Abstract
Purpose
Evaluate the safety, pharmacokinetic profile, pharmacodynamic effects, and antitumor activity of the first-in-class investigational NEDD8-activating enzyme (NAE) inhibitor pevonedistat (TAK-924/MLN4924) in patients with relapsed/refractory lymphoma or multiple myeloma.
Experimental Design
Patients with relapsed/refractory myeloma (n=17) or lymphoma (n=27) received intravenous pevonedistat 25–147 mg/m2 on days 1, 2, 8, 9 (schedule A; n=27) or 100–261 mg/m2 on days 1, 4, 8, 11 (schedule B; n=17) of 21-day cycles.
Results
Maximum tolerated doses were 110 mg/m2 (schedule A) and 196 mg/m2 (schedule B). Dose-limiting toxicities included febrile neutropenia, transaminase elevations, muscle cramps (schedule A), and thrombocytopenia (schedule B). Common adverse events included fatigue and nausea. Common grade ≥3 events were anemia (19%; schedule A), and neutropenia and pneumonia (12%; schedule B). Clinically significant myelosuppression was uncommon. There were no treatment-related deaths. Pevonedistat pharmacokinetics exhibited a biphasic disposition phase and approximate dose-proportional increases in systemic exposure. Consistent with the short mean elimination half-life of ~8.5 hours, little-to-no drug accumulation in plasma was seen after multiple dosing. Pharmacodynamic evidence of NAE inhibition included increased skin levels of CDT-1 and NRF-2 (substrates of NAE-dependent ubiquitin ligases), and increased NRF-2-regulated gene transcript levels in whole blood. Pevonedistat-NEDD8 adduct was detected in bone marrow aspirates, indicating pevonedistat target engagement in the bone marrow compartment. Three lymphoma patients had partial responses; 30 patients achieved stable disease.
Conclusions
Pevonedistat demonstrated anticipated pharmacodynamic effects in the clinical setting, a tolerable safety profile, and some preliminary evidence that may be suggestive of the potential for activity in relapsed/refractory lymphoma.
Keywords: Hematologic other, leukemias and lymphomas, phase I-III trials_hematologic other, phase I-III trials_leukemias and lymphomas, novel antitumor agents, pharmacokinetics/pharmacodynamics, pevonedistat, NEDD8-31 activating enzyme (NAE) inhibition
Introduction
Regulated protein turnover via the ubiquitin-proteasome system (UPS) is central to the control of a wide variety of cellular processes (1). UPS dysregulation can lead to unrestrained cellular proliferation and/or failure to undergo programmed cell death, and development of cancer (2). Targeting the UPS was initially validated clinically with proteasome inhibitors such as bortezomib and carfilzomib (3–5). Many proteins degraded by the UPS have important roles in cell signaling, cell cycle progression, and apoptosis (6). These proteins are targeted for destruction when modified with a polyubiquitin chain by E3 ubiquitin ligases. The largest family is the Cullin-RING E3 ubiquitin ligases (CRLs), the activity of which is regulated by conjugation of ubiquitin-like protein NEDD8 (neural precursor cell expressed, developmentally down-regulated 8) to the cullin proteins (7, 8). NEDD8-activating enzyme (NAE) controls NEDD8 conjugation and is required for CRL activity and proteasomal destruction of CRL substrates (7, 8).
Pevonedistat is a first-in-class investigational small-molecule inhibitor of NAE (9, 10). NAE inhibition with pevonedistat prevents NEDD8 conjugation to CRLs via formation of pevonedistat–NEDD8 adduct (9), and leads to accumulation of CRL substrates in cell culture studies and human tumor xenografts (10). Pevonedistat is cytotoxic to tumor cell lines including solid tumors and hematologic malignancies such as lymphoma, leukemia, and multiple myeloma (MM) (11–15), and has demonstrated antitumor activity in mouse xenograft models of several human malignancies (10–12, 15, 16), including acute myeloid leukemia (AML) (12), diffuse large B-cell lymphoma (DLBCL) (11), and MM (15). Preclinical studies have investigated pevonedistat in combination with other agents. Pevonedistat plus bortezomib, dexamethasone, or doxorubicin demonstrated at least additive cytotoxic activity in MM cell lines (13, 15), and pevonedistat significantly enhanced cytarabine (17) and azacitidine (18) cytotoxicity in AML cell lines.
In cell line studies, the primary phenotype observed following NAE inhibition with pevonedistat is a cell cycle defect associated with inhibited CDT-1 (chromatin licensing and DNA replication factor-1) degradation and consequent DNA re-replication and DNA damage, leading to apoptosis (10, 11, 19, 20). Elevations of CDT-1 and NRF-2 (nuclear factor (erythroid derived 2)-related factor 2) protein levels (both CRL substrates) have been used as pharmacodynamic markers of NAE inhibition in preclinical models (10, 11). A second phenotype described in models of activated B-cell-like DLBCL involved nuclear factor-κB inhibition, resulting in apoptosis induction (11).
This phase I study was undertaken to evaluate the safety, pharmacokinetic profile, pharmacodynamic effects (including validation of pharmacodynamic markers of NAE inhibition in the clinical setting), and antitumor activity of pevonedistat in patients with relapsed and/or refractory lymphoma or MM.
Patients and Methods
Patients
Patients were aged ≥18 years and had relapsed and/or refractory disease after ≥2 prior lines of therapy for MM, any B- or T-cell non-Hodgkin lymphoma (NHL), Hodgkin lymphoma (HL), or Waldenström macroglobulinemia. Other eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, life expectancy of >6 weeks, adequate bone marrow (absolute neutrophil count ≥1,000/mm3, platelet count ≥75,000/mm3), hepatic (bilirubin <upper limit of normal [ULN]; aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase ≤2.5 × ULN), renal (calculated creatinine clearance >50 mL/min), and cardiac (B-type natriuretic peptide ≤1.5 × ULN; left ventricular ejection fraction ≥45% or pulmonary artery systolic pressure ≤1.5 × ULN) function, and evaluable disease.
Patients were excluded if they had received corticosteroids within 7 days, systemic antineoplastic therapy within 21 days, rituximab within 2 months (unless evidence of progressive disease since last rituximab dose), any investigational products within 28 days, or major surgery, radiotherapy, or systemic antibiotic therapy within 14 days prior to the first dose of study treatment. Other exclusion criteria included use of moderate or strong CYP3A inhibitors or inducers or a serious infection within 14 days prior to the first dose, a prothrombin time (PT) or activated partial thromboplastin time (aPTT) >1.5 × ULN, a history of a coagulopathy or bleeding disorder, or a requirement for warfarin which could not be switched to low molecular weight heparin. Patients with left ventricular ejection fraction <45% or pulmonary artery systolic pressure >1.5 × ULN as assessed by echocardiogram with Doppler; uncontrolled cardiovascular conditions, including cardiac arrhythmias, congestive heart failure, angina, or myocardial infarction within the past 6 months; or abnormalities on 12-lead ECG such as changes in rhythm and intervals considered by the investigator to be clinically significant were also excluded.
Review boards at all participating institutions approved the study, which was conducted according to International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided written informed consent prior to enrollment onto the study.
Study Design
This open-label, multicenter, phase I, dose-escalation study (ClinicalTrials.gov: NCT00722488) was conducted at eight sites in the United States between June 2008 and June 2012 for patients reported herein. The primary objectives were to determine the maximum tolerated dose (MTD) and safety profile of pevonedistat, describe pevonedistat pharmacokinetic and pharmacodynamic profiles in blood, and investigate pharmacodynamic effects in skin biopsies and bone marrow aspirates (patients with bone marrow involvement only). Secondary objectives included evaluation of disease response and pharmacokinetic–pharmacodynamic relationships.
Patients received escalating doses of pevonedistat by intravenous infusion over 1 hour on days 1, 2, 8, and 9 (schedule A) or days 1, 4, 8, and 11 (schedule B) of 21-day cycles. Schedule A was selected based on a dosing schedule that had been used in preclinical in vivo investigations (10, 11). Subsequently, based on observed toxicities and data from other studies, Schedule B was selected, based on the standard dosing schedule for the proteasome inhibitor bortezomib (21), to give a break between doses. The maximum permitted duration of therapy was 12 months. In schedule A, dose escalation started at 25 mg/m2, followed by 50 mg/m2, 83 mg/m2, and then doses 1.33 times the prior dose level. Schedule B dose escalation started at the schedule A MTD, with subsequent dose levels 1.33 times the prior dose level. A minimum of two patients were to be treated at each dose level before escalating. MTD determination was based on a Bayesian continual reassessment method. Once established during schedule A, the MTD level was expanded to approximately 14 patients (seven MM, seven lymphoma).
Patients who received all four scheduled doses, or had a dose-limiting toxicity (DLT) in cycle 1, were included in the DLT-evaluable population. DLT was defined as: grade ≥3 neutropenia with fever/infection, or grade 4 neutropenia for >7 days; a platelet count <10,000/mm3, grade ≥3 thrombocytopenia with bleeding, or grade 4 thrombocytopenia for >7 days; grade ≥3 non-hematologic toxicity despite maximal supportive therapy, except arthralgia/myalgia, brief fatigue, or fever without neutropenia; grade ≥2 pevonedistat-related toxicities requiring dose reduction/discontinuation; or treatment delay >2 weeks due to lack of adequate recovery from toxicities.
Dose reduction was required for dose-limiting hematologic toxicity. For grade 3 non-hematologic toxicity, dosing was interrupted until resolution to grade ≤1, and then reinstated at the next lower dose level. Pevonedistat was discontinued for any grade 4 non-hematologic toxicity.
Assessments
Toxicities were recorded throughout the study until 30 days after last dose. Adverse events (AEs) were graded according to the National Cancer Institute’s Common Terminology Criteria for AEs (NCI CTCAE) version 3.0.
For lymphoma patients, computed tomography (CT) of the chest, abdomen, and pelvis, and a positron emission tomography (PET) scan from neck to mid-thighs were performed at screening and on day 21 of cycles 1, 3, and 5. From cycle 7, patients were evaluated using CT only every other cycle and at the end of study visit. Tumor response was assessed by the investigators using International Working Group criteria for lymphoma patients (22).
For MM patients, a skeletal survey was performed at screening and radiographs were performed as needed to evaluate for disease progression if signs or symptoms of increased or new bone lesions were present. CT or magnetic resonance imaging (MRI) scans were performed as clinically indicated, at baseline, at the end of cycle 1, and every other cycle thereafter, to evaluate suspected extramedullary disease. Quantitative M-protein assessment and serum and urine immunofixation were performed at baseline, and at the end of cycle 1 and every alternate cycle thereafter. Disease status was assessed using the International Myeloma Working Group Uniform Response Criteria (23).
Pharmacokinetic and pharmacodynamic analyses
For pharmacokinetic analyses, serial blood samples were obtained during cycle 1 for determination of pevonedistat plasma concentrations. On schedule A, samples were obtained within 1 hour before infusion and immediately after completion of infusion on days 1, 2, 8, and 9. Additionally, samples were obtained: on day 1 at 30 mins, 1, 2, 4, and 8 hours after completion of infusion; and on day 9 at 30 mins, 1, 2, 4, 6, 9, 24, 72, and 144 hours after completion of infusion. On schedule B, samples were collected on days 1, 4, 8, and 11 within 1 hour (day 1) or within 10 mins (days 4, 8, 11) before infusion as well as immediately after completion of infusion. Additionally, samples were obtained at 1, 2, 4, and 8 hours after completion of infusion and 24 and 48 hours after start of infusion on days 1 and 4. Bioanalytical assays were conducted at Tandem Labs (West Trenton, NJ). Plasma concentrations were measured using Good Laboratory Practice (GLP)-validated liquid chromatography with tandem mass spectrometry (LC/MS/MS). The dynamic range was 1–500 ng/mL for the low-range assay, and 75–7500 ng/mL for the high-range assay. Noncompartmental methods (WinNonlin software, vs 6.2, Pharsight Corporation, Cary, NC) were used to estimate pharmacokinetic parameters.
For pharmacodynamic analyses, whole blood was isolated in PAXgene tubes at screening, baseline, and during cycle 1: following pevonedistat administration on day 1 (at 4 and 8 hours), on day 2 pre-dose, and on day 5 (schedule A); or following pevonedistat administration on days 1 and 11 (at 4, 8, and 23 hours), pre-dose on days 4 and 11, and on day 15 (schedule B). Reverse-transcriptase-PCR was used to analyze expression in whole blood of eight genes shown to be induced by pevonedistat in preclinical studies (ATF-3, GCLM, GSR, MAG1, NQO1, SLC7A11, SRXN1, and TXNRD1; all except MAG1 known to be regulated by NRF-2(24)). Pharmacodynamic assays were performed at Asuragen (Austin, TX; reverse-transcriptase-PCR panel), and Millennium Pharmaceuticals, Inc. (neddylated cullins, CDT-1).
Raw data were transformed prior to calculation of percent change and assumptions were made for missing data. The percentage change from baseline in the relative expression of each of the eight genes of interest was calculated as (2−(post-baseline ΔCt − baseline ΔCt) − 1) × 100. The ΔCt was the mean Ct (cycle threshold, a measure of mRNA gene expression, over the replicates) for each gene of interest, minus the mean Ct for four housekeeping genes (18S, B2M, RPLP0, and UBC) calculated at each time point. A positive percent change was equivalent to an increase in expression after dosing with pevonedistat. For Ct values that were undetermined, the value 40 (the number of PCR cycles) was used for purposes of estimation of pharmacodynamic parameters and data summarization. Summary statistics were generated for the percent change from baseline at each time point for each gene.
Skin punch biopsies (2 mm) for CDT-1 and NRF-2 immunohistochemical assays were performed at screening, and 3–6 hours after the cycle 1, day 2 (schedule A) or day 4 (schedule B) dose. The rationale for these investigations was to determine if biologically active levels of pevonedistat were achieved outside of the blood compartment.
Immunohistochemistry assays were performed at Millennium Pharmaceuticals, Inc. on five micrometer sections using the Ventana XT® auto-stainer. Antigen retrieval consisted of incubation with Ventana’s CC1 antigen retrieval solution for 20 minutes and treatment with assay-specific antibodies. Antibodies to CDT-1 (Millennium Pharmaceuticals, Inc.) and NRF-2 (Epitomics Catalog #2178) were incubated for 1 hour at room temperature, labeled with Ventana’s alkaline phosphatase ultra secondary antibodies for 32 mins at room temperature, and developed with Ventana’s NBT/BCIP substrate system. Slides were counterstained and imaged at 20x magnification using Aperio’s whole-slide imaging scanner. Signal expression was calculated as percent stained area of the basal epithelial region, and quantified using Metamorph imaging software.
Bone marrow aspirates for pevonedistat–NEDD8 adduct and CD138 immunohistochemical assay were taken at screening and 2–6 hours after the cycle 1, day 2 (schedule A) or day 4 (schedule B) dose. Analyses were performed at Millennium Pharmaceuticals, Inc. Aspirates were allowed to clot and then formalin-fixed and paraffin-embedded. An antibody specific for pevonedistat–NEDD8 adduct (Millennium Pharmaceuticals, Inc.) was incubated for 1 hour at 37°C and labeled with Vector Laboratories goat anti-rabbit biotinylated secondary for 32 minutes at room temperature; Ventana’s DAB Map system was used for detection of the biotinylated secondary. Similarly, a CD138 antibody (Ventana catalog #760–4248) was incubated for 1 hour at 37°C and labeled with UltraMap™ HRP anti-mouse secondary antibody for 4 minutes at room temperature. Ventana’s ChromoMap DAB Map system was used for detection of the HRP secondary. All slides were counterstained and imaged at 20x magnification using Aperio’s whole-slide imaging scanner. CD138 staining was used to identify MM cells, and presence of pevonedistat–NEDD8 adduct showed mechanism-based NAE inhibition in the bone marrow compartment.
Statistical methods
The MTD for each schedule was defined as the dose level closest to that predicted to result in a 25% DLT rate. MTD was predicted using a Bayesian continual reassessment method (25) algorithm based on observed toxicities in all enrolled patients. The dose level at which the total number of enrolled patients reached six was to be considered the observed MTD. The dose–toxicity relationship was modeled by a single-parameter logistic model with a beta prior distribution. Cohorts of no more than two patients were dosed with pevonedistat at a given time. The decision to escalate, de-escalate, or expand the given dose was based on the model-estimated predicted MTD (PMTD). Patients were dosed at the dose level closest to the PMTD. Dose levels were pre-defined; skipping dose levels was not permitted. Descriptive statistics were used to summarize all data.
Results
Patient characteristics
Twenty seven patients received pevonedistat on schedule A, and 17 patients received pevonedistat on schedule B. Patient demographics and baseline disease characteristics are summarized in Table 1. Patients were heavily pretreated (Table A1, online-only appendix). All MM patients and 2 lymphoma patients had been previously treated with bortezomib.
Table 1.
Patient demographics and baseline characteristics in schedule A (days 1, 2, 8, 9 of 21-day cycles) and schedule B (days 1, 4, 8, 11 of 21-day cycles)
| Characteristics | Schedule A N=27 |
Schedule B N=17 |
|---|---|---|
| Median age, years (range) | 62.0 (34–90) | 58.0 (26–68) |
| Male, n (%) | 17 (63) | 13 (76) |
| Race, n (%) | ||
| White | 21 (78) | 11 (65) |
| Black or African American | 5 (19) | 4 (24) |
| American Indian or Alaskan native | 0 | 1 (6) |
| Not reported | 1 (4) | 1 (6) |
| ECOG performance status, n (%) | ||
| 0 | 11 (41) | 3 (18) |
| 1 | 13 (48) | 13 (76) |
| 2 | 2 (7) | 1 (6) |
| 3a | 1 (4) | |
| Patients with MM, n (%) | n=15 | n=2 |
| MM subtype, n (%)b | ||
| IgG | 10 (67) | 2 (100) |
| IgA | 4 (27) | 1 (50) |
| Other | 2 (13) | 0 |
| Median time since initial diagnosis, months (range) | 78.0 (18–111) | 81.5 (66–97) |
| Patients with lymphoma, n (%) | n=12 | n=15 |
| Lymphoma subtype, n (%) | ||
| Follicular lymphoma | 4 (33) | 1 (7) |
| DLBCL | 3 (25) | 7 (47) |
| Small lymphocytic lymphoma/chronic lymphocytic leukemia | 1 (8) | 1 (7) |
| Mantle cell lymphoma | 1 (8) | 0 |
| Lymphoplasmacytic lymphoma | 1 (8) | 0 |
| Hodgkin lymphoma | 1 (8) | 4 (27) |
| PTCL | 0 | 1 (7) |
| Splenic marginal zone B-cell lymphoma | 0 | 1 (7) |
| Other | 1 (8) | 0 |
| Bone marrow involvement, n (%) | 5 (42) | 3 (20) |
| Median time since primary diagnosis, months (range) | 75.5 (19–197) | 32.0 (1–102) |
Protocol deviation.
1 patient in schedule A recorded as having MM subtypes of IgG and other: oligosecretory; 1 patient in schedule B recorded as having MM subtypes of IgG and IgA.
DLBCL, diffuse large B-cell lymphoma; ECOG, Eastern Cooperative Oncology Group; Ig, immunoglobulin; PTCL, Peripheral T-cell lymphoma - not otherwise specified
Dose escalation and MTD determination
Schedule A
Patients were treated at six pevonedistat dose levels: 25 (n=3), 50 (n=2), 65 (n=3), 83 (n=2), 110 (n=14), and 147 (n=3) mg/m2. Twenty-two patients were DLT-evaluable; five were excluded due to not receiving all four doses in cycle 1. Three patients experienced DLTs. One patient at 65 mg/m2 had drug-related grade 4 febrile neutropenia on day 3, which resolved by day 10 following dose reduction to 50 mg/m2. One patient at 110 mg/m2 had drug-related grade 3 AST/ALT elevation on day 2, which resolved by day 8 following dose reduction to 83 mg/m2. One patient at 147 mg/m2 had grade 3 muscle cramps on day 2, which resolved within 48 hours following pevonedistat discontinuation. Due to the severity of this AE, it was decided to no longer dose patients above 110 mg/m2 in schedule A. Three additional patients were enrolled to the 110 mg/m2 cohort, and with 6 patients treated at this dose level the MTD was determined to be 110 mg/m2.
Schedule B
Patients were treated at five pevonedistat dose levels: 110 (n=2), 147 (n=2), 196 (n=8), 261 (n=4); one patient received 100 mg/m2 after this dose level was identified for further evaluation based on safety evaluation across all pevonedistat trials. Thirteen patients were DLT-evaluable. One patient at 110 mg/m2 experienced grade 3 thrombocytopenia on day 15; the patient discontinued after cycle 2, day 1 due to disease progression, and thrombocytopenia was ongoing at the end-of-study visit 12 days later. Following the occurrence of acute severe toxicities at high pevonedistat doses in a few patients in another study (NCT01011530), it was decided not to administer pevonedistat at doses >200 mg/m2. Therefore, although no DLTs were observed at 261 mg/m2, 196 mg/m2 was selected as the schedule B MTD. Unlike in schedule A, this cohort was not expanded to 14 patients per the sponsor’s decision, following a cross-program review, to move forward with studies of pevonedistat in combination with standard-of-care therapies.
Treatment exposure and safety
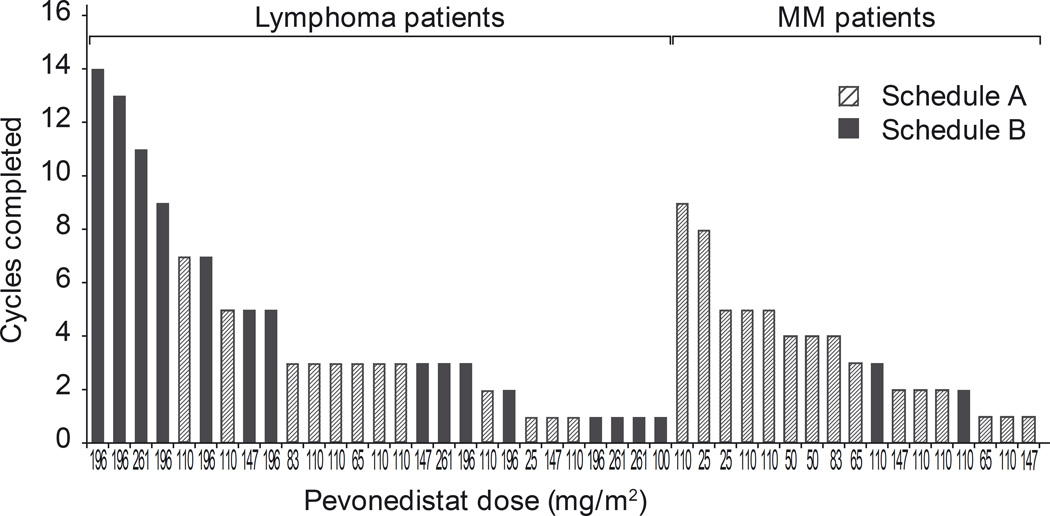
Patients received a median of 3 cycles of treatment on both schedules (schedule A, range 1–9; schedule B, range 1–14; Fig. 1). All patients have discontinued pevonedistat due to progressive disease/symptomatic deterioration (schedule A, n=19; schedule B, n=15), consent withdrawal (n=5; n=1), occurrence of an AE (n=2; n=1), or unsatisfactory therapeutic response (n=1, n=0).
Fig 1.
Treatment duration with pevonedistat, by dose level and tumor type. MM and lymphoma patients received a median of 3 (range 1–9) and 3 (range 1–14) cycles, respectively. Fourteen patients (7 in each schedule) received ≥5 treatment cycles.
AEs reported in ≥15% of patients and grade ≥3 AEs reported in >1 patient on either schedule are shown in Table 2. Transaminase elevations were clinically asymptomatic and reversible with dose modification. Myelosuppression was limited, with anemia the only hematologic toxicity reported in >20% of patients overall. There was no requirement for growth factor or transfusion support. Peripheral neuropathy was limited (11%) and similar between schedules (Table 2). No grade 4 AEs were reported at the MTD in either schedule.
Table 2.
The most common AEs of any grade reported in ≥15% of patients in either schedule and of grade ≥3 reported in >1 patient on either schedule
| AE, n (%) | Schedule All grades |
A,
n=27 Grade ≥3 |
Schedule All grades |
B,
n=17 Grade ≥3 |
|---|---|---|---|---|
| Any AE | 27 (100) | 16 (59) | 17 (100) | 12 (71) |
| Hematologic AEs | ||||
| Anemia | 7 (26) | 5 (19) | 3 (18) | 1 (6) |
| Thrombocytopenia | 3 (11) | 1 (4) | 3 (18) | 1 (6)a |
| Neutropenia | 2 (7) | 2 (7) | 2 (12) | 2 (12) |
| Non-hematologic AEs | ||||
| Fatigue | 18 (67) | 2 (7) | 12 (71) | 1 (6) |
| ALT increased | 12 (44) | 1 (4)a | 4 (24) | 1 (6) |
| AST increased | 12 (44) | 2 (7)a | 2 (12) | 0 |
| Nausea | 12 (44) | 0 | 13 (76) | 0 |
| Pyrexia | 11 (41) | 0 | 8 (47) | 1 (6) |
| Constipation | 10 (37) | 0 | 5 (29) | 0 |
| Myalgia | 10 (37) | 0 | 10 (59) | 0 |
| Diarrhea | 7 (26) | 1 (4) | 8 (47) | 0 |
| Headache | 7 (26) | 0 | 9 (53) | 0 |
| Vomiting | 7 (26) | 0 | 10 (59) | 0 |
| Blood alkaline phosphatase increased | 6 (22) | 0 | 1 (6) | 0 |
| Decreased appetite | 6 (22) | 0 | 10 (59) | 0 |
| Dizziness | 6 (22) | 0 | 8 (47) | 0 |
| Hypomagnesemia | 6 (22) | 0 | 1 (6) | 0 |
| Pain | 6 (22) | 1 (4) | 5 (29) | 1 (6) |
| Chills | 5 (19) | 0 | 6 (35) | 0 |
| Dyspnea | 5 (19) | 1 (4) | 4 (24) | 1 (6) |
| Hypercalcemia | 5 (19) | 2 (7) | 1 (6) | 0 |
| Hypophosphatemia | 5 (19) | 3 (11) | 1 (6) | 1 (6) |
| Vision blurred | 5 (19) | 0 | 0 | 0 |
| Blood creatinine increased | 4 (15) | 0 | 0 | 0 |
| Hyperkalemia | 4 (15) | 1 (4) | 0 | 0 |
| Muscle spasms | 4 (15) | 1 (4)a | 10 (59) | 1 (6) |
| Pain in extremity | 4 (15) | 0 | 5 (29) | 0 |
| Back pain | 3 (11) | 0 | 4 (24) | 0 |
| Insomnia | 3 (11) | 0 | 4 (24) | 0 |
| Peripheral edema | 3 (11) | 0 | 3 (18) | 0 |
| Upper respiratory tract infection | 3 (11) | 0 | 3 (18) | 1 (6) |
| Peripheral neuropathies NECb | 3 (11) | 0 | 2 (12) | 0 |
| Asthenia | 2 (7) | 0 | 3 (18) | 0 |
| Cough | 2 (7) | 0 | 5 (29) | 0 |
| Dehydration | 2 (7) | 0 | 3 (18) | 1 (6) |
| Paresthesia | 2 (7) | 0 | 5 (29) | 0 |
| Productive cough | 2 (7) | 0 | 3 (18) | 0 |
| Sinus tachycardia | 2 (7) | 1 (4) | 3 (18) | 0 |
| Abdominal discomfort | 1 (4) | 0 | 3 (18) | 0 |
| Hyperbilirubinemia | 1 (4) | 0 | 5 (29) | 1 (6) |
| Pneumonia | 1 (4) | 1 (4) | 3 (18) | 2 (12) |
| Weight decreased | 1 (4) | 0 | 3 (18) | 0 |
| Dyspepsia | 0 | 0 | 3 (18) | 0 |
| Dyspnea exertional | 0 | 0 | 3 (18) | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; NEC, not elsewhere classified
1 DLT;
High-level term, incorporating 3 ‘neuropathy peripheral’ (2 schedule A, 1 schedule B), 1 peripheral sensory neuropathy (schedule A), and 1 peripheral sensorimotor neuropathy (schedule B); 3 MM patients with prior bortezomib had peripheral neuropathy, plus 2 lymphoma patients without prior bortezomib
Eight patients each on schedule A (30%) and schedule B (47%) experienced serious AEs (SAEs). On schedule A, four patients had SAEs considered related to pevonedistat: grade 4 anemia and grade 3 generalized body pain; grade 4 febrile neutropenia (DLT); grade 2 pyrexia; and grade 2 orthostatic hypotension. On schedule B, two patients had drug-related SAEs: grade 4 neutropenia and grade 3 bacteremia; and grade 2 pyrexia and grade 2 sinus tachycardia. Two patients in schedule A died on study due to progressive disease; there were no treatment-related deaths.
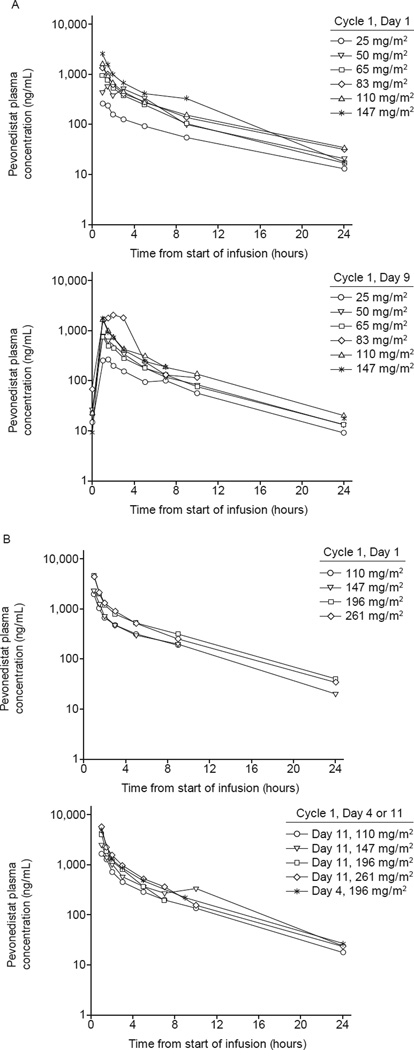
Pharmacokinetic profile
Mean plasma concentration–time profiles of pevonedistat exhibited a biphasic disposition phase (Fig. 2). In general, acknowledging the relatively small cohort sizes, approximate dose-proportional increases in mean Cmax and AUC0–24hr were seen across the 25–147 mg/m2 dose levels in schedule A (Table A2, online-only appendix) and the 100–261 mg/m2 dose levels in schedule B (Table A3, online-only appendix).
Fig 2.
Mean pevonedistat plasma concentration–time profiles on (A) days 1 and 9 of schedule A, and (B) days 1 and 4 (or, alternatively, day 11) of schedule B. Mean plasma concentration–time profiles of pevonedistat exhibited a biphasic disposition phase, characterized by an initial rapid decline at the end of the infusion, followed by a slower phase with plasma concentrations remaining quantifiable up to 24 hours (schedule A) or 48 hours (schedule B) post-infusion at all doses.
Pharmacokinetic parameters from 13 and 7 patients receiving the MTDs on schedules A and B, respectively, are summarized in Table 3. Terminal disposition phase was adequately characterized in five patients at the schedule B MTD; mean plasma elimination half-life was estimated to be 8.5 hours (range 7.7–9.1). Consistent with this, pevonedistat systemic exposures were similar between days 1 and 9 on schedule A and days 1 and 4 (or, alternatively, day 11) on schedule B, indicating little or no drug accumulation in plasma.
Table 3.
Pharmacokinetic parameters of pevonedistat during cycle 1 in patients receiving pevonedistat at the MTD determined in each schedule
| Schedule A 110 mg/m2 |
Schedule B 196 mg/m2 |
|||
|---|---|---|---|---|
| Parameter | Day 1, n=13 | Day 9, n=9 | Day 1, n=7 | Day 4, n=6 |
| Cmax, ng/mL | 1502 (33) | 1683 (28) | 4565 (24) | 3751 (53) |
| Tmax, hr | 1.02 (1.0–1.3) | 1.08 (1.0– 1.1) | 1.1 (1.0–1.1) | 1.02 (0.9– 1.1) |
| AUC0-24hr, nghr/mL | 4685 (23) | 4833 (19)a | 10,830 (19)b | 8309 (39) |
| AUC0-τ, nghr/mL | 4685 (23) | 4833 (19)a | 12,367 (13)c | NE |
| t½, hr | NE | NE | 8.5 (0.6)d | NE |
| CLp, L/hr | NE | NE | 28.3 (3)e | NE |
| Vss, L | NE | NE | 158 (9)e | NE |
Parameters are reported as geometric mean (% coefficient of variation), except for Tmax, which is reported as median (range), and t½, which is reported as the arithmetic mean (standard deviation).
n=7,
n=6,
n=4,
n=5,
n=3.
NE, not estimated. Due to limited time points in the terminal disposition phase, an estimate of pevonedistat elimination half-life could not be obtained for schedule A.
Cmax, maximum observed concentration; Tmax, time to reach Cmax; AUC0–24hr/AUC0-τ, area under the concentration–time curve from time zero to 24 hours / to the end of dosing interval, where the dosing interval, τ, is equal to 24 and 72 hours for schedules A and B, respectively; t½, terminal disposition half-life; CLp, plasma clearance; Vss, steady-state volume of distribution.
Pharmacodynamic effects
Pevonedistat induced the expected pharmacodynamic effects in whole blood, indicating NAE inhibition; expression of NRF-2-regulated genes (and MAG-1) was increased following pevonedistat administration on cycle 1, day 1 versus baseline across the 25–261 mg/m2 dose range (data at MTDs shown in Table A4, online-only appendix), notably NQO1, for which maximal changes from baseline ranged from approximately 250% to 400% and 700% to 800% at doses of 25–83 mg/m2 and 110–261 mg/m2, respectively (data not shown). These effects generally appeared to increase with increasing systemic exposure to pevonedistat.
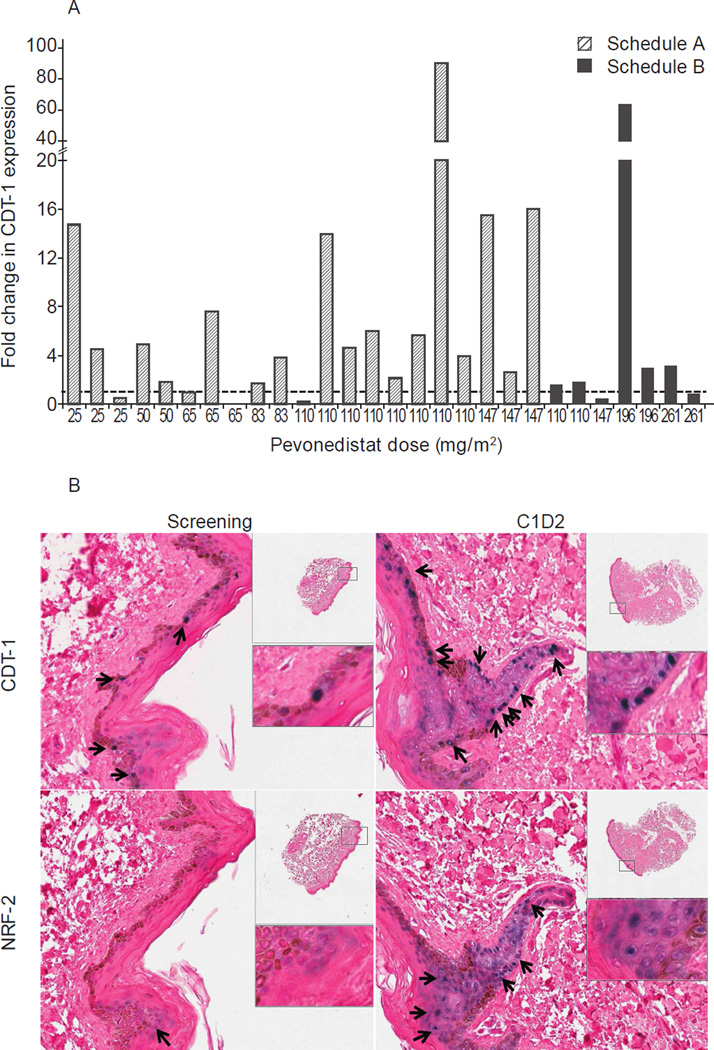
Immunohistochemical assessment of skin biopsies showed increases in CDT-1 and NRF-2 expression levels following the second dose of pevonedistat in cycle 1, compared to baseline, indicating NAE pathway inhibition by pevonedistat in peripheral tissue. Fig. 3 shows fold-change in CDT-1 expression versus baseline among evaluable patients, and representative immunohistochemistry images of CDT-1 and NRF-2 staining. For NRF-2, 19 patients treated on schedule A at pevonedistat 25–147 mg/m2 showed a mean 185-fold (standard deviation 356; median 39.4-fold [range 0–1483]) increase; 3 additional patients on schedule A had NRF-2 increases post-dose from a baseline value of zero (data not shown). No correlation could be found with individual CDT-1 or NRF-2 changes and pevonedistat systemic exposures.
Fig 3.
Pevonedistat treatment increased CDT-1 and NRF-2 levels compared to baseline. (A) Fold-change from baseline in CDT-1 expression in skin biopsies obtained 3–6 hours after the second dose of pevonedistat in cycle 1 (day 2 on schedule A, day 4 on schedule B). (B) Representative immunohistochemistry images of skin biopsy sections stained for CDT-1 and NRF-2 taken at baseline and on cycle 1, day 2 from a patient enrolled in the 83 mg/m2 dose level on schedule A. Arrows and blue/purple staining indicate CDT-1 or NRF-2 positive staining.
Immunohistochemical assays of bone marrow aspirates from patients with MM or lymphoma with bone marrow involvement demonstrated the presence of pevonedistat–NEDD8 adduct in post-dose samples from 11 of 13 evaluable patients on schedules A and B. In one patient treated at 25 mg/m2, pevonedistat–NEDD8 adduct was not detected in either the pre-dose or post-dose sample, and one pair of samples showed adduct in the pre-dose but not the post-dose sample, indicating a likely sample switch. A representative image is shown in Fig. A1 (online-only appendix), indicating pevonedistat target engagement in the bone marrow compartment.
Antitumor activity
One patient in schedule A and two in schedule B achieved partial responses (PRs). A 34-year-old male with relapsed nodular sclerosis HL achieved a PR with pevonedistat 110 mg/m2 (schedule A) after 5 cycles, and had progressive disease on cycle 7, day 21. A 47-year-old male with relapsed DLBCL achieved a PR after 3 cycles of pevonedistat 196 mg/m2 (schedule B) and remained on study to cycle 9, day 18, when he experienced disease progression. A 65-year-old female with relapsed peripheral T-cell lymphoma achieved a PR with pevonedistat 196 mg/m2 (schedule B) in cycle 1 (day 21) that lasted two cycles until disease progression. Further patient details are provided in Table A5 (online-only appendix).
An additional 30 of 42 (71%) response-evaluable patients (treated patients who had ≥1 post-baseline disease assessment; n=19, schedule A, n=11, schedule B; n=17 lymphoma, n=13 MM) achieved stable disease (SD), 12 of whom (n=6 in each schedule) received ≥5 cycles. Of these 12, 7 lymphoma patients had SD lasting 3.25–9.53 months and 5 MM patients had SD lasting 3.22–6.01 months (Table A6, online-only appendix).
Discussion
Targeting the UPS is an effective therapeutic approach in human cancers, as demonstrated by the clinical development of proteasome inhibitors (3–5). Other therapeutic targets within the UPS are being investigated with the aim of affecting specific substrate proteins and signaling pathways of importance in human cancers (26, 27), without disrupting degradation of all UPS-processed proteins, as occurs with 26S proteasome inhibition (28). Inhibition of the NEDD8 conjugation pathway has emerged as a feasible therapeutic target (29).
This is the first reported study of pevonedistat in MM and lymphoma. Pevonedistat had a generally manageable safety profile over a median treatment duration of 3 cycles. Approximately two-thirds of patients experienced grade ≥3 AEs, with only anemia and hypophosphatemia on schedule A and neutropenia and pneumonia on schedule B reported in >10% of patients. The commonly reported elevations in liver enzymes were generally clinically asymptomatic and resolved to baseline. Furthermore, limited myelosuppression may make pevonedistat a suitable agent for use in combination with cytotoxic chemotherapy, or a feasible therapy for patients unable or unwilling to receive standard supportive measures for hematologic toxicity. Notably, the peripheral neuropathy rate was limited, at 11%.
The pevonedistat MTD was 110 mg/m2 on schedule A and 196 mg/m2 on schedule B. DLTs were observed relatively infrequently; in schedule A three DLTs of febrile neutropenia, transaminase elevations, and muscle cramps were observed, and in schedule B a single DLT of thrombocytopenia was observed. During schedule A, the severity of grade 3 muscle cramps prompted a decision to no longer dose patients above 110 mg/m2, and in schedule B, while no DLTs were observed at 261 mg/m2, toxicities observed in another study of pevonedistat (NCT01011530) prompted a program-wide safety review and a subsequent decision not to continue dosing at this higher end of the dosing range employed across clinical trials. Additionally, as noted in the Results, a cross-program review resulted in the decision to move forward with studies of pevonedistat in combination with standard-of-care therapies, and thus schedule B was not expanded at the MTD. Of note, the MTD in a separate phase 1 study of pevonedistat in patients with AML and myelodysplastic syndromes (MDS), using the same dosing as schedule B (days 1, 4, 8, and 11), was determined to be 83 mg/m2 (30). This MTD may have been lower due to the population of relapsed/refractory AML/MDS patients being sicker than the lymphoma/MM patients in the present study or due to inherent differences between the diseases.
Pharmacokinetic analyses revealed a general increase in pevonedistat systemic exposure with increasing dose, and a half-life of 8.5 hours; consistent with this, little or no drug accumulation was seen following multiple dosing on either schedule. Analyses of whole blood, skin biopsies, and bone marrow aspirates demonstrated that pevonedistat exerted predicted pharmacodynamic effects, including increased levels of CDT-1 and NRF-2, and pevonedistat–NEDD8 adduct formation. Our findings represent important validation in the clinic of the pharmacodynamic markers of NAE inhibition and target engagement demonstrated in preclinical investigations (9–11).
Our study provides some preliminary evidence that may be suggestive of modest antitumor activity of single-agent pevonedistat in heavily pretreated patients in both dosing schedules, with 3 lymphoma patients achieving a PR. A further 71% of patients achieved SD, with 12 patients receiving ≥5 cycles. PRs were seen only in lymphoma patients, with best responses of only SD seen in MM patients. Of potential importance, all MM and 2 lymphoma patients had received prior bortezomib. Higher levels of proteasome maturation protein (POMP) have been seen in bortezomib-resistant cell lines, and over-expression of NRF-2 has been shown to induce POMP and increase proteasome chymotrypsin activity (31); thus, increased NRF-2 levels following NAE inhibition, together with prior proteasome inhibition, may be associated with compensatory increases in POMP and possibly resistance to further UPS inhibition. Furthermore, lack of response in MM patients with pevonedistat may possibly be associated with limited induction of the endoplasmic reticulum (ER) stress response (32), compared with proteasome inhibition with bortezomib or ixazomib, for which ER stress has been linked with anti-myeloma activity preclinically (33–35) and clinically (36).
In conclusion, this phase I study of pevonedistat demonstrated the validity of NAE inhibition as a therapeutic target and the anticipated pharmacodynamic effects in the clinical setting. We also report the safety profile of pevonedistat in MM and lymphoma patients and some preliminary evidence that is suggestive of the potential for modest activity in patients with relapsed/refractory lymphoma. Further to this single-agent activity, plus some modest clinical activity in patients with relapsed and/or refractory AML and MDS (30), rational combination strategies have been investigated including pevonedistat plus azacitidine in patients with AML considered unfit for intensive induction therapy (NCT01814826) (37); future clinical testing is expected to include lymphoma, AML, and MDS, while additional testing in MM may not be warranted without additional research to support enrollment of a specific patient population. In patients with advanced solid tumors, rational combination strategies are being investigated, including pevonedistat plus docetaxel, gemcitabine, or carboplatin-paclitaxel (NCT01862328).
Supplementary Material
Statement of translational relevance.
Targeting the ubiquitin–proteasome system (UPS), a key regulator of intracellular protein degradation, is an effective therapeutic approach in hematological cancers. Many proteins with roles in cell signaling and cell cycle progression are targeted for UPS degradation by the Cullin-RING E3 ubiquitin ligases (CRLs), the activity of which is regulated by conjugation of ubiquitin-like protein NEDD8 to the cullin proteins. NEDD8-activating enzyme (NAE) controls NEDD8 conjugation and is therefore essential for CRL activity making NAE inhibition a feasible therapeutic target. This study investigated pevonedistat (TAK-924/MLN4924), a novel investigational inhibitor of NAE, in patients with multiple myeloma or lymphoma, and demonstrated the validity of NAE inhibition as a therapeutic target and the anticipated pharmacodynamic effects in the clinical setting. The safety profile of single-agent pevonedistat in multiple myeloma and lymphoma patients was generally tolerable, and there was preliminary evidence that may be suggestive of modest antitumor activity in relapsed/refractory lymphoma.
Acknowledgments
The authors would like to thank Dr Stephen J Blakemore, a former employee of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd., for helpful discussions and critical review during the development of the manuscript. The authors would also like to thank Steve Hill of FireKite, an Ashfield business, part of UDG Healthcare plc, for writing support during the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc.
Financial support: This study was funded by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd. R.Z.O. would like to acknowledge support from The MD Anderson Cancer Center Support Grant (P30 CA016672).
Footnotes
Disclosures of conflicts of interest:
JJS: Consulting or Advisory Role. FORMA Therapeutics; Array BioPharma; Novartis; Celgene; Onyx; Takeda; Research Funding: Array Biopharma; Novartis; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd; Onyx; Celgene.
AJJ: Consultancy and honoraria: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd, Celgene, Centocor Ortho Biotech, Exelixis, Bristol-Myers Squibb, Onyx Pharmaceuticals; honoraria: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd, Celgene, Centocor Ortho Biotech, Exelixis, and Bristol-Myers Squibb; advisory committee member: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd, Centocor Ortho Biotech, Exelixis, Bristol-Myers Squibb, Celgene, Onyx Pharmaceuticals.
OAO: Research funding: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd, Celgene, TG Therapeutics, Spectrum, Acetylon. Scientific advisory boards: Mundipharma, Novartis; TG Therapeutics.
RZO: Research funding: Bristol-Myers Squibb, Celgene Corporation, Millennium Pharmaceuticals, Inc., Onyx Pharmaceuticals, Resverlogix. Honoraria: Array Biopharma, Bristol-Myers Squibb, Celgene Corporation, Genentech, Millennium Pharmaceuticals, Inc., Onyx Pharmaceuticals. Membership of advisory boards: Array Biopharma, Bristol-Myers Squibb, Celgene Corporation, Genentech, Merck, Millennium Pharmaceuticals, Inc., Onyx Pharmaceuticals.
RDH: Consulting or Advisory Role: BMS; Amgen; Research Funding: Eisai; Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd; Onyx; Novartis; Celgene; Sanofi; AstraZeneca; Calithera Biosciences; Acetylon Pharmaceuticals; AVEO; Agensys; Merck; Genzyme; ImClone Systems; Synta; Abbott Biotherapeutics
MRS: Research funding: AbbVIE, Celgene Corporation, Janssen, Millennium Pharmaceuticals, Inc., Seattle Genetics. Membership of advisory boards: Spectrum Pharmaceuticals, Inc.
DL: no conflicts of interest to disclose.
CD: Stock or Other Ownership: Gilead Sciences; Consulting or Advisory Role: Seattle Genetics; Janssen Oncology; Idera Pharmaceuticals; Celgene; Immunogen; Speakers’ Bureau: Seattle Genetics; Gilead Sciences; Research Funding: Seattle Genetics; Genentech; Gilead Sciences; Janssen Oncology; Molecular Templates; Incyte.
KK: no conflicts of interest to disclose.
ZH, AJB, GM, HF, ST, and BJD: employees of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Ltd
SL: Consultancy: Millennium Pharmaceuticals, Inc., Celgene, Novartis, Bristol-Myers Squibb, Onyx Pharmaceuticals, Merck.
ClinicalTrials.gov identifier: NCT00722488
Author contribution statement:
JJS, AJJ, OAO, RZO, RDH, MRS, AJB, GM, BJD, and SL designed the research. JJS, AJJ, OAO, RZO, RDH, MRS, DL, CD, KK, and SL performed the research. JJS, AJJ, OAO, RZO, RDH, MRS, DL, CD, KK, AJB, GM, HF, BJD, and SL collected data. JJS, ZH, AJB, GM, HF, ST, and BJD analyzed and interpreted data (pharmacokinetics: HF; pharmacodynamics: AJB, GM, ST). ZH performed statistical analysis. JJS, MRS, AJB, GM, and BJD wrote the draft manuscript. All authors contributed to the writing and reviewing of the manuscript, and approved the final manuscript for submission.
References
- 1.Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005;12:1191–1197. doi: 10.1038/sj.cdd.4401702. [DOI] [PubMed] [Google Scholar]
- 2.Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ. 2005;12:1178–1190. doi: 10.1038/sj.cdd.4401692. [DOI] [PubMed] [Google Scholar]
- 3.McBride A, Ryan PY. Proteasome inhibitors in the treatment of multiple myeloma. Expert Rev Anticancer Ther. 2013;13:339–358. doi: 10.1586/era.13.9. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor OA, Wright J, Moskowitz C, Muzzy J, Gregor-Cortelli B, Stubblefield M, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin's lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23:676–684. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor OA, Portlock C, Moskowitz C, Hamlin P, Straus D, Gerecitano J, et al. Time to treatment response in patients with follicular lymphoma treated with bortezomib is longer compared with other histologic subtypes. Clin Cancer Res. 2010;16:719–726. doi: 10.1158/1078-0432.CCR-08-2647. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann J, Lerman LO, Lerman A. Ubiquitin and ubiquitin-like proteins in protein regulation. Circ Res. 2007;100:1276–1291. doi: 10.1161/01.RES.0000264500.11888.f0. [DOI] [PubMed] [Google Scholar]
- 7.Podust VN, Brownell JE, Gladysheva TB, Luo RS, Wang C, Coggins MB, et al. A Nedd8 conjugation pathway is essential for proteolytic targeting of p27Kip1 by ubiquitination. Proc Natl Acad Sci U S A. 2000;97:4579–4584. doi: 10.1073/pnas.090465597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Read MA, Brownell JE, Gladysheva TB, Hottelet M, Parent LA, Coggins MB, et al. Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha. Mol Cell Biol. 2000;20:2326–2333. doi: 10.1128/mcb.20.7.2326-2333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brownell JE, Sintchak MD, Gavin JM, Liao H, Bruzzese FJ, Bump NJ, et al. Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell. 2010;37:102–111. doi: 10.1016/j.molcel.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 11.Milhollen MA, Traore T, Duffy J, Thomas MP, Berger AJ, Dang L, et al. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 12.Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E, et al. Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood. 2010;115:3796–3800. doi: 10.1182/blood-2009-11-254862. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Kaufman JL, Bernal L, Torre C, Matulis SM, Harvey RD, et al. MLN4924, an NAE inhibitor, suppresses AKT and mTOR signaling via upregulation of REDD1 in human myeloma cells. Blood. 2014;123:3269–3276. doi: 10.1182/blood-2013-08-521914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godbersen JC, Humphries LA, Danilova OV, Kebbekus PE, Brown JR, Eastman A, et al. The Nedd8-activating enzyme inhibitor MLN4924 thwarts microenvironment-driven NF-kappaB activation and induces apoptosis in chronic lymphocytic leukemia B cells. Clin Cancer Res. 2014;20:1576–1589. doi: 10.1158/1078-0432.CCR-13-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillin DW, Jacobs HM, Delmore JE, Buon L, Hunter ZR, Monrose V, et al. Molecular and cellular effects of NEDD8-activating enzyme inhibition in myeloma. Mol Cancer Ther. 2012;11:942–951. doi: 10.1158/1535-7163.MCT-11-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D, et al. The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res. 2012;72:3360–3371. doi: 10.1158/0008-5472.CAN-12-0388. [DOI] [PubMed] [Google Scholar]
- 17.Nawrocki ST, Kelly KR, Smith PG, Keaton M, Carraway H, Sekeres MA, et al. The NEDD8-Activating Enzyme Inhibitor MLN4924 Disrupts Nucleotide Metabolism and Augments the Efficacy of Cytarabine. Clin Cancer Res. 2015;21:439–447. doi: 10.1158/1078-0432.CCR-14-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith PG, Traore T, Grossman S, Narayanan U, Carew JS, Lublinsky AR, et al. Azacitidine/decitabine synergism with the NEDD8-activating enzyme inhibitor MLN4924 in pre-clinical AML models. Blood. 2011;118(578):578. [Google Scholar]
- 19.Milhollen MA, Narayanan U, Soucy TA, Veiby PO, Smith PG, Amidon B. Inhibition of NEDD8-activating enzyme induces rereplication and apoptosis in human tumor cells consistent with deregulating CDT1 turnover. Cancer Res. 2011;71:3042–3051. doi: 10.1158/0008-5472.CAN-10-2122. [DOI] [PubMed] [Google Scholar]
- 20.Lin JJ, Milhollen MA, Smith PG, Narayanan U, Dutta A. NEDD8-targeting drug MLN4924 elicits DNA rereplication by stabilizing Cdt1 in S phase, triggering checkpoint activation, apoptosis, and senescence in cancer cells. Cancer Res. 2010;70:10310–10320. doi: 10.1158/0008-5472.CAN-10-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 22.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 23.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 24.Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, et al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 26.Micel LN, Tentler JJ, Smith PG, Eckhardt GS. Role of ubiquitin ligases and the proteasome in oncogenesis: novel targets for anticancer therapies. J Clin Oncol. 2013;31:1231–1238. doi: 10.1200/JCO.2012.44.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal A, Young MA, Donato NJ. Emerging Potential of Therapeutic Targeting of Ubiquitin-Specific Proteases in the Treatment of Cancer. Cancer Res. 2014;74:4955–4966. doi: 10.1158/0008-5472.CAN-14-1211. [DOI] [PubMed] [Google Scholar]
- 28.Esseltine DL, Mulligan G. An historic perspective of proteasome inhibition. Semin Hematol. 2012;49:196–206. doi: 10.1053/j.seminhematol.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Soucy TA, Dick LR, Smith PG, Milhollen MA, Brownell JE. The NEDD8 Conjugation Pathway and Its Relevance in Cancer Biology and Therapy. Genes Cancer. 2010;1:708–716. doi: 10.1177/1947601910382898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swords RT, Erba HP, DeAngelo DJ, Bixby DL, Altman JK, Maris M, et al. Pevonedistat (MLN4924), a First-in-Class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol. 2015 doi: 10.1111/bjh.13323. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Wang H, Orlowski RZ. Proteasome maturation protein (pomp) is associated with proteasome inhibitor resistance in myeloma, and its suppression enhances the activity of bortezomib and carfilzomib. Blood. 2013;122(280):280. [Google Scholar]
- 32.Liao H, Liu XJ, Blank JL, Bouck DC, Bernard H, Garcia K, et al. Quantitative proteomic analysis of cellular protein modulation upon inhibition of the NEDD8-activating enzyme by MLN4924. Mol Cell Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chauhan D, Tian Z, Zhou B, Kuhn D, Orlowski R, Raje N, et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res. 2011;17:5311–5321. doi: 10.1158/1078-0432.CCR-11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraus M, Malenke E, Gogel J, Muller H, Ruckrich T, Overkleeft H, et al. Ritonavir induces endoplasmic reticulum stress and sensitizes sarcoma cells toward bortezomib-induced apoptosis. Mol Cancer Ther. 2008;7:1940–1948. doi: 10.1158/1535-7163.MCT-07-2375. [DOI] [PubMed] [Google Scholar]
- 35.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung-Hagesteijn C, Erdmann N, Cheung G, Keats JJ, Stewart AK, Reece DE, et al. Xbp1s–negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell. 2013;24:289–304. doi: 10.1016/j.ccr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swords RT, Savona MR, Maris MB, Erba HP, Hua Z, Faessel H, et al. First-in-class NAE inhibitor MLN4924 in combination with azacitidine for acute myeloid leukemia (AML) patients considered unfit for conventional chemotherapy: results from the C15009 trial. Haematologica. 2014;99(S650):223–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.