11.77±28.02 | 9.02±19.62 | 0.091 | 22.57±56.21 |
| TC mmol/L | 5.38±1.11 | 5.35±1.1 | 5.4±1.13 | 0.490 | 4.63±1.21 |
| TG mmol/L | 1.99±1.18 | 1.94±1.14 | 2.06±1.22 | 0.078 | 1.99±1.43 |
| HDL mmol/L | 1.3±0.31 | 1.25±0.32 | 1.22±0.29 | 0.095 | 1.23±0.53 |
| LDL mmol/L | 3.28±0.85 | 3.26±0.84 | 3.3±0.86 | 0.394 | 3±1.14 |
| SCr μmolL | 81.37±24.04 | 81.17±26.28 | 81.58±21.37 | 0.797 | 72.67±32 |
| HRV indices | |||||
| HR bpm | 75.11±10.41 | 74.77±10 | 75.52±10.78 | 0.110 | 73.26±11.41 |
| TP ms2 | 747.3±682.53 | 758.08±673.82 | 735.59±692.46 | 0.620 | 658.07±680.86 |
| LF ms2 | 166.57±225.93 | 170.15±215.56 | 162.67±236.87 | 0.618 | 145.83±225.52 |
| HF ms2 | 152.15±188.51 | 155.75±208.43 | 147.36±163.1 | 0.230 | 119.25±174.13 |
| LF/HF | 1.84±2.12 | 1.81±2.14 | 1.87±2.1 | 0.651 | 1.98±2.36 |
| Medical history | |||||
| Smoking yes, % | 89 (19.56%) | 43 (17.82%) | 46 (21.46%) | 0.145 | 13 (22.81%) |
| DM duration years | 5.24±6.45 | 5.25±6.72 | 5.24±6.14 | 0.972 | 10.57±8.23 |
| HTN yes, % | 291 (63.96%) | 157 (66.24%) | 134 (61.47%) | 0.134 | 67 (58.77%) |
| HTN duration years | 6.42±9.99 | 6.67±10.17 | 6.15±9.79 | 0.449 | 6.12±8.72 |
| MetS yes, % | 330 (72.53%) | 170 (71.31%) | 160 (73.88%) | 0.410 | 87 (75.65%) |
| DCAN yes, % | 132 (29.01%) | 68 (28.69%) | 64 (30.05%) | 0.192 | 47 (40.87%) |
Present the difference between exploratory set and validation set.
SBP – systolic blood pressure; DBP – diastolic blood pressure; FPG – fasting plasma glucose; PBG – plasma blood glucose; FINS – fasting blood insulin; TC – serum total cholesterol; TG – triglyceride; HDL – high-density lipoprotein cholesterol; LDL – low density lipoprotein cholesterol; SCr – serum creatinine; HR – heart rate; TP – total power of variance; LF – low frequency; HF – high frequency; MetS – metabolic syndrome; HTN – hypertension; DCAN – diabetic cardiovascular autonomic neuropathy.
Development and validation of the screening model
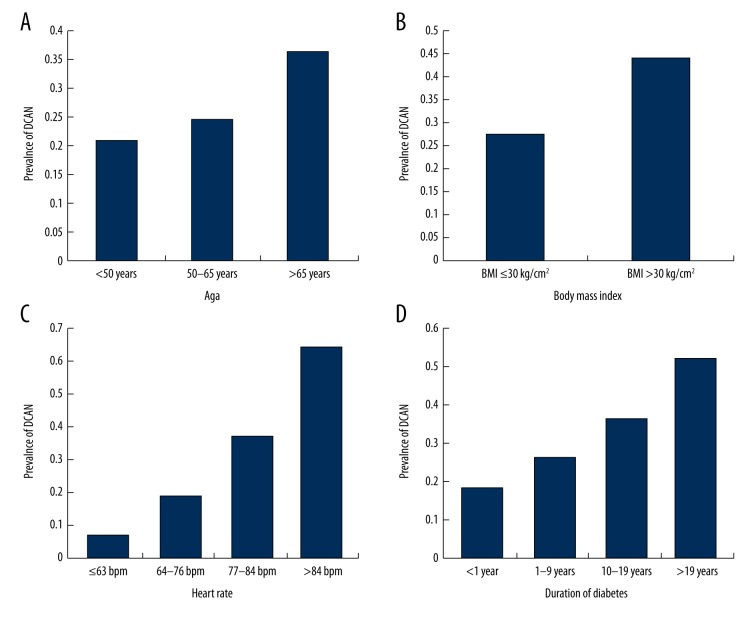
As was discussed in earlier studies [25–27], ULR analysis showed associated factors for DCAN, including age, BMI, FPG, PBG, FINS, TG, resting HR, DM duration, HTN duration, and MetS (P<0.05 for all). The simple association factors of age, BMI, DM duration, and HR were included in the final MLR model (P<0.05 for all; Table 2). The DCAN prevalence was 20.83%, 24.71%, and 36.36% for the total sample, validation set, and external set, respectively, according to age (Figure 1). There were no significant differences among the 3 groups (P<0.001 for difference and P<0.001 for a trend). There was a significant difference found in the DCAN prevalence between the 2 groups according to BMI (27.79% vs. 44.12%, P=0.004). The DCAN prevalence was 7.01%, 18.75%, 36.67%, and 64.29% in the 4 groups, respectively, according to resting HR (P<0.001 for difference and P<0.001 for a trend). There were significant differences in DCAN prevalence among the 4 groups according to DM duration (18.18%, 26.35%, 36.25%, 51.72%, respectively, with P<0.001 for difference and P<0.001 for a trend).
Table 2.
Simple risk score system for diabetic cardiovascular autonomic neuropathy in the exploratory set.
| Variable | β | P value | OR (95%CI) | Risk score* |
|---|---|---|---|---|
| Age (year) | ||||
| ≤50 years | 0.000 | – | 1.00 | 0 |
| 50–65 years | 0.452 | 0.006 | 1.57 (1.14–2.17) | 2 |
| ≥66 years | 0.912 | <0.001 | 2.48 (1.80–3.43) | 4 |
| BMI (kg/m2) | ||||
| ≤30.0 kg/m2 | 0.000 | – | 1.00 | 0 |
| >30.0 kg/m2 | 0.750 | 0.018 | 2.12 (1.14–3.95) | 3 |
| Diabetes duration | ||||
| <1 year | 0.000 | – | 1.00 | 0 |
| 1–9 years | 0.492 | 0.001 | 1.78 (1.26–2.51) | 2 |
| 10–19 years | 0.971 | <0.001 | 2.64 (2.16–3.22) | 4 |
| ≥20 years | 1.525 | <0.001 | 4.60 (3.76–5.16) | 6 |
| Heart Rate (beats/min) | ||||
| ≤63 bpm | 0.000 | – | 1.00 | 0 |
| 64–76 bpm | 1.039 | <0.001 | 2.83 (2.82–3.50) | 4 |
| 77–84 bpm | 1.891 | <0.001 | 6.23 (5.35–8.20) | 8 |
| ≥85 bpm | 2.836 | <0.001 | 17.06 (13.77–21.12) | 12 |
For each significant variable in the multiple logistic regression analysis, a risk score was calculated from the regression coefficients (β) dividing by a common factor (0.226) and rounding to the nearest integer.
Figure 1.
Comparison of prevalence of diabetic cardiovascular autonomic neuropathy (DCAN) according to risk factors. (A) Comparison of DCAN prevalence according to age. DCAN prevalence was 20.83%, 24.71% and 36.36% in the 3 groups, respectively. Additionally, there were significant differences among the 3 groups (P for difference <0.001 and P for a trend <0.001). (B) Comparison of DCAN prevalence according to body mass index (BMI). DCAN prevalence was 27.79%, and 44.12% between BMI ≤30 kg/cm2 and BMI >30 kg/cm2 group, respectively. There was a significant difference between the 2 groups (P=0.004). (C) Comparison of DCAN prevalence according to heart rate (HR). DCAN prevalence was 07.01%, 18.75%, 36.67% and 64.29% in the 4 groups, respectively. There were significant differences among the 4 groups (P for difference <0.001 and P for a trend <0.001). (D) Comparison of DCAN prevalence according to duration of diabetes (DM duration). DCAN prevalence was 18.18%, 26.35%, 36.25% and 51.72% in the 4 groups, respectively. There were significant differences among the 4 groups (P for difference <0.001 and P for a trend <0.001).
The maximum score possible in our screening model was 25. The optimal cutoff value was determined to be 12 and parameters were evaluated at this cutoff point (Sen=80.77%, Spe=68.21%, Youden Index=48.98%, and %Need=34.33%, Table 3; and AUC=0.779, Figure 2). There were no significant differences in the characteristics of the validation cohort and exploratory set (Table 1). The AUC for the total sample, validation set, and external set was 0.798 (95% CI 0.752–0.844; Figure 2), 0.756 (95% CI 0.705–0.808), and 0.729 (95% CI 0.601–0.857), respectively. The Spe, PPV, NPV, and%Need were similar among the 3 sets, whereas the Sen tended to be higher in the exploratory set than the validation set.
Table 3.
Performance of the risk score at cutoff point of 12 in the four datasets.
| Variable | Exploratory Set | Validation Set | Total sample | External dataset |
|---|---|---|---|---|
| Sensitivity (%) | 80.77 (74.05–87.49) | 80.36 (73.42–87.29) | 80.56 (74.07–87.05) | 61.54 (54.67–68.41) |
| Specificity (%) | 68.21 (57.85–78.57) | 58.27 (47.72–68.81) | 63.67 (52.94–74.4) | 85.02 (74.64–95.4) |
| Youden index (%) | 48.98 (40.47–57.5) | 38.62 (30.08–47.17) | 44.23 (35.96–52.5) | 46.56 (37.85–55.27) |
| PPV (%) | 50.55 (43.62–57.48) | 45.27 (39.27–51.27) | 47.54 (41.54–53.54) | 72.75 (66.72–78.78) |
| NPV (%) | 89.81 (86.07–93.55) | 87.35 (83.71–90.99) | 88.91 (85.63–92.19) | 77.28 (73.52–81.04) |
| Need testing (%)* | 34.33 (30.29–38.38) | 43.55 (39.04–48.07) | 38.7 (34.44–42.96) | 33.38 (28.67–38.09) |
PPV – positive predictive value; NPV – negative predictive value;
Proportion of the study sample with risk score above the cutoff value; The confidence intervals (CIs) for sensitivity, specificity, and predictive values were calculated using bootstrapping (1000).
Figure 2.
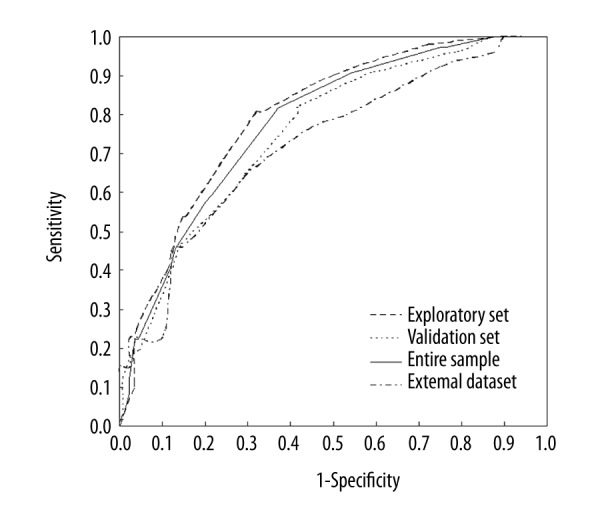
Receiver operating characteristic curves showed the performance of each cardiovascular autonomic neuropathy risk score (CRS) in predicting prevalence of diabetic cardiovascular autonomic neuropathy (DCAN) in the exploratory set, total sample, validation set and external dataset. The 95% confidence interval (CI) is given in parentheses. AUC – area under the curve. In exploratory set, AUC=0.779, 95%CI: 0.744–0.813, P<0.001; in total sample, AUC=0.798, 95%CI: 0.752–0.844, P<0.001; in validation set, AUC=0.756, 95%CI: 0.705–0.808, P<0.001; and in external dataset, AUC=0.729, 95%CI: 0.601–0.857, P=0.002.
Performance analysis for the screening model
For clinical practice, 2 cutoff points were selected to divide the total sample into 3 groups: a low risk group (score 0–7), a medium risk group (score 8–17), and a high-risk group (score 18–25) (Table 4). In the total sample, at the cutoff point of 7, the Sen and NPV were 97.22% and 95.69%, respectively. The percentage of individuals in the low risk group was 18.65% in the total sample. Additionally, in the total sample, at the cutoff point of 18, the Spe and PPV were 98.88% and 90.05%, respectively. The percentage of individuals in the high-risk group was 8.81% in the total sample.
Table 4.
Risk and predictive performance analysis of simple risk system for diabetic cardiovascular autonomic neuropathy in total sample an external dataset.
| Cutoff point | Total sample | External dataset | ||
|---|---|---|---|---|
| 7 (Score 0–7) | 18 (Score 18–25) | 7 (Score 0–7) | 18 (Score 18–25) | |
| Risk value | Low risk | High risk | Low risk | High risk |
| Sensitivity (%) | 97.22 (91.12–103.33) | 25.22 (24.99–25.45) | 96.15 (95.18–97.13) | 23.08 (22.97–23.18) |
| Specificity (%) | 24.82 (14.41–35.23) | 98.88 (88.21–109.55) | 31.4 (20.43–42.37) | 97.5 (87.23–107.77) |
| Youden Index (%) | 22.04 (13.48–30.6) | 24.1 (15.9–32.3) | 27.55 (18.83–36.28) | 20.58 (11.74–29.42) |
| PPV (%) | 34.22 (27.3–41.15) | 90.05 (83.47–96.63) | 47.67 (41.38–53.95) | 85.71 (79.34–92.09) |
| NPV (%) | 95.69 (92.19–99.19) | 76.67 (73.53–79.82) | 92.63 (89.07–96.19) | 66.11 (63.07–69.14) |
| % Total sample | 18.65 (13.72–23.59) | 8.81 (4.53–13.09) | 24.24 (19.68–28.8) | 10.61 (6.6–14.62) |
PPV – positive predictive value; NPV – negative predictive value; The confidence intervals (CIs) for sensitivity, specificity, and predictive values were calculated using bootstrapping (1000).
In the external dataset, the Sen at the cutoff point of 7 and the Spe at the cutoff point of 18 were 96.15% and 97.50%, respectively. In addition, the NPV was 92.63% at the cutoff point of 7, and the PPV was 85.71% at the cutoff point of 18. The 2 cutoff points have a high prediction rate for the risk of DCAN. This was the highest proportion of individuals with a score of 12–13 (18.36% in the total sample; Figure 3). The DCAN prevalence increased as the DCAN score increased in the total sample. The highest DCAN prevalence was more than 75.00% in subjects with a score of 19–25 (data not shown).
Figure 3.
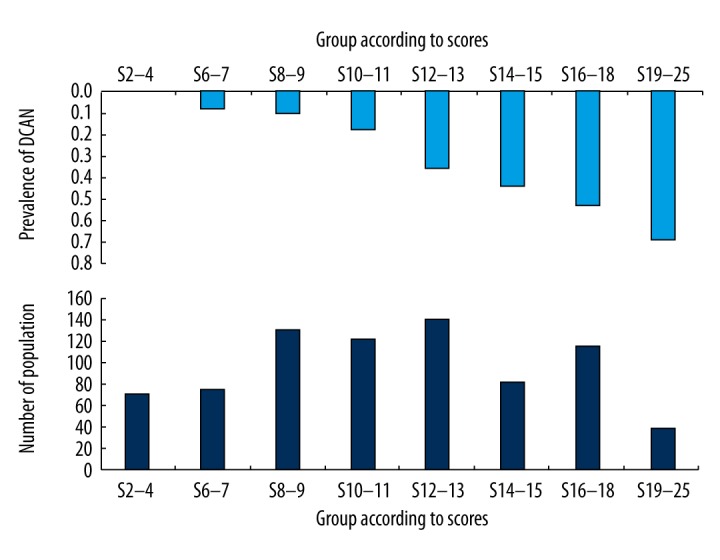
Distribution of diabetic cardiovascular autonomic dysfunction (CAN) risk score (bottom bars) and DCAN prevalence (upper bars) against DCAN risk score in the modeling dataset.
Comparison of DCAN subjects with low and high scores
There were significantly higher values for resting HR and DM duration in DCAN subjects with high scores as compared with those with low scores (P<0.001, Table 5), while there were lower HDL levels in DCAN subjects with high scores (P=0.001). No significant differences were found in the other variables between the 2 groups (P>0.05 for all).
Table 5.
Comparison of individuals with diabetic cardiovascular autonomic neuropathy in high- and low- score groups.
| Variable | High-score group (12–26) | Low-score group (0–11) | P value |
|---|---|---|---|
| N | 111 | 21 | – |
| Age years | 64.71±8.91 | 63.9±9.15 | 0.592 |
| Gender male,% | 57 (51.35%) | 8 (38.1%) | 0.115 |
| BMI kg/cm2 | 25.73±3.98 | 25.02±4.96 | 0.306 |
| SBP mmHg | 135.47±20.13 | 135.95±22.93 | 0.889 |
| DBP mmHg | 81.3±9.79 | 80.71±8.44 | 0.716 |
| FPG mmol/L | 7.88±2.91 | 7.87±4.38 | 0.994 |
| PBG mmol/L | 13±4.3 | 13.69±4.74 | 0.350 |
| FINS uml/L | 12±29.67 | 21.32±60.67 | 0.129 |
| TC mmol/L | 5.44±1.11 | 5.43±1.24 | 0.989 |
| TG mmol/L | 2.3±1.39 | 2.04±1.31 | 0.263 |
| HDL mmol/L | 1.26±0.27 | 1.41±0.32 | 0.001 |
| LDL mmol/L | 3.29±0.9 | 3.11±0.86 | 0.227 |
| HR bpm | 83.1±10.52 | 71.74±6.27 | <0.001 |
| HTN duration years | 7.88±10.88 | 5.25±8.63 | 0.150 |
| DM duration years | 9.11±8.62 | 3.62±3.98 | <0.001 |
| Smoking yes, % | 25 (22.52%) | 4 (19.05%) | 0.618 |
| HTN yes, % | 73 (65.77%) | 14 (66.67%) | 0.910 |
| MetS yes, % | 90 (81.08%) | 15 (71.43%) | 0.155 |
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; FPG – fasting plasma glucose; PBG – plasma blood glucose; TC – serum total cholesterol; TG – triglyceride; HDL – high-density lipoprotein cholesterol; LDL – low density lipoprotein cholesterol; HR – heart rate; MetS – metabolic syndrome; HTN – hypertension.
Discussion
In this community-based study, short-term HRV was measured non-invasively using a power spectral analysis to evaluate the cardiovascular autonomic (CA) function because this test has good reproducibility and is practical for use in clinical practice. More importantly, this study resulted, to the best of our knowledge, in the first risk score system for DCAN screening, which can be applied not only in a Chinese population but also globally. We employed MLR methods to develop the DCAN risk score system in the exploratory set and confirmed them in another validation set and in the total sample. Finally, a DCAN screening system was created for identifying DCAN in Chinese diabetic patients.
For convenience and low cost, our screening system includes age, BMI, DM duration, and resting HR, allowing people to evaluate their risk of DCAN through simple calculations. The highest DCAN prevalence was more than 75.00% in subjects with a score of 19–25. It has been recommended that ROC curves be employed to evaluate the performance of screening models and diagnostic tests, which we used [28]. At a cutoff score of 12, the screening system had a high Sen of 80.77% in the exploratory set and a low%Need of 34.33%. Importantly, the scoring system, which is derived from a simple model without blood tests, performed well when it was validated in an independent cohort. The results confirmed that our risk score system performs well in the prediction of DCAN. The optimal cutoff score determined for the external validation set was identical to the optimal cutoff score determined for the total sample, further confirming the utility of the cutoff score.
Several prior studies explored DM risk scores in various populations, and most of them were developed with Caucasian populations [14–19], while only a few risk scores were based on Asian populations, including Chinese populations [20–22,31]. However, no DCAN risk score systems have been developed for diabetic populations prior to this study. Previously, we reported that, in the absence of a gold standard, a short-term HRV test for DCAN diagnosis could be used with high Sen and Spe. In our previous study, Bayesian analysis was applied for estimating diagnostic parameters for DCAN in Chinese diabetic patients [13]. Our findings are important for the clinical diagnosis of DCAN in diabetic patients. Additionally, we previously developed a risk score system to predict people at high risk of CAN in a random sample of the Chinese population [28]. That screening system consists of simple parameters – age, BMI, HTN, and resting HR – that make it more convenient and less expensive than other models based on complex cardiovascular autonomic reflex tests. Our model enabled detection of 74.24% of individuals with previously diagnosed CAN who had risk scores between 16 and 37 and decreased%Need to 37.23% [24]. In the present study, the risk system had higher predictive performance for screening DCAN in the Chinese population.
In this study, the prevalence of DCAN ranged from 28% to 36% in our samples. The estimated DCAN prevalence in DM patients was found to be 30% to 60% in other studies [4,32], indicating that our results were consistent with these studies. Generally, a screening system that includes simple factors cannot have a 100% Sen [28]. This is partly because the variables of a screening system cannot completely represent differences between false-negative and true-positive individuals. Additionally, we found that overweight or obese DCAN patients can have normal resting HR due to both an impaired sympathetic and parasympathetic nervous system. The comparison analysis of true-positive and false-negative groups indicated that there were differences in the HDL levels.
The simple model that we developed to screen DCAN in Chinese diabetic patients is based on several simple variables, which are easy to assess in clinical practice. This screening system could also be conveniently used in primary care. The possibility of selection bias in this study was reduced by using a community-based population. The screening system was found to be stable, as demonstrated by internal validation. Our study showed that the screening system could efficiently identify diabetic patients with high risks of DCAN, depending on the risk score cutoff point. The screening system could be particularly useful as an effective and practical screening tool to enhance people’s awareness of DCAN. Diabetic patients with high-risk scores may benefit from receiving health education intervention with the opportunity to engage in lifestyle modifications that prevent or delay the onset of DCAN. Summarily, according to simple variables – age, BMI, diabetic duration and hypertension, physicians can make appropriate clinical decisions for diabetic patients to treat their DCAN complications.
However, several potential limitations of this study should be mentioned. Because a cross-sectional study was used to create the screening model, we were unable to confirm a direct causal relationship among association factors and DCAN. Additionally, because all participants were aged between 30 years and 80 years, our model may be less practical for younger or older diabetic patients.
Conclusions
This study offered evidence that our screening system has a high Sen and Spe for DCAN diagnosis in Chinese diabetic patients and can be applied as a self-assessment tool in primary medical care practice for identifying high-risk subjects with DCAN among Chinese diabetic patients.
Acknowledgments
We thank the grant from Institutes of Integrative Medicine of Fudan University to support the study.
Abbreviations
- BP
blood pressure
- BMI
body mass index
- CI
confidence intervals
- Cr
creatinine
- DM
diabetes
- DCAN
diabetic cardiovascular autonomic neuropathy
- FPG
fasting plasma glucose
- HDL
high-density lipoprotein cholesterol
- HRV
heart rate variability
- HTN
hypertension
- LDL
low-density lipoprotein cholesterol
- MetS
metabolic syndrome
- MLR
multivariable logistic linear regression
- OGTT
oral glucose tolerance test
- OR
odds ratios
- PBG
postprandial blood glucose
- RACE
rapid autonomic cardiovascular evaluation
- TC
serum total cholesterol
- TG
triglyceride
- UA
uric acid
Footnotes
Conflict of interest
None.
Source of support: Institutes of Integrative Medicine of Fudan University, Shanghai (ClinicalTrials.gov Identifier: NCT02461472); and Shanghai Development Project of Shanghai Peak Disciplines-Integrative Medicine (20150407); and China Postdoctoral Science Foundation funded project (2017M611461)
Reference
- 1.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–79. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 2.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: A meta-analysis. Diabetes Care. 2003;26:1895–901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 3.Hazari MA, Khan RT, Reddy BR, Hassan MA. Cardiovascular autonomic dysfunction in type 2 diabetes mellitus and essential hypertension in a South Indian population. Neurosciences. 2012;17:173–75. [PubMed] [Google Scholar]
- 4.Spallone V, Ziegler D, Freeman R, et al. Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–53. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 5.Banthia S, Bergner DW, Chicos AB, et al. Detection of cardiovascular autonomic neuropathy using exercise testing in patients with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:64–69. doi: 10.1016/j.jdiacomp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–97. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Wei D, Zhou Z, et al. Anti-PLA2R antibodies in Chinese patients with membranous nephropathy. Med Sci Monit. 2016;22:1630–36. doi: 10.12659/MSM.896090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li YD, Ji YT, Zhou XH, et al. TNNT2 gene polymorphisms are associated with susceptibility to idiopathic dilated cardiomyopathy in Kazak and Han Chinese. Med Sci Monit. 2015;21:3343–47. doi: 10.12659/MSM.894630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler D, Zentai C, Perz S, et al. KORA Study Group. Selective contribution of diabetes and other cardiovascular risk factors to cardiac autonomic dysfunction in the general population. Exp Clin Endocrinol Diabetes. 2006;114:153–59. doi: 10.1055/s-2006-924083. [DOI] [PubMed] [Google Scholar]
- 10.Kamphuis MH, Geerlings MI, Dekker JM, et al. Autonomic dysfunction: A link between depression and cardiovascular mortality? The FINE Study. Eur J Cardiovasc Prev Rehabil. 2007;14:796–802. doi: 10.1097/HJR.0b013e32829c7d0c. [DOI] [PubMed] [Google Scholar]
- 11.Min KB, Min JY, Paek D, et al. Is 5-minute heart rate variability a useful measure for monitoring the autonomic nervous system of workers? Int Heart J. 2008;49:175–81. doi: 10.1536/ihj.49.175. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Tang ZH, Zeng F, Zhou L. Associations between the severity of metabolic syndrome and cardiovascular autonomic function in a Chinese population. J Endocrinol Invest. 2013;36:993–99. doi: 10.3275/9005. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Yang SB, Liu J, Tang ZH. Diagnostic performance analysis for diabetic cardiovascular autonomic neuropathy based on short-term heart rate variability using Bayesian methods: Preliminary analysis. Diabetol Metab Syndr. 2015;7:74. doi: 10.1186/s13098-015-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindstrom J, Tuomilehto J. The diabetes risk score: A practical tool to predict type 2 diabetes risk. Diabetes Care. 2003;26:725–31. doi: 10.2337/diacare.26.3.725. [DOI] [PubMed] [Google Scholar]
- 15.Glumer C, Carstensen B, Sandbaek A, et al. inter99 study. A Danish diabetes risk score for targeted screening: the Inter99 study. Diabetes Care. 2004;27:727–33. doi: 10.2337/diacare.27.3.727. [DOI] [PubMed] [Google Scholar]
- 16.Schulze MB, Hoffmann K, Boeing H, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care. 2007;30:510–15. doi: 10.2337/dc06-2089. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MI, Duncan BB, Bang H, et al. Atherosclerosis Risk in Communities Investigators. Identifying individuals at high risk for diabetes: The Atherosclerosis Risk in Communities study. Diabetes Care. 2005;28:2013–18. doi: 10.2337/diacare.28.8.2013. [DOI] [PubMed] [Google Scholar]
- 18.Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: Do we need the oral glucose tolerance test? Ann Intern Med. 2002;136:575–81. doi: 10.7326/0003-4819-136-8-200204160-00006. [DOI] [PubMed] [Google Scholar]
- 19.Wilson PW, Meigs JB, Sullivan L, et al. Prediction of incident diabetes mellitus in middle-aged adults: The Framingham Offspring Study. Arch Intern Med. 2007;167:1068–74. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 20.Aekplakorn W, Bunnag P, Woodward M, et al. A risk score for predicting incident diabetes in the Thai population. Diabetes Care. 2006;29:1872–77. doi: 10.2337/dc05-2141. [DOI] [PubMed] [Google Scholar]
- 21.Sun F, Tao Q, Zhan S. An accurate risk score for estimation 5-year risk of type 2 diabetes based on a health screening population in Taiwan. Diabetes Res Clin Pract. 2009;85:228–34. doi: 10.1016/j.diabres.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Chien K, Cai T, Hsu H, et al. A prediction model for type 2 diabetes risk among Chinese people. Diabetologia. 2009;52:443–50. doi: 10.1007/s00125-008-1232-4. [DOI] [PubMed] [Google Scholar]
- 23.Ge X, Pan SM, Zeng F, et al. A simple Chinese risk score model for screening cardiovascular autonomic neuropathy. PLoS One. 2014;9:e89623. doi: 10.1371/journal.pone.0089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang ZH, Zeng F, Li Z, Zhou L. A risk score of cardiovascular autonomic dysfunction for targeted screening in the Chinese population. Int J Cardiol. 2013;168:4861–62. doi: 10.1016/j.ijcard.2013.07.062. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Zhou L, Tang Z. An association analysis of lipid profile and diabetic cardiovascular autonomic neuropathy in a Chinese sample. Lipids Health Dis. 2016;15:122. doi: 10.1186/s12944-016-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge X, Chen H, Zhang K, Tang ZH. The analysis of blood pressure profiles and their severity in relation to diabetic cardiovascular autonomic neuropathy in the Chinese population: preliminary analysis. J Endocrinol Invest. 2016;39:891–98. doi: 10.1007/s40618-016-0444-6. [DOI] [PubMed] [Google Scholar]
- 27.Fang P, Dong J, Zeng F, Tang Z. Analysis of the association between glucose profiles and beta cell function for diabetic cardiovascular autonomic neuropathy in China. J Diabetes Investig. 2017;8(3):354–62. doi: 10.1111/jdi.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang ZH, Zeng F, Yu X, Zhou L. Bayesian estimation of cardiovascular autonomic neuropathy diagnostic test based on baroreflex sensitivity in the absence of a gold standard. Int J Cardiol. 2014;171:e78–80. doi: 10.1016/j.ijcard.2013.11.100. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Tang ZH, Zeng F, et al. Artificial neural network models for prediction of cardiovascular autonomic dysfunction in general Chinese population. BMC Med Inform Decis Mak. 2013;13:80. doi: 10.1186/1472-6947-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su YC, Chen LL, Lin JD, et al. BCQ+: A body constitution questionnaire to assess Yang-Xu. Part I: establishment of a first final version through a Delphi process. Forsch Komplementmed. 2008;15:327–34. doi: 10.1159/000175938. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Pan C, Jin M. A Chinese diabetes risk score for screening of undiagnosed diabetes and abnormal glucose tolerance. Diabetes Technol Ther. 2011;13:501–7. doi: 10.1089/dia.2010.0106. [DOI] [PubMed] [Google Scholar]
- 32.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: A clinical perspective. Diabetes Care. 2010;33:434–41. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]