Abstract
The unique spectral signatures and biologically inert compositions of surface-enhanced (resonance) Raman scattering (SE(R)RS) nanoparticles make them promising contrast agents for in vivo cancer imaging. Subtle aspects of their preparation can shift their limit of detection by orders of magnitude. In this protocol, we present the optimized, step-by-step procedure for generating reproducible SERRS nanoparticles with femtomolar (10−15 M) limits of detection. We introduce several applications of these nanoprobes for biomedical research, with a focus on intraoperative cancer imaging via Raman imaging. A detailed account is provided for successful intravenous administration of SERRS nanoparticles such that delineation of cancerous lesions may be achieved without the need for specific biomarker targeting. The time estimate for this straightforward, yet comprehensive protocol from initial de novo gold nanoparticle synthesis to SE(R)RS nanoparticle contrast-enhanced preclinical Raman imaging in animal models is ~96 h.
INTRODUCTION
Raman imaging is an optical imaging modality that holds promise for both preclinical and clinical cancer imaging1,2. Raman imaging is based on an inelastic interaction of light with matter. While most photons scatter elastically (i.e. Rayleigh scattering), 1 in 107 photons either deposits (i.e. Stokes) or gains energy (i.e. anti-Stokes) by causing vibrational transitions within a molecule in a process known as Raman scattering3. Since these vibrational transitions are related to the respective molecular bonds, they are unique to the molecule and generate distinct, fingerprint-like Raman spectra. Label-free Raman imaging is currently being explored clinically to discriminate healthy from abnormal tissues based on differences in Raman fingerprints, particularly for cancer screening of the skin, cervix, and gastrointestinal tract2. However, since only a small fraction of the photons is Raman scattered, intrinsic Raman imaging requires long acquisition times. Accordingly, applications that require near real-time imaging must employ a means to amplify the signal intensity of the Raman spectra being tracked. One means of accomplishing this is to introduce a pump laser that stimulates enhanced emission of Raman scattering4,5. This process, called stimulated Raman scattering (SRS) shows promise for clinical applications, but the instrumentation required is not widely available. The other, more common approach to enable rapid in vivo Raman imaging is to introduce exogenous contrast agents that exhibit Raman spectra many orders of magnitude greater than any intrinsic signal6,7. These contrast agents exploit a phenomenon known as surface-enhanced Raman scattering (SERS), wherein a noble metal nanoparticle acts as an antenna to amplify the Raman scattering intensity of molecules adsorbed on its surface. The magnitude of this amplification is highly dependent on the properties of the Raman scattering molecule (called a “Raman reporter” molecule), the number of molecules that are directly adsorbed on the nanoparticle surface, and the size of the nanoparticle core with 50–60 nm gold nanoparticles providing the strongest enhancements8,9. In this protocol, we describe how to prepare, characterize, and conjugate SERRS nanoparticles to enable molecular imaging of lesions in preclinical cancer models using Raman imaging.
Development of the protocol
One of the first examples demonstrating sensitive detection and delineation of tumors using contrast-enhanced Raman imaging using SERS nanoparticles was performed in mouse models of glioblastoma6. The SERS nanoparticles were non-targeted and therefore their accumulation in the glioblastoma tissues resulted from a passive targeting mechanism known as the enhanced permeability and retention (EPR) effect10. Since this mechanism is ubiquitously observed in a variety of solid tumors, we expected that early generation SERS nanoparticles should accumulate in other solid tumors as well, albeit in concentrations below their limit of detection, which is in the low picomolar range6. Thus, we hypothesized that if we could develop a new generation of SE(R)RS nanoparticles optimized to achieve sufficiently low limits of detection, we would be able to detect a wide range of cancers using intravenously administered SE(R)RS nanoparticles.
From a theoretical perspective, it was known that maximizing the resonant interactions at the metal-molecule interface11,12 should enable markedly higher SERS intensities. Therefore, we incorporated Raman reporter molecules in resonance with the 785-nm laser used in our Raman imaging system, producing a metal-molecule system with greatly enhanced polarizability, and thus superior Raman scattering intensity with respect to the first-generation non-resonant SERS nanoparticles13. Moreover, we redesigned the synthesis protocol for encapsulation of the gold cores with silica. Silica not only stabilizes the gold core, but, because it also serves as a matrix for the resonant Raman reporter, it also prevents the desorption of the resonant Raman reporter from the gold core under physiological condition ensuring constancy of the Raman fingerprint. Unlike other protocols14–16, our redesigned silica encapsulation protocol obviates the need for stabilizing polymers or auxiliary surface primers at the gold surface, thereby promoting increased surface availability for adsorption of resonant Raman reporter molecules13.
Both steps proved critical in achieving orders-of-magnitude better limits of detection because the electromagnetic “antenna” effect provided by the gold core falls off with distance according to an r−12 dependence, and the SERS selection rules require electronic coupling between the reporter molecules and nanoparticle surface for optimal enhancement to Raman scattering13. Lastly, an increase of the affinity of the resonant Raman reporter for the gold surface by incorporation of sulfur-containing functional groups such as thiophenes resulted in lowering the limit of detection by another order-of-magnitude11,12,17. Collectively, the above-described improvements afforded SERRS nanoparticles with attomolar (10−18 M) detection limits under conditions amenable to in vivo imaging9.
Applications of SERRS nanoparticles
Nanotechnology holds great promise for cancer diagnostics and therapy. The (sub)femtomolar (≤10−15 M) limits of detection of SERRS nanoparticles and straightforward synthesis and surface modification described in this protocol makes SERRS nanoparticle-based Raman imaging an ideal platform to study the interaction of nanoparticles with biological systems. Since we were particularly interested in early and comprehensive cancer detection, we administered the optimized surface-enhanced resonance Raman scattering (SERRS) nanoparticles intravenously into a variety of mouse models bearing extracranial tumors. We had hypothesized previously that the increased sensitivity of the new generation of SERRS nanoparticles would overcome the barrier that previously prevented us from universal tumor imaging based on the EPR effect alone. This hypothesis was now confirmed in vivo, as the new SERRS nanoparticles, in their non-targeted form (i.e. poly(ethyleneglycol)-coating only) indeed enabled detection of tumors in all tested preclinical models including sarcomas, breast-, prostate-, and pancreatic cancer13. Moreover, we found that these non-targeted nanoparticles also delineated pre-cancerous lesions after intravenous administration13. This is of great clinical relevance because at this stage the patient would still be amenable to therapeutic intervention with curative intent. Another clinically relevant application is the use of SERRS nanoparticle-guided resection particularly in cancers where there is no room for wide safety margins such as glioblastoma or pancreatic cancer. We have shown that SERRS nanoparticles selectively accumulate in tumor- and tumor-associated tissue and has high tumor-to-background ratios. In this way, SERRS nanoparticle-based Raman imaging provided accurate delineation of the tumors. It has also enabled detection of lymph node metastases in a preclinical prostate cancer model18. Clinical Raman imaging endoscopes have been developed and that have been tested preclinically and even in human patients19. This, in combination with the fact that i) the SERRS produced with this protocol consist of inert materials, ii) other Raman nanoparticles of similar size and material composition have been shown to be nontoxic20, and iii) other gold or gold-silica nanoparticles have already advanced into clinical trials, suggests a viable path towards clinical translation of contrast-enhanced Raman imaging for cancer detection21.
Since different ‘flavors’ of SERRS nanoparticles can be produced that have equally low limits of detection but generate distinct fingerprint-like Raman spectra, SERRS-based Raman imaging offers tremendous capabilities for multiplexing. This, in combination with the straightforward silica surface modification of SERRS nanoparticles, allows for rapid and efficient conjugation of targeting moieties such as antibodies, nanobodies, peptides, and small molecules to enable actively targeted molecular imaging9,22. SERRS nanoparticles targeted towards widely established diagnostic markers can be applied ex vivo or in vitro to inform rapidly on the molecular profile of resected tissue or tumor cells. For instance, following tumor resection, the tumor can be surveyed with Raman imaging ex vivo to determine the margin status based on SERRS-signal positivity. Alternatively, the resected tissue could be incubated ex vivo with different flavors of SERRS nanoparticles targeted towards widely established diagnostic markers such as EGFR, HER2, MUC1, and EpCAM to provide an immediate molecular profile of the tumor23,24. Ratiometric Raman imaging of breast cancer lumpectomy tissue has demonstrated the viability of this multiplexed approach for ex vivo applications24, and the ability to discriminate tumor A431 cells from non-tumor 3T3 2.2 cells validated the utility of SERS multiplexing in vitro23. Multiplexed SERS nanoparticles have also been used to identify circulating tumor cells from unprocessed human blood25.
Lastly, SERRS nanoparticles have or can be given multimodal imaging properties6. For instance, the gold nanoparticle core serves as a contrast agent for CT and optoacoustic imaging, and the silica shell can be coated with gadolinium for contrast on magnetic resonance imaging (MRI)6. Additionally, the high affinity of silica for radioisotopes such as 89Zr and 68Ga can enable whole-body imaging capabilities using positron-emission tomography (PET)9,26. Apart from detection of premalignant and malignant lesions, nanoparticles have been shown to accumulate in atherosclerotic plaques and in ischemic lesions following myocardial infarction and therefore can play an important role in cardiovascular disease (CVD) imaging as well27,28.
Comparisons with other methods
Many methods exist for imaging cancer in vivo, each with qualities that make them advantageous for certain applications and disadvantageous for others. Well-established modalities include PET, MRI, and ultrasound imaging29,30. These techniques are preferable over optical approaches for deep tissue imaging and pre-operative staging. Optical imaging is generally better-suited for applications that require high spatial-resolution, such as intraoperative detection of small tumor deposits to facilitate complete surgical resection. Among optical technologies, fluorescence imaging has so far been the most widely used31. However, background tissue fluorescence (i.e., autofluorescence) can lead to false positive signals, and fluorescent contrast agents suffer from rapid degradation during imaging (i.e., photobleaching). SE(R)RS nanoparticles currently offer the lowest limits of detection (i.e., greatest sensitivity) and demonstrate stability with respect to photobleaching, making them ideal candidates for intraoperative imaging13,32.
Several alternative protocols exist for the preparation of SE(R)RS nanoparticles. The primary advantage of our method is the very low limit of detection, which to our knowledge is currently unmatched. This is afforded by the optimized metal-molecule system. The gold nanoparticle cores used in this protocol are not stabilized by auxiliary agents like surfactants or polymers. Such stabilization inhibits binding of Raman reporter molecules at the gold surface. Current alternative protocols rely upon, for example, thiolated polyethylene glycol (PEG)33–36, polyvinylpyrrolidone (PVP)37, mercaptoundecanoic acid (MUA)38, or sodium dodecyl sulfate and protein39 to provide sufficient stability to the gold nanoparticle core such that it can withstand the destabilizing effect of Raman reporter molecules adsorbing to the surface. The cost of stabilizing the gold surface prior to addition of the reporter molecules is that less of the surface is available for binding, which can decrease the SERS intensity of the final nanoparticle construct by orders of magnitude.
Instead of stabilizing the gold nanoparticles with surfactants or polymers prior to reporter molecule addition, we perform a single-step reaction that requires no auxiliary surface priming molecules. This approach relies on a careful manipulation of the encapsulation kinetics (i.e., silica shell formation) such that molecular clusters of silica begin condensing onto the metal-molecule interface precisely as the binding of reporter molecules destabilizes the colloidal dispersion. Since silica has relatively low affinity for the gold surface, it does not substantially displace the Raman reporter molecules, which have a higher affinity for the gold surface. Continued growth of the silica shell imparts lasting colloidal stability without decreasing the intensity of the SE(R)RS signal. We quench the silica growth at will, such that we can promote fast encapsulation kinetics while preventing unwanted formation of free silica nanoparticles (i.e., without gold nanoparticle cores) yielding narrowly dispersed SERS nanoparticle preparations with (sub)femtomolar limits of detection.
Experimental Design
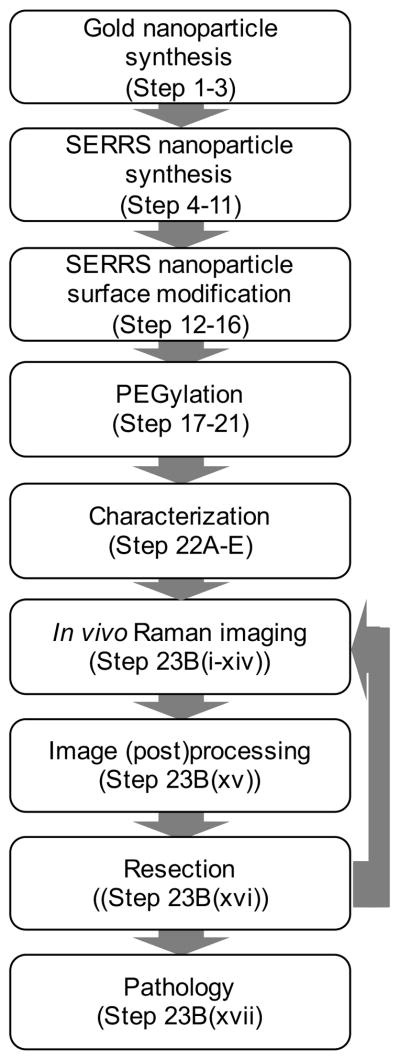
The workflow for preclinical contrast-enhanced Raman imaging of cancer using SERRS nanoparticles is depicted in Figure 1. A schematic representation of SERRS nanoparticle synthesis, which is described in this protocol, is shown in Figure 2.
Figure 1.
Flowchart of the workflow for SERRS-based Raman imaging of cancer. Firstly, the gold nanocores are synthesized and coated with silica in the presence of a dye that is resonant with the excitation laser wavelength (i.e. 785 nm) to afford the SERRS nanoparticles. The surface of SERRS nanoparticles can be modified to include PEG functionality. The functionalized SERRS nanoparticles are injected intravenously via tail vein in tumor-bearing animals and imaged using Raman imaging. The data is converted into an image and enables SERRS-based image-guided resection. Rescanning of the tumor-bed ensure adequate resection. If residual SERRS signal is detected more tissue can be resected. Finally, the resected tissues are processed for histological examination.
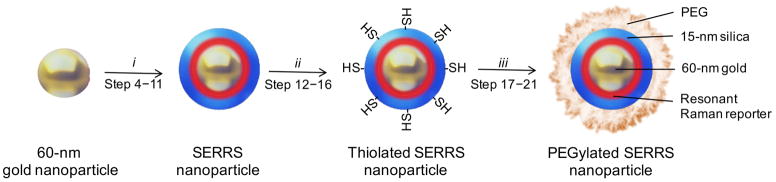
Figure 2.
Schematic representation of PEGylated SERRS nanoparticle synthesis. Dialyzed 60-nm gold nanoparticles are encapsulated with silica in the presence of a resonant Raman reporter. The as-synthesized SERRS nanoparticles are thiolated and subsequently coated with PEG via straightforward maleimide-chemistry. Reaction conditions: (i) 5.7 M water, 0.25 M NH3, 1 nM 60 nm gold nanoparticles, and 5–65 μM resonant Raman reporter (depending on the reporter) in isopropanol for 30 min.; (ii) 0.71 M water, 0.33 M NH3, 4 nM SERRS nanoparticles, and 0.49 M MPTMS in ethanol at 70 °C for 2 h; (iii) 0.5% (wt/vol) PEG-maleimide, 4 nM thiolated SERRS nanoparticles, in 10 mM MES buffer (pH 7.1) for 2 h.
We use a modified Turkevich method to synthesize gold nanoparticles with a size around 60 nm40. We obtain nanospheres by rapidly adding a citrate solution to a stirring, boiling solution of gold chloride. Alternatively, to obtain star-shaped gold nanoparticles of similar size, we rapidly add a gold chloride solution to an ice-cold solution of ascorbic acid. In both cases, we collect the as-synthesized gold nanoparticles by centrifugation and dialyze the gold nanoparticles to remove any residual salts. The size and shape of the as-synthesized nanoparticles can be measured on a transmission electron microscope (TEM). The size and concentration can be measured using nanoparticle tracking analysis (NTA). The NTA measurements are complementary to the TEM images, because the TEM provides information on the size, shape and integrity of the nanoparticles, while the NTA provides the concentration and size distribution of the nanoparticle dispersion.
We encapsulate the dialyzed gold nanoparticles with silica using a modified Stöber procedure41. Of note, commercially available gold nanoparticle dispersions are synthesized using proprietary procedures and often auxiliary agents are added to stabilize these gold nanoparticle dispersions. These are incompatible with the current protocol and therefore commercially available gold nanoparticle dispersions may not provide the expected results using this protocol. We perform silica encapsulation in the presence of a Raman reporter that is resonant with the excitation laser wavelength, which is 785 nm in the current protocol. Unlike other protocols14–16, the current SERRS nanoparticle synthesis protocol obviates the need for any commonly used auxiliary gold surface priming or stabilizing agents such as (3-mercaptopropyl)trimethoxysilane (MPTMS), (3-aminopropyl)-trimethoxysilane (APTMS), albumin, or polyethylene glycol (PEG)-thiol, meaning silica shells can be grown directly from the gold nanoparticles affording significantly higher SERRS signal sensitivities and lower limits of detection13. To prevent gold nanoparticle aggregation, the gold nanoparticles do need to be dialyzed prior to silica encapsulation to remove any residual salts42. Further, we have recently shown that unlike kosmotropic counter anions such as chloride, cationic dyes with chaotropic counter anions such as iodide, tetrafluoroborate (BF4−), hexafluorophosphate (PF6−), and perchlorate (ClO4−) stabilize the colloidal dispersion thereby minimizing gold nanoparticle aggregation during silica encapsulation9 yielding narrowly dispersed SERRS nanoparticles. While we monitored the integrity of the nanoparticle dispersions by eye or UV/VIS spectroscopy for the spherical gold nanoparticles, we recommend to use TEM and NTA to validate SERRS nanoparticle integrity, size distribution, and measure the concentration. Furthermore, we performed Raman spectroscopy to measure the intensity of the signal generated by the SERRS nanoparticles.
We functionalize the surface of SERRS nanoparticles by heating them in the presence of MPTMS or APTMS to introduce thiol- or amine functionality, respectively. However, since the introduction of amine groups switches the zeta-potential of the SERRS nanoparticles from negative to positive, the SERRS nanoparticles readily adsorb to the plastic container. In the current protocol, therefore, only surface functionalization of SERRS nanoparticles with MPTMS is described.
Once we have functionalized the SERRS nanoparticles with MPTMS, we use the introduced thiols for conjugation of PEG (Step 17–21) or targeting moieties (e.g. antibodies; Step 17–21) to the SERRS nanoparticle surface using maleimide-functionalized PEG or heterobifunctional maleimide functionalized linkers, respectively. We redisperse the functionalized SERRS nanoparticles in 10 mM 2-(N-morpholino)ethanesulfonic acid (pH 7.3).We evaluate the serum stability of the PEGylated SERRS nanoparticles in terms of colloidal stability and Raman signal intensity using NTA/TEM and Raman spectroscopy, respectively.
For Raman imaging, we inject the SERRS nanoparticles intravenously via the tail vein of tumor-bearing animals. After 3–18 h, we anesthetize or sacrifice the SERRS nanoparticle-injected, tumor-bearing animal and we place the animal on the platform of the Raman imaging system. As described in this protocol, we use a commercially available Raman imaging system (InVia, Renishaw Inc.). We perform a raster scan of the region of interest and generate a Raman image using a direct classical least square (DCLS) algorithm. We resect SERRS-positive tissue and process for histopathological examination. Following resection, another Raman scan can be performed to validate whether all SERRS-positive tissue has adequately been removed.
Limitations
Although the described properties of SERRS nanoparticles such as high signal sensitivity and specificity are highly sought-after features for molecular imaging agents both in a preclinical and clinical setting, currently Raman imaging is not widely applied. Important underlying reasons are that, unlike other optical imaging modalities such as fluorescence, SERRS nanoparticles are not commercially available and instrumentation for Raman imaging is currently not widespread. Our protocol offers a step-by-step procedure that yields injectable (targeted) SERRS nanoparticles within 6 hours. Regarding instrumentation, the instrument components are widely available and step-by-step protocols exist to build affordable Raman imaging systems19,43,44 that can be tailored to a specific (clinical) application such as endoscopy. Further, the SERRS nanoparticles described in this protocol are optimized for detection using a 785-nm excitation laser. Even at this laser wavelength in the near-infrared window tissue penetration depth is limited to several millimeters. This limitation can be addressed by spatially offset Raman spectroscopy (SORS)45, which is based on the detection of Raman-shifted photons offset from the excitation position. Application of SORS for detection of SERRS nanoparticles, which is known as surface-enhanced spatially offset Raman scattering (SESORS) reportedly enables the detection of SERRS nanoparticles at several centimeters tissue depth46.
MATERIALS
Reagents
Gold chloride trihydrate (HAuCl4 3H2O; ≥99.9% trace metal basis; Sigma-Aldrich, cat.no. 520918) !Caution Gold chloride causes discoloration of the skin. Wear gloves when handling gold chloride. Further gold chloride trihydrate is hygroscopic. Store gold chloride trihydrate in a desiccator.
Sodium citrate tribasic dihydrate (Sigma-Aldrich, cat no. S4641)
Ascorbic acid (AA; Sigma-Aldrich, cat.no. A5960)
Deionized water (18.2 MΩcm; Elga, Model Purelab Ultra Genetic)
Nitric acid (70%; Sigma-Aldrich, cat.no.438073). !Caution Nitric acid is corrosive. Wear gloves and eye protection when handling it.
Hydrochloric acid (36.5–38.0%; Sigma-Aldrich, cat.no.H1758) !Caution Hydrochloric acid is corrosive. Wear gloves and eye protection when handling it.
Sodium chloride (Sigma-Aldrich, cat.no. S7653)
Sodium bicarbonate (Sigma-Aldrich, cat.no. S5761)
Sodium hydroxide (NaOH; Sigma-Aldrich, cat.no.S5881). !Caution Sodium hydroxide is corrosive. Wear gloves and eye protection when handling it.
Ethyl alcohol (200 proof; Sigma-Aldrich, cat.no. E7023)
2-Propanol (Sigma-Aldrich, cat.no. I9030)
N,N-dimethylformamide (DMF; anhydrous, 99.8%; Sigma-Aldrich, cat.no. 227056) !Caution N,N-dimethylformamide is toxic. Limit exposure by wearing gloves and work in a fume hood when handling it.
Tetraethyl orthosilicate (TEOS; 99.999% trace metal basis; Sigma-Aldrich, cat.no. 333859)
Ammonium hydroxide solution (NH4OH; 28% NH3 in H2O, ≥99.99% trace metal basis; Sigma-Aldrich, cat.no. 338818). !Caution Ammonium hydroxide is corrosive. Wear gloves and eye protection when handling it.
IR-780 perchlorate (dye content 99%; Sigma-Aldrich, cat.no. 576409) CRITICAL
IR-792 perchlorate (dye content 99%; Sigma-Aldrich, cat.no. 425982) CRITICAL
(3-Mercaptopropyl)trimethoxysilane (MPTMS; 95%; Sigma-Aldrich, cat.no.175617)
Poly(ethylene glycol) methyl ether maleimide (mal-PEG2,000; Mn 2,000; Sigma-Aldrich, cat.no.731765)
5-(N-Ethyl-N-isopropyl)amiloride (EIPA; Sigma-Aldrich, cat.no. A3085)
Wortmannin (Sigma-Aldrich, cat.no. W1628)
NVP-BEZ235 (Cayman Chemical, cat.no.10565)
Cytochalasin D (MP Biomedicals, cat.no. 02150771)
Human cancer cell lines (e.g. Myc-CaP (ATCC CRL-3255), RAW264.7 (ATCC TIB-71)) !Caution The cell lines used for research should be regularly checked to ensure they are authentic, not cross-contaminated, and are not infected with mycoplasma. To check whether the cell lines are not known misidentified or cross-contaminated cell lines, go to http://iclac.org/databases/cross-contaminations/ for the most up to date list of these cells.
Dulbecco’s Modified Eagle Medium, high glucose (DMEM; Thermo Fisher Scientific, cat.no. 11965092)
Fetal bovine serum (FBS; Thermo Fisher Scientific, cat.no. 10082147)
Trypsin-EDTA (0.25%; Thermo Fisher Scientific, cat.no. 25200056)
Trypan Blue solution (0.4% (wt/vol); Thermo Fisher Scientific, cat.no.15250061)
2-(N-morpholino)ethanesulfonic acid (MES; Sigma-Aldrich, cat.no.M3671)
Mouse serum (azide free; AbD Serotec, cat.no. C11SCZ)
Hi-Myc mice (FVB-Tg(ARR2/Pbsn-MYC)) were provided by Dr. Charles Sawyers (Memorial Sloan Kettering Cancer Center). Another source of Hi-Myc animals is the National Cancer Institute (NCI) mouse repository. !Caution Any experiments involving live mice must conform to relevant Institutional and National regulation. Only trained and experienced personnel approved by the Institutional Animal Care and Use Committee (IACUC) can perform work with animals. All experiments have been approved by the Institutional Animal Care and Use Committees of Memorial Sloan Kettering Cancer Center (#06-07-011)
Ketamine hydrochloride (e.g. Vetalar®, Boehringer Ingelheim; generic, Putney Inc.). !Caution Ketamine is a controlled substance, so it should be maintained as required by DEA regulations under a locked double cabinet (both cabinets, inner and outer, shall have key-locked doors with separate keys). Detailed records of use will be maintained and provided to RARC as required by institutional policy. Only personnel approved by the Institutional Animal Care and Use Committee (IACUC) can perform work with this drug.
Xylazine (e.g. Rompun®, BayerDVM; generic, Putney Inc.). !Caution Xylazine should be mixed with ketamine as a cocktail.
Isoflurane (Forane®; Baxter)
Hair removal cream (Veet)
Paraformaldehyde solution (16%; methanol free; MP Biomedicals, cat.no. 0219998380). !Caution Paraformaldehyde is toxic. Wear appropriate gloves, masks and eye protection when handling it in a fume hood.
Phosphate-buffered saline (PBS; 10X; MP Biomedicals, cat.no. 091960454)
Equipment
Erlenmeyer, 2000 ml (Pyrex narrow-neck heavy-duty glass; Corning Inc., cat.no. 4980-2L)
Erlenmeyer, 125 ml (Pyrex narrow-neck heavy-duty glass; Corning Inc., cat.no. 4980-125)
Analytical balance (Mettler Toledo, Model AG204)
Graduated cylinder, 1000 ml (Nalgene propylene; Thermo Scientific, cat.no. 3664-1000)
Graduated cylinder, 100 ml (glass, Corning Inc. 70022100)
Vortexer (e.g. Fisher Scientific, cat.no.02-215-365)
Stirring hotplate (e.g. Fisher Scientific, cat.no. 11-300-49SHP)
Octagonal magnetic stir bar (e.g. Fisher Scientific, cat.no. 14-513-61)
Microcentrifuge tubes, 0.5 ml (e.g. Eppendorf, cat.no. 0030121023)
Microcentrifuge tubes, 1.5 ml (e.g. Eppendorf, cat.no. 0030120086)
Microcentrifuge tubes, 2.0 ml (e.g. Eppendorf, cat.no. 0030120094)
Centrifuge tubes, 50 ml (conical; polypropylene; Corning Inc., cat.no. 352098)
Dialysis cassette (Slide-A-Lyzer G2; MWCO 3,500 Da; 10 – 30 ml; Thermo Fisher, Scientific cat.no.87725)
Microcentrifuge (e.g. Eppendorf, Model 5417R)
Centrifuge (Thermo Fisher Scientific, Model Biofuge Primo R)
Incubator shaker (e.g. New Brunswick, Model Innova 4000)
Sonicator equipped with a cuphorn in a sound enclosure (Qsonica, Model Q700)
Thermomixer (Eppendorf, Thermomixer Compact, cat.no. 5384000020)
Micropipette (e.g. Eppendorf, Model Research Plus)
Micropoint pipette tips (e.g. Fisher Scientific, SureOne)
Pipet aid (Integra Biosciences, Model Pipetboy acu)
Serological pipettes (e.g. polystyrene; Corning Inc.)
pH meter (e.g. Mettler Toledo, Model S20 SevenEasy)
0.22μm disposable syringe filter (EMD Millipore, cat.no. SLGP033RB)
Black, clear bottom 384-well plate (e.g. Corning Inc., cat.no.3762)
White 1,536-well plate (e.g. Corning Inc., cat.no.3725)
Nanoparticle Tracking Analyzer (NTA; Malvern Instruments Ltd., Model NanoSight NS-500)
Dynamic Light Scattering (DLS; Malvern Instruments Ltd., Model Zetasizer Nano)
Transmission Electron Microscope (TEM; JEOL, Model 1200ex-II, 80kV acceleration voltage)
Carbon film coated TEM grids (Electron Microscopy Sciences, cat.no. CF300-CU)
12-well cell culture plates (Corning Inc., cat no. 353043)
Self-closing tweezers (Electron Microscopy Sciences, Style 2AX)
Insulin syringe (Becton Dickinson, cat.no.329461)
UV-VIS Spectrophotometer (Tecan Systems Inc., M1000Pro)
Raman microscopy system (Renishaw Inc, Model InVia). !Caution Ocular and skin exposure to laser radiation may pose the risk of eye injury and skin burns. Enclose the laser setup in a box and wear laser safety goggles at 785 nm when operating lasers. Only trained and licensed personnel can operate lasers.
Laser power meter (Edmund Optics, cat.no. 54-018)
Digital camera (e.g. Nikon, Model Coolpix AW100)
Scalpel (Bard-Parker®; Becton Dickinson, cat.no. 371610)
Surgical tool set (e.g. Fisher Scientific, cat.no. S17250)
Bottle (Costar, Corning Inc., cat.no. 8390)
Microtome (e.g. Leica, RM2155 rotary microtome)
REAGENT SETUP
Gold chloride stock solution
Prepare an aqueous 25 mM gold chloride stock solution by dissolving 491 mg gold chloride trihydrate in 50 ml deionized water. Vortex the stock solution. The gold chloride solution can be stored at room temperature (~18–20 °C) for several months.
Citrate solution
Prepare 0.5% (wt/vol) citrate water solution by dissolving 7.5 mg sodium citrate tribasic dihydrate in 15 ml deionized water. The citrate solution should be freshly prepared.
Ascorbate solution
Prepare a 40 mM ascorbate solution by dissolving 7.0 g of ascorbic acid in 1 l of ice-cold water. This solution should be made up fresh.
Aqua regia
Prepare the minimum volume (e.g. 10 ml) of aqua regia needed by carefully adding 1 part concentrated nitric acid to 3 parts concentrated hydrochloric acid. Aqua regia should be made up fresh.
!Caution aqua regia is highly corrosive. Wear gloves, lab coat, and eye protection and work in a fume hood when handling aqua regia. Do not store aqua regia for an extended length of time. Dispose of leftover aqua regia by pouring it over a large amount of ice. This mixture may be neutralized with a saturated sodium bicarbonate solution or 10% (wt/vol) sodium hydroxide. The neutralized solution may then be safely poured down the drain.
Sodium hydroxide
Prepare 50 mL 0.1 M NaOH by dissolving 0.2 g NaOH in 50 mL deionized water. !Caution Sodium hydroxide is corrosive. Wear gloves and eye protection when handling it. Sodium hydroxide solutions can be stored for at least a year at room temperature.
Resonant Raman reporter: IR780 and IR792 stock solutions
Dissolve 3.1 mg of IR-780 perchlorate or 26.5 mg IR-792 perchlorate in 1.0 ml DMF to obtain 5 mM or 37.5 mM stock concentrations of the respective resonant Raman reporters. The stock solutions can be stored for at least a week in the dark at 4 °C. !Caution N,N-dimethylformamide is toxic. Limit exposure by wearing gloves and work in a fume hood when handling it.
MES buffer
Prepare 100 mL 10 mM MES buffer by dissolving 195 mg MES in 95 mL water. Set the pH to 7.1 with 0.1 M NaOH. Transfer 50 ml of the 10 mM MES buffer (pH 7.1) to a new 50-ml centrifuge tube and label ‘10 mM MES buffer (pH 7.1)’. Set the pH of the remaining 10 mM MES buffer to 7.3 with 0.1 M NaOH. Filter-sterilize with a 0.22-μm-syringe filter and label ‘10 mM MES buffer (pH 7.3)’. MES buffers can be stored at room temperature for several months. !Caution Sodium hydroxide is corrosive. Wear gloves and eye protection when handling it.
Mouse anesthesia by ketamine/xylazine cocktail
Ketamine/xylazine combination is suitable for surgical procedures of short to moderate duration (< 20 minutes). Administer the cocktail (ketamine: 150 mg/kg; xylazine: 15 mg/kg) intraperitoneally at 0.1 ml/20 g mouse wt. Confirm depth of anesthesia by verifying an absence of response to ear, toe, and/or tail pinch. Evaluate response by monitoring withdrawal as well as an increase or change in respiratory rate and/or pattern. The cocktail should be freshly prepared.
Mouse anesthesia by inhalation of isoflurane
Use the volatile fluorocarbon isoflurane (1–4%) administered using a precision vaporizer (which has been calibrated and certified within the past 12 months) in an induction chamber followed by use of a nose cone. Scavenge waste anesthetic gas by using an activated carbon cannister. Confirm the depth of anesthesia by verifying an absence of response to ear, toe, and/or tail pinch. Evaluate response evaluated by monitoring withdrawal as well as an increase or change in respiratory rate and/or pattern.
EQUIPMENT SETUP
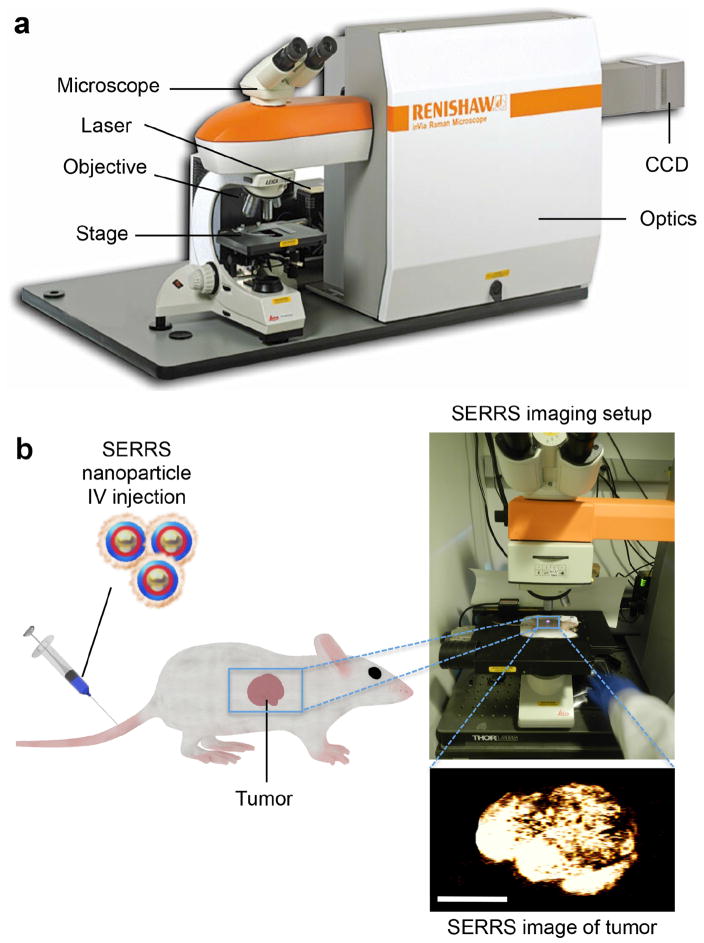
Raman spectroscopy and imaging setup
We use an InVia Raman microscopy system (Renishaw Inc.) equipped with a 300-mW 785-nm diode laser and a 1-inch charge-coupled device (CCD) detector with a spectral resolution of 1.07 cm−1 for SERRS nanoparticle characterization and small animal Raman imaging (Figure 3a). We collect the Raman spectra through a 5× objective (Leica). We measure laser output at the microscope objective with a handheld laser power meter and determine to be 100 mW when the laser is running at 100% laser power.
Figure 3.
Raman microscopy imaging setup. (a) The commercial InVia Raman imaging system (image adapted from Renishaw, Inc.) consists of a 785-nm laser, a box containing the optics, a microscope equipped with a motor controlled stage and a CCD camera. (b) Typical experiment using SERRS nanoparticles for preclinical cancer detection with Raman imaging. A tumor-bearing mouse was injected intravenously with PEGylated SERRS nanoparticles. Then the mouse was placed on the stage and the laser focused on the tumor, which was raster-scanned using Raman. A SERRS image of the region-of-interest was generated, which delineates the tumor with high sensitivity (scale bar, 500 μm). Appropriate institutional regulatory board permission was obtained.
To assess the SERRS intensity and Raman spectra of de novo synthesized SERRS nanoparticle dispersions, add a 10-μl 1-nM sample of the dispersion to a well of a 1,536-microwell plate. Place the microwell plate on the motor-controlled sample stage.
Focus the laser on the sample and obtain a Raman spectrum in the wavenumber range of 500–1,650 cm−1 using a 1-s acquisition time at 0.05% laser power to prevent the CCD from being saturated.
For in vivo Raman imaging, inject an animal intravenously with the SERRS nanoparticles, anesthetize or sacrifice and place on the motor-controlled stage (Figure 3b). Typically, we perform in vivo and ex vivo Raman scans at 10–100% laser power, 1.5-s acquisition time, in StreamLineTM high-speed acquisition mode. We generate and analyze the Raman maps using a direct classical least square (DCLS) algorithm (WiRE 3.4 software, Renishaw). However, other image-processing algorithms can be used instead as extensively discussed by Butler et al.47. After completion of the Raman imaging of the animal, process the scanned tissues for histopathological examination to validate the status of the SERRS-positive tissues.
PROCEDURE
Synthesis of 60 nm gold nanoparticles ● Timing 2 h
-
1|
Follow option A to synthesize spherical gold nanoparticles and option B to synthesis star-shaped gold nanoparticles. For Raman imaging applications, the spherical gold nanoparticles perform similarly to the star-shaped gold nanoparticles in terms of signal sensitivity, limit of detection, and tumor accumulation. Unlike the star-shaped gold nanoparticles which have an absorption maximum in the near-infrared (NIR) wavelength range, the absorption maximum of monomeric spherical gold nanoparticles is located at 540 nm, which red-shifts when the nanoparticles are aggregated. This allows visual monitoring of the colloidal stability during SERRS nanoparticle synthesis (see Figure 4), which is very helpful especially during optimization or evaluation of new resonant Raman reporters. Alternatively, by converting the absorbed NIR photons of the 785-nm excitation laser into heat via a process known as the photothermal effect, star-shaped gold nanoparticles enable Raman-guided photothermal therapy. However, since the current protocol focuses on SERRS nanoparticles for contrast-enhanced Raman imaging, we mainly discuss applications for spherical gold nanoparticles, while noting that star-shaped nanoparticles can be used interchangeably.
Figure 4.
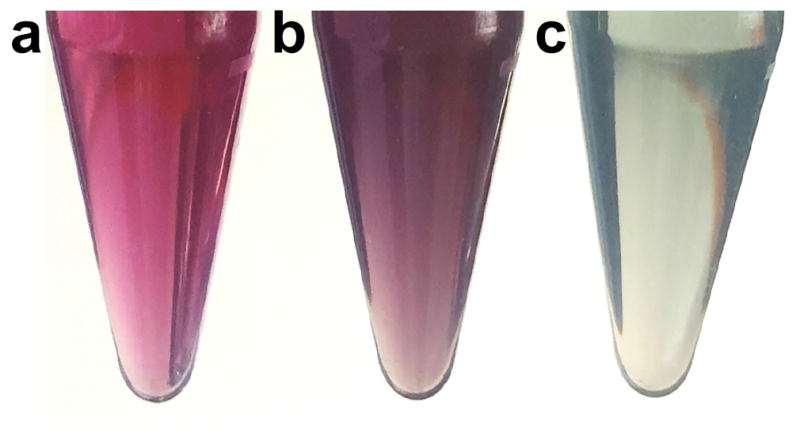
Visual monitoring of SERRS nanoparticle synthesis. (a) Gold nanoparticles in isopropanol have a pink color. (b) Gold nanoparticles during silica encapsulation in the presence of resonant Raman reporters have a purplish color. (c) Gold nanoparticles aggregation causes a grayish blue color.
(A) 60-nm, spherical gold nanoparticles ●Timing 2 h
Prepare a solution of 0.25 mM HAuCl4 by adding 10 ml of the 25 mM gold chloride stock solution to 975 ml deionized water in a 2-l Erlenmeyer flask.
Place the 0.25 mM HAuCl4 solution on a hotplate and bring the solution to a rolling boil while heating at >105 °C. Maintain the stirring speed at 300 rpm. !Caution Hot surface. Avoid contact with the heated parts without wearing heat-resistant gloves.
Rapidly add 15 ml 0.5% (v/v) sodium citrate solution. The color of the solution will go from yellow to black and finally to burgundy red. Leave it stirring for 10 min while heating.
Let the dispersion cool down to ambient temperature, separate the dispersion into 20 50-ml centrifuge tubes and centrifuge the dispersion for 10 min at 5,000 g.
Carefully discard ~48.5 ml of the supernatant of each 50-ml tube without disrupting the pelleted gold nanoparticles using a 50-ml pipette. !Caution Residual gold nanoparticles in the waste solutions generated in this section of the PROCEDURE can be recovered by treating the aqueous waste with an excess of sodium chloride to aggregate the gold nanoparticles. The precipitated aggregates can be collected, washed with water, and recycled to gold chloride using aqua regia. All glassware also can be rinsed with aqua regia. !Caution aqua regia is highly corrosive. Avoid contact with aqua regia by working in a fume hood while wearing gloves, lab coat, and eye protection. Always generate the minimal volume of aqua regia that is needed for a specific application. Dispose of leftover aqua regia by pouring it over a large amount of ice. This mixture may be neutralized with a saturated sodium bicarbonate solution or 10% sodium hydroxide. The neutralized solution may then be safely poured down the drain.
(B) 60-nm star-shaped gold nanoparticles ● Timing 2 h
Prepare 1 l of 40 mM AA solution by dissolving 7.0 g AA in 1-l ice-cold water.
Prepare a 10 ml 20 mM gold chloride solution by dissolving 78 mg gold (III) chloride trihydrate in 10 ml DI water.
Place the AA solution on a magnetic stirrer, add a magnetic stir bar, and thoroughly vortex the solution.
Use a 10-ml pipette to rapidly add the gold chloride solution to the AA solution while thoroughly vortexing. CRITICAL STEP It is important to add the gold chloride outside of the vortex to enable rapid distribution of the gold chloride. Therefore, do not add the gold chloride into the vortex.
After rapid addition of the gold chloride solution, the color of the AA solution should go from colorless to deep blue. This occurs if only nanostars have been synthesized. CRITICAL STEP If the solution transitions from no color to pink to deep blue, both spherical and star-shaped nanoparticles have been synthesized. Dispose of this dispersion and repeat the procedure.
Immediately separate the dispersion into 20 50-ml centrifuge tubes and centrifuge the dispersion for in a pre-chilled centrifuge for 10 min at 5,000 g at 4 °C. CRITICAL STEP It is important to keep the dispersion at 4 °C otherwise the nanostars will transform into spheres, which can be monitored by a transition from a dark blue to purple to eventually pink color of the dispersion. Only the dark blue dispersion produce narrowly disperse nanostar dispersions.
Carefully discard ~48.5 ml of the supernatant without disrupting the pelleted gold nanostars using a 50-ml pipette. !Caution Residual gold nanoparticles in the waste solutions generated in this section of the PROCEDURE can be recovered by treating the aqueous waste with an excess of sodium chloride to aggregate the gold nanoparticles. The precipitated aggregates can be collected, washed with water, and recycled to gold chloride using aqua regia. All glassware also can be rinsed with aqua regia. !Caution aqua regia is highly corrosive. Avoid contact with aqua regia by working in a fume hood while wearing gloves, lab coat, and eye protection. Always generate the minimal volume of aqua regia that is needed for a specific application. Dispose of leftover aqua regia by pouring it over a large amount of ice. This mixture may be neutralized with a saturated sodium bicarbonate solution or 10% sodium hydroxide. The neutralized solution may then be safely poured down the drain.
Dialysis of 60-nm gold nanoparticles ● Timing 5 d
-
2|
Collect, pool and transfer the remaining volume containing the gold nanoparticles to a 30-ml dialysis cassette (MWCO 3.5 kDa). Remove the air from the dialysis cassette to maximize the surface area-to-volume ratio and place the dialysis cassette in a container containing 5 l deionized water and a magnetic stir bar. Place the container on a magnetic stirrer and stir overnight at 350 rpm at ambient conditions. Replace the water daily for 5 days.
-
3|
Recover the dialyzed gold nanoparticle dispersion using a 10-ml pipette and transfer the dispersion to a 50-ml centrifuge tube.
CRITICAL STEP Transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and Raman spectroscopy, as described in step 22, can be performed on the nanoparticle dispersions to assess the size, shape, and concentration of the nanoparticles.
■ Pause Point The 60-nm, citrate-capped gold nanoparticles can be stored in deionized water at ambient conditions for at least 1 year.
Synthesis of surface-enhanced resonance Raman scattering (SERRS) nanoparticles ●Timing 1 h
-
4|
Prepare a basic dispersion of gold nanoparticles in isopropanol by adding 2.0 ml dialyzed 60-nm gold nanoparticles (2.0 nM), 2.0 ml deionized water and 600 μl ammonium hydroxide to 30 ml isopropanol in a 50-ml centrifuge tube and vortex.
-
5|
Prepare a solution of Raman reporter and silica precursor TEOS in isopropanol by adding 75 μl 5 mM IR780 or 37.5 mM IR792 in DMF and 1.2 ml TEOS to 9.0 ml isopropanol and vortex.
-
6|
Rapidly add the basic gold nanoparticle dispersion from step 4 to the Raman reporter/TEOS solution from step 5 and place the reaction mixture in an incubator shaker for 15 min at 25 °C at 350 rpm. The dispersion should have a purplish color (Figure 4). A successful reaction generates a purple dispersion. If, however, the dispersion turns blackish purple or grayish blue (Figure 4), the gold nanoparticles are aggregating and the reaction should be repeated with fresh reagents.
-
7|
Transfer 22.5 ml of the reaction mixture to a new 50 ml centrifuge tube, add 27.5 ml ethanol to both tubes to quench the silication reaction and centrifuge the quenched dispersions for 10 min at 4 °C at 5,000 g.
-
8|
Carefully remove all supernatant using a 50-ml pipette and add 500 μl ethanol to both tubes. Redisperse the de novo synthesized SERRS nanoparticles by placing the closed 50 ml tubes containing the SERRS nanoparticle pellets in a sonicator (5 s; amplitude 50). !Caution Close the tubes to prevent aerosol formation. Wear ear protection when performing sonication.
-
9|
Transfer the redispersed SERRS nanoparticles to 1.5-ml centrifugation tubes and centrifuge the dispersion for 5 min at 10,500 g in a microcentrifuge.
-
10|
Remove and discard the supernatant, add 1.0 ml ethanol and redisperse the SERRS nanoparticles using sonication.
-
11|
Repeat the wash steps (9–10) at least 3 times.
CRITICAL STEP Transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and Raman spectroscopy, as described in step 22, can be performed on the nanoparticles to assess the size, shape, concentration, as well as the SERRS spectrum and intensity of the nanoparticles dispersions.
■Pause Point The SERRS nanoparticles can be stored in ethanol for a couple of weeks.
SERRS nanoparticle surface modification ●Timing 2.5 h
-
12|
To 1.0 ml 4 nM SERRS nanoparticles in ethanol add 100 μl MPTMS and 20 μl ammonium hydroxide (28% vol/vol) and vortex. !Caution MPTMS has a very pungent odor. Work in a fume hood when handling MPTMS.
-
13|
Place the reaction mixture in a thermomixer for 2 h at 70 °C at 350 rpm.
-
14|
Centrifuge the dispersion for 5 min at 10,500 g. Remove and discard the supernatant, add 1.0 ml ethanol and redisperse the SERRS nanoparticles using sonication to wash the surface-modified SERRS nanoparticles.
-
15|
Repeat the wash step (14) at least 3 times.
-
16|
Repeat the wash step (14) at least 3 times with deionized water instead of ethanol.
CRITICAL STEP Transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and Raman spectroscopy, as described in step 22, can be performed on the nanoparticles to assess the size, shape, concentration, as well as the SERRS spectrum and intensity of the nanoparticles dispersions.
■Pause Point The SERRS nanoparticles can be stored in water for a couple of days.
PEGylation of ‘conjugation-ready’ SERS nanoparticles ●Timing 2.5 h
-
17|
Prepare a 1% (wt/vol) mal-PEG2,000 dispersion in a 2-ml microcentrifuge tube by adding 10 mg mal-PEG2000 to 1.0 ml 10 mM MES buffer (pH 7.1).
-
18|
Add 1 ml ‘conjugation-ready’ SERRS nanoparticles from step 16 in 1.0 ml deionized water to 1.0 ml 1% (w/v) mal-PEG2000 and vortex.
-
19|
After 2 h, centrifuge the dispersion for 5 min at 10,500 g.
-
20|
Remove and discard the supernatant and add 2 ml deionized water and redisperse the PEGylated SERRS nanoparticles using sonication.
-
21|
Repeat the wash steps (steps 19 and 20) at least 3 times. After the last wash redisperse the PEGylated SERRS nanoparticles in 1.0 ml filter sterilized 10 mM MES buffer (pH 7.3) unless you wish to store them, in which case resuspend in 1 ml deionized water.
■Pause Point The PEGylated SERRS nanoparticles (~4 nM) can be temporarily stored in 1.0 ml deionized water for a couple of days. Before injection, centrifuge the stored PEGylated SERRS nanoparticles, remove the aqueous supernatant, and redisperse the PEGylated SERRS nanoparticles in the injection buffer (e.g. 10 mM MES (pH 7.3)) using sonication
Nanoparticle Characterization
-
22|
Perform transmission electron microscopy (TEM, option A), nanoparticle tracking analysis (NTA, option B), and Raman spectroscopy (option D) on the nanoparticles to assess the size, shape, concentration, as well as the SERRS spectrum and intensity of the nanoparticles dispersions. We advise to perform TEM, NTA, Raman spectroscopy after every new SERRS nanoparticle batch synthesis to ensure reproducibility in terms of size, concentration, and Raman signal intensity. In addition, you can assess the absorption spectrum of SERRS nanoparticle dispersion using UV/VIS spectroscopy (option D) or In vitro characterization of the effect of serum components on SERRS nanoparticle stability (option E). Options A–C can also be performed at steps 3, 11 and 16.
(A) Transmission electron microscopy (TEM) characterization of the nanoparticles ●Timing 1 h
Put a 1-μl drop of the dispersions onto a carbon-coated TEM grid and air-dry it at room temperature for 5 – 10 min (depending on the dispersant).
Image the samples on a TEM (e.g. JEOL 1200ex-II; 80 kV). The spherical, bare gold nanoparticles (from Step 3) will appear spherical or slightly ellipsoid with a mean diameter of 60 nm. The SERRS nanoparticles (Step 11, 16, 21) will appear as a dense core that is encapsulated by a less-dense layer of amorphous silica (Figure 5). The silica layer should measure 15 – 20 nm in thickness. If the layer appears thinner than expected or if there is no layer at all, repeat the procedure again but with freshly ordered ammonium hydroxide (28% vol/vol). !Caution Ammonium hydroxide is corrosive. Wear gloves and eye protection when handling it.
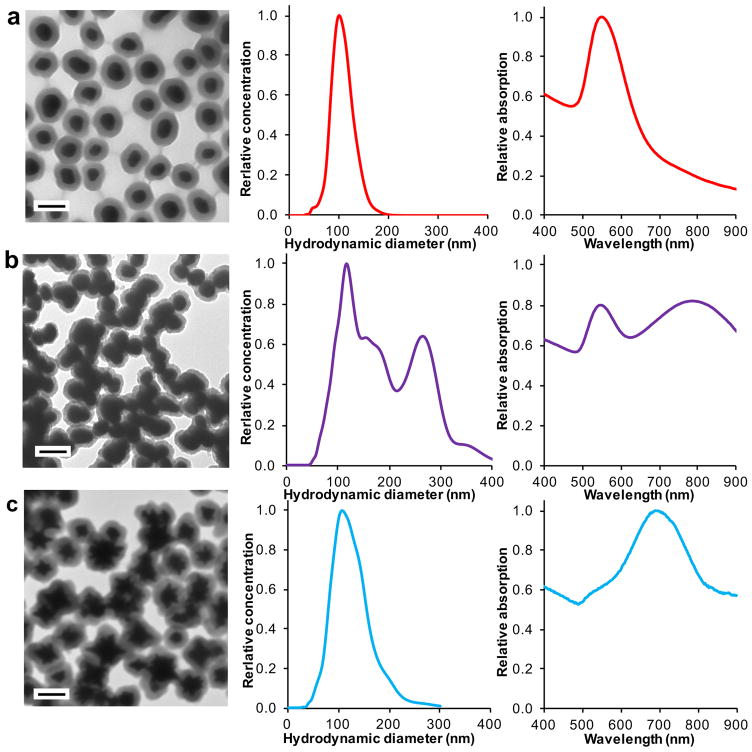
Figure 5.
SERRS nanoparticle characterization. Transmission electron micrographs, hydrodynamic diameters as measured by nanoparticle tracking analysis (NTA), and absorption spectra of (a) successful synthesis of SERRS nanoparticles. (b) gold nanoparticle aggregation during the silica encapsulation. (c) silica-encapsulated gold nanostars. Scale bars, 100 nm.
(B) Nanoparticle tracking analysis (NTA) – size distribution and concentration of the nanoparticle dispersions ●Timing 15 min
Add 1 μl of the dispersion to 999 μl deionized water and vortex.
Load the diluted dispersion into an NTA (e.g. NS500).
Measure the size distribution and determine the concentration of the nanoparticles.
(C) Raman spectroscopy ●Timing 15 min
Load 10 μl of the dispersion (Step 11, 16, 21) into the well of a white 1,536-well plate.
Place the 1,536-well plate onto the stage of the Raman microscopy system (Renishaw InVia).
Focus the laser on the sample. !Caution Ocular and skin exposure to laser radiation may pose the risk of eye injury and skin burns. Enclose the laser setup in a box and wear laser safety goggles at 785 nm when operating lasers. Only trained and licensed personnel can operate lasers.
Measure the Raman spectrum. If the detector saturates, lower the laser output power. If the background is curved this is due to fluorescent background generated by the dye. Wash the SERRS nanoparticles with ethanol to remove any residual free resonant Raman reporter. If the problem persists, repeat the SERRS nanoparticle synthesis procedure again (Step 4) using 1.5 ml of deionized water (instead of 2.0 ml). This will generate SERS nanoparticles with thinner silica shells, which will ensure that the near infrared fluorescent signal of the encapsulated resonant Raman reporters is quenched by the gold core.
(D) Absorption spectrum of SERRS nanoparticle dispersion using UV/VIS spectroscopy ●Timing 15 min
-
(i)
Add an appropriate volume of the gold nanoparticle or SERRS nanoparticle dispersion to 1 ml of water in a 1.5-ml microcentrifuge tube and vortex.
-
(ii)
Transfer an appropriate volume of the diluted dispersion to either a cuvette or a microwell plate.
-
(iii)
Place the cuvette or microwell plate in a UV/VIS spectrometer and record the absorbance in the wavelength range of 400–800 nm. The 60-nm gold nanoparticles and SERRS nanoparticles should have an absorption maximum around 540 nm. Increases in the absorption in the 600–800 nm wavelength range are indicative of aggregation (Figure 5).
-
(i)
In vitro characterization of the effect of serum components on SERRS nanoparticle stability ●Timing 48 hAdd 50 μl of a 2 nM SERRS nanoparticle dispersion in a 10 mM MES buffer (pH 7.3) to a 500 μl microcentrifuge tube containing 50 μl mouse serum; for each time point a separate tube.
-
(ii)
Vortex and incubate the tubes at 37 °C.
-
(iii)
At the appropriate time point, transfer 2 μl to 998 μl water (or 50% (vol/vol) mouse serum). Perform NTA to evaluate the degree of agglomeration due to SERRS nanoparticle-serum interactions (Step 22; option B; (ii)–(iii)).
-
(iv)
Collect the remaining SERRS nanoparticles by centrifugation (5 min at 10,500 g).
-
(v)
Remove and discard the supernatant, add 100 μl water, and redisperse the SERRS nanoparticles using sonication.
-
(vi)
Repeat the wash step (iv and v) at least 3 times
-
(vii)
Characterize the SERRS nanoparticles using TEM (Step 22; option A; (i)–(ii)) and Raman spectroscopy (Step 22; option C; (i) – (iv)).
Experiments with SERRS nanoparticles
-
23|
Various experiments can now be performed that utilise contrast-enhanced Raman imaging of cancer using SERRS nanoparticles. To look at uptake of nanoparticles by cells, follow option A. To use nanoparticles to detect and image malignant tumors in mice follow option B13.
(A) Uptake mechanisms of SERRS nanoparticles by cells ●Timing 10 h
Seed the cells of interest (e.g. Myc-CaP or RAW264.7) into 12-well-plates in the appropriate medium (e.g. 2 mL DMEM (high glucose) medium with 10% FBS, 2 mM L-glutamine and 1 mM sodium pyruvate) at 37 °C in 5% CO2.
Prepare the uptake inhibitors as 100X stock solutions. Typical uptake inhibitors are EIPA (7.5 mM), wortmannin (10 μg/ml), NVP-BEZ235 (200 μM) and cytochalasin D (1 mg/ml) in DMSO.
Add 20 μL of the 100X uptake inhibitor to 2 ml culture medium in a 15-ml centrifuge tube.
When the cells reached 70–80% confluence, aspirate the medium.
Wash the cell monolayers with PBS and aspirate the PBS.
Add the culture medium containing the uptake inhibitors to the wells.
Incubate for 30 min at 37 °C in 5% CO2.
Redisperse the SERRS nanoparticles in 10 mM MES buffer (pH 7.3) at a concentration of 200 pM.
After a 30-min pre-incubation with the uptake inhibitors for 30 min, add 20 μL SERRS nanoparticles to each well and incubate for an additional 3 h at 37 °C in 5% CO2.
At the endpoint, remove the culture medium from each well.
Add 0.5 mL 0.25% Trypsin/0.02% EDTA Solution to each well. Cells were observed under an inverted microscope to ensure the cell layer was suspended (usually within 5 to 10 minutes).
Add 1.5 mL of culture medium and aspirate cells by gently pipetting.
Centrifuge the cell suspensions at 700 g for 5 min at room temperature. Discard the supernatant and resuspend the cell pellets using 1 mL PBS buffer.
Repeat the washing procedure (step xiii) twice. Divide the cell suspension in 1ml PBS into two parts: 0.75 mL (Sample 1: for Raman imaging) and 0.25 mL (Sample 2: for cell counting).
Mix 10 μL of Sample 2 with 10 μL 0.4% trypan blue solution. Then load 10 μL mixture into the Countess cell counting chamber slide and measure by an automated cell counter (Invitrogen).
Centrifuge sample 1 at 700 g for 5 min at room temperature. Discard the supernatant and resuspend the cell pellets using 20 μL PBS buffer. Transfer the cell suspension into a well of a 384-well microplate.
Place the 384-well plate on the motor-controlled stage of the Raman microscopy system and fix the plate in place with tape.
Focus the laser at the bottom of the 384-well plate. !Caution Ocular and skin exposure to laser radiation may pose the risk of eye injury and skin burns. Enclose the laser setup in a box and wear laser safety goggles at 785 nm when operating lasers. Only trained and licensed personnel should operate lasers.
Select the FOV using the software controlling the Raman imaging system (e.g. Wire 3.4).
Scan the selected FOV at 10% laser power, 1.5-s acquisition time (in StreamLineTM mode; InVia, Renishaw) using an appropriate number of steps (100,000–350,000; this will determine the resolution of the Raman image).
Once the Raman scanning is finished, make sure the laser is turned off and save the data.
Analyze the data and generate a Raman image using an appropriate image analysis algorithm such as DCLS. Normalize the Raman intensity of Sample 1 to the cell number of Sample 2.
Normalize the mean values and standard deviations of inhibition effects by different inhibitors to DMSO-treated sample. All experiments were run in triplicate.
(B) Using SERRS nanoparticles to detect and image malignant tumors in mice ●Timing 6–26 h
Place a tumor-bearing mouse (e.g. subcutaneous xenograft model, orthotopically implanted model, or transgenic mouse model) under a heated lamp to dilate its tail-vein. !Caution monitor the animal while placing it under a heated lamp to prevent the animal from getting burns.
Immobilize the animal by placing the animal in a restrainer. Wipe its tail with a sterile alcohol wipe (70% (vol/vol)).
Aspirate 150 μl of a 3.5 nM PEGylated SERRS nanoparticle dispersion in 0.22-μm filter-sterilized 10 mM MES buffer (pH 7.3) in a 29G insulin syringe and thoroughly sonicate. Slowly inject the PEGylated SERRS nanoparticles into the dilated tail vein. This is the equivalent of a dose of 30 fmol/g SERRS nanoparticles. CRITICAL STEP The PEGylated SERRS nanoparticles must be thoroughly sonicated immediately before injection.
Once the injection is completed withdraw the needle and press the injection site with a sterile alcohol wipe (70% (vol/vol)).
Return the mouse to the housing room without any special treatment.
Allow 3–18 hours for sufficient circulation and homing of the nanoparticles.
For rapid in vivo Raman imaging (i.e. <20 min, small field of view (FOV)), anesthetize the animal via i.p. injection of a ketamine/xylazine cocktail. For longer Raman imaging (i.e.>20 min, wider field of view (FOV)), anesthetize the animal with at least 2% (vol/vol) isoflurane. For endpoint Raman imaging, sacrifice the animal using CO2 asphyxiation or any other procedure approved by the IACUC. In the current protocol, only endpoint Raman imaging will be discussed. To sacrifice the animal, expose the animal to 100% carbon dioxide at 5 PSI for a minimum of 3 minutes in a euthanasia chamber.
Confirm death by palpating for the absence of an apex heartbeat and a lack of respiration. After sacrificing the animal, if required due to the location of the tumor, expose the tumour tissue through an appropriate surgical procedure. Some samples (e.g. skin overlying the tumor, or adjacent tissues) can be paraffin-embedded prior to imaging, to permit imaging later. To do this carefully resect the tissues without disrupting the tumor. Fix the tissue overnight in 4% paraformaldehyde at 4 °C, and, subsequently, process for paraffin embedding. Prior to imaging remove from the fridge and expose the fixed tissue by removing a thin layer of the paraffin using a microtome.
Take a photo of the area to be imaged.
Place the animal or tissue block on the stage of the Raman microscopy system and fix the animal in place using tape.
Focus the laser onto the surface of the tissue. !Caution Ocular and skin exposure to laser radiation may pose the risk of eye injury and skin burns. Enclose the laser setup in a box and wear laser safety goggles at 785 nm when operating lasers. Only trained and licensed personnel can operate lasers.
Select the FOV using the software controlling the Raman imaging system (e.g. Wire 3.4).
Scan the selected FOV at 100% laser power, 1.5-s acquisition time (in StreamLineTM mode) using an appropriate number of steps (100,000–350,000; this will determine the resolution of the Raman image).
Once the Raman scanning is finished, make sure the laser is turned off and save the data.
Analyze the data and generate a Raman image using an appropriate image analysis algorithm such as DCLS.
If you have imaged an intact animal, perform either a Raman-guided resection or a blinded resection (i.e. blinded to the Raman image) of the tumor tissue.
Process the resected SERRS-positive tissue for histopathological examination
Repeat viii–xvi until no SERRS nanoparticle-positive signal is detected in the tumor bed.
Dispose of the mouse remains using an appropriate carcass disposal facility.
Troubleshooting
For troubleshooting guidance, see Table 1.
Table 1.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1A | Nanoparticle size is smaller or larger than 60 nm | The gold chloride:citrate ratio is smaller or larger, respectively, than 10:1 | Make sure the concentration of the stock solution are correct or prepare fresh stock solutions. |
| 1B | Dispersion slowly changes from blue to pink | Residual reagents are present in the dispersion | Dialyze the collected nanoparticles against DI water and keep the dispersion ice-cold. |
| 6 | Dark purple color appears | Gold nanoparticle aggregation due to high ionic strength | Lower dye concentration or dialyze the gold nanoparticles longer |
| 22A | Silica shell too thin | Ammonium hydroxide or water concentration too low | Use fresh ammonium hydroxide or add more water during step 4–6 |
| 22A | Free silica | Reaction time too long or reaction was not sufficiently quenched | Quench the reaction at 15 min reaction time with sufficient amounts of ethanol |
| 22D | Fluorescent interference | Residual resonant Raman reporter | Wash SERRS nanoparticles thoroughly |
| 23B | Difficult to inject | SERRS nanoparticle agglomeration | Sonicate the dispersion right before injection |
●Timing
Step 1, synthesis of the gold nanoparticle core: 2 h
Step 2–3, dialysis of gold nanoparticles: 5 d
Step 4–11, synthesis of SERRS nanoparticles: 1 h
Step 12–16, surface functionalization of SERRS nanoparticles: 2.5 h
Step 17–21, PEGylation of SERRS nanoparticles: 2.5 h
Step 22A, SERRS nanoparticle characterization using TEM: 1 h
Step 22B, SERRS nanoparticle characterization and concentration measurement using NTA: 15 min
Step 22C, UV/VIS absorption of SERRS nanoparticle dispersion: 15 min
Step 22D, Raman spectroscopy of SERRS nanoparticles: 15 min
Step 22E, In vitro characterization of the effect of serum components on SERRS nanoparticle stability: 48 h
Step 23A, Cellular uptake mechanisms of SERRS nanoparticles: 10 h
Step 23B, In vivo cancer imaging using SERRS nanoparticles: 1–26 h
ANTICIPATED RESULTS
Gold nanoparticle synthesis
The gold nanoparticle synthesis (Step 1A) will reproducibly yield spherical gold nanoparticles with a size of 60 nm (coefficient of variation (CV): <1%; n=3). The factors that are critical for obtaining 60-nm, spherical gold nanoparticles are the gold chloride:citrate ratio and the temperature of the reaction. A gold chloride:citrate ratio of 10:1 produces 60-nm gold nanoparticles. Decreasing this ratio will result in smaller gold nanoparticles, while increasing the ratio will result in larger gold nanoparticles. However, since the enhancement effect is the most significant for gold nanoparticle of size 50–60 nm8, we strongly discourage the use of other sizes, because this will affect the SERRS signal sensitivity and limits of detection. For the gold nanostars, it is important to maintain the temperature before dialysis at ≤4 °C to prevent the gold nanostars from transforming into spheres. This can be monitored visually, because color of the gold nanostar dispersion will turn from blue to purple to red. However, once the gold nanostars have been dialyzed, they will remain stable at ambient conditions for prolonged periods of time.
SERRS nanoparticle synthesis
Prior to synthesis, the basic 60-nm, spherical gold nanoparticle dispersion will have a pink color (Step 4; Figure 4a) and the resonant Raman reporter containing solution has a green color (Step 5). After rapidly combining the two (Step 6), the resulting dispersion should appear purple (Figure 4b). However, if the resulting dispersion (Step 11) appears grayish blue or black (Figure 4c) the gold nanoparticles have aggregated and the procedure described in Step 4–11 should be repeated. Of note, visual monitoring is not possible for the nanostars during silica encapsulation, because aggregation causes a red-shift of the nanostars’ localized surface plasmon resonance beyond the spectral sensitivity of the human eye (>700 nm). In the event of gold nanoparticle aggregation during silica encapsulation, decreasing the resonant Raman reporter concentration or longer dialysis of the gold nanoparticles will prevent aggregation. After centrifugation (Step 7), successful encapsulation of spherical or star-shape gold nanoparticles will yield a red pellet or blue pellet, respectively, at the bottom of the centrifugation tubes with a clear, green supernatant.
The SERRS nanoparticle morphology and dispersity is assessed using TEM, NTA, and UV/VIS spectroscopy (Step 22A–C). Successful SERRS nanoparticle synthesis results in monomeric nanoparticle species that appear as a dense gold core surrounded by a less dense silica shell measuring 15–20 nm on TEM (Figure 5a). If the silica shells appear thinner, replace the ammonium hydroxide solution with a fresh batch. The hydrodynamic diameter of the SERRS nanoparticles is centered on 100 nm and the concentration should be ~4.0 nM as measured by NTA (Figure 5a). A typical absorption spectrum of an as-synthesized SERRS nanoparticle dispersion has an absorption maximum around 540–550 nm (depending on the silica shell thickness and the size and the shape of the gold nanoparticle core; Figure 5a). When the dispersion turns grayish blue or black during SERRS nanoparticle synthesis, large gold nanoparticle aggregates surrounded by silica are observed (Figure 5b). Large aggregates have hydrodynamic diameters of >200 nm and demonstrate an increase in absorption in the 700–900 nm wavelength range (Figure 5b). Aggregated nanoparticle dispersions should be discarded and should not be used for in vivo cancer imaging. If gold nanoparticle aggregation occurs during silica encapsulation, lower the dye concentrations. Only use cationic dyes with chaotropic counter-anions such as iodide, tetrafluoroborate (BF4−), hexafluorophosphate (PF6−), and perchlorate (ClO4−) and avoid kosmotropic counter-anions such as chloride and fluoride. If the problem persists, re-dialyze the gold nanoparticle dispersion. Successful SERRS nanostar synthesis results in monomeric silica-coated gold nanostars with a hydrodynamic diameter centered around 100 nm as measured by NTA and an absorption maximum in the near infrared range (i.e. ~700 nm; Figure 5c).
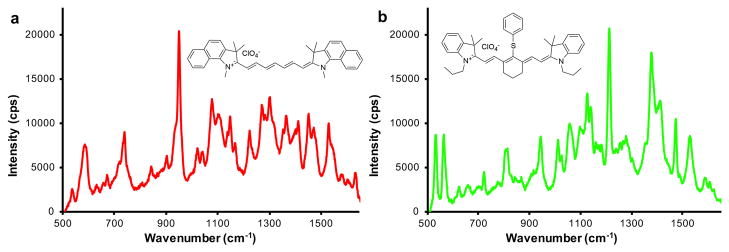
In Figure 6, the Raman spectra of two different flavors of SERRS nanoparticles is shown. SERRS nanoparticle with IR780 perchlorate as the resonant Raman reporter produce a spectrum with a diagnostic band at 950 cm−1 (Figure 6a), while IR792 perchlorate produces a diagnostic band at 1200 cm−1 (Figure 6b). However, when the background is curved, residual free resonant Raman reporter is present in the SERRS nanoparticle dispersion causing fluorescence. Wash the SERRS nanoparticles with ethanol to remove any residual free resonant Raman reporter. If the problem persists, repeat the SERRS nanoparticle synthesis procedure using less water present during the silica encapsulation. This will generate SESRS nanoparticles with thinner silica shells, which will ensure that the near infrared fluorescent signal of the encapsulated resonant Raman reporters is quenched by the gold core. Further, this will also prevent the generation of resonant Raman reporter-embedded free silica (without a gold core), which also produces a fluorescent signal. Further, avoid using anionic resonant Raman reporters such as sulfonated NIR dyes (e.g. IR783 or ICG).
Figure 6.
Raman spectra of two SERRS nanoparticles of different ‘flavor’. (a) Raman spectrum of SERRS nanoparticle with the resonant Raman reporter IR780 perchlorate. (b) Raman spectrum of SERRS nanoparticle with the resonant Raman reporter IR792 perchlorate. The spectra were obtained from a 10 μl dispersion of 1 nM SERRS nanoparticles on a Raman microscopy system equipped with a 785-nm 300-mW laser operating at 0.05% laser power, 1-s acquisition time, with a 5× objective.
Surface modification is achieved by coating the SERRS nanoparticles with (3-mercaptopropyl)trimethoxysilane (MPTMS) at 70 °C for at least 1 h in a basic ethanolic solution. Following surface modification, the SERRS nanoparticles should be thoroughly washed to remove all residual MPTMS. Thiolation efficiency can be assessed using Ellman’s reagent48. Typically, the thiolated SERRS nanoparticles are added to Ellman’s reagent in a microcentrifuge tube and after an appropriate amount of time (i.e. 5–10 min) the samples are centrifuged for 5 min at 5,000 g. Subsequently, the supernatant is transferred to a 96-well microplate and the absorption is measured on a UV/VIS spectrometer at 412 nm. Typically, the SERRS nanoparticles should contain on average 35,000–50,000 thiol groups per nanoparticle49,50. Next, the introduced thiol functionality is used to conjugate PEG to afford non-specific PEGylated SERRS nanoparticles or, in a similar manner, targeting moiety-functionalized PEG linkers to afford targeted SERRS nanoparticles.
When intravenously injected via tail vein into a tumor-bearing animal, the PEGylated SERRS nanoparticles target the tumor within several hours. Typically, no noticeable signs of discomfort or adverse reactions following injection of narrowly dispersed 100-nm PEGylated SERRS nanoparticles should be observed. To avoid any potential larger nanoparticle aggregates in the injectate, we recommend to, filter a diluted (i.e. 1.0 nM) SERRS nanoparticle dispersion through a 0.22-μm filter before injection.
For Raman imaging in living animals, estimate and consider the needed imaging time in advance. For instance, if imaging will take <20 min, i.p. injection of a ketamine/xylazine cocktail is sufficient to anesthetize the animal, while anesthetization using isoflurane is the preferred method for Raman imaging of longer duration. Make sure to lubricate the eyes with a lubricant (e.g. Duratears, Alcon) and monitor the vital signs of the animal, adjust anesthesia accordingly. Raman imaging is highly dependent on the laser focus. An unfocused laser can lead to markedly decreased Raman signal intensity. While this is less of an issue for samples with flat surfaces such as tissue blocks or well-plates, the curvature of tissues in situ may lead to signal loss on the edge of the tissue where the laser is no longer in focus. While this can be mitigated by performing multiple Raman scans at different focal planes, systems (or system updates) are available that focus the laser in real-time avoiding signal loss due to out-of-focus issues.
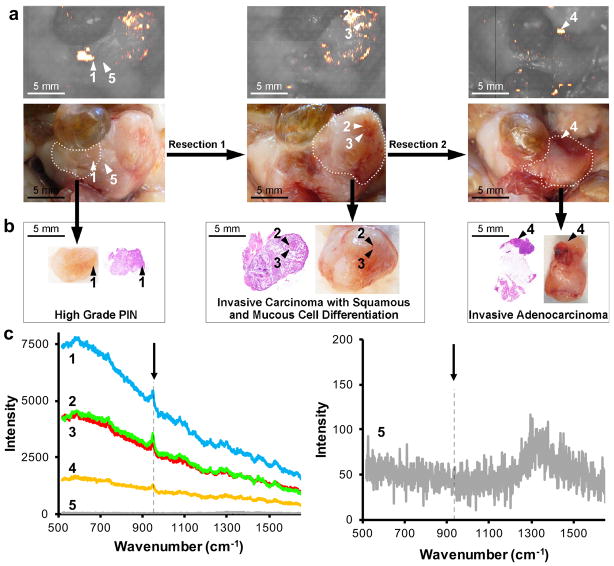
As shown in Figure 713, in situ Raman imaging of the prostate of a Hi-Myc mouse injected with SERRS nanoparticles identified a focal, SERRS-positive ‘lesion 1’ (Figure 7a), which was graded as high-grade prostate intraepithelial neoplasia (PIN) – a precursor lesion to prostate cancer – by a veterinary pathologist (Figure 7b). After Raman-guided resection of lesion 1, secondary Raman imaging was performed followed by Raman-guided resection of ‘lesions 2,3’, which was found to be invasive carcinoma with squamous and mucous cell differentiation. Following resection 2, a final Raman scan was performed. Residual SERRS signal was found and ‘lesion 4’, biopsied, and confirmed to be invasive adenocarcinoma. As shown in Figure 7c, the diagnostic 950 cm−1 band of the SERRS nanoparticles was found in all identified premalignant and malignant prostate lesions, but not in normal prostate tissue (indicated by arrow head 5).
Figure 7.
Contrast-enhanced Raman imaging of prostate neoplasia. (a) Raman image and photo of in situ prostate neoplasia detection using contrast-enhanced Raman imaging in a Hi-Myc mouse that was injected intravenously with PEGylated SERRS nanoparticles (30 fmol/g). A Raman-guided resection was performed of lesion 1. After resection 1, Raman imaging was performed and a second Raman-guided resection was performed along the dotted line. To screen for residual disease, Raman imaging was performed after resection 2. A residual lesion ‘4’ was found and biopsied. (b) Histopathological examination of H&E section of the excised tissues 1–4 identified lesion 1 as high-grade prostate intraepithelial neoplasia (PIN; a precursor lesion to prostate cancer), and lesion 2–4 as advanced prostate cancer. (c) Left panel shows the Raman spectra of the indicated lesion 1–4 and normal prostate tissue ‘5’ adjacent to lesion 1. The arrow indicates the diagnostic 950 cm−1 band of the injected SERRS nanoparticles. In the right panel the intensity is scaled between 0–200 to show the Raman spectrum of ‘5’. Reproduced and adapted with permission from Ref13. Appropriate institutional regulatory board permission was obtained.
Overall, the current protocol describes the synthesis of PEGylated SERRS nanoparticles that when administered intravenously in preclinical cancer models enable highly sensitive tumor detection independent of type and stage. Apart from biomedical research, our protocols will also be of interest to material scientists, because with minor modifications, the same synthesis protocols could be applied to different metal nanoparticles or with different encapsulation materials such as, for instance, titanium oxide instead of silica, thereby opening entirely new avenues for applications in photocatalysis and photovoltaics51–53.
Acknowledgments
We thank Travis Shaffer, PhD (Department of Radiology, Stanford University) for verifying and reproducing our results using the described protocol. We further acknowledge the MSKCC Electron Microscopy and Molecular Cytology core facilities for technical support, and Chrysafis Andreou, PhD (MSKCC) for helpful suggestions. The following funding sources are acknowledged: NIH R01 EB017748 (M.F.K.); NIH K08 CA16396 (M.F.K.); M.F.K. is a Damon Runyon-Rachleff Innovator supported (in part) by the Damon Runyon Cancer Research Foundation (DRR-29-14); Pershing Square Sohn Prize by the Pershing Square Sohn Cancer Research Alliance (M.F.K.); The Dana Foundation Brain and Immuno-Imaging Grant (M.F.K.); Dana Neuroscience Scholar Award (M.F.K.); MSKCC Brain Tumor Center Grant (M.F.K.); MSKCC Center for Molecular Imaging and Nanotechnology Grant (M.F.K.); MSKCC Technology Development Grant (M.F.K.); “Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research” and “The Center for Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center” (M.F.K.). Geoffrey Beene Cancer Research Center at MSKCC Grant and Shared Resources Award (M.F.K.); RSNA Research Scholar Grant (M.F.K.); Society of MSKCC Research Grant (M.F.K.); the National Natural Science Foundation (81401461, R.H.). Acknowledgments are also extended to the grant-funding support provided by the MSKCC NIH Core Grant (P30-CA008748).
Footnotes
AUTHOR CONTRIBUTIONS S.H. and M.A.W. designed the protocol, performed the experiments, and wrote the paper. R.H. performed the animal experiments and tissue processing and wrote the paper. M.F.K. supervised the researchers and edited the paper.
COMPETING FINANCIAL INTERESTS S.H., M.A.W., and M.F.K. have filed patent applications focused on SERRS nanoparticle composition, synthesis method(s), and Raman detection hardware. M.F.K. is a co-founder of the startup company RIO Imaging Inc., which has licensed these patents.
TWEET
How to make surface-enhanced resonance Raman scattering nanoparticles and use them to image cancer.
References
- 1.Andreou C, Kishore SA, Kircher MF. Surface-Enhanced Raman Spectroscopy: A New Modality for Cancer Imaging. J Nucl Med. 2015;56:1295–1299. doi: 10.2967/jnumed.115.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pence I, Mahadevan-Jansen A. Clinical instrumentation and applications of Raman spectroscopy. Chem Soc Rev. 2016;45:1958–1979. doi: 10.1039/c5cs00581g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long DA. Raman Spectroscopy. McGraw-Hill; 1977. [Google Scholar]
- 4.Freudiger CW, et al. Label-Free Biomedical Imaging with High Sensitivity by Stimulated Raman Scattering Microscopy. Science. 2008;322:1857–1861. doi: 10.1126/science.1165758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saar BG, et al. Video-Rate Molecular Imaging in Vivo with Stimulated Raman Scattering. Science. 2010;330:1368–1370. doi: 10.1126/science.1197236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kircher MF, et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat Med. 2012;18:829–834. doi: 10.1038/nm.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang YQ, Yan B, Chen LX. SERS Tags: Novel Optical Nanoprobes for Bioanalysis. Chem Rev. 2013;113:1391–1428. doi: 10.1021/cr300120g. [DOI] [PubMed] [Google Scholar]
- 8.Hong SM, Li X. Optimal Size of Gold Nanoparticles for Surface-Enhanced Raman Spectroscopy under Different Conditions. J Nanomater. 2013 doi: 10.1155/2013/790323. Artn 790323. [DOI] [Google Scholar]
- 9.Shaffer TM, et al. Silica nanoparticles as substrates for chelator-free labeling of oxophilic radioisotopes. Nano Lett. 2015;15:864–868. doi: 10.1021/nl503522y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 11.Lombardi JR, Birke RL. The theory of surface-enhanced Raman scattering. J Chem Phys. 2012;136 doi: 10.1063/1.3698292. Artn 144704. [DOI] [PubMed] [Google Scholar]
- 12.Lombardi JR, Birke RL. A unified view of surface-enhanced Raman scattering. Acc Chem Res. 2009;42:734–742. doi: 10.1021/ar800249y. [DOI] [PubMed] [Google Scholar]
- 13.Harmsen S, et al. Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Sci Transl Med. 2015;7:271ra277. doi: 10.1126/scitranslmed.3010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fales AM, Yuan H, Vo-Dinh T. Silica-coated gold nanostars for combined surface-enhanced Raman scattering (SERS) detection and singlet-oxygen generation: a potential nanoplatform for theranostics. Langmuir. 2011;27:12186–12190. doi: 10.1021/la202602q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulvaney SP, Musick MD, Keating CD, Natan MJ. Glass-coated, analyte-tagged nanoparticles: A new tagging system based on detection with surface-enhanced Raman scattering. Langmuir. 2003;19:4784–4790. doi: 10.1021/la026706j. [DOI] [Google Scholar]
- 16.Qian XM, Nie SM. Single-molecule and single-nanoparticle SERS: from fundamental mechanisms to biomedical applications. Chem Soc Rev. 2008;37:912–920. doi: 10.1039/b708839f. [DOI] [PubMed] [Google Scholar]
- 17.Campion A, Kambhampati P. Surface-enhanced Raman scattering. Chem Soc Rev. 1998;27:241–250. doi: 10.1039/a827241z. [DOI] [Google Scholar]
- 18.Spaliviero M, et al. Detection of Lymph Node Metastases with SERRS Nanoparticles. Mol Imaging Biol. 2016;18:677–685. doi: 10.1007/s11307-016-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garai E, et al. High-sensitivity, real-time, ratiometric imaging of surface-enhanced Raman scattering nanoparticles with a clinically translatable Raman endoscope device. J Biomed Opt. 2013;18:096008. doi: 10.1117/1.JBO.18.9.096008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakor AS, et al. The fate and toxicity of Raman-active silica-gold nanoparticles in mice. Sci Transl Med. 2011;3:79ra33. doi: 10.1126/scitranslmed.3001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakor AS, Gambhir SS. Nanooncology: the future of cancer diagnosis and therapy. CA Cancer J Clin. 2013;63:395–418. doi: 10.3322/caac.21199. [DOI] [PubMed] [Google Scholar]
- 22.Huang R, et al. High Precision Imaging of Microscopic Spread of Glioblastoma with a Targeted Ultrasensitive SERRS Molecular Imaging Probe. Theranostics. 2016;6:1075–1084. doi: 10.7150/thno.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodelon G, et al. Au@pNIPAM SERRS Tags for Multiplex Immunophenotyping Cellular Receptors and Imaging Tumor Cells. Small. 2015;11:4149–4157. doi: 10.1002/smll.201500269. [DOI] [PubMed] [Google Scholar]
- 24.Wang YW, et al. Rapid ratiometric biomarker detection with topically applied SERS nanoparticles. Technology (Singap World Sci) 2014;2:118–132. doi: 10.1142/S2339547814500125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nima ZA, et al. Circulating tumor cell identification by functionalized silver-gold nanorods with multicolor, super-enhanced SERS and photothermal resonances. Sci Rep. 2014;4:4752. doi: 10.1038/srep04752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaffer TM, et al. Stable Radiolabeling of Sulfur-Functionalized Silica Nanoparticles with Copper-64. Nano Lett. 2016;16:5601–5604. doi: 10.1021/acs.nanolett.6b02161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulder WJ, Jaffer FA, Fayad ZA, Nahrendorf M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci Transl Med. 2014;6:239sr231. doi: 10.1126/scitranslmed.3005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suarez S, Almutairi A, Christman KL. Micro- and nanoparticles for treating cardiovascular disease. Biomater Sci-Uk. 2015;3:564–580. doi: 10.1039/c4bm00441h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kircher MF, Willmann JK. Molecular body imaging: MR imaging, CT, and US. Part II. Applications. Radiology. 2012;264:349–368. doi: 10.1148/radiol.12111703. [DOI] [PubMed] [Google Scholar]
- 30.Kircher MF, Willmann JK. Molecular body imaging: MR imaging, CT, and US. part I. principles. Radiology. 2012;263:633–643. doi: 10.1148/radiol.12102394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Boer E, et al. Optical innovations in surgery. Br J Surg. 2015;102:e56–72. doi: 10.1002/bjs.9713. [DOI] [PubMed] [Google Scholar]
- 32.Andreou C, et al. Imaging of Liver Tumors Using Surface-Enhanced Raman Scattering Nanoparticles. ACS Nano. 2016;10:5015–5026. doi: 10.1021/acsnano.5b07200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian X, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 34.von Maltzahn G, et al. SERS-Coded Gold Nanorods as a Multifunctional Platform for Densely Multiplexed Near-infrared Imaging and Photothermal Heating. Adv Mater. 2009;21:3175. doi: 10.1002/adma.200803464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Lopez C, et al. Highly controlled silica coating of PEG-capped metal nanoparticles and preparation of SERS-encoded particles. Langmuir. 2009;25:13894–13899. doi: 10.1021/la9016454. [DOI] [PubMed] [Google Scholar]
- 36.Fales AM, Yuan H, Vo-Dinh T. Development of Hybrid Silver-Coated Gold Nanostars for Nonaggregated Surface-Enhanced Raman Scattering. J Phys Chem C Nanomater Interfaces. 2014;118:3708–3715. doi: 10.1021/jp4091393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury CG, Vo-Dinh T. Gold Nanostars For Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J Phys Chem C. 2008;112:18849–18859. doi: 10.1021/jp8054747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mir-Simon B, Reche-Perez I, Guerrini L, Pazos-Perez N, Alvarez-Puebla RA. Universal One-Pot and Scalable Synthesis of SERS Encoded Nanoparticles. Chem Mater. 2015;27:950–958. doi: 10.1021/cm504251h. [DOI] [Google Scholar]
- 39.Yuan H, et al. Quantitative surface-enhanced resonant Raman scattering multiplexing of biocompatible gold nanostars for in vitro and ex vivo detection. Anal Chem. 2013;85:208–212. doi: 10.1021/ac302510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turkevich J, Stevenson PC, Hillier J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss Faraday Soc. 1951;55 doi: 10.1039/df9511100055. [DOI] [Google Scholar]
- 41.Stober W, Fink A, Bohn E. Controlled Growth of Monodisperse Silica Spheres in Micron Size Range. J Colloid Interf Sci. 1968;26:62. doi: 10.1016/0021-9797(68)90272-5. [DOI] [Google Scholar]
- 42.Han X, Goebl J, Lu Z, Yin Y. Role of salt in the spontaneous assembly of charged gold nanoparticles in ethanol. Langmuir. 2011;27:5282–5289. doi: 10.1021/la200459t. [DOI] [PubMed] [Google Scholar]
- 43.Bohndiek SE, et al. A small animal Raman instrument for rapid, wide-area, spectroscopic imaging. Proc Natl Acad Sci U S A. 2013;110:12408–12413. doi: 10.1073/pnas.1301379110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palonpon AF, et al. Raman and SERS microscopy for molecular imaging of live cells. Nat Protoc. 2013;8:677–692. doi: 10.1038/nprot.2013.030. [DOI] [PubMed] [Google Scholar]
- 45.Matousek P, et al. Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl Spectrosc. 2005;59:393–400. doi: 10.1366/0003702053641450. [DOI] [PubMed] [Google Scholar]
- 46.Stone N, et al. Surface enhanced spatially offset Raman spectroscopic (SESORS) imaging - the next dimension. Chem Sci. 2011;2:776–780. doi: 10.1039/c0sc00570c. [DOI] [Google Scholar]
- 47.Butler HJ, et al. Using Raman spectroscopy to characterize biological materials. Nat Protoc. 2016;11:664–687. doi: 10.1038/nprot.2016.036. [DOI] [PubMed] [Google Scholar]
- 48.Ellman GL. Tissue Sulfhydryl Groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 49.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond) 2011;6:715–728. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jokerst JV, Miao Z, Zavaleta C, Cheng Z, Gambhir SS. Affibody-functionalized gold-silica nanoparticles for Raman molecular imaging of the epidermal growth factor receptor. Small. 2011;7:625–633. doi: 10.1002/smll.201002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cushing SK, et al. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J Am Chem Soc. 2012;134:15033–15041. doi: 10.1021/ja305603t. [DOI] [PubMed] [Google Scholar]
- 52.Pandikumar A, et al. Titania@gold plasmonic nanoarchitectures: An ideal photoanode for dye-sensitized solar cells. Renew Sust Energ Rev. 2016;60:408–420. doi: 10.1016/j.rser.2016.01.107. [DOI] [Google Scholar]
- 53.Zhou N, et al. Plasmon-enhanced light harvesting: applications in enhanced photocatalysis, photodynamic therapy and photovoltaics. Rsc Adv. 2015;5:29076–29097. doi: 10.1039/c5ra01819f. [DOI] [Google Scholar]