Abstract
Introduction
Thrombelastography Platelet Mapping (TEG-PM) is a useful assay to assess antiplatelet therapy. Inhibited response to the ADP receptor on platelets occurs early following injury but recent work suggests this alteration occurs even with minor trauma. However, the utility of TEG-PM, specifically the percent of ADP receptor inhibition (%ADP-INH), in predicting outcomes and guiding platelet transfusion in trauma-induced coagulopathy (TIC) remains unknown. We assessed the role of %ADP-INH in predicting survival, requirement for massive transfusion or platelet transfusion in patients at risk for TIC.
Methods
TEG-PM was assessed in 303 trauma activation patients from 2014–2016 and in 89 healthy volunteers. %ADP-INH is presented as median and interquartile range (IQR). We compared the area under the receiver operating characteristic curve (AUROC) of %ADP-INH, platelet count, and rTEG Maximum amplitude (MA) for in-hospital mortality, massive transfusion (>10 RBC or death/6 hours, and platelet transfusion (>0 platelet units or death/6hrs).
Results
Overall, 35 (11.5%) patient died, 27 (8.9%) required massive transfusion and 46, platelet transfusions (15.2%). Median %ADP-INH was 42.5% (IQR: 22.4–69.1%), compared to 4.3 % (IQR: 0–13.5%) in healthy volunteers (p<0.0001). Patients that died, had a massive transfusion, or platelet transfusion had higher %ADP-INH than those that did not (p<0.05 for all). However, %ADP-INH did not add significantly to the predictive performance of MA or platelet count for any of the three outcomes, after adjustment for confounders. Subgroup analyses by severe traumatic brain injury, severe injury and requirement of RBCs showed similar results.
Conclusion
ADP receptor inhibition did not add predictive value to predicting mortality, massive transfusion, or platelet transfusion. Thus, the role of TEG-PM as a solitary tool to guide platelet transfusions in trauma requires continued refinement.
Keywords: platelet dysfunction, trauma-induced coagulopathy, massive transfusion, platelet transfusion, TEG-PM, platelet mapping
Introduction
Uncontrolled hemorrhage is the leading causes of preventable death from trauma, and occurs predominantly within two hours of injury [1]. An endogenous trauma-induced coagulopathy (TIC) is the driving mechanism and, if present at admission, is associated with a fourfold increase in mortality [2]. TIC is a multifocal process attributed to reduced thrombin generation, fibrinogen depletion, platelet dysfunction, and systemic fibrinolysis [3–5]. Consequently, early blood component administration is now standard in patients at risk for TIC [5]. However, the optimal composition of blood components remains debated. A large multicenter randomized controlled trial, PROPPR, indicated no survival benefit of empiric immediate platelet transfusion [6].
Our recent goal-directed resuscitation trial based on viscoelastic assays indicated a 50% reduction in mortality, but current thrombelastography (TEG) and thromboelastometry (ROTEM) assays do not include a measurement specific for platelet transfusion [7]. While these devices are capable of measuring platelet receptor inhibition to monitor antiplatelet therapy [8], their role in guiding platelets transfusions for TIC remains unclear. The current TEG-PM assays represent the responses to arachidonic acid (AA), which serves as substrate for cyclooxygenase dependent thromboxane A2 that binds to the thromboxane (TP) receptor, or adenosine diphosphate (ADP), which binds to platelet P2Y1 and P2Y12 receptors [9]. ADP is released from the dense granules of platelets. Simplistically, the inner core of a blood clot (primary hemostasis) is composed of activated platelets in an environment abundant in thrombin and fibrin; whereas, platelet released TXA2 and ADP are believed to be important in the formation of the subsequent outer core of less active platelets [9,10].
Our previous work has shown that inhibited platelet response to AA following trauma is non-specific [3]. Consequently, our group and others have focused on the ADP response in TEG-PM to determine its role in guiding platelet transfusion [3,11]. While inhibited platelet response to ADP following injury has been suggested to represent an exhausted platelet, recent work does not support this concept [12]. Specifically, trauma patients maintain their dense granules even in the presence of ADP receptor inhibition contradicting the theory of platelet granule exhaustion as the etiology for platelet dysfunction [12]. Furthermore, other studies have documented a significant inhibited response to ADP following minor trauma, suggesting receptor status may be a biomarker of injury rather than a mediator of TIC [13]. Therefore, we propose to assess the role of platelet ADP receptor dysfunction, measured by TEG –PM mapping in predicting mortality, requirement of massive transfusion and platelet transfusion among patients at risk for TIC.
Methods
Study Design
This is an analysis of prospectively collected data from our Trauma Activation Protocol from 2014 to 2016 database (TAP database), which includes patients who met criteria for the highest level of trauma team activation at Denver Health Medical Center (DHMC), an American College of Surgeons verified and Colorado state certified Level 1 trauma center affiliated with the University of Colorado Denver. The Colorado Multiple Institutional Review Board (COMIRB) approved all studies included in the TAP database. We did not exclude patients who were taking medications affecting coagulation or platelet function, as this information is rarely available or reliable upon admission.
Clinical data were collected by trained research professional assistants (PRAs) and included: age, gender, mechanism, body mass index (BMI), new injury severity score (NISS), field and hospital arrival systolic blood pressure (SBP), heart rate (HR), Glasgow Coma Scale (GCS), international normalized ratio (INR), partial thromboplastin time (PTT), fibrinogen, base deficit (BD), as well as number of units of blood products transfused (red blood cells (RBCs), fresh frozen plasma (FFP), platelets, cryoprecipitate) and volume of crystalloid infused. Severe traumatic brain injury (TBI) was defined as AIS-Head greater than or equal to 3.
Outcome variables: The outcomes of this study were: 1) in-hospital death within 28 days postinjury; 2) massive transfusion, defined as greater than 10 units of RBCs or death within 6 hours, [3,4–6]; and 3) platelet transfusion: defined as platelet transfusion or death within 6 hours of injury. The death criterion was added to both massive transfusion and platelet transfusion to minimize survivor bias (i.e., non-survivors did not have the “opportunity” to receive transfusions).
Main effect variables: the main effects in this study were: 1) % ADP INH: 2) platelet count; 3) rTEG MA (MA). All these variables were obtained within 1 hour after injury.
The protocol for massive transfusion of blood products has been described previously and includes initial empiric initial transfusion of FFP:RBC 1:2 [14] followed by a standardized rTEG guided hemostatic resuscitation [15]. Transfusion was triggered if the patient was perceived to have ongoing blood loss and had the following TEG-derive parameters; FFP for ACT>128, cryoprecipitate for Angle <65, platelets for MA < 55 and, tranexamic acid for LY30>5%, as described before [15]. PRBCs were transfused to maintain a hemoglobin at least 10g/dL while bleeding was ongoing. Massive transfusion was stopped as clinically indicated, once control of bleeding was achieved and the patient was hemodynamically stable.
Blood Samples from Healthy Volunteers
Trained study staff collected samples from volunteers at an outpatient clinic after obtaining an informed consent. The study was open to patients and hospital staff not taking antiplatelet or anticoagulant medications. Volunteers with diabetes, morbid obesity, renal disease, or liver disease were excluded. Blood samples were collected in tubes containing sodium citrate (3.5mL, 3.2% sodium citrate, Greiner Bio-One) or lithium heparin (4.0mL, Greiner Bio-One).
Blood Samples from Trauma Patients
Samples were collected during trauma activations in the field by trained paramedics or upon arrival to Emergency Department (ED) to evaluate the role of TEG in the management injury. Samples were collected in tubes containing sodium citrate and lithium heparin. A team of trained PRAs on 24/7 schedule for prospective studies completed viscoelastic assays within two hours after blood collection. Citrated blood samples were analyzed using the TEG 5000 Thrombelastography Hemostasis Analyzer (Haemonetics, Niles, IL, USA). The following indices were obtained from the tracings of the TEG: activated clotting time (ACT [sec]), angle (°), maximum amplitude (MA [mm]), and lysis 30 min after MA (LY30 [%]).
TEG Platelet Mapping
Whole blood collected in heparin (19 U mL−1) was analyzed with the TEG Platelet Mapping assay (Haemonetics, Niles, IL, USA)), and was mixed with a solution containing reptilase and FXIIIa to stimulate thrombin independent fibrin polymerization, and then activated with 2 milimolar ADP. The percent ADP receptor inhibition (%ADP-INH) was used to quantify platelet dysfunction.
Statistical Analysis
Continuous variables were expressed as median and interquartile range (IQR) and the Wilcoxon non-parametric test was used for univariate comparisons. The Chi-square and Fisher Exact tests were used to compare categorical variables. Covariates with p value <0.01 on univariate analysis and with minimal collinearity (Rho<|0.30|) were entered into logistic regression models for the defined outcomes and significant covariates were identified using stepwise selection. The performance of these models was assessed via measures of model discrimination (area under the receiver operating characteristic curves, AUROC, with 95% confidence intervals, 95% CI) and calibration (Hosmer-Lemeshow statistics, in which higher p-values indicate better calibration). We then added the three effects of interest (%) to these models and compared the area under the receiver operating characteristic curves (AUROC) for the above described outcomes using the DeLong et al method [16]. We repeated these analyses in the subgroups with severe injuries (NISS>=25), with TBI (AIS head/neck >=3) and assessed the predictive performance of %ADP-INH and platelet count among patients with MA<=55mm. The optimal cutoffs for %ADP-INH, platelet count and TEG MA were estimated by the maximum value of the Youden Index (sensitivity + specificity -1) calculated using the ROC curves described above.
Sample size and power: assuming a 50% positive test (for %ADPINH, platelet count and rTEG MA), a moderate baseline AUROC of 0.72, and a higher than anticipated correlation of 0.60 or less between the effects of interest, a sample of 303 patients achieved 80% power to detect a difference of 0.09 in the AUROCs using a two-sided at a significance level of 0.05 (PASS 14 Power Analysis and Sample Size Software (2015). NCSS, LLC. Kaysville, Utah, USA). All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). All tests were two-tailed with significance declared at p<0.05.
Results
Patient Characteristics
Of 436 consecutive trauma activation patients enrolled in our trauma activation database from 2014 to 2016, 303 (69.5%) had %ADP-INH measured within one hour of injury. Table 1 depicts the characteristics and injury severity for all patients and stratified by the three major outcomes (in-hospital death, massive transfusion, platelet transfusion). Overall, these were severely injured patients with a median NISS of 17 and base deficit (BD) −6 mEq/L. Mortality was 11.6%, massive transfusion was required in 8.9% and platelet transfusion in 15.2%. Patients with these adverse outcomes had significantly higher %ADP-INH, lower platelet count and lower rTEG MA, as well as worse levels of other indicators of injury severity (Table 1).
Table 1.
Demographics of Patients Trauma Activation Patients with Platelet Function Measured
| Variable | All patients n=303 |
Survivor | Non–Survivor | MTx or death/6 hrs | No MTx | Platelet Tx or death/6 hrs | No Platelet Tx |
|---|---|---|---|---|---|---|---|
| n=268 | n=35 | n=27 | n=276 | n=46 | n=257 | ||
| Age (years) | 34.1 (26.1–48.7) | 34.2 (258–48.1) | 36.8 (28–54) | 28.3 (25–48.7) | 34.4 (26.1–48.5) | 33.9 (25.2–51.7) | 34.1 (26.1–48) |
| Male | 78.22% | 77.99% | 80% | 74.07% | 78.62% | 69.57% | 79.77% |
| BMI (kg/m2) | 26.31 (23.86–30.15) | 26.3 (23.8–30.3) | 26 (23.9–30.2) | 25.9 (24.2–29.4) | 26.3 (23.8–30.6) | 26.3 (24.2–28.4) | 26.4 (23.8–30.7) |
| Blunt Mechanism | 54.79% | 53.36% | 65.71% | 40.74% | 56.16% | 60.87% | 53.70% |
| NISS | 17 (6–34) | 17 (5–29)* | 50 (38–59) | 50 (34–57)** | 17 (5–29) | 50 (34–59)*** | 17 (4.5–27) |
| TBI | 26.40% | 12.69%* | 54.29% | 22.22% | 17.03% | 28.26%*** | 15.56% |
| Max AIS Head and Neck | 0 (0–3) | 0 (0–2)* | 5 (0–5) | 0 (0–3) | 0 (0–3) | 0 (0–5)### | 0 (0–2) |
| Max AIS Chest | 0 (0–3) | 0 (0–0)# | 3 (0–3) | 3 (0–5)** | 0 (0–3) | 3 (0–4)*** | 0 (0–3) |
| Max AIS Abdomen and Pelvis | 0 (0–2) | 0 (0–2) | 0 (0–2) | 2 (0–4)** | 0 (0–2) | 0 (0–3)### | 0 (0–2) |
| Max AIS Extremities | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–4) | 0 (0–2) | 2 (0–4)### | 0 (0–2) |
| ED SBP (mmHg) | 117 (90–140) | 118 (92–140)# | 104 (58–136) | 75 (0–90)** | 121 (92–140) | 82 (55–110)*** | 122 (94–140) |
| ED HR (BPM) | 100 (78–116) | 100 (83–115) | 77 (53–119) | 116.5 (63–133) | 99.5 (80–114) | 112 (70–130) | 100 (81–113) |
| ED GCS | 15 (7–15) | 15 (12–15)* | 3 (3–3) | 3 (3–13)** | 15 (10–15) | 3 (3–14)*** | 15 (11–15) |
| ED Temp (Celsius) | 36.6 (36.3–36.8) | 36.6 (36.3–36.8) | 36 (35.7–36.7) | 36 (36–36.6) | 36.6 (36.3–36.9) | 36.4 (36–36.6)### | 36.6 (36.3–36.9) |
| Calcium (mg/dL) | 8.1 (7.6–8.5) | 8.1 (7.6–8.5) | 7.9 (7.3–8.7) | 8.3 (7.5–9.3) | 8 (7.6–8.5) | 7.9 (7.2–8.6) | 8.1 (7.6–8.5) |
| Lactate (mg/dL) | 3.5 (2.6–5.8) | 3.4 (2.4–5.3)# | 4.3 (3–8.7) | 10.1 (4.9–16)** | 3.3 (2.4–5.2) | 5.1 (3.6–10.9)*** | 3.3 (2.4–5.2) |
| Base Deficit (BD) | −6 (−3 to −10) | −6 (−3 to −9)# | −8 (−5 to −13) | −15 (−10.8 to −20)** | −6 (−3 to −9) | −9.9 (−7 to −17)*** | −5.3 (−3 to −9) |
| Platelet Count (10*9/L) | 257 (205–309) | 261 (212–311)* | 192 (168–259) | 143 (86–205)** | 261 (214–310) | 181.5 (114–232)*** | 264 (220–315) |
| INR | 1.1 (1.02–1.23) | 1.1 (1–1.2)* | 1.4 (1.1–1.8) | 1.7 (1.4–2.1)** | 1.1 (1–1.2) | 1.4 (1.3–1.8)*** | 1.1 (1–1.2) |
| PTT (seconds) | 27.5 (24.6–31.4) | 27 (24.3–29.7)* | 38.7 (32.1–47.3) | 46.1 (38.2–61.3)** | 27.1 (24.3–30.2) | 38.3 (31.2–47.7)*** | 26.8 (24.1–29.5) |
| Fibrinogen (mg/dL) | 196 (144–263) | 207 (155–267)# | 141 (98–158) | 134 (98–178)** | 205 (158–265) | 134 (106–193)*** | 216 (162–267) |
| D-Dimer (ng/mL) | 3.36 (1.24–17.19) | 2.8 (0.8–17.2) | 10 (3.4–20) | 15.5 (3.9–20)** | 2.5 (0.8–11) | 17.7 (5.5–20)*** | 2.2 (0.6–7.5) |
| %ADP-INH (%) | 42.5 (22.4–69.1)# | 38.7 (21.5–67.2) | 59.4 (36.4–82.5) | 65.3 (33.7–92.4)** | 39.2 (21.5–66.2) | 72.9 (46.2–92.4)*** | 36.6 (20.5–60.7) |
| r-TEG ACT (seconds) | 121 (113–136)# | 121 (113–128) | 128 (113–152) | 136 (121–160)** | 121 (113–128) | 128 (121–152)*** | 121 (113–128) |
| r-TEG Angle (degrees) | 71.9 (66–75.6)* | 72.3 (66.5–75.6) | 67.3 (57.1–72.7) | 60.8 (47–68.6)** | 72.5 (66.9–75.6) | 62.9 (53.8–70.3)*** | 72.7 (67.2–75.6) |
| r-TEG MA (mm) | 62 (56.5–66.5)* | 62.5 (57.5–67) | 59 (41–62) | 47.5 (32.5–54.5)** | 62.5 (58–66.8) | 51 (40–57.5)*** | 63.5 (58.5–67.5) |
| r-TEG LY30 (%) | 1.8 (0.9–2.9) | 1.9 (1–2.9) | 1.1 (0.1–3.1) | 2.2 (0.1–24.4) | 1.8 (1–2.8) | 1.7 (0.5–4)*** | 1.8 (1–2.8) |
Demographic characteristics of all patients and patients stratified by the three major outcomes (in-hospital death, massive transfusion, platelet transfusion). Data are presented as median with lower and upper quartile in parentheses or as percentage (%).
=p<0.01,
=p<0.05 comparing survivors to non-survivors.
=p<0.01 comparing Massive Tx to no massive Tx.
=p<0.01,
=p<0.05 comparing platelet tx to no platelet tx. Abbreviations: BMI (body mass index), NISS (new injury severity score) AIS (abbreviated injury scale), ED (Emergency Department), SBP (systolic blood pressure), HR (heart rate), GCS (Glasgow Coma Scale), INR (International Normalized Ratio), PTT (Partial Thromboplastin Time), r-TEG (Rapid Thrombelastography), ACT (Activated Clotting Time), MA (Maximum Amplitude).
Comparison between %ADP-INH, platelet count and rTEG-MA as predictors of death, massive transfusion and platelet transfusion, adjusted for significant covariates
Table 2 shows the significant covariates for the three selected outcomes. The models had excellent discrimination as indicated by the AUROCs and calibration (Hosmer-Lemeshow statistics with high p-values). The three effects of interest were then added to these models initially one at a time (Table 3). Regarding death and massive transfusion, %ADP-INH, platelet count and MA did not add to the predictive performance of the predictors shown in Table 2. However, both platelet count and MA significantly added to the other covariates in predicting the need for platelet transfusion, in contrast to %ADP-INH, which did not improve prediction.
Table 2.
Predictive Models of Post Injury Death, Massive Transfusion and Platelet Transfusion
| Estimate | Standard Error | Wald Chi-Square | Pr > Chi Sq | Odds ratios | 95% Confidence Limits | ||
|---|---|---|---|---|---|---|---|
| Outcome=death | |||||||
| Intercept | −7.1435 | 1.0717 | 44.4295 | <.0001 | |||
| TBI | 1.7151 | 0.6184 | 7.691 | 0.0055 | 5.557 | 1.654 | 18.674 |
| NISS | 0.0581 | 0.0151 | 14.85 | 0.0001 | 1.06 | 1.029 | 1.092 |
| INR | 1.6715 | 0.4263 | 15.3713 | <.0001 | 5.32 | 2.307 | 12.269 |
| AUROC (95% CI) | 0.9177 (0.8773–0.9581) | ||||||
| Hosmer-Lemeshow Statistics p-value | 0.9325 | ||||||
| Outcome: massive transfusion | |||||||
| Intercept | −11.763 | 2.1709 | 29.3595 | <.0001 | |||
| NISS | 0.0616 | 0.0201 | 9.4147 | 0.0022 | 1.064 | 1.023 | 1.106 |
| Base Deficit | −0.2295 | 0.0661 | 12.0508 | 0.0005 | 0.795 | 0.698 | 0.905 |
| PTT | 0.1396 | 0.0404 | 11.9478 | 0.0005 | 1.15 | 1.062 | 1.244 |
| AUROC (95% CI) | 0.9697 (0.9475–0.992) | ||||||
| Hosmer-Lemeshow Statistics p-value | 0.9991 | ||||||
| Outcome=platelets transfusion | |||||||
| Intercept | −8.252 | 1.2094 | 46.5586 | <.0001 | |||
| NISS | 0.0596 | 0.0134 | 19.7262 | <.0001 | 1.061 | 1.034 | 1.09 |
| Base Deficit | −0.1395 | 0.0454 | 9.4283 | 0.0021 | 0.87 | 0.796 | 0.951 |
| PTT | 0.105 | 0.0297 | 12.5024 | 0.0004 | 1.111 | 1.048 | 1.177 |
| AUROC (95% CI) | 0.923 (0.8797–0.9663) | ||||||
| Hosmer-Lemeshow Statistics p-value | 0.722 | ||||||
Covariates with p value <0.01 on univariate analysis and with minimal co-linearity (Rho<|0.30|) were entered into logistic regression models for the defined outcomes and significant covariates were identified using stepwise selection. The performance of these models was assessed via measures of model discrimination (AUROC curves) and calibration (Hosmer-Lemeshow statistics, in which higher p-values indicate better calibration). TBI, NISS, and INR were the significant covariates identified for death while NISS, base deficit and PTT were the covariates identified as the best predictors of massive transfusion and platelet transfusion.
Table 3.
Predictive Performance of Platelet Count, rTEG MA, % ADP INH for Post Injury Death, Massive Transfusion and Platelets Transfusion, Adjusted for Significant Covariates
| Outcome=Death | AUROC | 95% Wald Confidence Limits |
Comparison with Covariates only model p-value | |
|---|---|---|---|---|
| % ADP-INH + Covariates | 0.9286 | 0.8947 | 0.9624 | 0.9825 |
| Platelet Count + Covariates | 0.9340 | 0.8983 | 0.9697 | 0.5361 |
| rTEG-MA + Covariates | 0.9297 | 0.8967 | 0.9627 | 0.6432 |
| Covariates only | 0.9285 | 0.8953 | 0.9617 | |
| Outcome=Massive Transfusion | ||||
| % ADP-INH +Covariates | 0.9704 | 0.9491 | 0.9917 | 0.5689 |
| Platelet Count +Covariates | 0.9744 | 0.9490 | 0.9997 | 0.4326 |
| rTEG-MA +Covariates | 0.9695 | 0.9400 | 0.9989 | 0.8753 |
| Covariates only | 0.9682 | 0.9450 | 0.9914 | |
| Outcome=Platelet Transfusion | ||||
| % ADP-INH +Covariates | 0.9264 | 0.8792 | 0.9737 | 0.5671 |
| Platelet Count +Covariates | 0.9533 | 0.9225 | 0.9842 | 0.0345 |
| rTEG-MA +Covariates | 0.9464 | 0.9149 | 0.9778 | 0.0468 |
| Covariates only | 0.9208 | 0.8765 | 0.9652 | |
Following multivariate analysis of predictors of study outcomes, the %ADP-INH, platelet count, and rTEG MA were added with the previously determined covariates to evaluate their ability to improve predictability of death, massive transfusion, or platelet transfusion. Neither %ADP-INH, platelet count, nor rTEG MA added predictive power to predict death or massive transfusion compared to the covariates alone. However, both platelet count and rTEG MA had statistically significant improvements in predicting platelet transfusion while %ADP-INH did not.
When these three effects of interest were simultaneously entered into the models with the covariates shown in Table 2, %ADP-INH did not show an independent association with any of the three outcomes. In contrast, low platelet count significantly predicted all three outcomes, and MA predicted massive transfusion (Table 4).
Table 4.
Models with Platelet count, rTEG MA, %ADP-INH, Adjusted for Significant Covariates
| Outcome=Death | Estimate | Standard Error |
Wald Chi-Square |
P-value | Odds Ratios | 95% CI | |
|---|---|---|---|---|---|---|---|
| %ADP-INH | 0.00562 | 0.00911 | 0.3796 | 0.5378 | 1.006 | 0.988 | 1.024 |
| Platelet Count | −0.0097 | 0.00401 | 5.8406 | 0.0157 | 0.99 | 0.983 | 0.998 |
| rTEG MA | 0.015 | 0.0274 | 0.3009 | 0.5833 | 1.015 | 0.962 | 1.071 |
| AUROC (95% CI) | 0.9335 (0.8969–0.9700) | ||||||
| H-L stats p value | 0.5046 | ||||||
| Outcome=Massive Transfusion | |||||||
| %ADP-NH | −0.0312 | 0.0181 | 2.9863 | 0.084 | 0.969 | 0.936 | 1.004 |
| Platelet Count | −0.0124 | 0.00568 | 4.7479 | 0.0293 | 0.988 | 0.977 | 0.999 |
| rTEG MA | −0.0643 | 0.0314 | 4.1865 | 0.0407 | 0.938 | 0.882 | 0.997 |
| AUROC (95% CI) | 0.98 (0.9575–1.000) | ||||||
| H-L stats p value | 0.9712 | ||||||
| Outcome=Platelet Transfusion | |||||||
| %ADP-INH | 0.0182 | 0.0109 | 2.7678 | 0.0962 | 1.018 | 0.997 | 1.040 |
| Platelet Count | −0.0159 | 0.00508 | 9.7483 | 0.0018 | 0.984 | 0.974 | 0.994 |
| rTEG MA | −0.066 | 0.0337 | 3.8261 | 0.0505 | 0.936 | 0.876 | 1.000 |
| AUROC (95% CI) | 0.9625 (0.9341–0.9908) | ||||||
| H-L stats p value | 0.1248 | ||||||
Once adjusted for significant covariates, %ADP-INH, platelet count and rTEG MA’s performances were compared for three outcomes (death, massive transfusion, and platelet transfusion). Platelet count was a significant predictor of death and platelet transfusion while platelet count and rTEG MA were significant predictors of massive transfusion. % ADP-INH was not a significant predictor of any of the primary outcomes measured.
Table 5 shows a subgroup analysis of patients with NISS>=25 (N=124, 40.9%) and those with TBI (N=80, 26.4%). In both subgroups, none of the three effects of interest were significantly superior than the model with the covariates in Table 2. Patients with NISS >=25 had significantly higher %ADP-INH than those with NISS<25 [36.5% (19.4–62.9) vs 50.8% (30.4–82.0), p=0.0015]. The %ADP-INH was also higher among TBI patients compared to those without significant TBI, albeit not significantly [38.9% (21.1–68.1) vs 49.6% (27.7–78.4), p=0.17].
Table 5.
Predictive performance of Platelet Count, rTEG MA, % ADP INH for postinjury death, massive transfusion and platelets transfusion, adjusted for significant covariates in the subgroups with severe injury (NISS>=25) and with TBI (AIS head/neck >=3)
| Outcome=death | NISS>=25 | TBI | ||||||
|---|---|---|---|---|---|---|---|---|
| AUROC | 95% Wald | Comparison with Covariates | AUROC | 95% Wald | Comparison with Covariates p-value | |||
| Confidence Limits | p-value | Confidence Limits | ||||||
| % ADP-INH + Covariates | 0.8306 | 0.7532 | 0.9081 | 0.5213 | 0.8746 | 0.7737 | 0.9755 | 0.4098 |
| Platelet Count + Covariates | 0.8485 | 0.7645 | 0.9326 | 0.5916 | 0.8693 | 0.7637 | 0.9749 | 0.8758 |
| rTEG-MA + Covariates | 0.8348 | 0.7585 | 0.9111 | 0.9061 | 0.8746 | 0.7759 | 0.9732 | 0.6582 |
| Covariates only | 0.8342 | 0.7575 | 0.9109 | 0.8728 | 0.7718 | 0.9738 | ||
| Outcome=massive transfusion | ||||||||
| % ADP-INH +Covariates | 0.95 | 0.9066 | 0.9934 | 0.58 | NA | NA | NA | NA |
| Platelet Count +Covariates | 0.9541 | 0.9072 | 1 | 0.6284 | NA | NA | NA | NA |
| rTEG-MA +Covariates | 0.9453 | 0.8894 | 1 | 0.9709 | NA | NA | NA | NA |
| Covariates only | 0.9448 | 0.8957 | 0.9939 | |||||
| Outcome=platelet transfusion | ||||||||
| % ADP-INH +Covariates | 0.8942 | 0.827 | 0.9614 | 0.574 | 0.8734 | 0.7888 | 0.958 | 0.1655 |
| Platelet Count +Covariates | 0.923 | 0.866 | 0.98 | 0.1362 | 0.8889 | 0.8006 | 0.9771 | 0.1804 |
| rTEG-MA +Covariates | 0.9213 | 0.8649 | 0.9777 | 0.1413 | 0.8597 | 0.7587 | 0.9608 | 0.3455 |
| Covariates only | 0.8843 | 0.8186 | 0.95 | 0.8315 | 0.7335 | 0.9296 | ||
Subgroup analysis of patients with NISS>=25 and those with TBI. In both subgroups, none of the three effects of interest were significantly superior than the model with the covariates in Table 2. Patients with NISS >=25 had significantly higher %ADP-INH than those with NISS<25 [36.5% (19.4–62.9) vs 50.8% (30.4–82.0), p=0.0015]. The %ADP-INH was also higher among TBI patients compared to those without significant TBI, albeit not significantly [38.9% (21.1–68.1) vs 49.6% (27.7–78.4), p=0.17].
Among patients with MA<=55mm (N=53, 20.8%), %ADP-INH was higher among non-survivors than survivors [77.6% (46.2–90.5) vs 55.0% (32.1–83.8)], but this difference did not reach significance (p=0.22). In contrast, platelet count was significantly lower among non-survivors than survivors [86,000/ml (46,000–179,000) vs 204,500/ml (142,000–275,50000), p=0.0013]. No multivariate adjustment was possible for the patients with MA<=55mm due to the small sample size.
Youden Index (optimal cutoff) for %ADP-INH, platelet count and MA for death, massive transfusion and platelet transfusion
The death Youden Index was 46% for %ADP-INH, 217,000/ml for platelet count and 56mm for MA. Regarding prediction of massive transfusion, we estimated 80% for %ADP-INH, 183,000/mL for platelet count and 56mm for MA. For platelet transfusion, these indices were: 50% for %ADP-INH, 217,000/ml for platelet count and 56mm for MA.
%ADP-INH, platelet count and MA as prognostic indicators among patients who received platelet transfusion
Forty (13%) patients received platelet transfusion within 6 hours of injury. Of these, 16 (4–%) died. There were only slight, non-significant differences between non-survivors and survivors regarding levels of %ADP-INH [80.3% (48.1–91.0) vs 72.9% (46.8–96.9), p=0.86], platelet count [183,500/ml(84,000–228,000) vs 189,000/ml (114,000–241,000), p=0.74] and MA [(56.5mm (40.5–61.2) vs. 51.2mm(47–56), p=0.50]. Again, it was not possible to adjust using multivariate modeling due to the small sample size.
Discussion
The purpose of this study was to assess whether platelet ADP receptor dysfunction, measured by TEG–PM, adds additional information in determining the need for massive transfusion, platelet transfusion or predicts mortality. This study confirmed that platelet dysfunction, as measured by %ADP-INH, occurs early following trauma and is associated with mortality, massive transfusion, or platelet transfusion. However, %ADP-INH was not a better predictor of these outcomes than platelet count, rTEG MA or even clinical indicators of injury severity and coagulation. Conversely, the prediction by platelet count and rTEG-MA added significantly to clinical indicators in the prediction of requirements of platelet transfusion.
TEG-PM was originally developed to assess antiplatelet therapy in cardiac patients taking salicylic acid or clopidogrel [8], and recently has been suggested as a tool to evaluate platelet function in trauma patients [3]. Early studies measuring TEG-PM in trauma showed significant correlations between traumatic brain injury (TBI) and degree of ADP receptor inhibition with increased risk of mortality [17,18]. In our study, the lack of significant association between %ADP-INH with TBI could be due to a type 2 error. Alternatively, it is possible that the increase in %ADP-INH may not be specific to TBI, rather it may be related to the severity of the injury. Indeed, NISS>=25 is significantly associated with %ADP-INH.
The absence of increased predictive value by %ADP-INH may suggest %ADP-INH is simply a biomarker of injury severity rather than a direct mediator of TIC. Although the ADP receptor appears to have a major role in the secondary platelet response to maintain clot stability [9], other agonists are also critical in the platelet response including arachadonic acid, thrombin, collagen, serotonin, epinephrine, Von Willebrand factor, histone, and HMGBI [9,10,19]. In addition, these other platelet cell surface receptors may act to compensate the dysfunction through the ADP receptor pathway, maintaining global platelet function in the injured patient and should be evaluated further in the future. For example, platelets with a high degree of ADP inhibition continue to maintain their dense granules indicating their maintained functional capacity in the injured patient and argue against the theory of platelet exhaustion as an etiology of platelet dysfunction [12].
A liberal threshold (>100,000/ml) for platelet transfusions to trauma patients has been proposed [20]. While high ratios of platelets to RBC in retrospective trauma studies have suggested improvement in survival, randomized controlled trial data suggests that a platelet:RBC transfusion ratio higher than 1:2 does not improve survival [6]. Furthermore, the dangers of excessive platelet transfusions for intracranial bleeding were suggested in the PATCH-3 study in which empiric platelet transfusion in hemorrhagic stroke patients was associated with increased mortality [20]. While platelet count predicts outcome, transfusing platelets based on platelet count to “normalize” platelet count does not seem to improve outcomes, and may even harm patients, if the internal environment is hostile [21]. The appropriate transfusion trigger for platelets in injured patients remains to be elucidated but goal-directed platelet transfusions with TEG MA have been associated with a reduction in platelet transfusions and a reduction in mortality in patients undergoing massive transfusion [7,22,23].
There are several limitations to our study. Our work with TEG-PM has been confined to assessment of the response to ADP. In addition, these data reflect a single point in time of dynamic process and does not take into account the temporal changes of the coagulation process. Furthermore, it is possible that different injury patterns and severity induce specific platelet function responses. The current sample is not large enough to examine the effect of %ADP-INH in specific injuries. As we continue to enroll patients in our TAP database and collaborate with other investigators to produce larger, multicenter samples, we will be able to determine whether platelet dysfunction tests have a role in specific subgroups, for example more severely injured patients or patients with TBI.
In conclusion, %ADP receptor inhibition does not add predictive value for mortality, massive transfusion, or platelet transfusion in injured patients compared to platelet count or rTEG MA, thus does not appear to provide assistance in determining the need for platelet transfusion. This study illustrates the limitations of TEG-PM as a diagnostic tool to help discriminate those that are at risk for death, massive transfusion and platelet transfusion. The utility of TEG-PM as a solitary tool to guide clinical decisions continues to require refinement. The status of other platelet surface receptors may provide more useful in determining the ultimate need for platelet transfusions in injured patients.
Figure 1.
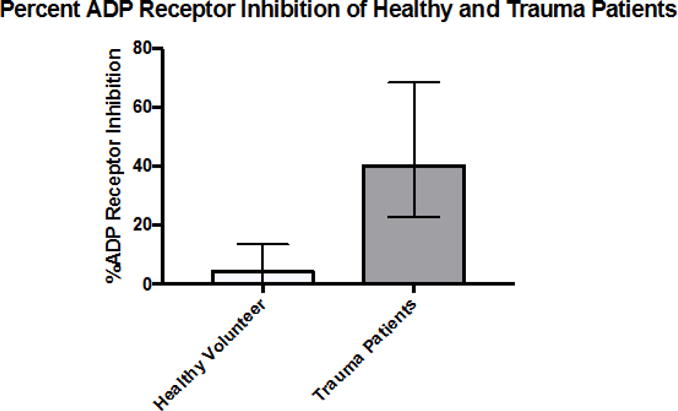
Percent ADP receptor inhibition for healthy volunteers and injured patients. The median %ADP-INH was 42.5% (IQR: 22.4–69.1%) in trauma patients compared to 4.3 % (IQR: 0–13.5%) in healthy volunteers. This ADP receptor inhibition was present at the time of first blood draw (field or arrival in ED) indicating that platelet inhibition occurs very early in injured patients. Data are presented as median +/− IQR.
Acknowledgments
Disclosure: Research reported in this publication was supported in part by the National Institute of General Medical Sciences grants: T32-GM008315 and P50-GM49222, the National Heart Lung and Blood Institute UM1-HL120877, in addition to the Department of Defense USAMRAA and W81XWH-12-2-0028. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Heart, Lung, and Blood institute, or the Department of Defense. Additional research support was provided by Haemonetics with shared intellectual property.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 12th Annual Academic Surgical Congress; Las Vegas NV, February 7–9 2017
Author Contribution: G.R.S implemented the study, interpreted data, drafted and critically revised the manuscript. H.B.M interpreted data, and drafted and critically revised the manuscript. G.R.N. interpreted data, and drafted and critically revised the manuscript. B.J.H. interpreted data, and drafted and critically revised the manuscript. P.E. interpreted data, and drafted and critically revised the manuscript. A.G. implemented the study, oversaw data collection, drafted and critically revised the manuscript. E.E.M, C.C.S, A.B, and A.S. are principal investigators, were responsible for study conception and design, implementation of study, completion of study, interpretation of data, manuscript drafting, and critical revision.
References
- 1.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of Trauma Deaths. The Journal of Trauma: Injury, Infection, and Critical Care. 1995;38(2):185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Moore HB, Moore EE, Gonzalez E, Chapman MP, Chin TL, Silliman CC, Banerjee A, Sauaia A. Hyperfibrinolysis, Physiologic Fibrinolysis, and Fibrinolysis Shutdown. Journal of Trauma and Acute Care Surgery. 2014;77(6):811–17. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, Silliman CC, Ploplis V, Castellino FJ, Walsh M. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–746. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashuk JL, Moore EE, Sawyer M, Le T, Johnson J, Biffl WL, Cothren CC, Barnett C, Stahel P, Sillman CC, Sauaia A, Banerjee A. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann Surg. 2010;251:604–614. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 5.Moore HB, Moore EE, Chapman MP, Gonzalez E, Slaughter AL, Morton AP, D’Alessandro A, Hansen KC, Sauaia A, Banerjee A, Silliman CC. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13:1878–87. doi: 10.1111/jth.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–82. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez E, Moore EE, Moore HB, Chapman MP, Chin TL, Ghasabyan A, Wohlauer MV, et al. Goal-directed Hemostatic Resuscitation of Trauma-induced Coagulopathy: A Pragmatic Randomized Clinical Trial Comparing a Viscoelastic Assay to Conventional Coagulation Assays. Annals of Surgery. 2016;263(6):1051–9. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobson AR, Agarwala RA, Swallow RA, Dawkins KD, Curzen NP. Thrombelastography: current clinical applications and its potential role in interventional cardiology. Platelets. 2006;17(8):509–518. doi: 10.1080/09537100600935259. [DOI] [PubMed] [Google Scholar]
- 9.Furie B, Furie BC. Thrombus formation in vivo. Journal of Clinical Investigaiton. 2005;115(12):3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li R, Elmongy H, Sims C, Diamond SL. Ex vivo recapitulation of trauma-induced coagulopathy and preliminary assessment of trauma patient platelet function under flow using microfluidic technology. Journal of Trauma and Acute Care Surgery. 2016;80(3):440–449. doi: 10.1097/TA.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutcher ME, Redick BJ, McCreery RC, Crane IM, Greenberg MD, Cachola LM, et al. Characterization of platelet dysfunction after trauma. Journal of Trauma and Acute Care Surgery. 2012;73:13–19. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartels AN, Johnson C, Lewis J, Clevenger JW, Barnes SL, Hammer RD, et al. Platelet Adenosine Diphosphate Inhibition in Trauma Patients by Thromboelastography Correlates with Paradoxical Increase in Platelet Dense Granule Content by Flow Cytometry. Surgery. 2016;160(4):954–59. doi: 10.1016/j.surg.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Sirajuddin S, Valdez C, DePalma L, Maluso P, Singhal R, Schroeder M, et al. Inhibition of platelet function is common following even minor injury. Journal of Trauma and Acute Care Surgery. 2016;81(2):328–32. doi: 10.1097/TA.0000000000001057. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez E, Pieracci FP, Moore EE, Kashuk JL. Coagulation Abnormalities in the Trauma Patient: The Role of Point-of-Care Thromboelastography. Semin Thromb Hemost. 2010;36(7):723–737. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Einersen PM, Moore EE, Chapman MP, Moore HB, Gonzalez E, Silliman CC, Banerjee A, Sauaia A. Rapid thrombelastography thresholds for goal-directed resuscitation of patients at risk for massive transfusion. Journal of Trauma and Acute Care Surgery. 2017;82(1):114–19. doi: 10.1097/TA.0000000000001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 17.Castellino FJ, Chapman MP, Donahue DL, Thomas S, Moore EE, Wohlauer MV, et al. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. Journal of Trauma and Acute Care Surgery. 2014;76:1169–76. doi: 10.1097/TA.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nekludov M, Bellander BM, Blomback M, Wallen HN. Platelet dysfunction in patients with severe traumatic brain injury. J Neurotrauma. 2007;24(11):1699–1706. doi: 10.1089/neu.2007.0322. [DOI] [PubMed] [Google Scholar]
- 19.Clemetson KJ. Platelets and Primary Haemostasis. Thrombosis Research. 2012;129:220–224. doi: 10.1016/j.thromres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Baharoglu MI, Cordonnier C, Salman RA, de Gans D, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387:2605–13. doi: 10.1016/S0140-6736(16)30392-0. [DOI] [PubMed] [Google Scholar]
- 21.Vulliamy P, Gillespie S, Gall LS, Green L, Brohi K, Davenport RA. Platelet transfusions reduce fibrinolysis but do not restore platelet function during trauma hemorrhage. J Trauma Acute Care Surg. 2017;27 doi: 10.1097/TA.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 22.Brown LM, Call MS, Margaret Knudson M, Cohen MJ, Trauma Outcomes Group. Holcomb JB, Wade CE, et al. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. The Journal of Trauma. 2011;71:S337–42. doi: 10.1097/TA.0b013e318227f67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikura H, Kitamura T. Trauma-induced coagulopathy and critical bleeding: the role of plasma and platelet transfusion. Journal of Intensive Care. 2017;5(2) doi: 10.1186/s40560-016-0203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


