Abstract
Background
Altered bone microarchitecture and higher marrow adipose tissue (MAT) may reduce bone strength. High resolution pQCT (HRpQCT) allows assessment of volumetric BMD (vBMD), and size and microarchitecture parameters of bone, while 1H-magnetic resonance spectroscopy (1H-MRS) allows MAT evaluation. We have reported impaired microarchitecture at the non-weight bearing radius in adolescents with anorexia nervosa (AN) and that these changes may precede aBMD deficits. Data are lacking regarding effects of AN on microarchitecture and strength at the weight-bearing tibia in adolescents and young adults, and the impact of changes in microarchitecture and MAT on strength estimates.
Objective
To compare strength estimates at the distal tibia in adolescents/young adults with AN and controls in relation to vBMD, bone size and microarchitecture, and spine MAT.
Design and Methods
This was a cross-sectional study of 47 adolescents/young adults with AN and 55 controls 14–24 years old that assessed aBMD and body composition using DXA, and distal tibia vBMD, size, microarchitecture and strength estimates using HRpQCT, extended cortical analysis, individual trabecular segmentation, and finite element analysis. Lumbar spine MAT (1H-MRS) was assessed in a subset of 19 AN and 22 controls.
Results
Areal BMD Z-scores were lower in AN than controls. At the tibia, AN had greater cortical porosity, lower total and cortical vBMD, cortical area and thickness, trabecular number, and strength estimates than controls. Within AN, strength estimates were positively associated with lean mass, aBMD, vBMD, bone size and microarchitectural parameters. MAT was higher in AN, and associated inversely with strength estimates.
Conclusions
Adolescents/young adults with AN have impaired microarchitecture at the weight-bearing tibia and higher spine MAT, associated with reduced bone strength.
Keywords: Anorexia Nervosa, Strength Estimates, Distal tibia, Marrow Fat, Microarchitecture, Adolescents
1. INTRODUCTION
Anorexia nervosa (AN) is a common psychiatric condition which often begins during adolescence, a time of peak bone mass accrual [1]. We have previously reported that adolescent girls and young adult women with AN have low bone mineral density (BMD) at multiple sites and are at an increased risk of fracture [2, 3].
Areal BMD (aBMD) assessed by dual-energy x-ray absorptiometry (DXA) is the clinical tool used to evaluate and monitor bone health. However, the reporting of aBMD may be impacted by changes in body composition (particularly changes in fat mass), which is dramatically altered in AN. High resolution peripheral quantitative computed tomography (HRpQCT) allows assessment of volumetric BMD (vBMD) unaffected by alterations in body composition and comprehensive microarchitectural evaluation of cortical and trabecular bone compartments.
Importantly, we have previously reported that microarchitectural changes may precede measurable deficits in aBMD in girls with AN [4]. At the distal radius, a non-weight bearing site, we have previously demonstrated increased cortical porosity, and reduced total and trabecular vBMD, cortical area and thickness, and lower strength estimates in girls with AN compared to controls [2]. However, data are limited regarding the impact of AN on bone at weight bearing sites, and it is unclear whether the beneficial effects of weight bearing overcome the detrimental effects of AN at such sites. One group has reported lower trabecular but higher cortical vBMD at the tibia using pQCT in young women with AN [5]. However, trabecular microarchitectural parameters, and strength estimates assessed using microfinite element analysis (FEA) remain to be defined for this site. Importantly, trabecular microarchitecture and FEA-derived strength estimates better predict fracture risk than aBMD alone [6, 7]. Recognition of microarchitectural features that alter bone strength in adolescents with AN may help target therapy for the bone compartment most affected by this condition, and with maximum impact on fracture risk.
Higher prevalent fracture risk is also associated with higher marrow adipose tissue (MAT), which is known to increase in conditions associated with lower aBMD [8, 9]. Further, higher MAT is associated with impaired cortical and trabecular bone parameters in other populations [9, 10]. There are reports of increased MAT in adult women with AN, but MAT has not been quantified in adolescents with AN; a time when healthy adolescents also have physiological increases in MAT [11, 12]. We thus investigated alterations in MAT in adolescents with AN in relation to changes in tibial microarchitecture and strength estimates to determine whether changes in MAT are associated with decreased strength estimates.
We hypothesized that MAT and cortical and trabecular vBMD, area and microarchitecture would be adversely affected at the distal tibia, a weight-bearing site, in AN, and that these alterations would predict alterations in strength estimates.
2. METHODS
This study was approved by the Partners Healthcare Institutional Review Board. Written informed assent and consent were obtained from all subjects and/or their parents.
2.1. Study participants
We enrolled 102 participants aged 14–24 years, 47 subjects with AN and 55 normal-weight healthy controls. AN subjects met DSM-V criteria, were recruited from regional eating disorder centers or outpatient clinics, and were enrolled in ongoing treatment programs. We did not recruit subjects with co-existing bulimia. All subjects had a bone age (BA) ≥ 14 years. None of the participants had a delayed bone age (i.e. bone age for all patients was within 1 SD of chronological age). Controls had a body mass index (BMI) between the 10th and 90th percentiles for age, no current or past history of an eating disorder, and were eumenorrheic (≥ nine menses in the preceding year with cycle length between 21 and 35 days). None of the controls had a past history of amenorrhea. Twenty of the 47 AN subjects were amenorrheic and two of these 20 had primary amenorrhea. Exclusion criteria for both AN subjects and controls included conditions other than AN that may affect bone (rheumatological conditions, skeletal dysplasias, hyper/hypothyroidism, growth hormone deficiency), use of medications known to affect bone (glucocorticoids, gonadal steroids, selective estrogen receptor modulators, depot medroxy progesterone acetate and fluoride) within two months of the study, an abnormal TSH or an elevated FSH level (to rule out thyroid dysfunction or premature ovarian insufficiency as causes of amenorrhea), or a history of pregnancy. None of the study participants were on oral contraceptives; however twenty-four AN participants were on selective serotonin reuptake inhibitors (SSRIs). We did not exclude AN girls on SSRIs as studies suggest no effect of SSRIs on bone loss and many patients in this population are on these medications and excluding participants on SSRIs would result in a sample not representative of a typical AN population. [13, 14]. A subset of this cohort underwent 1H-MRS for MAT evaluation at the lumbar spine. The lumbar spine is an established and reproducible site for assessment of marrow adiposity using 1H-MRS [15] and is more dynamic at this age compared to the adiposity of long bones [16]. All participants were offered this assessment, but only 19 AN participants and 22 controls received this test. We examined the group that had the 1H-MRS assessment vs. the group of AN girls who did not have this assessment for clinical characteristics and the groups did not differ for age, weight, BMI and percent median BMI (%mBMI).
2.2. Experimental Protocol
A detailed history was obtained, including assessment of menarchal age, presence/absence of amenorrhea (defined as absence of menarche in those ≥15 years old, or absence of menstruation for ≥ three months preceding the study in post-menarchal girls). Gynecologic age was calculated as time since menarche. Tanner staging was performed to assess pubertal status, and an X-ray of the left hand and wrist was obtained to assess bone age [17]. Participants were weighed on a calibrated electronic scale wearing a hospital gown, and height was measured in triplicate on a stadiometer. BMI was calculated using the formula: weight (kg)/[height (m)]2 and the 50th percentile (median) of BMI for age were used to calculate % of median BMI (%mBMI). Validated food frequency questionnaires were used to calculate daily calcium and vitamin D intake in a subset of AN and controls [18].
2.2.1. Dual-energy x-ray absorptiometry (DXA)
DXA was used to assess areal BMD (aBMD) of the total hip, lumbar spine and whole body, and also body composition, including measures of fat and lean mass. The same scanner (Hologic 4500 A, Waltham, MA) was used for all subjects. Z-scores were generated using race and age specific normative databases (NHANES 2008) [19].
2.2.2. High resolution peripheral quantitative computed tomography (HRpQCT)
HRpQCT was used to assess bone size parameters, volumetric BMD (vBMD), and microarchitecture at the distal tibia (Xtreme CT; Scanco Medical AG, Bassendorf, Switzerland) with an isotropic voxel size of 82 μm3. 2D scout views were obtained to locate the distal CT slice site 22.5 mm from the tibial endplate; the scanned section was 9.02 mm in length. A fixed distance was used given that >98% of linear growth is complete in girls with a bone age of ≥14 years [20]. The precision for short-term repeated measurements at our center is 0.7–1.5% for bone density and 2.5–4.4% for trabecular and cortical microarchitecture.
2.2.3. Extended cortical analysis (ECA)
ECA images and quantifies the pores within cortical bone in vivo as previously described, and evaluates cortical parameters [2].
2.2.4. Individual trabecula segmentation (ITS)
All HR-pQCT images of the trabecular bone compartment underwent ITS-based morphological analysis, as previously described to define plate, rod and plate-rod junction characteristics [21].
2.2.5. Micro-finite element analysis (FEA)
FEA estimates the biomechanical properties of the bone in the setting of simulated axial compression. Failure load (kN) was estimated by scaling the resultant load from a 1% apparent compressive strain until 2% of all elements reached an effective strain > 7000 μstrain, per previously published methods [22]. FEA-derived estimates of failure load are strongly correlated (r2 = 0.75) with experimentally measured failure loads that produce Colles’ fractures in human cadaveric radii [23]. We also report measures of bone stiffness and von Mises strain (stress at which bone deformation is likely to occur).
2.2.6. Marrow Adipose Tissue Analysis
A subset of subjects underwent single voxel proton magnetic resonance spectroscopy (1H-MRS) of the L4 vertebral body to determine MAT content using a 3.0T MR imaging system (Siemens Trio, Siemens Medical Systems, Erlangen, Germany). A voxel measuring 15×15×15mm (3.4 ml) was placed within the L4 vertebral body. Data were acquired using point-resolved spatially localized spectroscopy (PRESS) pulse sequence without water suppression. Automated procedures for optimization of gradient shimming and transmit and receive gain were used. MAT is reported as the quotient of the lipid peak to the water peak. The coefficient of variation for MAT quantification is 5%.[24]
2.3. Statistical analysis
Statistical analysis was performed using JMP software. Normally distributed data are reported as means ± standard deviation (mean ± SD). The Student t-test was used to determine differences between groups when data were normally distributed. In the case of non-normally distributed data, the median and interquartile range are reported, and differences between groups were assessed using the Wilcoxon test. Statistical significance was set at a p-value of < 0.05. The False Discovery Rate method was used to determine whether the statistical significance of each conducted test held after controlling for the multiple tests performed [25, 26]. Pearson or Spearman correlations (depending on the distribution of the data) were used to determine univariate associations of strength estimates with clinical and bone parameters. Multivariable regression was used to control for possible confounders.
3. RESULTS
3.1. Clinical Characteristics and aBMD
Subjects with AN did not differ from controls for age, height, menarchal and gynecological age (Table 1). As expected, AN subjects had lower weight, BMI, and fat and lean mass than controls. 25OHD concentrations and dietary calcium intake were higher among subjects with AN. The racial distribution differed between groups, and therefore, further analyses are adjusted for race. Areal BMD was lower in AN vs. controls at the spine, hip, and whole body; differences persisted after controlling for age and race but not after controlling for BMI. Total body less head (TBLH) BMD was 0.8677 (0.0622) gm/cm2 in AN and 0.9445 (0.0690) gm/cm2 in controls (p <0.0001).
Table 1.
Clinical Characteristics, Body Composition, Areal Bone Mineral Density and Marrow Adipose Tissue in Adolescents and Young Adults with Anorexia Nervosa and Controls
| Anorexia Nervosa (n= 47) | Controls (n= 55) | p | |
|---|---|---|---|
| Age (years) | 19.6 ± 2.1 | 19.5 ± 1.9 | 0.90 |
| Weight (kg) | 48.4 (44.6–52.3) | 58.5 (54.0–64.0) | <0.0001 |
| Height (cm) | 164.6 ± 6.3 | 163.7 ± 6.6 | 0.49 |
| BMI (kg/m2) | 18.4 ± 1.7 | 22.1 ± 2.2 | <0.0001 |
| % Ideal BMI | 85.2 (78.9–89) | 100.6 (94.5–108.8) | <0.0001 |
| % median BMI | 85.25 (78.90–88.94) | 100.58 (94.47–108.83) | <0.0001 |
| BMI Z-Score | −1.28 (−2.11 – −0.90) | 0.089 (−0.411–0.597) | <0.0001 |
| Race (n,%) | 0.08 | ||
| Black | 1 (2.2) | 4 (9.1) | |
| Non-Blacks | 46 (97.9) | 51 (92.7) | |
| Age of menarche (years) | 13 (12–14) | 13 (12–13) | 0.17 |
| Gynecological age (years) | 6.9 ± 2.3 | 7.1 ± 2.5 | 0.60 |
| Duration since diagnosis (months) | 18.5 (8.3–51) | NA | NA |
| Percent with amenorrhea | 43 | 0 | |
| Duration of amenorrhea a (months) | 8 (4.25–18) | NA | NA |
| 25OHD (ng/ml) | 32.8 (27–41.2) | 24.2 (18.3–31.3) | 0.0003 |
| Daily calcium intake (mg) | 1381.4 ± 658.0 (n=17) | 915.6 ± 355.6 (n=41) | 0.0009 |
| Body Composition (DXA) | |||
| Total fat mass (kg) | 12.4 (9.7–15.0) | 15.1 (6.2–19.0) | 0.05 |
| Total lean mass (kg) | 36.3 (34.6–39.6) | 39.4 (9.5–42.9) | 0.16 |
| Total mass (kg) | 50.8 (46.9–54.8) | 57.7 (15.7–64.5) | 0.007 |
| % Fat mass | 24.1 ± 4.9 | 30.3 ± 5.5 | <0.0001 |
| % Lean mass | 72.3 ± 4.6 | 67.2 ± 4.9 | <0.0001 |
| Areal BMD (DXA) | |||
| Total Hip BMD Z-Score | −0.85 ± 0.83 | 0.29 ± 0.87 | <0.0001# |
| Lumbar spine BMD Z-score | −1.3 ± 1.1 | −0.3 ± 0.8 | <0.0001# |
| Whole body BMD Z-score | −1.39 ± 0.88 | −0.63 ± 0.84 | <0.0001# |
| Marrow Fat (MRI/MRS) | |||
| n=19 | n=22 | ||
| L4 MAT (Lipid/Water) | 0.89 ± 0.42 | 0.52 ± 0.24 | 0.001# |
Data are reported as mean ± SD or median (interquartile range).
Quantified only in subjects who had amenorrhea;
Significant after controlling for age and race
BMD: bone mineral density; MAT: marrow adipose tissue; 25OHD: 25-hydroxyvitamin D
The difference between aBMD measures and L4MAT held after controlling for multiple comparisons
3.2. HRpQCT Measures
Girls with AN had 9.3% lower mean total vBMD compared to controls, with no difference between groups for total cross-sectional area of the distal tibia (Table 3).
Table 3.
Volumetric Bone Mineral Density, Size Estimates and Cortical Microarchitecture of the Distal Tibia (Using Standard and Extended Cortical Analyses) in Adolescents and Young Adults with Anorexia Nervosa versus Controls
| Anorexia Nervosa (N=47) |
Controls (N=55) |
p | |
|---|---|---|---|
| Total cross-sectional area (mm2) | 639.4 (589.6–691.9) | 668.9 (574.2–706.5) | 0.22 |
| Cortical perimeter (mm) | 99.2 (95.7–103.0) | 101.6 (94.5–104.5) | 0.17 |
| Endocortical perimeter (mm) | 93.1 (88.6–96.6) | 94.3 (86.6–97.7) | 0.65 |
| Cortical area (mm2) | 101.7 ± 16.3 | 121.4 ± 23.3 | <0.0001# |
| Cortical thickness (mm) | 1.04 ± 0.17 | 1.23 ± 0.24 | <0.0001# |
| Cortical total volume (mm3) | 906.3 ± 128.0 | 1011.6 ± 156.3 | 0.0004# |
| Cortical bone volume (mm3) | 852.3 ± 956.8 | 962.0 ± 149.7 | 0.0001# |
| Cortical porosity (%) | 2.6 (1.8–3.6) | 1.6 (1.0–2.8) | 0.0009# |
| Cortical vBMD (mg HA/cm3) | 868.0 (844.1–887.9) | 882.2 (865.7–911.1) | 0.01# |
| Total vBMD (mg HA/cm3) | 297.5 ± 35.5 | 328.0 ± 52.7 | 0.001# |
Data are reported as mean ± SD or median (interquartile range)
Significant after controlling for age and race
All significances held after controlling for multiple comparisons
vBMD: volumetric bone mineral density
3.2.1. Cortical Compartment
Differences between AN and control groups for cortical and endocortical perimeter were not significant. However, despite these non-significant differences in periosteal and endosteal perimeter, cortical thickness was 16.7% lower and cross-sectional area 4.4% lower in the AN group compared with controls (p<0.05). The total volume of the cortical component was 10.4% lower in AN subjects than in controls, and cortical vBMD was 1.6% lower in AN. Cortical porosity was higher in AN than controls (2.6% compared with 1.6% in controls).Differences between groups, except for cortical porosity, were no longer significant after controlling for BMI.
3.2.2. Trabecular Compartment
Trabecular vBMD in AN was comparable to controls (Table 4). However, trabecular number was 5.3% lower and trabecular separation 11.6% higher in AN. On ITS, the ratio of bone volume to total volume of trabecular bone was 14.8% lower in AN. Morphology: There was no difference in trabecular plate thickness but plate number density was lower by 3% in AN girls compared with controls. AN did not differ from controls for trabecular rod number but mean trabecular rod thickness was higher by 2.3%. Connectivity: AN girls had lower rod-plate and plate-plate junction densities [9.1% and 9% respectively], but did not differ for rod-rod junction density. Differences between groups were no longer significant after controlling for BMI.
Table 4.
Trabecular Microarchitecture at the Tibia (Using Standard Analysis and Individual Trabecula Segmentation) in Adolescents and Young Adults with Anorexia Nervosa and Controls
| Anorexia Nervosa (N=47) | Controls (N=55) | p | |
|---|---|---|---|
| Standard Analysis | |||
| Trabecular Area (mm2) | 528.1 ± 73.2 | 532.8 ± 105.1 | 0.80 |
| Number of trabeculae(1/mm) | 1.78 ± 0.23 | 1.95 ± 0.21 | 0.0003# |
| Trabecular thickness (mm) | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.35 |
| Trabecular separation (mm) | 0.48 (0.44–0.52) | 0.43 (0.39–0.46) | 0.0002# |
| Inhomogeneity of network (mm) | 0.21 (0.19–0.24) | 0.19 (0.17–0.21) | 0.002# |
| Trabecular vBMD (mg HA/cm3) | 184.3 ± 28.6 | 196.5 ± 34.5 | 0.06 |
| Individual Trabecular Segmentation | |||
| Total bone volume fraction (%) | 0.29 ± 0.03 | 0.32 ± 0.04 | 0.004# |
| Plate bone volume fraction (%) | 0.16 ± 0.05 | 0.18 ± 0.05 | 0.12 |
| Rod bone volume fraction (%) | 0.13 (0.11–0.14) | 0.14 (0.11–0.16) | 0.16 |
| Axial bone volume fraction (%) | 0.15 ± 0.03 | 0.16 ± 0.04 | 0.30 |
| Plate number (1/mm) | 1.59 (1.51–1.65) | 1.64 (1.55–1.72) | 0.03 |
| Rod number (1/mm) | 1.68 ± 0.16 | 1.74 ± 0.16 | 0.08 |
| Plate trabecular thickness (mm) | 0.223 ± 0.010 | 0.225 ± 0.012 | 0.42 |
| Rod trabecular thickness (mm) | 0.221 (0.217–0.228) | 0.216 (0.211–0.221) | 0.0002# |
| Rod-rod junction density(1/mm3) | 1.81 (1.42–2.11) | 2.07 (1.59–2.65) | 0.12 |
| Plate-rod junction density (1/mm3) | 3.93 ± 0.72 | 4.32 ± 0.79 | 0.004# |
| Plate-plate junction density (1/mm3) | 2.44 ± 0.49 | 2.68 ± 0.50 | 0.01# |
Data are reported as mean ± SD or median (interquartile range).
Significant after controlling for age and race
All significances held after controlling for multiple comparisons
vBMD: volumetric bone mineral density
3.2.3. Estimates of Bone Strength (FEA)
Estimated failure load and stiffness, surrogate markers of bone strength, were lower in AN than controls (Table 2). The cortical (but not trabecular) compartment in AN could be deformed at lower stress than controls (von Mises stress). Between-group differences in failure load and stiffness remained significant after controlling for age and race, but not after controlling for BMI and hip aBMD Z-scores. Of the various sites for which aBMD measures were obtained, the distribution of cortical and trabecular bone at the total hip is most similar to that at the tibia; as both are lower extremity weight-bearing sites.
Table 2.
Finite- Element Strength Estimates at the Tibia in Adolescents and Young Adults with Anorexia Nervosa and Healthy Controls
| Anorexia Nervosa (n= 47) |
Controls (n= 55) |
p | |
|---|---|---|---|
| Stiffness (kN/mm) | 197.2 ± 33.1 | 222.4 ± 40.4 | 0.0009# |
| Failure load (kN) | 9.9 ± 1.6 | 11.2 ± 2.0 | 0.0006# |
| Cortical von Mises Stress (N/mm2) | 89.5 (88.7–90.5) | 90.2 (89.3–91.3) | 0.01 |
| Trabecular von Mises Stress (N/mm2) | 63.0 (60.0–64.8) | 62.3 (57.9–65.0) | 0.62 |
Data are reported as mean ± SD or median (interquartile range).
Significant after controlling for age and race
All significances held after controlling for multiple comparisons
3.3. Amenorrheic vs. Eumenorrheic AN
We conducted a post hoc analysis to evaluate the impact of hypogonadism on bone in girls with AN. AN girls who were amenorrheic for at least 3 months had lower lumbar BMD Z-scores than eumenorrheic AN girls, but did not differ for other aBMD measures, HRpQCT measures of vBMD, and bone size or microarchitecture (data not shown). The lack of expected difference between the two groups could stem from a relatively short median duration of amenorrhea in our cohort and also because assessing menses for only the preceding three months does not describe long-term gonadal status.
3.4. Marrow Adipose Tissue
Mean MAT at L4 was higher in the AN group compared to controls (Table 1), and persisted so after controlling for age and race but not after controlling for BMI.
3.5. Associations of Strength Estimates with Clinical Characteristics, aBMD, HRpQCT parameters and MAT
We used stiffness as a strength estimate for these analyses; however our results were similar with failure load (not reported).
3.5.1. Clinical Characteristics
Within the AN group, there was no correlation of tibial strength estimates (data for stiffness are shown in Table 5) with age, age at diagnosis, time since diagnosis, gynecologic age, duration of amenorrhea and fat mass. Of note, BMI and fat mass were positively associated with stiffness in controls but not in the AN group. In contrast, total lean mass was positively associated with strength estimates in both AN and controls. These results suggest that factors other than those mentioned above likely impact bone strength at the distal tibia (such as the nature and frequency of weight bearing activities).
Table 5.
Correlations of Distal Tibia Stiffness with Clinical Characteristics and DXA and HRpQCT Parameters in Adolescents and Young Adults with Anorexia Nervosa and Healthy Controls
| Distal Tibia Stiffness | ||||
|---|---|---|---|---|
| Anorexia Nervosa | Controls | |||
| Correlation Coefficient | p-value | Correlation Coefficient | p-value | |
| Clinical Characteristics and Body Composition | ||||
| Age (years) | 0.07 | 0.66 | −0.09 | 0.50 |
| Age at AN diagnosis (years) | 0.15 | 0.32 | NA | NA |
| Duration since diagnosis (years) | −0.10 | 0.49 | NA | NA |
| Duration of amenorrhea (months) | 0.02 | 0.89 | −0.19 | 0.16 |
| Body mass index (kg/m2) | 0.24 | 0.10 | 0.55 | <0.0001 |
| Total lean mass | 0.72 | <0.0001 | 0.44 | 0.0005 |
| Total fat mass | 0.05 | 0.72 | 0.35 | 0.007 |
| DXA: Areal Bone Mineral Density | ||||
| Total hip BMD Z-score | 0.68 | <0.0001 | 0.75 | <0.0001 |
| Whole body BMD Z-score | 0.65 | <0.0001 | 0.69 | 0.001 |
| Lumbar spine BMD Z-score | 0.59 | <0.0001 | 0.53 | <0.0001 |
| HRpQCT: Standard Analysis (Total and Cortical Compartment) | ||||
| Total cross-sectional area (mm2) | 0.61 | <0.0001 | 0.56 | <0.0001 |
| Cortical area (mm2) | 0.68 | <0.0001 | 0.78 | <0.0001 |
| Cortical thickness (mm) | 0.46 | 0.001 | 0.49 | <0.0001 |
| Cortical vBMD (mgHA/cm3) | 0.11 | 0.48 | 0.01 | 0.92 |
| Total vBMD (mgHA/cm3) | 0.66 | <0.0001 | 0.44 | 0.0006 |
| HRpQCT: Extended Cortical Analysis | ||||
| Cortical porosity (%) | 0.46 | 0.001 | 0.27 | 0.04* |
| Cortical perimeter (mm) | 0.62 | <0.0001 | 0.44 | 0.0007 |
| Endocortical perimeter (mm) | 0.55 | <0.0001 | 0.41 | 0.002 |
| HRpQCT: Standard Analysis (Trabecular Compartment) | ||||
| Bone volume fraction (%) | 0.81 | <0.0001 | 0.59 | <0.0001 |
| Trabecular area (mm2) | 0.49 | 0.0004 | 0.43 | 0.0008 |
| Trabecular number (1/mm) | 0.21 | 0.15 | 0.39 | 0.002 |
| Trabecular thickness (mm) | 0.63 | <0.0001 | 0.38 | 0.003 |
| Trabecular separation (mm) | −0.37 | 0.009 | −0.44 | 0.0005 |
| Trabecular vBMD (mgHA/cm3) | 0.81 | <0.0001 | 0.66 | <0.0001 |
| HRpQCT: Individual Trabecula Segmentation | ||||
| Plate trabecular fraction (%) | 0.77 | <0.0001 | 0.73 | <0.0001 |
| Rod trabecular fraction (%) | −0.31 | 0.03 | −0.26 | 0.046* |
| Axial trabecular fraction (%) | 0.75 | <0.0001 | 0.70 | <0.0001 |
| Plate trabecular number (1/mm) | 0.79 | <0.0001 | 0.72 | <0.0001 |
| Plate trabecular thickness (mm) | 0.38 | 0.007 | 0.39 | 0.002 |
| Rod trabecular thickness (mm) | 0.09 | 0.54 | −0.25 | 0.06 |
| Rod-rod junction density (1/mm3) | −0.39 | 0.006 | −0.29 | 0.03 |
| Rod-plate junction density (1/mm3) | 0.49 | 0.0003 | 0.31 | 0.02 |
| Plate-plate junction density (1/mm3) | 0.79 | <0.0001 | 0.71 | <0.0001 |
Not significant after adjusting for multiple tests using the false discovery rate adjustment
3.5.2. Areal BMD
Stiffness was positively associated with total hip, lumbar spine and whole body BMD Z-scores in AN and controls (Table 5).
3.5.3. HRpQCT Measures
Stiffness was associated positively with total and trabecular vBMD but not cortical vBMD in both groups (Table 5). Bone stiffness was also positively associated with total area, cortical area and thickness, and periosteal and endocortical perimeter. Bone strength estimates were associated positively with trabecular bone fraction, area and thickness and inversely with trabecular separation. Bone stiffness correlated positively with trabecular number in controls, but not in AN. Furthermore, plate trabecular parameters (% fraction, number, thickness, plate-plate junction and plate-rod junction) were positively associated with stiffness in both AN and controls, and rod trabecular fraction and rod-rod junction density were associated negatively with stiffness.
To further evaluate the relative impact of bone parameters on strength estimates in AN (bone stiffness), we performed stepwise regression analysis with the following covariates: a DXA measure of aBMD (hip BMD Z-score, as the hip is another lower extremity weight-bearing site), relevant bone size parameters (total cross sectional area and cortical thickness, known determinants of bone strength), and a measure each of cortical and trabecular microarchitecture (cortical porosity and trabecular bone volume) that were associated with bone stiffness on univariate analysis. Trabecular bone volume, total cross-sectional area and cortical thickness contributed to 64%, 15% and 10% of the variance in bone stiffness respectively, and together explained 89.7% of the total variance in stiffness at the distal tibia.
3.5.4. Marrow Adipose Tissue
When evaluating the entire group, stiffness at the distal tibia and trabecular von Mises stress were negatively associated with MAT at the lumbar spine. This association was no longer significant after controlling for total vBMD. When AN and controls were assessed separately, we did not observe significant correlations, likely because of the small sample size. We also found negative associations of total hip and lumbar spine aBMD, total tibial vBMD, plate and axial trabecular fraction, plate number, thickness and plate-plate junction density with MAT (Table 6).
Table 6.
Associations of Spine Marrow Adipose Tissue (MAT) with Distal Tibia Bone Strength Estimates, Size, Volumetric BMD and Microarchitecture Parameters (in all 41 participants with MAT assessment)
| DXA: Areal BMD | ||
|---|---|---|
| Corr. Coeff | P value | |
| Total hip BMD Z-score | −0.40 | 0.006 |
| Whole body BMD Z-score | 0.07 | 0.76 |
| Lumbar spine BMD Z-score | −0.44 | 0.003 |
| HRpQCT: Standard Analysis | ||
| Total vBMD (mgHA/cm3) | −0.35 | 0.03 |
| Cortical vBMD (mgHA/cm3) | −0.10 | 0.51 |
| Trabecular vBMD (mgHA/cm3) | −0.30 | 0.05 |
| Total area (mm2) | −0.14 | 0.38 |
| Cortical area (mm2) | −0.25 | 0.11 |
| HRpQCT: Individual Trabecula Segmentation | ||
| Trabecular bone volume fraction (%) | −0.34 | 0.03 |
| Plate trabecular fraction (%) | −0.38 | 0.01 |
| Rod trabecular fraction (%) | 0.23 | 0.16 |
| Axial trabecular fraction (%) | −0.37 | 0.02 |
| Rod-rod junction density (1/mm3) | 0.29 | 0.07 |
| Rod-plate junction density (1/mm3) | −0.15 | 0.37 |
| Plate-plate junction density (1/mm3) | −0.33 | 0.04* |
| Plate number (1/mm) | −0.36 | 0.02 |
| Plate thickness (mm) | −0.39 | 0.01 |
| Finite Element Analysis: Strength estimates | ||
| Stiffness (kN/mm) | −0.37 | 0.02 |
| Trabecular von Mises Stress (N/mm2) | −0.37 | 0.02 |
| Cortical von Mises Stress (N/mm2) | −0.15 | 0.36 |
Not significant after adjusting for multiple tests using the false discovery rate adjustment
4. Discussion
Women with AN have an increase in fracture risk both during adolescence [3] and adulthood [27]. This incidence is increased even without significant reductions in aBMD, suggesting that aBMD does not capture all the detrimental changes affecting bone. Of importance, studies in post-menopausal women show that bone strength is altered by differences in bone size and microarchitecture, and differences in bone microarchitecture predict fracture risk even after controlling for aBMD [28]. Strength estimates derived from finite element analysis using images from HRpQCT predict fracture risk in elderly men and women and hence are considered surrogates for fracture risk, otherwise difficult to assess in a young population and also for this diagnosis. We have previously reported decreased bone strength estimates in adolescents with AN at the distal radius, a non-weight bearing site [2]. To our knowledge, this is the first study evaluating both bone microarchitecture and strength estimates using HRpQCT in adolescent girls and young adults with AN at a weight bearing site. We have also investigated compartment specific microarchitectural changes that may affect strength estimates, and changes in MAT. Our findings could help target therapies to the compartment most affected in AN and most likely to have an impact on bone strength, and to monitor treatment in order to optimize bone health in young women with AN.
We have previously reported that adolescents and young adult women with AN have lower strength estimates at the non-weight-bearing radius [2] similar to adults [29]. We now report reductions in strength estimates at the weight-bearing tibia. Strength estimates in postmenopausal women and young oligoamenorrheic athletes assessed using finite element analysis are known to be associated with prevalent fracture risk [7, 30], and may be impacted by changes in both the cortical and trabecular compartments of bone.
In our study, girls with AN had lower total vBMD, cortical vBMD and plate-like trabecular density than controls. Our data differ from those in a recently published paper reporting lower total trabecular vBMD but higher cortical vBMD as assessed by peripheral QCT (pQCT) [5]. This could be the result of different sites assessed by these different techniques and also from differences in severity and duration of AN in the two studies. Our study participants had lower BMI Z-scores, longer duration of AN and longer duration of amenorrhea than reported in the pQCT study. In contrast to this report, our data support the adverse effects of AN on cortical bone at the distal tibia.
On further assessment of the cortical compartment of bone, adolescents and young adults with AN had thinner and more porous cortices than controls. This is likely consequent to decreased mineralization of bone from nutritional deficiency, hypogonadism, low IGF-1 or high cortisol levels, as previously reported in patients with AN [31]. However, on post hoc analysis, we found no differences in these parameters between currently amenorrheic versus eumenorrheic subjects with AN, arguing against a significant impact of hypogonadism. This should, however, be interpreted with caution as we did not have lifetime duration of amenorrhea in our subjects. Also, in contrast to our expectations of increased endosteal resorption in AN from hypogonadism [32], there was no difference between groups for their endocortical perimeter. In fact, the two groups did not differ for periosteal or endosteal circumference, although the combined effect of subtle changes in these measures resulted in lower cortical thickness in AN. Cortical bone grows by coalescence of peripheral trabeculae and subsequent mineralization. A halt or delay in this process, likely from lower IGF-1 levels or elevated cortisol as seen in AN, could explain the higher cortical porosity and decreased cortical vBMD in this group [33, 34].
Trabecular bone volume fraction was lower in AN with lower trabecular number and increased trabecular spacing. In addition, there appears to be a preferential preservation of the rod (versus plate) fraction of trabecular bone in AN. This is particularly relevant as studies have shown that a transition from plate-like to rod-like trabeculae may represent bone that is more prone to damage [35]. Consistent with this, lower plate number and plate-plate junctions are associated with fragility fractures in osteopenic women [36]. The contrasting associations of plate and rod trabecular fractions with strength estimates are consistent with the literature, which suggests that plate like (but not rod like) trabeculae are beneficial to bone strength [37].
Both cortical and trabecular parameters predicted bone strength estimates similarly in girls with AN and controls. The positive association of cortical porosity and endocortical perimeter with strength estimates in both AN and control groups suggests that the porosity is likely from higher numbers of large, asymmetrical, drifting osteons which have giant canals, and reflects active bone modeling [38]. Unlike in adults, cortical porosity may indicate active bone modeling and remodeling in this age group, and thus greater cortical porosity may not necessarily be deleterious to bone [39]. Our observation is similar to that of Nishiyama et al. who reported that teenage boys had greater strength estimates despite higher cortical porosity than girls [40].
Areal BMD, and cortical and trabecular parameters were important predictors of tibial bone strength estimates on univariate analysis, and remained so after controlling for age and race. However, aBMD was no longer an independent predictor of bone strength after controlling for HRpQCT parameters (total area, cortical thickness, cortical porosity and trabecular bone volume). Trabecular bone volume was the strongest predictor of bone strength in this model, followed by total cross-sectional area and cortical thickness. Most differences between groups were no longer significant after controlling for BMI, which underscores the critical impact of low weight on bone parameters.
Lumbar spine MAT was higher in AN than controls. This is consistent with published literature in adults with AN and other groups with osteoporosis, such as in postmenopausal women or adults with type 2 diabetes [11, 41]. Higher MAT in AN and negative associations with bone parameters suggest increased differentiation of the mesenchymal progenitor stem cell in marrow along the adipogenic lineage than the osteoblastic lineage [42]. Further, to our knowledge, this is the first study highlighting the negative association of MAT with strength estimates, especially trabecular bone strength at the distal tibia. These associations were lost after controlling for vBMD, suggesting that a common factor, such as hypoestrogenism, likely affects both variables. Conversely, the negative impact of MAT on bone strength may be mediated by changes in vBMD.
In AN participants, correlations of bone strength estimates with lean mass (in the absence of correlations with BMI and fat mass) stress the importance of lean mass as a determinant of bone strength. Gains in lean mass usually accompany gains in BMI and fat mass. Yet, a prospective study by Milos et al. in adults with AN showed that improvements in BMI were not necessarily associated with improved BMD [43] while in other studies, changes in BMI correlated with changes in BMD at spine and total hip [44]. The lack of association of BMI with strength estimates in our study is consistent with the previous report. However, differences between groups did not persist after controlling for BMI.
Our study has certain limitations. It is a cross sectional study and thus cannot determine causality or follow the progression of changes in bone microarchitecture following recovery from AN. We did not assess hormone levels, which may drive changes in bone microarchitecture in AN. Also, although strength estimates using finite element analysis are highly correlated with ex-vivo cadaveric bone strength estimates, these experiments have not been conducted specifically in patients with AN [45], thus a reduction in strength estimates may not necessarily translate to an increase in fracture risk. Further, we conducted multiple tests on the dataset, but did control for multiple tests using the false discovery rate method. Importantly, this is the first study to evaluate vBMD, bone size and microarchitecture at a weight-bearing site in adolescents and young women with AN in relation to MAT, and the impact of these measures on bone strength estimates.
In conclusion, this is the first study showing significant cortical and trabecular alterations at the weight-bearing tibia in adolescent girls and young adults with AN, associated with increases in MAT and reduced strength estimates. We also show that in subjects with AN, bone strength estimates are independently associated with cortical and trabecular microarchitectural parameters, while areal BMD is no longer associated with strength estimates after controlling for these parameters. This emphasizes the importance of considering bone microarchitecture in studies evaluating bone outcomes in AN.
Figure 1.
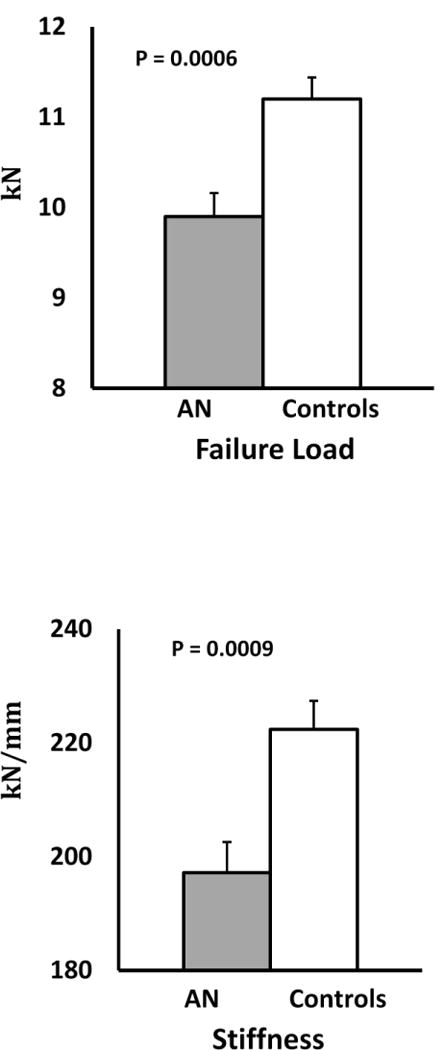
Strength estimates at the weight-bearing tibia in adolescents and young adults with anorexia nervosa and normal weight controls
Figure 2.
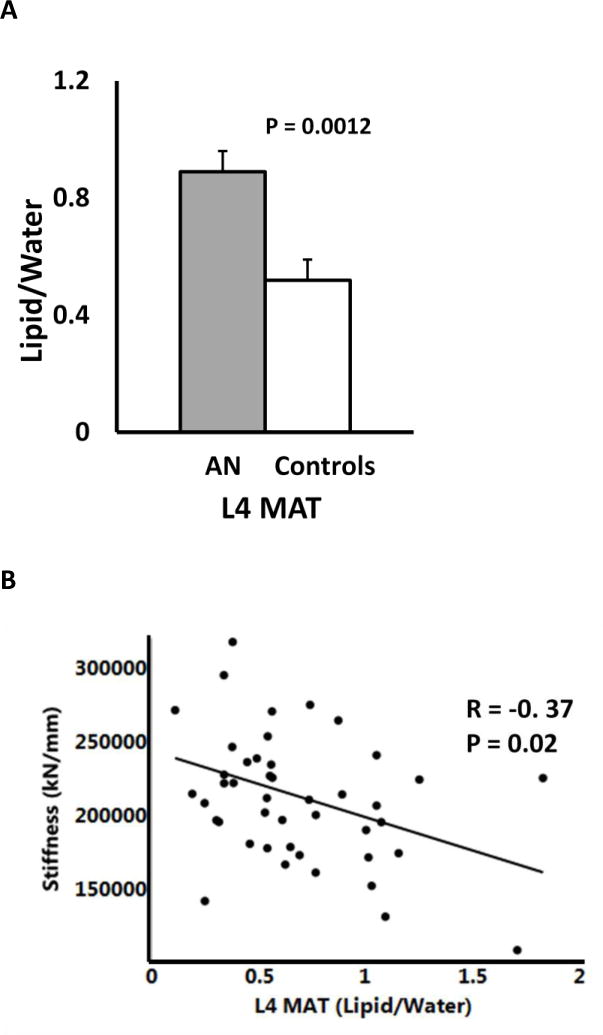
(A) Lumbar spine marrow adipose tissue in adolescents and young adults with anorexia nervosa and normal weight controls; (B) Relationship of lumbar spine marrow adipose tissue with strength estimates at the weight-bearing bone tibia
Figure 3.
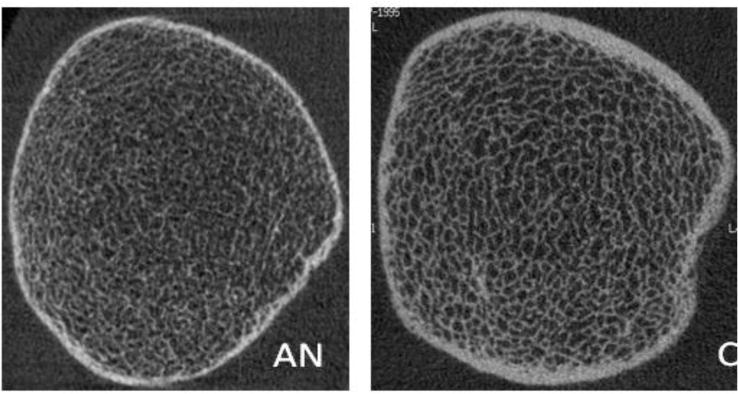
Representative HRpQCT images of Tibia in 18 year old patient with anorexia nervosa (AN) and normal weight control (C)
HIGHLIGHTS.
Adolescents and young adults with anorexia nervosa have impaired bone strength estimates at the weight-bearing tibia
Adolescents and young adults with anorexia nervosa have higher marrow fat at lumbar vertebrae
Bone strength estimates were positively associated with lean mass, areal BMD assessed by DXA, and microarchitectural parameters assessed by HRpQCT (independent of aBMD)
Bone strength estimates at tibia were negatively associated with marrow adipose tissue at lumbar spine
Acknowledgments
Sources of Funding: This work was funded by NIH grants R01 DK062249–09; T32 HD052961–08, K24HD07184, the Global Foundation for Eating Disorders, and the Davis Foundation.
Abbreviations
- aBMD
areal bone mineral density
- MAT
marrow adipose tissue
- HRpQCT
high resolution peripheral quantitative computed tomography
- AN
anorexia nervosa
- FEA
finite element analysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have no relevant conflicts of interest to disclose
References
- 1.Matkovic V, et al. Timing of peak bone mass in Caucasian females and its implication for the prevention of osteoporosis. Inference from a cross-sectional model. J Clin Invest. 1994;93(2):799–808. doi: 10.1172/JCI117034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faje AT, et al. Adolescent girls with anorexia nervosa have impaired cortical and trabecular microarchitecture and lower estimated bone strength at the distal radius. J Clin Endocrinol Metab. 2013;98(5):1923–9. doi: 10.1210/jc.2012-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faje AT, et al. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014;47(5):458–66. doi: 10.1002/eat.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bredella MA, et al. Distal radius in adolescent girls with anorexia nervosa: trabecular structure analysis with high-resolution flat-panel volume CT. Radiology. 2008;249(3):938–46. doi: 10.1148/radiol.2492080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiVasta AD, et al. Skeletal outcomes by peripheral quantitative computed tomography and dual-energy X-ray absorptiometry in adolescent girls with anorexia nervosa. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutroy S, et al. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–15. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 7.Crawford RP, Cann CE, Keaveny TM. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone. 2003;33(4):744–50. doi: 10.1016/s8756-3282(03)00210-2. [DOI] [PubMed] [Google Scholar]
- 8.Li X, et al. Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. J Magn Reson Imaging. 2011;33(4):974–9. doi: 10.1002/jmri.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz AV, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294–300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wren TA, et al. Bone marrow fat is inversely related to cortical bone in young and old subjects. J Clin Endocrinol Metab. 2011;96(3):782–6. doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 11.Bredella MA, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kugel H, et al. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. J Magn Reson Imaging. 2001;13(2):263–8. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 13.Diem SJ, et al. Effects of escitalopram on markers of bone turnover: a randomized clinical trial. J Clin Endocrinol Metab. 2014;99(9):E1732–7. doi: 10.1210/jc.2014-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diem SJ, et al. Rates of bone loss among women initiating antidepressant medication use in midlife. J Clin Endocrinol Metab. 2013;98(11):4355–63. doi: 10.1210/jc.2013-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singhal V, et al. Short- and long-term reproducibility of marrow adipose tissue quantification by 1H-MR spectroscopy. Skeletal Radiol. 2016;45(2):221–5. doi: 10.1007/s00256-015-2292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kricun ME. Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol. 1985;14(1):10–9. doi: 10.1007/BF00361188. [DOI] [PubMed] [Google Scholar]
- 17.Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. 2nd. Stanford University Press; Stanford: 1959. [Google Scholar]
- 18.Taylor C, et al. Validation of a food frequency questionnaire for determining calcium and vitamin D intake by adolescent girls with anorexia nervosa. J Am Diet Assoc. 2009;109(3):479–85. 485 e1–3. doi: 10.1016/j.jada.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40(4):423–41. doi: 10.1016/s0022-3476(52)80205-7. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell DM, et al. Altered trabecular bone morphology in adolescent and young adult athletes with menstrual dysfunction. Bone. 2015;81:24–30. doi: 10.1016/j.bone.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pistoia W, et al. High-resolution three-dimensional-pQCT images can be an adequate basis for in-vivo microFE analysis of bone. J Biomech Eng. 2001;123(2):176–83. doi: 10.1115/1.1352734. [DOI] [PubMed] [Google Scholar]
- 23.Boutroy S, et al. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23(3):392–9. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 24.Bredella MA, et al. Marrow fat composition in anorexia nervosa. Bone. 2014;66:199–204. doi: 10.1016/j.bone.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811–8. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hechtlinger Y. Discussion: An estimate of the science-wise false discovery rate and applications to top medical journals by Jager and Leek. Biostatistics. 2014;15(1):13–6. doi: 10.1093/biostatistics/kxt032. discussion 39–45. [DOI] [PubMed] [Google Scholar]
- 27.Lucas AR, et al. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clin Proc. 1999;74(10):972–7. doi: 10.4065/74.10.972. [DOI] [PubMed] [Google Scholar]
- 28.Sornay-Rendu E, et al. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22(3):425–33. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 29.Fazeli PK, et al. Serum FGF-21 levels are associated with worsened radial trabecular bone microarchitecture and decreased radial bone strength in women with anorexia nervosa. Bone. 2015;77:6–11. doi: 10.1016/j.bone.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackerman KE, et al. Fractures in Relation to Menstrual Status and Bone Parameters in Young Athletes. Med Sci Sports Exerc. 2015;47(8):1577–86. doi: 10.1249/MSS.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howgate DJ, et al. Bone metabolism in anorexia nervosa: molecular pathways and current treatment modalities. Osteoporos Int. 2013;24(2):407–21. doi: 10.1007/s00198-012-2095-6. [DOI] [PubMed] [Google Scholar]
- 32.Seeman E. Estrogen, androgen, and the pathogenesis of bone fragility in women and men. Curr Osteoporos Rep. 2004;2(3):90–6. doi: 10.1007/s11914-004-0016-0. [DOI] [PubMed] [Google Scholar]
- 33.Misra M, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2004;89(10):4972–4980. doi: 10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 34.van Dam EWCM, et al. Low amplitude and disorderly spontaneous growth hormone release in obese women with or without polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(9):4225–4230. doi: 10.1210/jc.2002-012006. [DOI] [PubMed] [Google Scholar]
- 35.Kreipke TC, et al. The roles of architecture and estrogen depletion in microdamage risk in trabecular bone. J Biomech. 2016 doi: 10.1016/j.jbiomech.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein EM, et al. Skeletal structure in postmenopausal women with osteopenia and fractures is characterized by abnormal trabecular plates and cortical thinning. J Bone Miner Res. 2014;29(5):1101–9. doi: 10.1002/jbmr.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, et al. Deterioration of trabecular plate-rod and cortical microarchitecture and reduced bone stiffness at distal radius and tibia in postmenopausal women with vertebral fractures. Bone. 2016;88:39–46. doi: 10.1016/j.bone.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnitzler CM, Mesquita JM. Cortical porosity in children is determined by age-dependent osteonal morphology. Bone. 2013;55(2):476–86. doi: 10.1016/j.bone.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzler CM. Childhood cortical porosity is related to microstructural properties of the bone-muscle junction. J Bone Miner Res. 2015;30(1):144–55. doi: 10.1002/jbmr.2312. [DOI] [PubMed] [Google Scholar]
- 40.Nishiyama KK, et al. Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study. J Bone Miner Res. 2012;27(2):273–82. doi: 10.1002/jbmr.552. [DOI] [PubMed] [Google Scholar]
- 41.Devlin MJ. Bone marrow composition, diabetes, and fracture risk: more bad news for saturated fat. J Bone Miner Res. 2013;28(8):1718–20. doi: 10.1002/jbmr.2013. [DOI] [PubMed] [Google Scholar]
- 42.Limonard EJ, et al. Short-Term Effect of Estrogen on Human Bone Marrow Fat. J Bone Miner Res. 2015;30(11):2058–66. doi: 10.1002/jbmr.2557. [DOI] [PubMed] [Google Scholar]
- 43.Milos G, et al. Does weight gain induce cortical and trabecular bone regain in anorexia nervosa? A two-year prospective study. Bone. 2007;41(5):869–74. doi: 10.1016/j.bone.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Legroux-Gerot I, et al. Factors influencing changes in bone mineral density in patients with anorexia nervosa-related osteoporosis: the effect of hormone replacement therapy. Calcif Tissue Int. 2008;83(5):315–23. doi: 10.1007/s00223-008-9173-y. [DOI] [PubMed] [Google Scholar]
- 45.Liu XS, et al. Accuracy of high-resolution in vivo micro magnetic resonance imaging for measurements of microstructural and mechanical properties of human distal tibial bone. J Bone Miner Res. 2010;25(9):2039–50. doi: 10.1002/jbmr.92. [DOI] [PMC free article] [PubMed] [Google Scholar]


