Abstract
High levels of reactive oxygen species (ROS) contributes to muscle cell death in aging and disuse. We have previously found that resveratrol can reduce oxidative stress in response to aging and hindlimb unloading in rodents in vivo, but it was not known if resveratrol would protect muscle stem cells during repair or regeneration when oxidative stress is high. To test the protective role of resveratrol on muscle stem cells directly, we treated the C2C12 mouse myoblast cell line with moderate (100 μM) or very high (1 mM) levels of H2O2; in the presence or absence of resveratrol. The p21 promoter activity declined in myoblasts in response to high ROS and this was accompanied a greater nuclear to cytoplasmic translocation of p21 in a dose dependent matter in myoblasts as compared to myotubes. Apoptosis, as indicated by TdT-mediated dUTP nick-end (TUNEL) labeling was greater in C2C12 myoblasts as compared to myotubes (P< 0.05) after treatment with H2O2. Caspase-9, -8, and -3 activities were elevated significantly (P< 0.05) in myoblasts treated with H2O2. Myoblasts were more susceptible to ROS-induced oxidative stress than myotubes. We treated C2C12 myoblasts with 50 μM of resveratrol for periods up to 48 h to determine if myoblasts could be rescued from high ROS-induced apoptosis by resveratrol. Resveratrol reduced the apoptotic index, and significantly reduced the ROS-induced caspase-9, -8, and -3 activity in myoblasts. Furthermore, Bcl-2 and the Bax/Bcl-2 ratio were partially rescued in myoblasts by resveratrol treatment. Similarly, muscle stem cells isolated from mouse skeletal muscles showed reduced Sirt1 protein abundance with H2O2 treatment but this could be reversed by resveratrol. Reduced apoptotic susceptibility in myoblasts as compared to myotubes to ROS is regulated at least in part, by enhanced p21 promoter activity and nuclear p21 location in myotubes. Resveratrol confers further protection against ROS by improving Sirt1 levels and increasing antioxidant production, which reduces mitochondrial associated apoptotic signaling, and cell death in myoblasts.
Keywords: myogenesis, oxidative stress, myoblasts, myotubes, apoptosis, reactive oxygen species
Introduction
Satellite cells are muscle stem cells that are critical for repair from muscle injury. When skeletal muscle damage occurs, satellite cells are activated and proliferate to become myogenic precursor cells or myoblasts, which migrate to the injury site, then differentiate to form new muscle. These events are similar to processes that regulate myogenesis and they can be studied in culture model systems. Reactive oxygen species (ROS) regulate cell signal transduction pathways and many cellular functions [40,41] including myogenesis [26]. However, excessive ROS levels both initiate and mediate the dysfunction in a variety of cells including muscle cells. This includes disruption of cell signaling, metabolism, transcription, and apoptosis and therefore ROS may impair myogenesis [2,21,34,58].
Aging is associated with high levels of muscle ROS in vivo, which may contribute to increased apoptosis and cell death and reduced myoblast differentiation leading to poor muscle repair [20]. High levels of ROS are associated with metabolic diseases like diabetes [16,20,36], which may contribute to the loss of myoblast function, increase myoblast cell death [33] and further exacerbate muscle repair in aging. Fulle and colleagues [25] have demonstrated that a high percentage of the myogenic precursor cells from elderly muscles undergo apoptosis triggered by mitochondrial-associated caspase-9 and this appears to be closely linked to the high ROS levels that are found in aged muscles [20]. Thus, we predict that strategies to attenuate high ROS levels should reduce apoptosis in myoblasts and improve muscle differentiation/repair in aging and in other diseases that have elevated ROS levels.
In vitro studies have shown that resveratrol (3,5,4′-trihydroxystilbene) increases antioxidants to reduce the impact of ROS, increases protein synthesis [47], inhibits protein degradation, and attenuates atrophy of skeletal muscle fibers [1,56,68,69]. A high dose of resveratrol that was fed to rodents (400 mg/kg/day) was shown to reduce muscle fiber atrophy after hindlimb unloading [46]. We also have found that resveratrol that was given at 12.5 mg/kg/day [32] tended (p=0.06) to blunt atrophy in fast contracting muscles in response to the high ROS environment associated with hindlimb unloading in aged rodents but it did not improve muscle stem cell (satellite cell) activation/proliferation in old animals after reloading [11]. The blunting of satellite cell activity in response to muscle reloading in old animals by resveratrol [11] could have been due to the direct inhibitory effects of excessive ROS levels in muscle and systemically, and potentially, the apoptotic events that occur in muscle in aged hosts. In this paper we tested the hypothesis that resveratrol would directly improve myoblast survival by reducing mitochondrial-associated apoptotic signaling in myoblasts and myotubes in response to a high ROS environment.
Alternatively, resistance to cell death in myoblasts and myotubes in a high ROS environment may occur through cell signaling that is independent from buffering ROS. Several studies suggest that ROS may alter p21 levels and protein localization in a variety of cell lines [9,12,30]. Specifically, phosphorylation of Ser153 in p21 is thought to induce its translocation from the nucleus to the cytosol, thereby blocking the cell cycle inhibitory activity in C2C12 myoblasts [45]. Other data [30] show that oxidative stress, induces p21 cytoplasmic localization and ubiquitination associated degradation. Thus, a secondary purpose of this study was to determine the effect of ROS on the p21 promoter activity in myoblasts and myotubes, and to investigate whether p21 promoter activity and protein abundance is associated with apoptotic resistance. In this study, we report that myotubes are more resistant to ROS-induced apoptosis than myoblasts, and the reduction of p21 promoter activity and nuclear loss of p21 co-localization is associated with apoptotic resistance within myoblasts. ROS treatment reduced silent mating type information regulation 2 homolog (Sirt1) in myoblasts, which is a putative target for resveratrol. Furthermore, resveratrol provided protection against high ROS induced apoptosis and apoptotic signaling proteins in ROS sensitive myoblasts, potentially through a p21 and/or Sirt1 mediated antioxidant mechanism.
Materials and methods
Cell culture
Murine derived C2C12 myoblasts were obtained from the American Type Cell Culture Collection (ATCC, Manassa, VA). The myoblasts were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) and antibiotics (100 U/ml penicillin G, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B; Mediatech. Inc., Herndon, VA). The cells were incubated at 37°C in a water-saturated atmosphere of 95% ambient air and 5% CO2. To induce myotube formation, C2C12 myoblasts were plated at an initial density 1 × 105 cells/well in six-well culture dishes. After reaching 70–80% confluency, the growth medium was replaced with, DMEM supplemented with 2% heat-inactivated horse serum and antibiotics (differentiation medium) to induce myotubes formation. The media was replaced with fresh media each day. Myotubes were used for experiments after 6 days of incubation in differentiation medium.
Myoblasts and myotubes were treated with 0 μM, 0.1 mM, or 1mM H2O2 for 6, 12, 24 or 48 hours, then were harvested in ice-cold lysis buffer [55].
Resveratrol treatment
Myoblasts or myotubes were transferred to fresh media containing 0, 10, 25 or 50 μM of resveratrol. After 24 hours of resveratrol treatment, H2O2 was added to the medium to make a final concentration of 0 mM, 0.1 mM, or 1 mM H2O2. Myoblasts or myotubes were transferred to fresh media containing 0–50 μM of resveratrol and 0–1 mM H2O2 each day. The cells were then harvested 6, 12, 24 or 48 h. after treatment with H2O2.
Detection of apoptotic cell death
DNA cleavage, which characteristically occurs in apoptotic cells, was measured by TdT-mediated dUTP nick-end labeling (TUNEL) (Roche Applied Science, Indianapolis, IN). The C2C12 cells were grown on glass cover slips, fixed in 4% paraformaldehyde in PBS (pH 7.4), and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate. The cells were incubated with TdT and fluorescein-dUTP at 37°C for 1 h. Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole, Vectashield® mounting medium, Vector Laboratories, Burlingame, CA). The cells were visualized with an ECLIPSE E800 fluorescence microscope (Nikon Instruments, Melville, NY). The apoptotic index was defined as the percentage of TUNEL-positive cells to DAPI-positive cells.
Fluorometric caspase-activity assay
Caspase activity was measured as previously reported [52]. Briefly, the cytoplasmic protein fraction without a protease inhibitor was incubated in assay buffer (50 mM PIPES, 0.1 mM EDTA, 10% glycerol, and 10 mM DTT, pH 7.2) with 1mM fluorogenic 7-amino-4-trifluoromethyl coumarin (AFC)-conjugated substrate (Ac-DEVD-AFC for caspase-3, Ac-LEHD-AFC for caspase-9, Ac-IETD-AMC for caspase-8; Alexis, San Diego, CA) at 37°C for 2 h. The change in fluorescence was measured on a spectrofluorometric (CytoFluor; Applied Biosystems, Foster City, CA) with an excitation wavelength of 390/20 nm and an emission wavelength of 530/25 nm for caspase-3 and -9 and 460/40 nm for caspase-8 before and after the 2-h incubation. Caspase activity was estimated as the change in arbitrary fluorescence units normalized to micrograms of protein used in the assay.
Immunoblot analysis
C2C12 cells or myoblasts were harvested and fractionated by the method described by the Rothermel et al. [55]. Immunodetection was performed using established methods as described previously [3,15]. Protein concentrations of samples were determined by the Lowry method, and the purity of each fraction was confirmed as reported previously [62]. Tissue lysates were separated on 12% sodium dodecyl sulfate-polyacrylamide gels by SDS-PAGE, followed by electroblotting to a nitrocellulose membrane. The membranes were blocked with 5% milk in TBST, then incubated overnight (1:1000) in antibodies purchased from Cell Signaling Technology, Boston, MA including: Bcl-2 (#2876), Bax (#2772), cleaved caspase-3 (#9664), cleaved caspase-9 (#9509) AIF (#4642), p21 (#2946) and histone H3 (#5192) at 4°C. Finally, the membranes were incubated with the appropriate secondary antibody conjugated with HRP (1:50,000). The immunopositive bands were detected with ECL Advance (Amersham Biosciences, Piscataway, NJ). The protein content of each band was quantified by densitometric analysis (Image J, NIH).
Immunohistochemistry analysis
Immunostaining for myosin heavy chain was visualized in myotubes by immunofluorescence as described previously [63]. In brief, cells were plated on glass cover slips in six-well dishes. After 24 or 48 hours of H2O2 treatment, the cells were washed with PBS and fixed in 4% formaldehyde in PBS. MF20 (Developmental Hybridoma Bank, IA) was used to detect the cellular location of this protein on myotubes, whereas desmin was used to identify myoblasts. The primary antibodies were followed by Alexa 546 conjugated anti-mouse IgG (Molecular Probes, Eugene, OR) and nuclei were stained with (DAPI). Samples were visualized using an Epi-fluorescence microscope (Nikon, Inc., Melville, NY), and images were obtained using a SPORT RT camera (Diagnostic Instruments, Sterling Height, MI).
Luciferase assay
A luciferase assay was used to determine the affect of H2O2 on p21 promoter activity. The myoblasts were maintained in DMEM supplemented with 10% fetal bovine serum at 37°C in 95% ambient air and 5% CO2. Approximately 2 × 106 C2C12 myoblasts cells per sample were transfected with a luciferase reporter containing p21 promoter (a kind gift from Dr. Yonghua Yang, Department of Pathology and Cell Biology, University of South Florida College of Medicine) using Nucleofector Kit V (Amaxa, Gaithersburg, MD) in a 0.2 cm gap cuvette (BioRad, Hercules, CA), with four 200V pulses each lasting 5 ms duration. After the electroporation, the cells were suspended in 500μl of MEM (ATCC, Manassas, VA) for 5 min at 37°C then plated into 10 cm plates and incubated in 5% CO2 at 37°C for 24 h before any treatment. 24 h after electroporation the cells were treated with 0 μM, 0.1 mM, or 1mM H2O2, and then harvested 24 h after treatment with H2O2. A pGL2 vector containing no promoter was used as a negative control for each experiment.
Isolation of mouse muscle stem cells
To follow up the studies from the C2C12 model, we tested the impact of ROS on skeletal muscle cells isolated from mice. Skeletal muscle satellite cells, which reside beneath the basal lamina of mature muscle fibers, function as muscle stem cells (C2C12 cells are a model for muscle stem cells), but they exhibit substantial phenotypic and functional heterogeneity. Although we have established the methods to isolate a population of MSCs which are CD45-,Sca-1-Mac-1-, CXCR4+b1-, integrin+ [19,43], in our hands this is a very small population (3% of all muscle stem cells) and would take a great number of mice to obtain a usable sample. Instead, we have opted to use Fluorescence Activated Cell Sorting (FACS) to isolate and examine the properties of syndecan-4 (Syn4) positive muscle stem cells [14], which, in our hands, represent ~20% of MSC cells. Muscle stem cells were isolated from 6 adult mouse (C57BL6) gastrocnemius muscles, aged 3 months via percoll gradient centrifugation, and incubated with anti-Syn4 (7.5ug/ml-based on optimization experiments) using published sorting methods [14]. The cells were sorted by FACS using gating strategies for side scatter, forward scatter, and singlet gating.
Sirt1 activity assays
Syn4 cells were obtained from hindlimb muscles of C57BL/6 mice, aged 3 months. The cells were cultured for 24 h then switched to 1 μM NAD MEM (normal DMEM has 30 μM NAD). Syn4 cells were treated with H2O2 (1 mM), and/or FK866 (50nM), a Nampt inhibitor which induces significant NAD+ intracellular reduction which would reduce NAD+ dependent Sirt1 activity, and/or 50 μM of resveratrol (a Sirt1 activator) for 48 h. Whole cell extracts were obtained using a mild lysis buffer (50 mM Tris-HCl, pH 8.0) with 1 mM PMSF and protease inhibitors. 30 μg of extracts was used in an in vitro deacetylation assay using the Fluor de Lys-Sirt1 substrate (Biomol) as previously reported [11]. The assay’s fluorescence signal is generated in proportion to the amount of deacetylation of the lysine corresponding to Lys-382, a known in vivo target of Sirt1 activity. Data were recorded from three experiments and expressed as the change in fluorescent units.
Data Analysis
Results are presented as mean ± SEM. Data were analyzed using the Sigma Stat 3.0 statistical software. A two-way analysis of variance (ANOVA) was performed for comparisons between experimental groups, followed by the Student-Newman-Keuls post hoc test to determine differences among treatments. Significant level was set at P<0.05.
Results
Myotubes but not myoblasts are resistant to H2O2-induced protein loss
The loss of total cellular protein in myoblasts and myotubes occurred in a H2O2 dose dependent manner (Figure 1A). Marked protein loss (as determined from the protein assay) occurred at 1 mM H2O2 in myoblasts, but differentiated myotubes had a lower loss of total protein when incubated in the same concentration of H2O2. The total protein loss in myoblasts and myotubes was not only H2O2 dose dependent, but also was regulated by the duration of incubation.
Figure 1. Myotubes are more resistant to oxidative stress than myotubes.
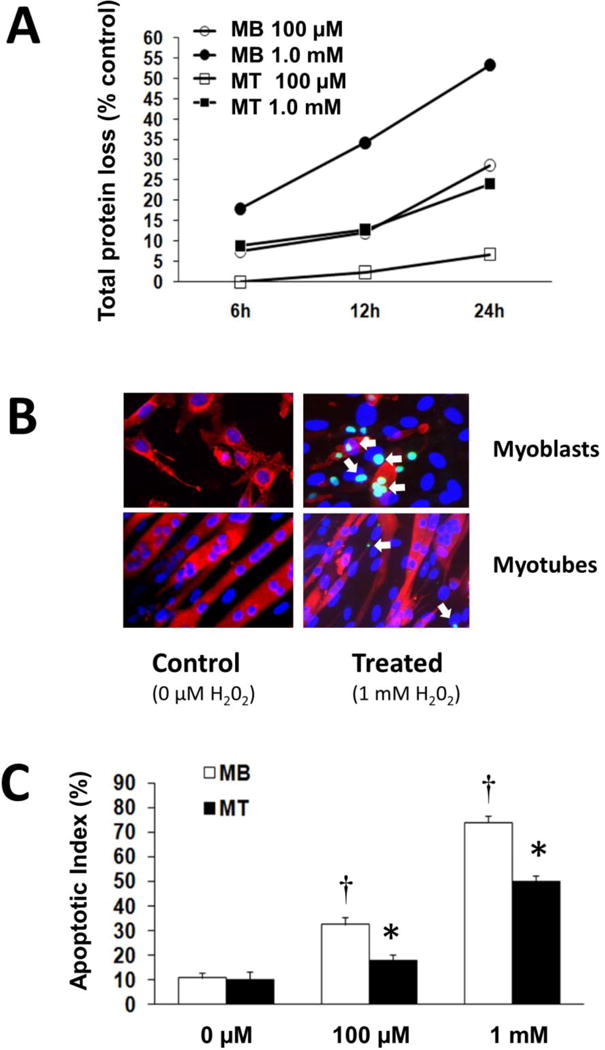
A. The percent of total protein loss is shown relative to control cells (0 μM) for each H2O2 treatment. C2C12 myoblasts (Mb) or myotubes (Mt) were incubated for 6, 12, or 24 h in 100 μM or 1.0 mM of H2O2.
B. Examples of myoblasts and myotubes that were incubated with 0 μM or 1.0 mM of H2O2 for 24 hours. TUNEL positive nuclei (green) are noted especially in myoblasts after treatment with H2O2. Examples of TUNEL positive cells are shown by the white arrows. Myoblasts and myotubes were incubated in antibodies to desmin and myosin heavy chain respectively, followed by incubation and staining with a secondary Alexa 546 conjugated anti-mouse IgG (red).
C. The apoptotic index is expressed as the number of positive TUNEL to the number of total cells. *, different from the corresponding treatment conditions (P< 0.05). †, difference from the control cells (P < 0.05).
Myoblasts have greater abundance of apoptotic nuclei than myotubes
The loss of total protein was due at least in part from apoptosis-induced cell death. Apoptosis was determined by quantifying the number of TUNEL positive nuclei (Figure 1B, green nuclei) and expressed relative to total nuclei, which were identified by DAPI staining (Figure 1B, blue nuclei). An example of myotubes and myoblasts in control and treated (1 mM H2O2) cells that were stained with desmin (myoblasts) or myosin heavy chain (myotubes) is shown in Figure 1B. The high TUNEL labeling in myoblasts is evident after the high ROS treatment. There was a greater apoptotic index in myoblasts as compared to myotubes (P<0.05) after incubation in either 0.1 mM or 1 mM H2O2 (Figure 1C).
Oxidant stress induced greater caspase activity in myoblasts than myotubes
Incubation of C2C12 cells in H2O2 as an oxidant stress is thought to increase cell death [22]; however, it is not known whether H2O2 per se triggers intrinsic and/or extrinsic apoptosis pathways in C2C12 cells or if one of these pathways is favored.
Caspase-9 is primarily associated with mitochondrial associated apoptotic signaling. Caspase-9 activity was 89.1 ± 17.5% (P<0.05) and 317.6 ± 13.7% (P<0.05) higher in C2C12 myoblasts that were treated with 0.1 mM or 1 mM of H2O2, respectively, compared with control myoblasts (P<0.05) (Figure 2A). After 6 h of incubation, caspase-9 activity was 19.0 ± 2.1% (P<0.05) and 57.7 ± 4.3% (P<0.05) greater in myotubes that were incubated in 0.1 mM or 1 mM of H2O2 respectively, as compared to control myoblasts incubated in 0 mM of H2O2. Furthermore, significantly lower caspase-9 activity was found in myotubes as compared to myoblasts that were incubated for 6 h in 0.1 mM or 1 mM of H2O2 (P<0.05). Caspase-9 activity was decreased in the myoblasts after 24 h of treatment with 1 mM of H2O2 but caspase-9 activity was greater in myotubes after 24 h of H2O2 treatment (Figure 2B).
Figure 2. Altered caspase activities of myoblasts and myotubes following indicated H2O2 treatment.
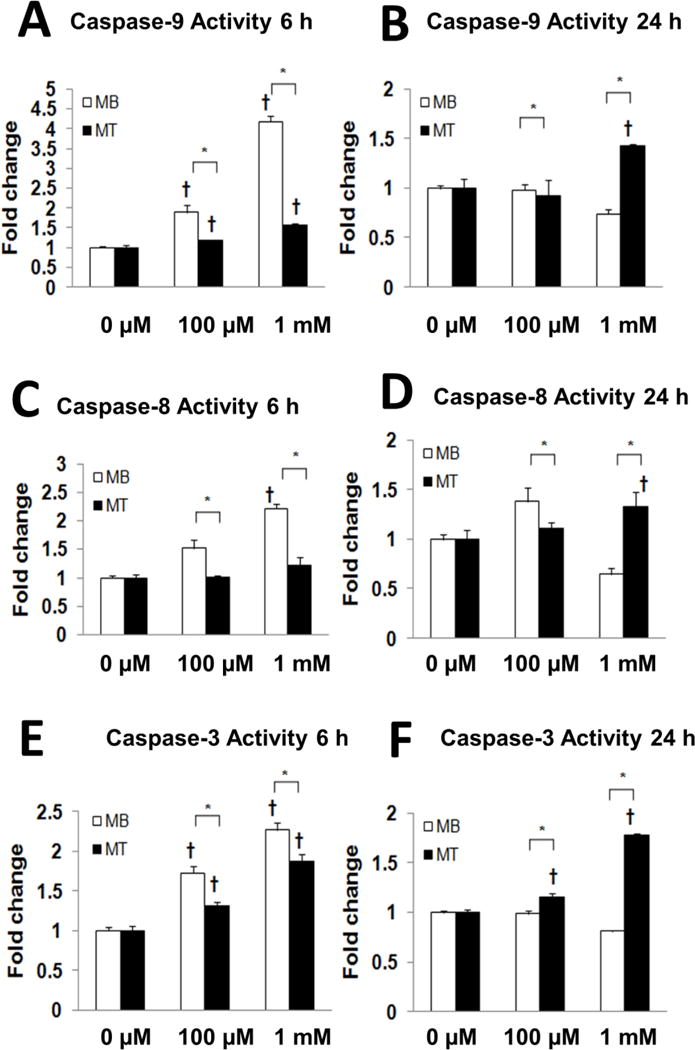
Caspase activity is shown for caspase-9 (A,B), caspase-8 (C,D) and caspase-3 (E,F) after 6 h (A,C,E) and 24 h (D,E,F) of incubation in 0 μM, 0.1 mM or 1.0 mM H2O2. H2O2-induced caspase activity is presented as a fold change relative to the corresponding control values. Note the different axes scales for 6 and 24 h *, myoblasts are different from myotubes (P<0.05). †, different from control cells (P < 0.05).
Caspase-8 is considered to represent the non-mitochondrial apoptotic pathway. Interestingly, caspase-8 activity was 51.9 ± 1.5% (P<0.05) and 32.0 ± 1.6% (P<0.05) higher in myoblasts after 6 h of H2O2 incubation in 0.1 mM and 1 mM of H2O2, respectively (P<0.05) as compared to control cells that were incubated in 0 μM of H2O2. However, caspase-8 activity did not increase in myotubes after 6 h of treatment with either the low or high dose of H2O2 (Figure 2C). While caspase-8 activity remained elevated in the myoblasts vs. myotubes after 24 hours of incubation in 0.1 μM of H2O2, caspase-8 activity increased only in the myotube group after treatment with 1 mM of H2O2, relative to control myotubes treated with 0 μM of H2O2 (Figure 2D). In general, these data are consistent with our TUNEL staining results, which suggest that myotubes are more resistant to apoptosis signaling that was induced via a high oxidant environment.
Compared to the control myoblasts, caspase-3 activity as the effector caspase was 73.0 ± 8.6% (P<0.05) and 126.4 ± 9.5% (P<0.05) higher after 6 h of incubation in myoblasts treated with 0.1 mM and 1 mM of H2O2 respectively, as compared with control myoblasts (P<0.05) (Figure 2E). Similarly, caspase-3 activity was 30.5 ± 5.7% (P<0.05) and 87.5 ± 8.5% (P<0.05) higher in myotubes after incubation in 0.1 mM and 1 mM of H2O2, respectively (P<0.05) after 6 h of treatment compared with the control myotubes. Although there was an increase in caspase-3 activity in myotubes after 6 h of incubation in 0.1 mM and 1 mM of H2O2 this was lower than for myoblasts. Interesting, there was only a small increase in caspase-3 activity in myoblasts after 24 h of incubation in 0.1 mM or 1 mM of H2O2. Caspase-3 activity was an 83.4 ± 3.2% greater (P<0.05) in myotubes after 24 h of treatment as compared to control myotubes (Figure 2F) although this might be reflective of both apoptosis and non-apoptotic signaling that occurs during differentiation of myotubes [23]. In general, the activity of all three caspases tended to decrease in myotubes after 24 h of incubation in 1mM of H2O2 as compared to control cells or and shorter incubation times. This observation likely reflects the greater loss of cells from apoptosis at the higher oxidant level and longer incubation period.
The effect of H2O2 treatment on p21 promoter activity
Previous studies [30,45] have shown a down regulation of p21 by H2O2 in C2C12 myoblasts. Thus, we investigated whether the difference in apoptotic resistance and loss of protein after treatment with H2O2 might related to different p21 promoter activity between myoblasts and myotubes. A luciferase assay indicated that the p21 promoter activity level declined in a H2O2 dose dependent matter in both myoblasts and myotubes (Figure 3A). The immunoblot data show a dose dependent decline of nuclear p21 content in myoblasts and myotubes after 24 h of incubation (Figure 3B). The alteration of p21 content in the nuclear protein fraction of myoblasts and myotubes had already begun to decline after only 6 h of incubation in H2O2 and this is shown in representative western blots (Figure 3C). Generally, the decline in p21 protein abundance corresponded with the decline in its promoter activity.
Figure 3. The effect of H2O2 treatment on p21 promoter activity and protein abundance.
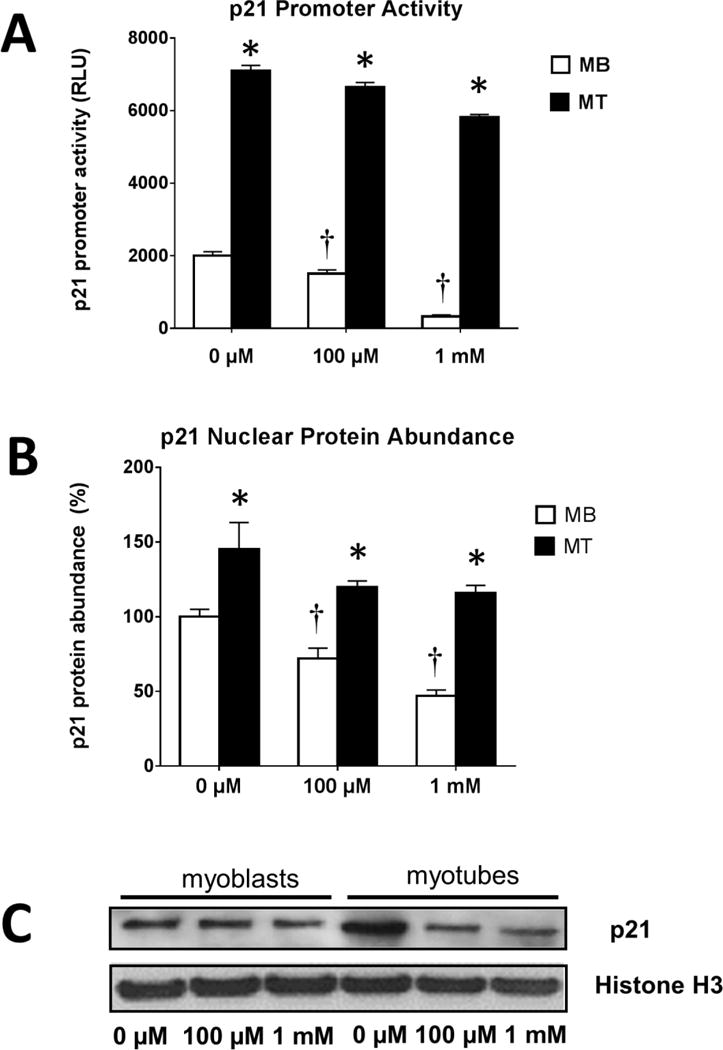
A. Luciferase activity is shown as relative luciferase light units (firefly/Renilla) and represents means ± SEM for four samples 24 h after incubation in 0 μM, 100 μM or 1.0 mM of H2O2. *, myoblasts are different from myotubes in the same group (P<0.05). †, different from control cells (P < 0.05).
B. Quantification of protein abundance by western blots for p21 nuclear content after 24 hours of incubation after 0 μM, 100 μM or 1.0 mM of H2O2. Results are presented relative to the corresponding controls. *, myoblasts are different from myotubes in the same group (P<0.05). †, different from control cells (P < 0.05).
C. Representative immunoblots of p21 content in nuclear protein fractions performed after 6 hours of H2O2 treatment.
H2O2 promotes loss of nuclear p21
Immunocytochemistry was used to confirm the H2O2-induced co-localization of p21 with myonuclei. p21 (red) was co-labeled with the nuclei (blue) in control treatments without H2O2 (co-labeled nuclei are pink), but the cytoplasm also reflected p21 labeling (Figure 4). However, p21 labeling in the nuclei appeared to decrease following 1 mM H2O2 treatment in both myoblasts and myotubes (Figure 4) as seen by less co-labeling of the p21 label (red) and clearer DAPI stained nuclei (blue). This suggests that p21 loss and/or potentially a relocation of p21 from the nuclear to the non-nuclear compartment occurred in response to high ROS and this was associated with greater apoptotic signaling in muscle cells.
Figure 4. Localization of p21 protein in the myonuclei.
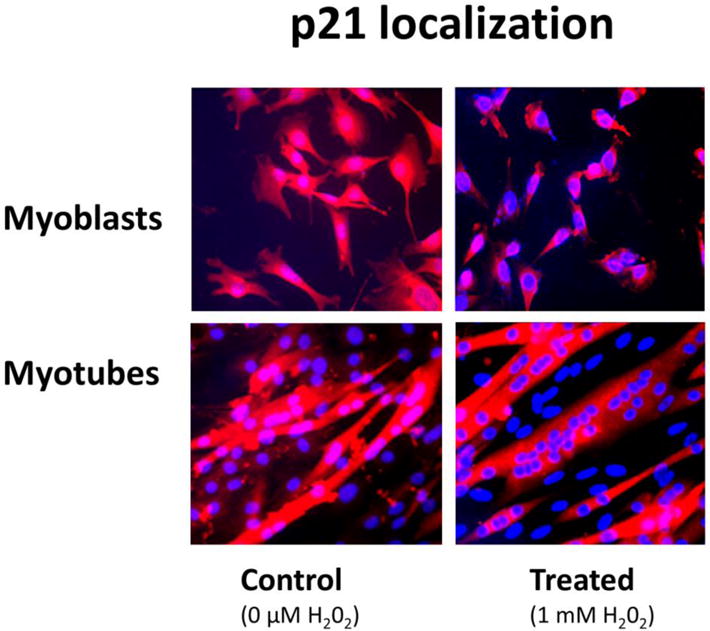
Immunohistochemical staining showing localization of p21 (red) in nuclei and cytoplasm of myoblasts (MB) and myotubes (MT) after treatment with H2O2 for 24 hours. Nuclei are counterstained with DAPI (blue). The data show a loss of p21 (red staining) over the nuclei after treatment and several nuclei with no p21 after treatment with H2O2. Less p21 staining is associated with the nucleus (DAPI) after 24 hours of treatment.
Resveratrol reduces but does not prevent apoptosis in myoblasts
To determine if resveratrol reduced ROS-induced cell death in myoblasts, we first measured the loss of total protein in myoblasts, which was the most sensitive to H2O2 treatment. Incubation in 0.1 mM or 1 mM H2O2 induced marked protein loss, but resveratrol treatment lessened the loss of protein in a dose dependent fashion (Figure 5A). However, resveratrol could not prevent all of the loss of protein that was induced by H2O2. Resveratrol reduced the apoptotic index in a dose dependent fashion in response to 0.1 or 1 mM of H2O2 but resveratrol treatment failed to abolish all of the apoptosis in the myoblasts after 24 h of treatment (Figure 5B; Supplemental Figure 1).
Figure 5. Effects of resveratrol on protein loss and apoptotic signaling in response to oxidative stress.
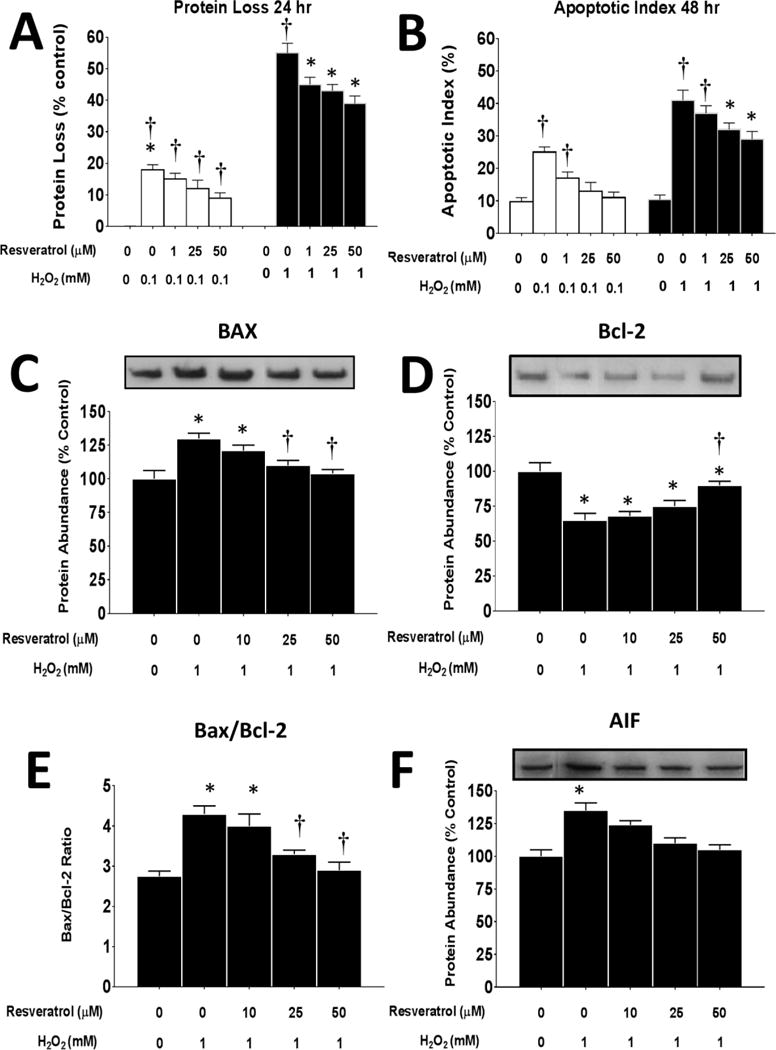
A. Total protein loss in myoblasts (expressed relative to control untreated cells) after 24 h or 48 h of incubation in 0 or 0.1 mM (white bars) and 0 or 1.0 mM (black bars) of H2O2 and 0–50 μM of resveratrol. *P<0.05 significantly different from non-treatment control myoblasts (0 mM resveratrol and 0 mM and H2O2) at 24 h or 48 h of incubation, respectively. †, P<0.05, Treatment group is significantly different from 0.1 mM or 1 mM H2O2 + 0 mM resveratrol at 24 or 48 h of incubation.
B. Apoptotic index (expressed as a percent of total nuclei) myoblasts after 24 h of incubation in 0 or 1 mM (white bars) or 1.0 mM (black bars) of H2O2 and 0–50 μM of resveratrol. *P<0.05 significantly different from non-treatment control myoblasts (0 mM resveratrol and 0 mM and H2O2) at 6 or 12 h of incubation, respectively. †, P<0.05, Treatment group is significantly different from 0.1 mM or 1 mM H2O2 + 0 mM resveratrol at 6 or 12 h of incubation.
Bax (C), Bcl-2 (D) and AIF (F) protein levels expressed relative to untreated control cells incubated in 0 or 1 mM of H2O2 and 0–50 μM of resveratrol for 24 h. E. The Bax/Bcl-2 ratio. P<0.05 significantly different from non-treatment control myoblasts (0 mM resveratrol and 0 mM and H2O2) at 6 or 12 h of incubation, respectively. †, P<0.05, Treatment group is significantly different from 1 mM H2O2 + 0 mM resveratrol at 6 or 12 h of incubation.
Consistent with the apoptotic index, the pro-apoptotic protein Bax increased in myoblasts with the high oxidative stress load (Figure 5C). Although 10 μM of resveratrol did not significantly reduce the total Bax protein abundance, higher doses of resveratrol (25 and 50 μM) did result in a significant reduction in Bax, and in fact, returned it to control levels (Supplemental Figure 2).
In a similar pattern, Bcl-2 decreased with the high ROS treatment; however, only the highest resveratrol level was able to significantly increase Bcl-2, to levels that were similar to 0 μM of H2O2 0 μM of resveratrol, but Bcl-2 protein abundance was still suppressed relative to the control levels (Figure 5D). The Bax/Bcl-2 ratio, was increased when myoblasts were treated with 1 mM of H2O2 but it had returned to control levels in myoblasts that were treated with 25 or 50 μM of resveratrol (Figure 5E). Similar to Bax, AIF was elevated when the myoblasts were treated with 1 mM of H2O2 but resveratrol blunted this increase such that the AIF protein abundance had returned to control levels in myoblasts treated with either 25 or 50 μM of resveratrol for 24 h (Figure 5F, Supplemental Figure 2). These data suggest that resveratrol was able to blunt but not prevent apoptosis protein signaling.
Effects of resveratrol on ROS-induced caspase activation
As AIF, Bax and Bcl-2 are important in mitochondrial apoptotic signaling, we wanted to determine if other markers of mitochondrial apoptosis and/or non-mitochondrial apoptosis signaling that occurs during the high ROS environment, were also reduced by resveratrol. To do this we evaluated caspase activity after both short duration and long duration H2O2 incubations that were between 6–48 h of high oxidative stress treatment. Caspase-9, a marker of the mitochondrial apoptotic pathway increased with both 0.1 and 1 mM of H2O2. Caspase-9 activity was blunted, relative to the respective ROS treatment by 25 or 50 μM of resveratrol after 6 h of incubation (Figure 6A) or by 10, 25 and 50 μM of resveratrol 12 h (Figure 6B) of incubation. This suggests that mitochondrial associated apoptosis induced by high oxidative stress levels, is blunted by resveratrol but apoptosis is not prevented after short duration resveratrol treatment.
Figure 6. Resveratrol reduces caspase activity in myoblasts after 6 or 12 hours of H2O2-induced oxidative stress.
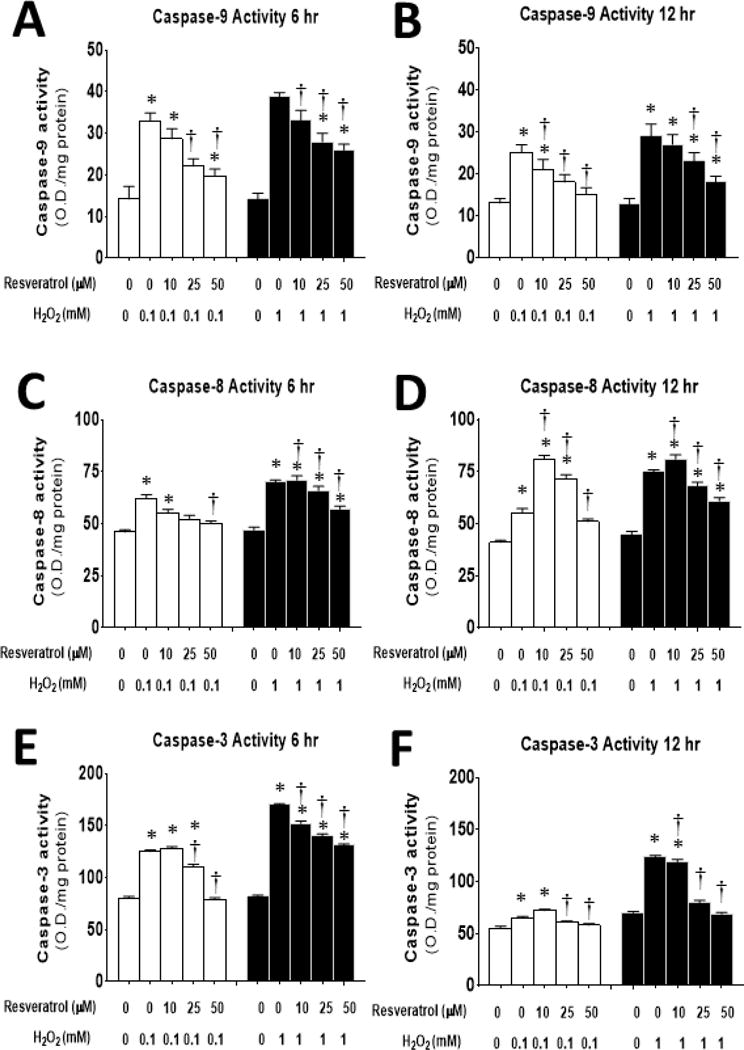
The activity of caspase-9 (A,B), caspase-8 (C,D) and caspase-3 (E,F), was determined after 6 (A,C,E) or 12 (B,D,F) hours of incubation in 0–1 mM of H2O2 and 0–50 μM of resveratrol. *P<0.05 significantly different from non-treatment control myoblasts (0 mM resveratrol and 0 mM and H2O2) at 6 or 12 h of incubation, respectively. †, P<0.05, Treatment group is significantly different from 1 mM H2O2 + 0 mM resveratrol at 6 or 12 h of incubation.
Caspase-8, is a non-mitochondrial extrinsic apoptotic pathway marker. Caspase-8 activity increased after both 0.1 and 1 mM of H2O2. Resveratrol reduced caspase-8 activity in cells that were treated in 0.1 mM of H2O2 for 6 h (Figure 6C). 12 h of treatment in 10, 25 and 50 μM of resveratrol blunted the 0.1 mM H2O2-induced increase caspase-8 activity relative to treatment with 0 μM of resveratrol. However, while 10, 25 and 50 μM of resveratrol were able to blunt caspase-8 activity in cells treated with 1 mM of H2O2 after 6 h (Figure 6C) or 12 h of incubation (Figure 6D), in each case, caspase-8 activity remained elevated in the resveratrol-treated cells as compared to the cells that received 0 mM of H2O2. These data suggest that the extrinsic apoptotic pathway was activated by the ROS treatments, and that resveratrol was partially effective in reducing signaling in this pathway.
Caspase-3 is the effector caspase, which is the convergence point for both mitochondrial and extrinsic apoptotic signaling cascade pathways. Caspase-3 activity increased by treatments in 0.1 and 1 mM of H2O2 and resveratrol blunted this increase in a dose dependent fashion (Figure 6E). 25 μM and 50 μM of resveratrol were able to blunt caspase-3 activity in cells that were incubated in 0.1 mM of H2O2 for 12 h (Figure 6F, Supplemental Figure 2).
Incubation of myoblasts in resveratrol and H2O2 for the longer durations of 24 and 48 hours (Figure 7) showed similar patterns as the acute studies (Figure 6). Of note, the magnitude of the caspase activity level tended to be greater after the longer incubation times than after 6 or 12 hours of incubation even in control cells. In general, caspase-9 (Figure 7A,B), caspase-8 (Figure 7C,D), and caspase-3 (Figure 7E,F), all increased with the longer duration of incubations and resveratrol decreased the caspase activities in myoblasts after both 24 and 48 h relative to treatment with 0 mM resveratrol. While the highest ROS dose was blunted by 50 μM of resveratrol after both 24 and 48 h of incubation, caspase-9 activity remained elevated relative to control untreated myoblasts in the 48 h incubation groups. Resveratrol was able to blunt caspase-8 levels to control levels after incubation in 0.1 or 1 mM of H2O2 for 24 h (Figure 7C). While caspase-8 activity was partially blunted by resveratrol treatment in myoblasts after 48 h of incubation, caspase-8 remained elevated relative to untreated control myoblasts (Figure 7D). In a similar fashion, resveratrol blunted the H2O2-induced elevation of caspase-3 activity in myoblasts after both 24h (Figure 7E) and 48 h (Figure 7F) incubation periods.
Figure 7. Resveratrol suppresses caspase activity in myoblasts after 24 or 48 hours of oxidative stress induced by H2O2.
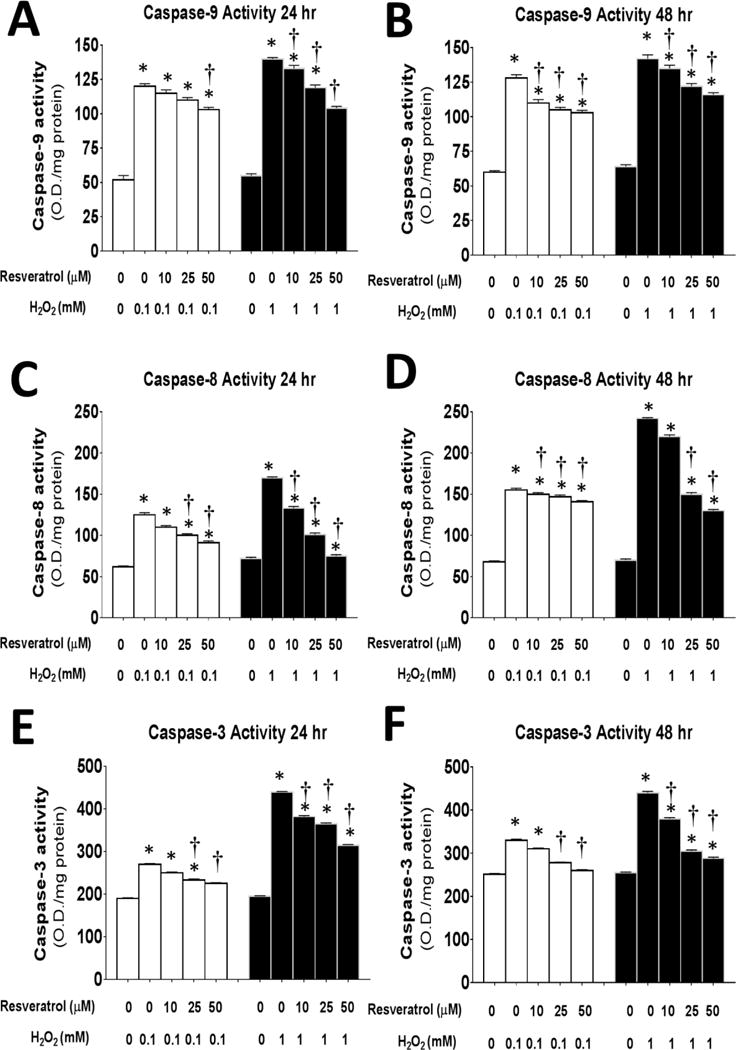
The activity of caspase-9 (A,B), caspase-8 (C,D) and caspase-3 (E,F), was determined after 24 (A,C,E) or 48 (B,D,F) hours of incubation in 0–1 mM of H2O2 and 0–50 μM of resveratrol.*P<0.05 significantly different from non-treatment control myoblasts (0 mM resveratrol and 0 mM and H2O2) at 6 or 12 h of incubation, respectively. †, P<0.05, Treatment group is significantly different from 1 mM H2O2 + 0 mM resveratrol at 24 or 48 h of incubation.
Other proteins in the mitochondrial pathway were similarly affected by resveratrol. For example, the protein abundance of Apaf-1, a protein that is part of the apoptosome was increased in myoblasts after exposure to 1 mM H2O2, but resveratrol blunted this response (Supplemental Figure 2). However, resveratrol failed to markedly reduce the protein abundance Poly (ADP-ribose) polymerase (PARP), which is involved in DNA repair, in myoblasts that were incubated with 1 mM of H2O2. This suggests that resveratrol did not fully prevent apoptotic DNA fragmentation in myoblasts in response to high ROS levels.
Resveratrol prevents the ROS-induced loss of Sirtuin-1 (Sirt1) in myoblasts
Sirt1 is a histone deacetylase that has many important physiological roles including protection against oxidative stress and apoptosis [28,29]. Sirt1 is reduced in aging and muscle wasting [28,42]. Although the mechanism for the loss of Sirt1 in aging is not known, we speculate that high levels of oxidative stress contribute to this loss in Sirt1. Indeed, muscle wasting is attenuated when Sirt1 activity is increased via caloric restriction [51] or overexpression [39]. Sirt1 is increased and activated by resveratrol [10,54,60]. It is important to note that a resveratrol-induced increase in Sirt1 activity elevates the transcription of mitochondrial antioxidant enzymes such as manganese superoxide dismutase (MnSOD) [50]. In addition, in a recent study we found that resveratrol treatment promoted anti-apoptotic and anti-oxidant changes in muscles of elderly subjects who exercised as compared to placebo treated subjects [6] and resveratrol feeding reduced oxidative stress in muscles of aged rodents [32,57]. To test the hypothesis that ROS is a prime inhibitor of muscle Sirt1 in conditions such as aging where oxidative stress is high, we evaluated if H2O2 would reduce Sirt1 protein levels in myoblasts and myotubes and if resveratrol treatment would rescue a H2O2-induced reduction in Sirt1 protein abundance. In these experiments, we conducted western blot analysis of myoblasts that were incubated for 24 h in 0–50 μM of resveratrol and were treated with 0.1 or 1 mM of H2O2. Consistent with our hypothesis, the data (Figure 8) suggest that high concentrations of H2O2 reduced Sirt1 protein abundance in myoblasts but this could be rescued at the highest concentrations of resveratrol. The resveratrol-induced increase in Sirt1 protein occurred in concert with a reduced apoptotic and caspase signaling. While we anticipated that Sirt1 would improve anti-oxidants in myoblasts and myotubes in culture as it does in muscle in vivo in response to ROS production [32,57], we were only able to detect a modest decrease in catalase protein abundance by H2O2 and a modest, albeit significant recovery in the loss of catalase protein abundance. However, there was a marked increase of MnSOD and glutathione peroxidase 1 (GPx1) in myoblasts after treatment with resveratrol (Supplemental Figures 2 and 3). This suggests that at least part of the manner in which resveratrol is protective against apoptotic signaling and cell death in myoblasts might be due to its regulation of antioxidant proteins through Sirt1 to prevent ROS damage in mitochondria, and in doing so, reduce mitochondrial induced apoptotic signaling (Figures 5–7).
Figure 8. Regulation of Sirt1 protein by oxidative stress.
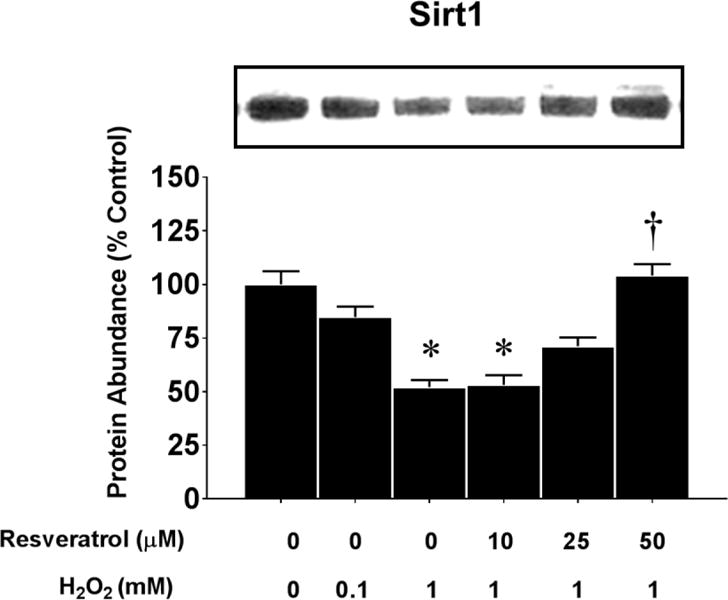
The figure shows representative blots of Sirt1 protein abundance after 24 h incubation in 0 mM or 1 mM of H2O2 and 0–50 μM of resveratrol. The protein abundance (expressed relative to untreated control cells) of Sirt1 was determined by western blots. The myoblasts were incubated in 0 or 1 mM of H2O2 and 0–50 μM of resveratrol. The protein bands was normalized to GAPDH/*P<0.05 significantly different from non-treatment control myoblasts (0 mM resveratrol and 0 mM and H2O2) at 6 or 12 h of incubation, respectively. †, P<0.05, Treatment group is significantly different from 0.1 mM or 1 mM H2O2 + 0 mM resveratrol at 24 or 48 hours of incubation.
Regulation of Sirt1 deacetylase activity oxidative stress
To confirm that resveratrol mediated ROS effects on Sirt1 in primary cultures, we isolated muscle stem cells (MSC) from mouse muscles. The pre-sort FACS data from isolated muscle stem cells (Figure 9A) show that 20.2% of the gated population of MSCs isolated from mouse gastrocnemius muscles expressed syndecan-4 (Syn4). The post-sort data show that the population was 97% pure. Figure 9B shows that Syn4 MSCs that were exposed to oxidative stress (H2O2) had lower Sirt1 activity and FK866 further reduced Sirt1 activity by ~40% relative to control conditions. Resveratrol restored Sirt1 activity in Syn4 positive MSCs, which protected the cells against oxidative stress-induced apoptosis. This likely occurred via a mechanism involving stabilization of mitochondria in part by increasing antioxidants to counter the effects of ROS inducing mitochondrial damage to induce apoptotic signaling.
Figure 9. Fluorescence Activated Cell Sorting (FACS) separation of the Syn4 population of MSCs from mouse muscle.
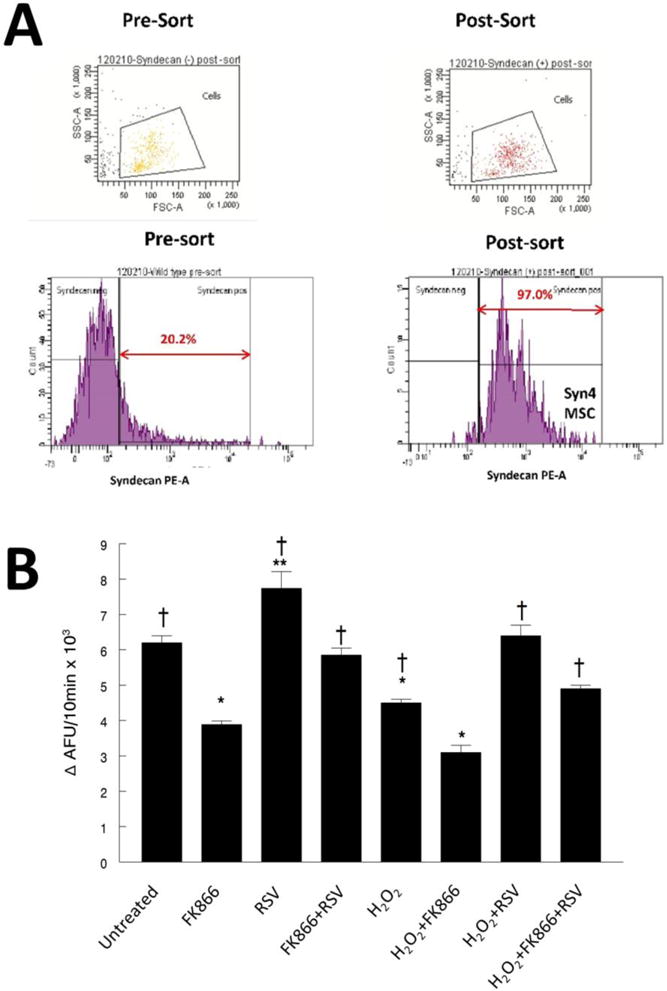
A. The pre-sort FACS data show that 20.2% of the gated population of MSC cells isolated from mouse gastrocnemius muscles expressed syndecan-4 (Syn4). The post-sort data show that the population was 97% pure. These represent a relevant muscle stem cell (MSC) population. Post-sort verification showed that 98.2% of the cells were negative for Syn4 in population sorted to be syndecan-4 negative (data not shown). Post-sort analysis of sorted cells which were identified from the 20.2% population of pre-sorted cells) as Syn4 positive, were 97.0% positive for Syn4. These data show that we have established the methods to isolate and evaluate Syn4 MSC cells from mouse skeletal muscles.
B. Sirt1 activity in Syn4 myotubes. Syn4 cells were examined in response to H2O2 (1 mM), and/or FK866 (50nM), and/or resveratrol for 48 hours. * P<0.05, significantly different from control untreated conditions. **, NMN treatment was greater than all other conditions. †, P<0.05, significantly different from FK866 treated cells.
Discussion
ROS mediated apoptosis
Cell cycle control and apoptosis are precisely regulated during myogenic differentiation [17,66,67], where single cell myoblasts fuse to form multinucleated myotubes. A large elevation in ROS is thought to impede myogenesis, and some reports suggest that this impact is greater in myoblasts than myotubes [24]. However, this is not a universal finding because apoptotic resistance has been reported to be greater in myotubes in some studies [22,67]. Similar to a report by Escobedo et al. [22], our data show that the loss of total cellular protein in myoblasts and myotubes occurred in a H2O2 dose dependent manner (Figure 1A). In this study, we show that myotubes were resistant to moderate and high ROS mediated H2O2-induced apoptosis and this is supported by the observation that myotubes lost less protein under high ROS conditions than myoblasts. This was confirmed by TUNEL staining data that showed greater DNA fragmentation and greater total protein loss after H2O2 treatment in myoblasts as compared with myotubes (Figure 1). Although H2O2-increased apoptotic signaling in both the intrinsic (mitochondrial) and extrinsic pathways, and also increased the effector caspase-3 activity in both myoblasts and myotubes relative to untreated control cells, (Figure 2, Supplemental Figures 1, 2, and 3) apoptotic signaling was greater in myoblasts than myotubes for the same concentration and exposure period to H2O2. Thus, our data show that myoblasts are more sensitive to changes in the ROS environment, and this has important implications for potentially reducing muscle repair or recovery by interfering with satellite cell/myoblast function under aging or disuse conditions, where the basal ROS levels are high [32,57].
Potential role of p21 in resistance to ROS-induced apoptosis
The level of proteins that are involved in cell cycle function and apoptosis are precisely regulated. For example, cyclin-cyclin dependent kinase (CDK) complexes tightly regulate the progression of the cell cycle, and the CDK inhibitor p21, controls cyclin-CDK complexes under a variety of environmental stresses [59]. The function of p21 is quite complex, in that it is involved in cell cycle regulation, differentiation, and apoptosis [59]. As such, ectopic p21 expression is correlated with apoptotic resistance in myotubes [67]. We speculated that if p21 localization and degradation was associated with apoptosis, the activation of apoptosis in myoblasts and myotubes should be associated with different apoptotic pathways. As our data showed that excessive ROS could initiate apoptosis and activate apoptosis associated proteins, the differences in ROS-induced apoptosis in myoblasts and myotubes may provide important insights into the mechanisms underlying greater apoptotic resistance in myotubes. Our novel data suggest that the greater p21 promoter activity and p21 protein abundance in myotubes (Figure 3) and increased co-localizations of p21 with nuclei as compared to myoblasts (Figure 4) is temporally associated with the increased resistance of myotubes to ROS-induced apoptosis. Our current data extends previous observations [67] by showing that p21 may regulate, at least in part, ROS-induced apoptotic resistance in myotubes. An important novel finding of our study was that the p21 promoter activity was decreased in a dose-dependent manner with H2O2 treatment in both myoblasts and myotubes. However, myotubes were more resistant to a H2O2-inhibition of the p21 promoter activity (Figure 3). Our results are consistent with previous reports that demonstrated a H2O2 mediated nuclear to cytoplasmic translocation of p21 via a nuclear export signal with p21 degradation [30]. Furthermore, Mirk-dependent p21 cytosolic translocation has been shown to block apoptosis in myoblasts during the myogenic differentiation [45]. Although experimental differences may exist between several published studies (i.e., different cell lines, treatment doses and periods of cell treatment), our current data together with previously published reports, suggest that cellular defenses against oxidative stress may include regulating p21 degradation and localization (i.e., nuclear vs. cytosolic). It is not clear at what levels p21 may directly or indirectly regulate both intrinsic and extrinsic apoptotic signaling pathways, but both pathways appear to be affected by H2O2 in myotubes and myoblasts. Furthermore, elevated levels of inhibitor of differentiation-2 (Id2) proteins in muscles of aged animals [4] have been implicated in sarcopenia and apoptosis [5]. We have previously speculated that the subcellular localization of Id2 may be important in regulating apoptosis in skeletal muscle [61,64]. Additionally, nuclear localization of Id2 is critical for the downregulation of p21 promoter activity [38] and in this study, we show that ROS-induced apoptosis is associated with a loss of nuclear p21 and a likely nuclear to cytoplasmic translocation of p21. We speculate that the higher levels of Id2 in muscle nuclei may invoke apoptosis in part through inhibition of p21 promoter activity [38]. The present study does not identify the mechanism whereby differences in ROS-associated apoptotic resistance are associated with the altered p21 expression levels, and addressing this will require addition experiments.
Resveratrol mediated ROS resistance in myoblasts
Given that excessive oxidative stress occurs with aging in many tissues including skeletal muscles [8,49,53], excessive ROS is likely involved in impaired myogenic differentiation in response to loading or injury repair. Lower populations of muscle stem cells (satellite cells) and increased apoptosis in nuclei and satellite cells of aged muscle has been suggested as one reason for the age associated suppression of muscle repair and may also contribute to sarcopenia [7]. Recent data suggested that a low dose of resveratrol could reduce H2O2 induced reductions in C2C12 muscle myoblast mobility; whereas a higher dose of resveratrol impaired myoblast regeneration and mitochondrial enzyme activity in culture [13]. Our lab and other investigators have found that in vivo, resveratrol of moderate doses improved muscle function [54,57], and reduced oxidative stress in skeletal muscle of old rodents [11,32,57]. Muscle function is highly dependent upon muscle stem cells (satellite cells/myoblasts) to regulate muscle mass, especially in response to injury and repair. To determine if resveratrol mediated a dose-dependent response on apoptotic signaling in C2C12 cells, a murine myoblasts line that is a model for satellite cells in vitro, we tested the impact of 0,10, 25 and 50 μM of resveratrol to mediate apoptotic responses to moderate (0.1 mM H2O2) and high ROS (1 mM H2O2) in C2C12 cells. In our study, we found that rather than reducing cell viability in response to a high ROS load as was found in another study [13], high concentrations of resveratrol reduced cell loss (therefore improving cell viability) relative to no treatment with resveratrol (Figure 5). This improved cell viability was at least partially accounted by a lower apoptotic index, and reduced Bax, Bax/Bcl-2 ratio, AIF, caspase-9 and caspase-3 activities and an increase in Bcl-2 protein abundance. This supports the idea that resveratrol treatment in high ROS conditions prevents mitochondrial-associated apoptosis signaling. The reduced apoptotic signaling likely occurred by reducing mitochondrial permeability to reduce caspase-9, Apaf1 and AIF levels, which would imply that mitochondrial “health” and integrity were improved. Although not tested in this study, a resveratrol-mediated improvement in mitochondria function may not have occurred through PGC1α or increased mitochondrial biogenesis. Rather we speculate that elevations of mitochondrial specific antioxidants such as MnSOD and GPx1 and by reducing H2O2 inside mitochondria, which we have shown to occur in muscles of aged rodents fed resveratrol [31]. This contrasts to the work of Bostti and Degens [13] who reported poorer mitochondrial function in high resveratrol treatments under correspondingly high ROS levels. Although the reason for the differences in observations between these studies is not known, we cannot rule out that potential variations in culture conditions (vendors for media and buffer composition, resveratrol sources etc.) could have partially accounted for these differences.
Resveratrol, Sirt1, and ROS
We have previously found that resveratrol treatment in vivo increased Sirt1, a putative resveratrol target that has been reported to have a role in several important physiological functions, including the regulation of oxidative stress. We found that resveratrol rescued the H2O2-mediated decrease in Sirt1 protein in myoblasts (Figure 8) and in muscle stem cells (Figure 9). This is consistent with observations that Sirt1 activation upregulates the transcription of antioxidant genes including MnSOD [48,65], and catalase [27,37,48] and reduces ROS-induced apoptosis [27]. In this study, we found that resveratrol treatment increased antioxidants catalase, GPx1, Supplemental Figure 2), and MnSOD (Supplemental Figure 3), in myoblasts exposed to ROS.
Our data suggest that the antioxidant buffering of ROS protected mitochondria against ROS-induced apoptosis. As mitochondrial damage (from ROS) is a primary initiator of apoptotic signaling, the data support the hypothesis that resveratrol mediated a Sirt1 induced antioxidant protection for mitochondria that reduced mitochondrial apoptotic signaling (Figures 5–7, Supplemental Figure 2).
Conclusion
Our results show that high ROS loads are better tolerated in myotubes than in myoblasts, but both cells undergo mitochondrial-associated apoptotic signaling and cell loss, as a result of a high ROS load, which presumably damages mitochondria to initiate apoptotic signaling. Furthermore, our data show that resistance to oxidative stress might involve modulation of p21 promoter activity in myotubes vs. myoblast because the p21 promoter is regulated in a dose dependent manner in both myoblasts and myotubes by H2O2. Furthermore, proteins that were not assessed in this study such as the role(s) that small heat shock proteins may play regulating apoptosis the differentiated myoblasts [22,35], has not been fully defined under conditions of high ROS. Finally, we have found that resveratrol is protective against ROS damage in part by increasing antioxidant production via a Sirt1 mediated mechanism, which suppresses apoptotic signaling from intrinsic and extrinsic mediated pathways, although the intrinsic mitochondrial pathway appears to be more important in myoblasts under a high ROS load. Our data are consistent with studies that have shown that resveratrol mediates antioxidant and anti-apoptotic protection against pathologies including renal cell damage [70], ischemia in neural cells [44], and ischemia-reperfusion in cardiac cells [71], and skeletal muscle cells [18]. We cannot rule out the possibility that myoblasts and myotubes may have different capabilities or regulation of their antioxidant systems with or without resveratrol treatment [22]. Although Sirt1 is strongly implicated, additional work is warranted to determine if Sirt1 is required for resveratrol to confer protection against ROS induced myoblast survival and apoptosis signaling in myoblasts, and to determine if Sirt1 is essential for regulating the resistance to ROS damage by myotubes as compared to myoblasts or perhaps if this is via a Sirt1-p21 mediated mechanism. Nevertheless, our data in muscle myoblasts and isolated mouse muscle stem cells are consistent with reports from other cell lines that support the probability that protection against a high ROS load by resveratrol is likely Sirt1 dependent. Furthermore, resveratrol may be a key nutritional approach that will improve muscle function, reduce frailty [54], and improve muscle repair in aging.
Supplementary Material
Acknowledgments
This work was partially supported by a WVU PSCoR grant to S.E. Alway. We also would like to acknowledge the West Virginia University Microscope Imaging Facility, which is supported by the Mary Babb Randolph Cancer Center and NIH grant 5P20RR016440, P30RR032138 and P20RR016477 and the WVU Flow Cytometry & Single Cell Core, which is supported by GM103488/RR032138, GM104942, GM103434, and Fortessa S10 grant: OD016165. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alamdari N, Aversa Z, Castillero E, Gurav A, Petkova V, Tizio S, et al. Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochem Biophys Res Commun. 2012;417:528–533. doi: 10.1016/j.bbrc.2011.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 3.Alway SE, Bennett BT, Wilson JC, Sperringer J, Mohamed JS, Edens NK, et al. Green tea extract attenuates muscle loss and improves muscle function during disuse, but fails to improve muscle recovery following unloading in aged rats. J App Physiol. 2015;118:319–330. doi: 10.1152/japplphysiol.00674.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol. 2002;283:C66–C76. doi: 10.1152/ajpcell.00598.2001. [DOI] [PubMed] [Google Scholar]
- 5.Alway SE, Degens H, Lowe DA, Krishnamurthy G. Increased myogenic repressor Id mRNA and protein levels in hindlimb muscles of aged rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R411–R422. doi: 10.1152/ajpregu.00332.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alway SE, McCrory JL, Kearcher K, Vickers A, Frear B, Gilleland DL, et al. Resveratrol enhances exercise-induced cellular and functional adaptions of skeletal muscle in older men and women. J Gerontol A Biol Sci Med Sci. 2017 doi: 10.1093/gerona/glx089. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alway SE, Siu PM. Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev. 2008;36:51–57. doi: 10.1097/JES.0b013e318168e9dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asano S, Rice KM, Kakarla S, Katta A, Desai DH, Walker EM, et al. Aging influences multiple indices of oxidative stress in the heart of the Fischer 344/NNia x Brown Norway/BiNia rat. Redox Rep. 2007;12:167–180. doi: 10.1179/135100007X200254. [DOI] [PubMed] [Google Scholar]
- 9.Barnouin K, Dubuisson ML, Child ES, Fernandez dM, Glassford J, Medema RH, et al. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J Biol Chem. 2002;277:13761–13770. doi: 10.1074/jbc.M111123200. [DOI] [PubMed] [Google Scholar]
- 10.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett BT, Mohamed JS, Alway SE. Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS One. 2013;8:e83518. doi: 10.1371/journal.pone.0083518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boncoeur E, Tabary O, Bonvin E, Muselet C, Fritah A, Lefait E, et al. Oxidative stress response results in increased p21WAF1/CIP1 degradation in cystic fibrosis lung epithelial cells. Free Radic Biol Med. 2006;40:75–86. doi: 10.1016/j.freeradbiomed.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Bosutti A, Degens H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci Rep. 2015;5:8093. doi: 10.1038/srep08093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 15.Butler DC, Haramizu S, Williamson DL, Alway SE. Phospho-ablated Id2 is growth suppressive and pro-apoptotic in proliferating myoblasts. PLoS One. 2009;4:e6302. doi: 10.1371/journal.pone.0006302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C-mediated caspase-3 activation pathway. Diabetes. 2002;51:1938–1948. doi: 10.2337/diabetes.51.6.1938. [DOI] [PubMed] [Google Scholar]
- 17.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, Di S, Fan C, Cai L, Gao C, Jiang P, et al. SIRT1 activation by pterostilbene attenuates the skeletal muscle oxidative stress injury and mitochondrial dysfunction induced by ischemia reperfusion injury. Apoptosis. 2016;21:905–916. doi: 10.1007/s10495-016-1258-x. [DOI] [PubMed] [Google Scholar]
- 19.Conboy MJ, Cerletti M, Wagers AJ, Conboy IM. Immuno-analysis and FACS sorting of adult muscle fiber-associated stem/precursor cells. Methods Mol Biol. 2010;621:165–173. doi: 10.1007/978-1-60761-063-2_11. [DOI] [PubMed] [Google Scholar]
- 20.Di Filippo ES, Mancinelli R, Pietrangelo T, La Rovere RM, Quattrocelli M, Sampaolesi M, et al. Myomir dysregulation and reactive oxygen species in aged human satellite cells. Biochem Biophys Res Commun. 2016;473:462–470. doi: 10.1016/j.bbrc.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escobedo J, Pucci AM, Koh TJ. HSP25 protects skeletal muscle cells against oxidative stress. Free Radic Biol Med. 2004;37:1455–1462. doi: 10.1016/j.freeradbiomed.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci U S A. 2002;99:11025–11030. doi: 10.1073/pnas.162172899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27:1122–1132. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- 25.Fulle S, Sancilio S, Mancinelli R, Gatta V, Di PR. Dual role of the caspase enzymes in satellite cells from aged and young subjects. Cell Death Dis. 2013;4:e955. doi: 10.1038/cddis.2013.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen JM, Klass M, Harris C, Csete M. A reducing redox environment promotes C2C12 myogenesis: implications for regeneration in aged muscle. Cell Biol Int. 2007;31:546–553. doi: 10.1016/j.cellbi.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa K, Wakino S, Yoshioka K, Tatematsu S, Hara Y, Minakuchi H, et al. Sirt1 protects against oxidative stress-induced renal tubular cell apoptosis by the bidirectional regulation of catalase expression. Biochem Biophys Res Commun. 2008;372:51–56. doi: 10.1016/j.bbrc.2008.04.176. [DOI] [PubMed] [Google Scholar]
- 28.Hsu CP, Odewale I, Alcendor RR, Sadoshima J. Sirt1 protects the heart from aging and stress. Biol Chem. 2008;389:221–231. doi: 10.1515/BC.2008.032. [DOI] [PubMed] [Google Scholar]
- 29.Hsu YJ, Hsu SC, Hsu CP, Chen YH, Chang YL, Sadoshima J, et al. Sirtuin 1 protects the aging heart from contractile dysfunction mediated through the inhibition of endoplasmic reticulum stress-mediated apoptosis in cardiac-specific Sirtuin 1 knockout mouse model. Int J Cardiol. 2017;228:543–552. doi: 10.1016/j.ijcard.2016.11.247. [DOI] [PubMed] [Google Scholar]
- 30.Hwang CY, Kim IY, Kwon KS. Cytoplasmic localization and ubiquitination of p21(Cip1) by reactive oxygen species. Biochem Biophys Res Commun. 2007;358:219–225. doi: 10.1016/j.bbrc.2007.04.120. [DOI] [PubMed] [Google Scholar]
- 31.Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66:751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson JR, Ryan MJ, Hao Y, Alway SE. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1572–R1581. doi: 10.1152/ajpregu.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji LL. Antioxidant signaling in skeletal muscle: a brief review. Exp Gerontol. 2007;42:582–593. doi: 10.1016/j.exger.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 35.Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- 36.Kazama K, Okada M, Yamawaki H. Adipocytokine, omentin inhibits doxorubicin-induced H9c2 cardiomyoblasts apoptosis through the inhibition of mitochondrial reactive oxygen species. Biochem Biophys Res Commun. 2015;457:602–607. doi: 10.1016/j.bbrc.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 37.Kim HN, Han L, Iyer S, de CR, Zhao H, O’Brien CA, et al. Sirtuin1 Suppresses Osteoclastogenesis by Deacetylating FoxOs. Mol Endocrinol. 2015;29:1498–1509. doi: 10.1210/me.2015-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim NS, Kim HJ, Koo BK, Kwon MC, Kim YW, Cho Y, et al. Receptor activator of NF-kappaB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol Cell Biol. 2006;26:1002–1013. doi: 10.1128/MCB.26.3.1002-1013.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J Biol Chem. 2013;288:30515–30526. doi: 10.1074/jbc.M113.489716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leloup C, Magnan C, Benani A, Bonnet E, Alquier T, Offer G, et al. Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes. 2006;55:2084–2090. doi: 10.2337/db06-0086. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Colavitti R, Rovira II, Finkel T. Redox-dependent transcriptional regulation. Circ Res. 2005;97:967–974. doi: 10.1161/01.RES.0000188210.72062.10. [DOI] [PubMed] [Google Scholar]
- 42.Lu TM, Tsai JY, Chen YC, Huang CY, Hsu HL, Weng CF, et al. Downregulation of Sirt1 as aging change in advanced heart failure. J Biomed Sci. 2014;21:57. doi: 10.1186/1423-0127-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maesner CC, Almada AE, Wagers AJ. Established cell surface markers efficiently isolate highly overlapping populations of skeletal muscle satellite cells by fluorescence-activated cell sorting. Skelet Muscle. 2016;6:35. doi: 10.1186/s13395-016-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng Z, Li J, Zhao H, Liu H, Zhang G, Wang L, et al. Resveratrol relieves ischemia-induced oxidative stress in the hippocampus by activating SIRT1. Exp Ther Med. 2015;10:525–530. doi: 10.3892/etm.2015.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercer SE, Ewton DZ, Deng X, Lim S, Mazur TR, Friedman E. Mirk/Dyrk1B mediates survival during the differentiation of C2C12 myoblasts. J Biol Chem. 2005;280:25788–25801. doi: 10.1074/jbc.M413594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25:3646–3660. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- 47.Montesano A, Luzi L, Senesi P, Mazzocchi N, Terruzzi I. Resveratrol promotes myogenesis and hypertrophy in murine myoblasts. J Transl Med. 2013;11:310. doi: 10.1186/1479-5876-11-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olmos Y, Sanchez-Gomez FJ, Wild B, Garcia-Quintans N, Cabezudo S, Lamas S, et al. SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1alpha complex. Antioxid Redox Signal. 2013;19:1507–1521. doi: 10.1089/ars.2012.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pansarasa O, Castagna L, Colombi B, Vecchiet J, Felzani G, Marzatico F. Age and sex differences in human skeletal muscle: role of reactive oxygen species. Free Radic Res. 2000;33:287–293. doi: 10.1080/10715760000301451. [DOI] [PubMed] [Google Scholar]
- 50.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 52.Pistilli EE, Jackson JR, Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis. 2006;11:2115–2126. doi: 10.1007/s10495-006-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rice KM, Preston DL, Walker EM, Blough ER. Aging influences multiple incidices of oxidative stress in the aortic media of the Fischer 344/NNiaxBrown Norway/BiNia rat. Free Radic Res. 2006;40:185–197. doi: 10.1080/10715760500464957. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Bies E, Tung BT, Navas P, Lopez-Lluch G. Resveratrol primes the effects of physical activity in old mice. Br J Nutr. 2016;116:979–988. doi: 10.1017/S0007114516002920. [DOI] [PubMed] [Google Scholar]
- 55.Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- 56.Russell ST, Wyke SM, Tisdale MJ. Mechanism of induction of muscle protein degradation by angiotensin II. Cell Signal. 2006;18:1087–1096. doi: 10.1016/j.cellsig.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 57.Ryan MJ, Jackson JR, Hao Y, Williamson CL, Dabkowski ER, Hollander JM, et al. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65:815–831. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandiford SD, Kennedy KA, Xie X, Pickering JG, Li SS. Dual oxidase maturation factor 1 (DUOXA1) overexpression increases reactive oxygen species production and inhibits murine muscle satellite cell differentiation. Cell Commun Signal. 2014;12:5. doi: 10.1186/1478-811X-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 60.Sin TK, Yu AP, Yung BY, Yip SP, Chan LW, Wong CS, et al. Effects of long-term resveratrol-induced SIRT1 activation on insulin and apoptotic signalling in aged skeletal muscle. Acta Diabetol. 2015;52:1063–1075. doi: 10.1007/s00592-015-0767-3. [DOI] [PubMed] [Google Scholar]
- 61.Siu PM, Alway SE. Id2 and p53 participate in apoptosis during unloading-induced muscle atrophy. Am J Physiol Cell Physiol. 2005;288:C1058–C1073. doi: 10.1152/ajpcell.00495.2004. [DOI] [PubMed] [Google Scholar]
- 62.Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2005;288:C338–C349. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- 63.Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2005;288:C338–C349. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- 64.Siu PM, Pistilli EE, Murlasits Z, Alway SE. Hindlimb unloading increases muscle content of cytosolic but not nuclear Id2 and p53 proteins in young adult and aged rats. J Appl Physiol. 2006;100:907–916. doi: 10.1152/japplphysiol.01012.2005. [DOI] [PubMed] [Google Scholar]
- 65.Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, et al. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang J, Guo K, Wills KN, Walsh K. Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res. 1997;57:351–354. [PubMed] [Google Scholar]
- 67.Wang J, Walsh K. Resistance to apoptosis conferred by Cdk inhibitors during myocyte differentiation. Science. 1996;273:359–361. doi: 10.1126/science.273.5273.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br J Cancer. 2004;91:1742–1750. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wyke SM, Tisdale MJ. Induction of protein degradation in skeletal muscle by a phorbol ester involves upregulation of the ubiquitin-proteasome proteolytic pathway. Life Sci. 2006;78:2898–2910. doi: 10.1016/j.lfs.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 70.Xiao Z, Chen C, Meng T, Zhang W, Zhou Q. Resveratrol attenuates renal injury and fibrosis by inhibiting transforming growth factor-beta pathway on matrix metalloproteinase 7. Exp Biol Med (Maywood) 2016;241:140–146. doi: 10.1177/1535370215598401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamoto T, Tamaki K, Shirakawa K, Ito K, Yan X, Katsumata Y, et al. Cardiac Sirt1 mediates the cardioprotective effect of caloric restriction by suppressing local complement system activation after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2016;310:H1003–H1014. doi: 10.1152/ajpheart.00676.2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


