Abstract
Background
Breast and cervical cancer incidence vary by urbanicity, and several ecological factors could contribute to these patterns. In particular, cancer screening or other sociodemographic and healthcare system variables could explain geographic disparities in cancer incidence.
Methods
Governmental and research sources provided data on 612 counties in the Surveillance, Epidemiology, and End Results program for rural-urban continuum code, socioeconomic status (SES) quintile, percent non-Hispanic white residents, density of primary care physicians, cancer screening, and breast and cervical cancer incidence rates (2009–2013). Ecological mediation analyses used weighted least squares regression to examine whether candidate mediators explained the relationship between urbanicity and cancer incidence.
Results
As urbanicity increased, so did breast cancer incidence (β̂=0.23, p<.001). SES quintile and density of primary care physicians mediated this relationship, while percent non-Hispanic white suppressed it (all p<.05); county-level mammography levels did not contribute to the relationship. After controlling for these variables, urbanicity and breast cancer incidence were no longer associated (β̂=0.11, p>.05). In contrast, as urbanicity increased, cervical cancer incidence decreased (β̂=−0.33, p<.001). SES quintile and density of primary care physicians mediated this relationship (both p<.05); percent non-Hispanic white and Pap screening levels did not contribute to the relationship. After controlling for these variables, the relationship between urbanicity and cervical cancer incidence was still statistically significant (β̂=−0.13, p<.05).
Conclusions
County-level SES and density of primary care physicians explained the relationships between urbanicity and breast and cervical cancer incidence. Improving these factors in more rural counties could ameliorate geographic disparities in breast and cervical cancer incidence.
Keywords: breast cancer, cervical cancer, rural, urban, disparities
Urban/rural differences in breast and cervical cancer incidence: The mediating roles of socioeconomic status and provider density1
The Surveillance, Epidemiology, and End Results (SEER) program estimated that, in 2013, annual age-standardized breast cancer incidence was 125 per 100,000 women and cervical cancer incidence was 8 per 100,000 women (Howlader et al., 2016). However, the burden of these cancers is not distributed equally across the United States: breast cancer incidence is higher, and cervical cancer incidence is lower, in urban areas compared to rural areas (Fogleman, Mueller, & Jenkins, 2015; Singh, 2011). One recent study found that, compared to rates in rural areas, breast cancer incidence rates were 9% higher in urban areas and cervical cancer incidence rates were 15% lower in urban areas (Blake, Moss, Gaysynsky, Srinivasan, & Croyle, 2017). Contrasting the associations of these cancers with potential local influences on health, such as sociodemographics (Pruitt, Shim, Mullen, Vernon, & Amick, 2009; Singh, Williams, Siahpush, & Mulhollen, 2011) and healthcare factors (Belasco, Gong, Pence, & Wilkes, 2014; Doescher & Jackson, 2009), can inform interventions aiming to reduce urban/rural disparities in cancer outcomes and improve overall population health (Wells & Horm, 1998).
While both breast and cervical cancer have screening tests, their associations with cancer incidence differ. In the short term, mammography screening is associated with increased breast cancer incidence (through (1) earlier diagnosis of pre-symptomatic cancers and (2) overdiagnosis of indolent breast cancer tumors that would have never progressed (Bleyer & Welch, 2012; Marcus, Prorok, Miller, DeVoto, & Kramer, 2015)), although this relationship is dynamic over time and generally reaches a steady state after screening has disseminated in the population (Feuer & Wun, 1992). In the long term, mammography screening is associated with decreased breast cancer mortality (Das, Feuer, & Mariotto, 2005; Nelson et al., 2016). In contrast, Pap screening is associated with reduced cervical cancer incidence ((1) through earlier detection of pre-symptomatic cancers and (2) by allowing the removal of cervical lesions before they develop into cancer) (Smith, Cokkinides, Brooks, Saslow, & Brawley, 2010). Like cancer incidence rates, cancer screening also varies geographically, with higher participation in mammography and Pap screening among women living in urban areas compared to rural areas (Casey, Thiede Call, & Klingner, 2001; Coughlin, Thompson, Hall, Logan, & Uhler, 2002; Doescher & Jackson, 2009). Currently, the U.S. Preventive Services Task Force recommends biennial mammography screening for women ages 50–74 years and triennial Pap screening for women ages 21–65 years (with other options for cervical cancer screening including human papillomavirus testing) (U. S. Preventive Services Task Force, 2016; U.S. Preventive Services Task Force, 2016).
Other area-level factors that may influence urban/rural differences in cancer incidence include local socioeconomic status (SES) (Pruitt et al., 2009; Singh et al., 2011), racial/ethnic composition (Singh et al., 2011), and access to primary care providers (Belasco et al., 2014; Doescher & Jackson, 2009). Area sociodemographics and healthcare system factors are associated with cancer outcomes (Kish, Yu, Percy-Laurry, & Altekruse, 2014; Kohler et al., 2015; Singh, Miller, Hankey, & Edwards, 2004) and screening (Continelli, McGinnis, & Holmes, 2010; Phillips, Kerlikowske, Baker, Chang, & Brown, 1998), but most previous studies have evaluated these factors in isolation and have not evaluated whether they explain urban/rural differences in cancer incidence.
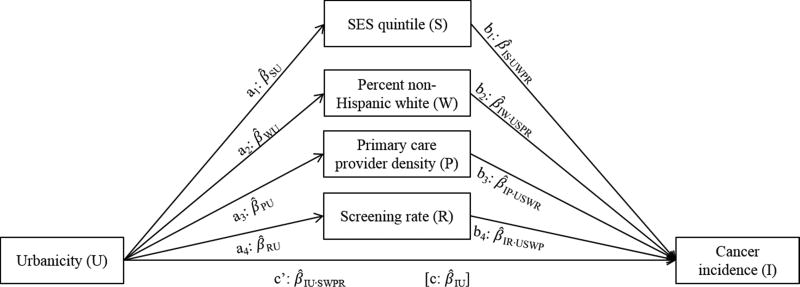
Thus, we hypothesized that county-level (1) SES, (2) racial/ethnic composition, (3) density of primary care providers (a measure of access to preventive services (Continelli et al., 2010)), and (4) cancer screening rates would mediate the relationship between urbanicity and breast or cervical cancer incidence. Figure 1 illustrates the full paths and interrelationships of the potential mediation analysis. Examining how area-level variables work together to influence disparities in cancer screening and outcomes could support the development of locally-targeted interventions (Andrews et al., 1994; Kish et al., 2016; Wells & Horm, 1998). Evidence supporting each of these candidate mediation pathways would highlight different targets for interventions aiming to ameliorate urban/rural differences in counties’ cancer incidence rates.
Figure 1.
Conceptual model for complex mediation analysis of the association between county urbanicity and cancer incidence rates, without and with adjustment for four simultaneous mediators: socioeconomic status (SES) quintile, percent non-Hispanic white population, physician density per 1,000 residents, and cancer screening rate. Subscripts of pathway estimates reflect coefficients of the association between dependent and independent variables, and control variables, if applicable (e.g., β̂IU·SWPR is the coefficient for cancer incidence regressed on urbanicity, adjusted for SES quintile, non-Hispanic white density, physician density, and screening rate).
Materials and Methods
Data sources and measures
Independent variable: Urbanicity
After each Census, United States Department of Agriculture (USDA) (2013) develops a 9-point rural-urban continuum to summarize urbanicity in each county, based on population, urbanization, and adjacency to metro areas. The 2013 codes reflect data derived from the 2010 Census. The present analysis reverse-coded the USDA codes such that higher values reflected greater urbanicity. The USDA continuum codes can be treated as continuous or categorical (1–3=metropolitan/urban, and 4–9=non-metropolitan/rural) indicators (United States Department of Agriculture, 2013). Previous studies of the USDA continuum codes have analyzed the association between urbanicity and cancer outcomes, including screening (Litaker & Tomolo, 2007), incidence (Bernard, Cooper Robbins, McCaffery, Scott, & Skinner, 2011), stage of diagnosis (Paquette & Finlayson, 2007), and survival (Modesitt, Huang, Shelton, & Wyatt, 2006), treating the codes as either continuous or categorical.
Mediating variables
We accessed data on four potential county-level mediators: SES, racial/ethnic composition, primary care physician density, and cancer screening.
SES was measured with an index in SEER drawing upon seven county-level indicators from the 2010 Census (Yost, Perkins, Cohen, Morris, & Wright, 2001; Yu, Tatalovich, Gibson, & Cronin, 2014) including median household income, proportion working class, and an index of education. Counties were assigned to quintiles based on the U.S. distribution to reflect relative positioning in the SES hierarchy, with higher quintiles reflecting higher SES. Each SES quintile contains approximately equal population (i.e., not equal numbers of counties, although each cell contains >5% of the observations). To capture the ordinal nature of this variable, we treated SES quintile as continuous.
To summarize county racial/ethnic composition, we gathered the percent of residents who self-identified as non-Hispanic white in the 2010 Census (U.S. Census Bureau, 2014). Thus, higher levels of the proportion of non-Hispanic whites in a county indicates lower concentrations of racial/ethnic minorities in the population.
Density of primary care providers came from the 2010 Area Health Resource File (2016) and reflected the number of non-federal primary care physicians per 1,000 people in the county, with higher values reflecting higher physician density. Previous studies have evaluated physician density as a proxy for healthcare access, demonstrating associations with health indicators (Belasco et al., 2014; Continelli et al., 2010; Macinko, Starfield, & Shi, 2007). In addition, the density of primary care providers is associated with other area-level measures of healthcare access and utilization (Kravet et al., 2008; Rabinowitz & Paynter, 2002).
Finally, cancer screening rates came from the Small Area Estimates for Cancer Risk Factors & Screening Behaviors (National Cancer Institute, Division of Cancer Control and Population Sciences, Statistical Methodology & Applications Branch, 2016; Raghunathan et al., 2007) developed by National Cancer Institute. These estimates draw on multiple years of data (2008–2010) from two population-based health surveys to account for survey biases and develop stable estimates at the county level (Raghunathan et al., 2007). Estimates used a weighted average of local direct estimates to the extent available, but increasingly substituted modeled estimates as a function of county covariates as the direct estimates became more unstable. From the Small Area Estimates, we gathered data on county mammography levels (i.e., the estimated percentage of women age 40 or older who received a mammogram in the previous two years) and Pap screening levels (i.e., the estimated percentage of women age 18 or older who received a Pap test in the previous three years).
Dependent variables: Cancer incidence rates
Data on breast and cervical cancer incidence rates came from SEER (National Cancer Institute, 2016). Established in 1973, SEER now includes 18 cancer registries covering 30% of the U.S. population (National Cancer Institute, 2016). We generated five-year (2009–2013) age-standardized estimates of breast cancer incidence and cervical cancer incidence per 100,000 women for each county in SEER, excluding the Alaska Native Tumor Registry (which does not include information on counties) (k=612) and suppressing data for counties with fewer than 5 cases.
We matched counties’ variables using Federal Information Processing Standard (FIPS) codes, developed by U.S. Census Bureau (2015) to uniquely identify each U.S. county.
Statistical analysis
As a preliminary step, we examined the distribution of characteristics of SEER counties by generating the minimum, Q1 (i.e., 25th percentile), mean, median, Q3 (i.e., 75th percentile), and maximum of each study variable. In addition, we generated the frequency and percentage of counties at each level of urbanicity and SES.
Next, we conducted simple mediation analyses using ecological weighted least squares (WLS) (Cohen, Cohen, West, & Aiken, 2003) regressions to test whether the candidate mediator variables could individually explain the relationship between urbanicity and breast or cervical cancer incidence rates. Specifically, these analyses examined the bivariate relationship between urbanicity and cancer incidence (estimating the unadjusted c path, or total effect). Then, we examined the relationship between urbanicity and cancer incidence when controlling for each mediator (estimating the adjusted c’ path, or direct effect). As part of this analysis, we also estimated the associations between urbanicity and each mediator (a paths) and between each mediator and cancer incidence (b paths). Then, we determined if controlling for the mediators affected the strength and/or direction of the main association by calculating whether the difference between the c and c’ paths (i.e., the indirect effect) was statistically significant (Hayes, 2009). Finally, we conducted complex mediation analyses modeling all four candidate mediators simultaneously, separately for breast or cervical cancer incidence. For each of the component mediators, we calculated their indirect effect as the product of the respective a and b paths.
To evaluate the statistical significance of each indirect effect, we repeated the WLS regression analyses with 10,000 bootstrapped datasets (Hayes, 2009) (generated with PROC SURVEYSELECT in SAS, sampled with replacement) and ordered the resulting estimates of each indirect effect by magnitude. We examined the observations corresponding to the upper and lower bounds of the 95%, 99%, and 99.9% confidence intervals to determine if they contained 0 (i.e., indicated statistical non-significance at the .05, .01, and .001 p value, respectively). Bootstrapping is preferred to other methods of evaluating indirect effects in mediation because it requires fewer assumptions about the distributional properties of the estimates (Hayes, 2009). In addition, for complex mediation models, we summarized the relative contribution of each mediation pathway to explaining the relationship between urbanicity and cancer incidence by dividing the estimate of each indirect effect by the sum of all the indirect effects, expressed as percentages (MacKinnon, 2008).
WLS regression models weighted observations by the inverse measure of error associated with the dependent variable (Cohen et al., 2003). Models estimating a paths versus c paths or c’ and b paths used different dependent variables; thus, we weighted models predicting SES, racial/ethnic composition, and primary care provider density by county population; screening rate by the inverse of the variance associated with small area estimates; and cancer rate by the inverse of the variance associated with each county’s estimates of breast or cervical cancer incidence. Previous research has demonstrated that mediation analysis is robust to using different model specifications (MacKinnon, 2008).
Sensitivity analyses probed the robustness of our findings. We reran the mediation analyses (1) using one weight for all models (the inverse of the variance of the cancer incidence rates) and excluding counties (2) in the top 10% of population (k=61) (these counties likely were more heterogeneous than remaining counties) and (3) in the bottom 10% of population (k=61) (estimates of these counties’ cancer screening levels were based more on modeled rather than observed data, given their small sample size).
We present standardized regression coefficients (β̂) to summarize the relationships among study variables and evaluate statistical mediation; these coefficients are equivalent to partial correlation coefficients in that they range from −1 to +1. Hereafter, “mediation” refers to indirect effects that attenuate (or explain away) the magnitude of the c path coefficient by producing c’ path coefficients closer to 0, and “suppression” refers to indirect effects that increase the magnitude of the c path coefficient. We treated all study variables as continuous. Mediation analyses used complete case analysis, excluding counties with suppressed cancer data. County cancer incidence rates were calculated using SEER* Stat (Surveillance Research Program, National Cancer Institute). All analyses were conducted using SAS version 9.3 (Cary, NC).
Results
Counties in the analytic sample spanned the entire 9-point rural-urban continuum and distribution of SES quintiles (Table 1). Across counties, 73.8% of the population was non-Hispanic white, with 0.44 primary care providers per 1,000 residents. On average, 67.1% of (age- and sex-appropriate) residents had received a recent mammogram, and 73.2% had received a recent Pap screening.
Table 1.
Descriptive statistics for county characteristics and breast and cervical cancer incidence rates (k=612).
| Minimum | Q1 | Mean | Median | Q3 | Maximum | |
|---|---|---|---|---|---|---|
|
|
||||||
| Urbanicity code1, 2010 | 1.00 | 3.00 | 5.29 | 5.00 | 8.00 | 9.00 |
| SES quintile2, 2010 | 1.00 | 1.00 | 2.11 | 1.00 | 3.00 | 5.00 |
| Percent non-Hispanic white, 2010 | 10.3 | 58.5 | 73.8 | 79.3 | 92.9 | 98.6 |
| Primary care providers per 1,000 residents, 2010 | 0.00 | 0.25 | 0.44 | 0.42 | 0.60 | 1.51 |
| Mammography %, 2008–2010 | 34.3 | 63.5 | 67.1 | 67.7 | 71.4 | 89.6 |
| Pap screening %, 2008–2010 | 42.8 | 90.3 | 73.2 | 73.7 | 76.9 | 88.7 |
| Breast cancer incidence rate3, 2009–2013 | 0.0 | 104.0 | 116.3 | 117.8 | 130.3 | 205.2 |
| Cervical cancer incidence rate3, 2009–2013 | 0.0 | 6.0 | 8.4 | 8.1 | 10.5 | 32.9 |
|
| ||||||
| Urbanicity code1, 2000 | Frequency | Percent4 | ||||
|
|
||||||
| 1 (nonmetro, population <2,500) | 58 | 9% | ||||
| 2 (adjacent to metro area, population <2,500) | 41 | 7% | ||||
| 3 (nonmetro, population 2,500–19,999) | 89 | 15% | ||||
| 4 (adjacent to metro area, population 2,500–19,999) | 113 | 18% | ||||
| 5 (nonmetro, population ≥20,000) | 22 | 4% | ||||
| 6 (adjacent to metro area, population ≥20,000) | 29 | 5% | ||||
| 7 (metro, population <250,000) | 77 | 13% | ||||
| 8 (metro, population 250,000–1 mil) | 89 | 15% | ||||
| 9 (metro, population ≥1mil) | 94 | 15% | ||||
| SES quintile2, 2000 | ||||||
| 1 (lowest SES) | 307 | 51% | ||||
| 2 | 112 | 19% | ||||
| 3 | 50 | 8% | ||||
| 4 | 84 | 14% | ||||
| 5 (highest SES) | 51 | 8% | ||||
Note.
Reverse-coded from USDA rural-urban continuum codes (ref. (United States Department of Agriculture, 2013))
Quintiles constructed to include 20% of US population (not counties) per category
Per 100,000 women; excludes counties with fewer than 5 cases
Percents reflect the counties, not the population, in each category.
Breast cancer incidence
County age-adjusted breast cancer incidence rates ranged from 0 to 205.2 cases per 100,000 women per year; on average, breast cancer incidence was 116.3 per 100,000 (Table 1). As urbanicity increased, so did breast cancer incidence (c path: β̂BU=0.23, p<.001) (Table 2).
Table 2.
Estimates of indirect (mediated) effects of county socioeconomic status quintile, percent non-Hispanic white population, physician density per 1,000 residents, and cancer screening rate (separately) on the associations between urbanicity and breast and cervical cancer incidence rates.
| c path | c′ path | Indirect effect | ||
|---|---|---|---|---|
| Est. | 95% CI | |||
| Mediator | Breast cancer incidence | |||
| β̂BS | β̂BS·M | |||
|
|
||||
| SES quintile | 0.23 *** | 0.11 * | 0.12 | (0.09, 0.15) |
| Percent non-Hispanic white | 0.23 *** | 0.26 ** | −0.03 | (−0.05, −0.01) |
| Primary care provider density | 0.23 *** | 0.17 ** | 0.06 | (0.04, 0.09) |
| Mammography rate | 0.23 *** | 0.21 *** | 0.02 | (0.00, 0.05) |
|
|
||||
| Cervical cancer incidence | ||||
| β̂CS | β̂CS·M | |||
|
|
||||
| SES quintile | −0.33 *** | −0.12 | −0.20 | (−0.25, −0.16) |
| Percent non-Hispanic white | −0.33 *** | −0.34 *** | 0.01 | (−0.01, 0.03) |
| Primary care provider density | −0.33 *** | −0.28 ** | −0.05 | (−0.07, −0.03) |
| Pap screening rate | −0.33 *** | −0.30 *** | −0.03 | (−0.05, −0.00) |
Note. Beta coefficients are standardized coefficients of the association between county urbanicity and cancer incidence rates; c path estimates do not adjust for the mediator, and c’ path estimates adjust for the mediator. Estimates of the indirect effects are the difference between c and c’ path estimates. All models control for clustering within states and are weighted by the inverse variance of the cancer incidence rate.
p<.05
p<.01
p<.001.
Est.=estimate; CI=confidence interval; SES=socioeconomic status; β̂=standardized betas (subscripts reflect dependent and independent variables, respectively, and control variables, if applicable).
In simple mediation analysis, SES quintile, primary care provider density, and mammography rate mediated the relationship between urbanicity and breast cancer incidence (all p<.05) (Table 2). For example, after controlling for SES quintile, the association between urbanicity and breast cancer incidence changed from 0.23 (c path) to 0.11 (c’ path; p<.05). Thus, the indirect effect (i.e., difference between the c path and c’ path estimates) was 0.12 (95% confidence interval[CI]=0.09, 0.15). That is, controlling for SES quintile reduced the relationship between urbanicity and breast cancer incidence. In contrast, percent non-Hispanic white suppressed the relationship between urbanicity and breast cancer incidence. After controlling for this variable, the association between urbanicity and breast cancer incidence changed from 0.23 (c path) to 0.26 (c’ path; p<.001); thus, the indirect effect was −0.03 (95% CI=−0.05, −0.01). That is, controlling for percent non-Hispanic white strengthened the relationship between urbanicity and breast cancer incidence.
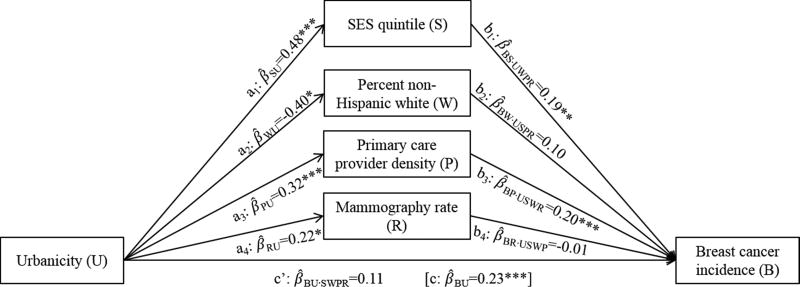
In complex mediation analysis, after controlling for all four candidate mediators, the relationship between urbanicity and breast cancer incidence changed from 0.23 to 0.11 (p>.05; β̂BU·SWPR in Figure 2). SES quintile and primary care provider density maintained their mediating effects on this relationship, while percent non-Hispanic white again had a suppression effect (Table 3). Specifically, urbanicity was positively associated with SES quintile, which in turn was positively associated with breast cancer incidence (Figure 2); controlling for SES quintile changed the main effect between urbanicity and breast cancer by 0.09 (95% CI=0.05, 0.13), or 81% of the total indirect effect (Table 3). Similarly, urbanicity was positively associated with primary care provider density, which was positively associated with breast cancer incidence; controlling for this variable changed the main effect by 0.06 (95% CI=0.04, 0.09), or 57%. In contrast, urbanicity was negatively associated with percent non-Hispanic white, which was not associated with breast cancer incidence; controlling for this variable changed the main effect by −0.04 (95% CI=−0.07, −0.01), or −36%. Mammography rate was not a significant mediator of the relationship between urbanicity and breast cancer incidence when controlling for the other variables.
Figure 2.
Complex mediation analysis depicting the association between county urbanicity and breast cancer incidence rates, without and with adjustment for four simultaneous mediators: socioeconomic status (SES) quintile, percent non-Hispanic white population, physician density per 1,000 residents, and mammography screening rate. Pathways estimates are standardized coefficients of the association between the indicated variables, with subscripts reflecting dependent and independent variables, respectively, and control variables, if applicable (e.g., β̂BU·SWPR is the coefficient for breast cancer incidence rate regressed on urbanicity, adjusted for SES quintile, non-Hispanic white density, physician density, and mammography rate). All models control for clustering within states and are weighted by variance associated with the dependent variable. *p<.05; **p<.01; ***p<.001.
Table 3.
Estimates of indirect (mediated) effects of county socioeconomic status quintile, percent non-Hispanic white population, physician density per 1,000 residents, and cancer screening rate (modeled simultaneously) on the associations between urbanicity and breast and cervical cancer incidence rates.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Indirect effect est. |
95% CI | % total indirect effect |
|||||
|
|
|||||||
| Mediator | Breast cancer incidence | ||||||
|
|
|||||||
| SES quintile | 0.09 | (0.05, 0.13) *** | 81% | ||||
| Percent non-Hispanic white | −0.04 | (−0.07, −0.01) * | −36% | ||||
| Primary care provider density | 0.06 | (0.04, 0.09) *** | 57% | ||||
| Mammography rate | 0.00 | (−0.02, 0.02) | −2% | ||||
| Total | 0.12 | (0.07, 0.16) *** | 100% | ||||
|
|
|||||||
| Cervical cancer incidence | |||||||
|
|
|||||||
| SES quintile | −0.13 | (−0.18, −0.09) *** | 82% | ||||
| Percent non-Hispanic white | 0.01 | (−0.01, 0.04) | −7% | ||||
| Primary care provider density | −0.05 | (−0.08, −0.03) *** | 32% | ||||
| Pap screening rate | 0.01 | (−0.02, 0.05) | −7% | ||||
| Total | −0.19 | (−0.25, −0.13) *** | 100% | ||||
|
| |||||||
Note. Estimates of the indirect effects are the difference between standardized estimates of the c path (unadjusted association between urbanicity and cancer incidence rate) and c’ path (association between urbanicity and cancer incidence rate adjusting for all four mediators). The “Total” of the indirect effects is the sum of the component indirect effects (rounded). All models control for clustering within states and are weighted by variance associated with the dependent variable.
p<.05
p<.01
p<.001.
Est.=estimate; CI=confidence interval; SES=socioeconomic status.
Cervical cancer incidence
County age-adjusted cervical cancer incidence rates ranged from 0 to 32.9 cases per 100,000 women per year; on average, cervical cancer incidence was 8.4 per 100,000 (Table 1). As urbanicity increased, cervical cancer incidence decreased (c path: β̂CU=−0.33, p<.001) (Table 2).
In simple mediation analysis, SES quintile, primary care provider density, and Pap screening rate mediated the relationship between urbanicity and cervical cancer incidence (all p<.05) (Table 2). For example, after controlling for SES quintile, the association between urbanicity and cervical cancer incidence changed from −0.33 (c path) to −0.12 (c’ path; p>.05). Thus, the indirect effect was −0.20 (95% CI=−0.25, −0.16). That is, controlling for SES quintile reduced the relationship between urbanicity and cervical cancer incidence. Percent non-Hispanic white was not a significant mediator of the relationship between urbanicity and cervical cancer incidence.
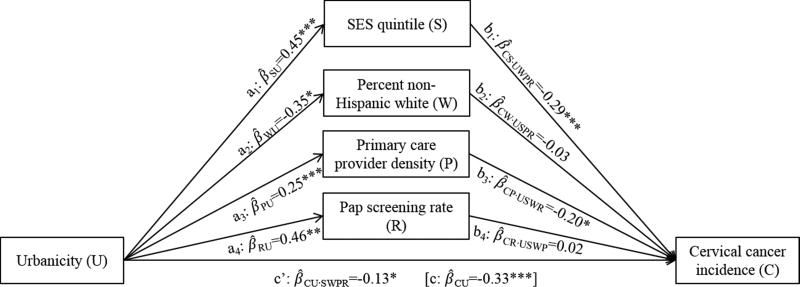
In complex mediation analysis, after controlling for all four candidate mediators, the relationship between urbanicity and cervical cancer incidence changed from −0.33 to −0.13 (p<.05; β̂CU·SWPR in Figure 3). SES quintile and primary care provider density maintained their mediating effects on this relationship (Table 3). Specifically, urbanicity was positively associated with SES quintile, which in turn was negatively associated with cervical cancer incidence (Figure 3); controlling for SES quintile changed the main effect between urbanicity and cervical cancer by −0.13 (95% CI=−0.18, −0.09), or 82% of the total indirect effect (Table 3). Similarly, urbanicity was positively associated with primary care provider density, which was negatively associated with cervical cancer incidence; controlling for this variable changed the main effect by −0.05 (95% CI=−0.08, −0.03), or 32%. Percent non-Hispanic white and Pap screening rate were not significant mediators of the relationship between urbanicity and cervical cancer incidence when controlling for the other variables.
Figure 3.
Complex mediation analysis depicting the association between county urbanicity and cervical cancer incidence rates, without and with adjustment for four simultaneous mediators: socioeconomic status (SES) quintile, percent non-Hispanic white population, physician density per 1,000 residents, and Pap screening rate. Pathways estimates are standardized coefficients of the association between the indicated variables, with subscripts reflecting dependent and independent variables, respectively, and control variables, if applicable (e.g., β̂CU·SWPR is the coefficient for cervical cancer incidence rate regressed on urbanicity, adjusted for SES quintile, non-Hispanic white density, physician density, and Pap screening rate). All models control for clustering within states and are weighted by variance associated with the dependent variable. *p<.05; **p<.01; ***p<.001.
Sensitivity analysis
When repeating the complex mediation analyses (1) using one weight for all models and excluding counties (2) in the top 10% of population and (3) in the bottom 10% of population, the direction, magnitude, and statistical significance of the observed relationships generally remained the same (Supplementary Table S1).
Discussion
In ecological mediation analysis of 612 counties, we found that compared to more rural counties, more urban counties tended to have higher rates of breast cancer incidence and lower rates of cervical cancer incidence. Researchers have increasingly documented the role of geography in health disparities research, both independent from and in conjunction with more traditional health disparities by race/ethnicity and socioeconomic status (Bell, Hoskins, Pickle, & Wartenberg, 2006; Chandra & Skinner, 2004; Diez Roux & Mair, 2010; Fogleman et al., 2015). Geographic health disparities, such as the differences in cancer incidence by urbanicity, point to potential modifiable influences on health that can affect entire regions and potentially also affect other health disparities (Andrews et al., 1994; Kish et al., 2016; Wells & Horm, 1998). The observed associations between urbanicity and breast and cervical cancer incidence were partly mediated by county-level SES and the density of primary care providers, both of which tended to be higher in urban areas. The urban/rural differences in cancer incidence can be partly (for breast cancer) or completely (for cervical cancer) explained by these latter variables. In addition, the percent of non-Hispanic white residents suppressed the relationship between urbanicity and breast cancer, indicating that differences in breast cancer by urbanicity could be even greater if racial/ethnic composition were equal across counties. County rates of cancer screening emerged as mediators in the simple models, but after controlling for the other variables, screening was no longer a significant mediator of the relationship between urbanicity and incidence. These analyses demonstrated potential explanations for the established relationships between urbanicity and breast and cervical cancer incidence rates.
These ecological analyses complement studies on other factors related to geographic differences in breast and cervical cancer incidence, including individual-level and contextual risk factors. For example, attitudes and perceptions related to cancer vary by urbanicity (Bryant & Mah, 1992; Lantz, Weigers, & House, 1997), and these psychosocial differences could drive lower rates of screening in rural areas compared to urban areas. Other individual-level characteristics relevant to screening also vary by urbanicity, such as health literacy (Zahnd, Scaife, & Francis, 2009) and insurance status (Hartley, Quam, & Lurie, 1994; Larson & Hill, 2005). Additional contextual risk factors related to screening and urbanicity were outside the scope of the present paper, including transportation issues (Arcury et al., 2005; Belasco et al., 2014; Khan-Gates, Ersek, Eberth, Adams, & Pruitt, 2015), medical infrastructure (Brems, Johnson, Warner, & Roberts, 2006; Peipins et al., 2012; Yabroff et al., 2005), and environmental exposures (Snedeker, 2001).
Implications for practice and/or policy
County-level differences in breast cancer incidence across the rural-urban continuum were mediated by SES quintile and density of primary care providers. Specifically, compared to rural counties, urban counties had higher SES and density of providers, and these factors were in turn associated with higher breast cancer incidence. These two variables explained significant portions of the main effect between urbanicity and breast cancer incidence, and controlling for them reduced the magnitude of this association. Previous studies have linked sociodemographics (such as SES) and healthcare access to breast cancer rates (Kish et al., 2014; Kohler et al., 2015; Pruitt et al., 2009; Singh et al., 2011), but to our knowledge, no studies have used these variables simultaneously to explain geographic differences in breast cancer incidence. At the individual level, SES (Adler, Boyce, Chesney, Folkman, & Syme, 1993) and healthcare access (Baker, Sudano, Albert, Borawski, & Dor, 2001) are typically associated with reductions in disease, although there is some evidence that SES (measured at the census tract level) and some subtypes of breast cancer incidence are positively correlated (Akinyemiju, Pisu, Waterbor, & Altekruse, 2015). The present findings, at the county level, suggest that contextual factors denoted by higher SES and healthcare access might account for the excess burden of breast cancer in urban versus rural areas (although screening was also correlated with SES and physician density). In contrast, compared to rural counties, urban counties had lower concentrations of non-Hispanic whites, which in turn had a non-significant positive association with breast cancer incidence. This measure of racial/ethnic composition suppressed the association between urbanicity and breast cancer incidence: If the county-level concentration of non-Hispanic whites were equal across counties, urbanicity and breast cancer incidence might be even more positively associated than the presently-observed relationship. Individual-level differences in breast cancer by race may contribute to these ecological associations (Kohler et al., 2015). Taken together, urban/rural differences in SES, percent non-Hispanic white, and primary care provider density completely explained the association between urbanicity and breast cancer incidence at the county level.
The relationships observed for cervical cancer were slightly different than for breast cancer. First, the main effect between urbanicity and cancer was reversed: breast cancer incidence was higher, while cervical cancer incidence was lower, in more urban versus more rural areas (Blake et al., 2017; Singh, 2011). Second, in complex mediation analysis, only SES and primary care provider density mediated the relationship between urbanicity and cancer incidence; neither percent non-Hispanic white nor Pap screening rate contributed to the ecological associations (although Pap screening rate did mediate the association in the preliminary models). Again, the county-level SES and healthcare access context appear to explain the urban/rural differences in cancer incidence, but these factors were associated with overall reductions in cervical cancer incidence (versus increases in breast cancer incidence). Third, even after controlling for all four candidate mediators, the association between urbanicity and cervical cancer incidence was still statistically significant (while the association for breast cancer incidence was not), indicating that significant variation in cervical cancer across the rural-urban continuum remained. In complex mediation analyses, these relationships lost statistical significance. Taken together, urban/rural differences in SES (Coughlin, King, Richards, & Ekwueme, 2006; Liu, Wang, Waterbor, Weiss, & Soong, 1998) and primary care provider density (Coughlin, Leadbetter, Richards, & Sabatino, 2008) partially explained the association between urbanicity and cervical cancer incidence at the county level.
Strengths and limitations
The present study had several strengths and limitations. In terms of strengths, we used several high-quality data sources to contextualize the sociodemographic and healthcare environments in counties across the U.S. In particular, SEER is a virtually complete, population-based collection of high-quality cancer registries. The study also used a 9-level continuum of urbanicity; many studies use dichotomous indicators of urbanicity, but using the continuum allowed more fine-grained examination of differences in cancer incidence across geographic differences. Finally, our findings were robust to sensitivity analyses.
In terms of limitations, the unit of analysis was the county, which means that (1) inferences cannot extend to the individual level, and (2) the distribution of study variables could be misleading because they reflect characteristics of counties regardless of the population size contained within. Intra-county heterogeneity could lead to overgeneralizations of ecological associations, and other studies have used smaller units, such as census tracts (Meilleur et al., 2013); however, data availability precluded this approach in the current study. Using the USDA rural-urban continuum codes to measure urbanicity could be problematic in that the nine categories may not truly represent a continuous construct; however, previous studies have evaluated this continuum as a continuous measure, and across studies of urbanicity, studies using this indicator find similar results to studies using other indicators, such as rural-urban commuting area codes (Meilleur et al., 2013). As noted above, our analyses covered only the SEER areas, so the findings may not be generalizable to the rest of the country. Of note, SEER prioritizes geographic areas with high proportions of people from underrepresented groups (such as Hispanics and Native Hawaiian/Pacific Islander) (National Cancer Institute, 2016), so the results may be more germane for racial/ethnic minorities than for non-Hispanic whites. In addition, we used a self-reported measures of cancer screening, and validation studies have indicated that the sensitivity and specificity of self-report versus clinical records are suboptimal (Rauscher, Johnson, Cho, & Walk, 2008). In addition, we could not discern between screening and diagnostic mammograms/Pap tests. These latter two limitations could have led to an overestimation of screening rates. Residual confounding is another limitation of this analysis; like all research studies, we could not control for all potential variables that could influence the primary relationship. Further, the relationship between cancer screening and incidence rates is very dynamic (Feuer & Wun, 1992), and our models may have obscured some of this nuance. Finally, our analysis focused only on main effects among independent, mediating, and dependent variables. While we tested for interactions between urbanicity and the mediating variables in their associations with cancer rates, only one out of eight terms was statistically significant, and we decided to limit the current study to main effects. Future studies may probe further for complex relationships among ecological variables related to urban/rural differences in cancer incidence.
Conclusions
County-level SES and primary care provider density explained the urban/rural differences in breast and cervical cancer incidence rates. Improving SES and increasing primary care provider density in rural areas, perhaps through supplemental programs such as student loan relief to physicians who practice in these counties, could help ameliorate the geographic disparities in cancer incidence. While such interventions could result in decreases in cervical cancer incidence in rural areas, based on the observed pathways in Figure 2, they may have the unintended negative consequence of increasing breast cancer incidence in rural areas, potentially as a result of overdiagnosis, earlier diagnosis, or other factors. Caution will be necessary in developing such interventions and, if necessary, expanding oncology capacity in these areas. These interventions could reduce the geographic disparities in breast and cervical cancer incidence rates currently observed across the rural-urban continuum.
Supplementary Material
Acknowledgments
Funding: Research was completed as part of official duty at NIH.
Biographies
Jennifer L. Moss, PhD, is a Cancer Prevention Fellow in the Surveillance Research Program at the National Cancer Institute. Her research focuses on geographic disparities in cancer prevention behaviors, especially for genital and reproductive cancers.
Benmei Liu, PhD, is a Mathematical Statistician in the Surveillance Research Program at the National Cancer Institute. Her research includes developing methods for surveys and analysis, small area estimation for cancer risk factors and screening, and imputation for complex survey data.
Eric (Rocky) Feuer, PhD, is Chief of the Statistical Research and Applications Branch in the Surveillance Research Program at the National Cancer Institute. His research includes cancer control modeling and statistical methods for analysis, interpretation, and presentation of cancer statistics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: CI=Confidence interval; FIPS=Federal Information Processing Standard; SEER=Surveillance, Epidemiology, and End Results; SES=Socioeconomic status; USDA=United States Department of Agriculture; WLS=Weighted least squares
COI: Authors have no conflicts of interest to report.
Author contributions: Conceptualization, JLM, BL, & EFJ; methodology, JLM, BL, & EFJ; formal analysis, JLM; resources, BL & EFJ; writing-original draft, JLM; writing-review & editing, JLM, BL, & EFJ; supervision, BL & EFJ.
References
- Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: No easy solution. Jama. 1993;269(24):3140–3145. [PubMed] [Google Scholar]
- Akinyemiju TF, Pisu M, Waterbor JW, Altekruse SF. Socioeconomic status and incidence of breast cancer by hormone receptor subtype. SpringerPlus. 2015;4 doi: 10.1186/s40064-015-1282-2. 508-015-1282-2. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews HF, Kerner JF, Zauber AG, Mandelblatt J, Pittman J, Struening E. Using census and mortality data to target small areas for breast, colorectal, and cervical cancer screening. American Journal of Public Health. 1994;84(1):56–61. doi: 10.2105/ajph.84.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcury TA, Gesler WM, Preisser JS, Sherman J, Spencer J, Perin J. The effects of geography and spatial behavior on health care utilization among the residents of a rural region. Health Services Research. 2005;40(1):135–155. doi: 10.1111/j.1475-6773.2005.00346.x. doi:HESR346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DW, Sudano JJ, Albert JM, Borawski EA, Dor A. Lack of health insurance and decline in overall health in late middle age. New England Journal of Medicine. 2001;345(15):1106–1112. doi: 10.1056/NEJMsa002887. [DOI] [PubMed] [Google Scholar]
- Belasco EJ, Gong G, Pence B, Wilkes E. The impact of rural health care accessibility on cancer-related behaviors and outcomes. Applied Health Economics and Health Policy. 2014;12(4):461–470. doi: 10.1007/s40258-014-0099-4. [DOI] [PubMed] [Google Scholar]
- Bell BS, Hoskins RE, Pickle LW, Wartenberg D. Current practices in spatial analysis of cancer data: Mapping health statistics to inform policymakers and the public. International Journal of Health Geographics. 2006;5:49. doi: 10.1186/1476-072X-5-49. doi:1476-072X-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard DM, Cooper Robbins SC, McCaffery KJ, Scott CM, Skinner SR. The domino effect: Adolescent girls’ response to human papillomavirus vaccination. The Medical Journal of Australia. 2011;194(6):297–300. doi: 10.5694/j.1326-5377.2011.tb02978.x. doi:ber10870_fm. [DOI] [PubMed] [Google Scholar]
- Blake KD, Moss JL, Gaysynsky A, Srinivasan S, Croyle RT. Making the case for investment in rural cancer control: An analysis of rural cancer incidence, mortality, and funding trends. Cancer Epidemiol Biomarkers Prev. 2017 doi: 10.1158/1055-9965.EPI-17-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. The New England Journal of Medicine. 2012;367(21):1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- Brems C, Johnson ME, Warner TD, Roberts LW. Barriers to healthcare as reported by rural and urban interprofessional providers. Journal of Interprofessional Care. 2006;20(2):105–118. doi: 10.1080/13561820600622208. doi:V647W200M450H812. [DOI] [PubMed] [Google Scholar]
- Bryant H, Mah Z. Breast cancer screening attitudes and behaviors of rural and urban women. Preventive Medicine. 1992;21(4):405–418. doi: 10.1016/0091-7435(92)90050-r. [DOI] [PubMed] [Google Scholar]
- Casey MM, Thiede Call K, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? American Journal of Preventive Medicine. 2001;21(3):182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- Chandra A, Skinner J. Geography and racial health disparities. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical perspectives on racial and ethnic differences in health in late life. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Third. London: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- Continelli T, McGinnis S, Holmes T. The effect of local primary care physician supply on the utilization of preventive health services in the United States. Health & Place. 2010;16(5):942–951. doi: 10.1016/j.healthplace.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, King J, Richards TB, Ekwueme DU. Cervical cancer screening among women in metropolitan areas of the United States by individual-level and area-based measures of socioeconomic status, 2000 to 2002. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2006;15(11):2154–2159. doi: 10.1158/1055-9965.EPI-05-0914. doi:15/11/2154. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Leadbetter S, Richards T, Sabatino SA. Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Social Science & Medicine (1982) 2008;66(2):260–275. doi: 10.1016/j.socscimed.2007.09.009. doi:S0277-9536(07)00509-6. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Thompson TD, Hall HI, Logan P, Uhler RJ. Breast and cervical carcinoma screening practices among women in rural and nonrural areas of the United States, 1998-1999. Cancer. 2002;94(11):2801–2812. doi: 10.1002/cncr.10577. [DOI] [PubMed] [Google Scholar]
- Das B, Feuer EJ, Mariotto A. Geographic association between mammography use and mortality reduction in the US. Cancer Causes & Control : CCC. 2005;16(6):691–699. doi: 10.1007/s10552-005-1991-x. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Mair C. Neighborhoods and health. Annals of the New York Academy of Sciences. 2010;1186(1):125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- Doescher MP, Jackson JE. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. Journal of Public Health Management and Practice : JPHMP. 2009;15(3):200–209. doi: 10.1097/PHH.0b013e3181a117da. [DOI] [PubMed] [Google Scholar]
- Feuer EJ, Wun LM. How much of the recent rise in breast cancer incidence can be explained by increases in mammography utilization? A dynamic population model approach. American Journal of Epidemiology. 1992;136(12):1423–1436. doi: 10.1093/oxfordjournals.aje.a116463. [DOI] [PubMed] [Google Scholar]
- Fogleman AJ, Mueller GS, Jenkins WD. Does where you live play an important role in cancer incidence in the U.S.? American Journal of Cancer Research. 2015;5(7):2314–2319. [PMC free article] [PubMed] [Google Scholar]
- Hartley D, Quam L, Lurie N. Urban and rural differences in health insurance and access to care. The Journal of Rural Health. 1994;10(2):98–108. doi: 10.1111/j.1748-0361.1994.tb00216.x. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76(4):408–720. doi: 10.1080/03637750903310360. [DOI] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Cronin KA. SEER cancer statistics review, 1975–2013. 2016 Retrieved from http://seer.cancer.gov/csr/1975_2013/
- Khan-Gates JA, Ersek JL, Eberth JM, Adams SA, Pruitt SL. Geographic access to mammography and its relationship to breast cancer screening and stage at diagnosis: A systematic review. Women’s Health Issues: Official Publication of the Jacobs Institute of Women’s Health. 2015;25(5):482–493. doi: 10.1016/j.whi.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish JK, Rolin AI, Zou Z, Cucinelli JE, Tatalovich Z, Saraiya M, Altekruse SF. Prioritizing US cervical cancer prevention with results from a geospatial model. J Glob Oncol. 2016 doi: 10.1200/JGO.2015.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish JK, Yu M, Percy-Laurry A, Altekruse SF. Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in surveillance, epidemiology, and end results (SEER) registries. Journal of the National Cancer Institute.Monographs. 2014;2014(49):236–243. doi: 10.1093/jncimonographs/lgu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Penberthy L. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. Journal of the National Cancer Institute. 2015;107(6) doi: 10.1093/jnci/djv048. djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravet SJ, Shore AD, Miller R, Green GB, Kolodner K, Wright SM. Health care utilization and the proportion of primary care physicians. The American Journal of Medicine. 2008;121(2):142–148. doi: 10.1016/j.amjmed.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Lantz PM, Weigers ME, House JS. Education and income differentials in breast and cervical cancer screening: Policy implications for rural women. Medical Care. 1997;35(3):219–236. doi: 10.1097/00005650-199703000-00004. [DOI] [PubMed] [Google Scholar]
- Larson SL, Hill SC. Rural-Urban differences in Employment-Related health insurance. The Journal of Rural Health. 2005;21(1):21–30. doi: 10.1111/j.1748-0361.2005.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Litaker D, Tomolo A. Association of contextual factors and breast cancer screening: Finding new targets to promote early detection. Journal of Women’s Health. 2007;16(1):36–45. doi: 10.1089/jwh.2006.0090. [DOI] [PubMed] [Google Scholar]
- Liu T, Wang X, Waterbor JW, Weiss HL, Soong SJ. Relationships between socioeconomic status and race-specific cervical cancer incidence in the United States, 1973–1992. Journal of Health Care for the Poor and Underserved. 1998;9(4):420–432. doi: 10.1353/hpu.2010.0482. [DOI] [PubMed] [Google Scholar]
- Macinko J, Starfield B, Shi L. Quantifying the health benefits of primary care physician supply in the United States. International Journal of Health Services: Planning, Administration, Evaluation. 2007;37(1):111–126. doi: 10.2190/3431-G6T7-37M8-P224. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. New York, NY: Lawrence Erlbaum and Associates; 2008. [Google Scholar]
- Marcus PM, Prorok PC, Miller AB, DeVoto EJ, Kramer BS. Conceptualizing overdiagnosis in cancer screening. Journal of the National Cancer Institute. 2015;107(4) doi: 10.1093/jnci/djv014[doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the United States: Issues and challenges. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2013;22(10):1657–1667. doi: 10.1158/1055-9965.EPI-13-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesitt SC, Huang B, Shelton BJ, Wyatt S. Endometrial cancer in Kentucky: The impact of age, smoking status, and rural residence. Gynecologic Oncology. 2006;103(1):300–306. doi: 10.1016/j.ygyno.2006.03.009. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Surveillance, epidemiology, and end results program. 2016 Retrieved from http://seer.cancer.gov/
- National Cancer Institute, Division of Cancer Control and Population Sciences, Statistical Methodology & Applications Branch. Small area estimates for cancer risk factors & screening behaviors. 2016 Retrieved from http://sae.cancer.gov/
- Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. preventive services task force recommendation. Annals of Internal Medicine. 2016;164(4):244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- Paquette I, Finlayson SR. Rural versus urban colorectal and lung cancer patients: Differences in stage at presentation. Journal of the American College of Surgeons. 2007;205(5):636–641. doi: 10.1016/j.jamcollsurg.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Peipins LA, Miller J, Richards TB, Bobo JK, Liu T, White MC, Ekwueme DU. Characteristics of US counties with no mammography capacity. Journal of Community Health. 2012;37(6):1239–1248. doi: 10.1007/s10900-012-9562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Kerlikowske K, Baker LC, Chang SW, Brown ML. Factors associated with women’s adherence to mammography screening guidelines. Health Services Research. 1998;33(1):29–53. [PMC free article] [PubMed] [Google Scholar]
- Pruitt SL, Shim MJ, Mullen PD, Vernon SW, Amick BC., 3rd Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: A systematic review. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2009;18(10):2579–2599. doi: 10.1158/1055-9965.EPI-09-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz HK, Paynter NP. The rural vs urban practice decision. Jama. 2002;287(1):113–113. [PubMed] [Google Scholar]
- Raghunathan TE, Xie D, Schenker N, Parsons VL, Davis WW, Dodd KW, Feuer EJ. Combining information from two surveys to estimate county-level prevalence rates of cancer risk factors and screening. Journal of the American Statistical Association. 2007;102(478):474–486. [Google Scholar]
- Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: A meta-analysis. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2008;17(4):748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- Singh GK. Rural-urban trends and patterns in cervical cancer mortality, incidence, stage, and survival in the United States, 1950–2008. Journal of Community Health. 2011 doi: 10.1007/s10900-011-9439-6. [DOI] [PubMed] [Google Scholar]
- Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer. 2004;101(5):1051–1057. doi: 10.1002/cncr.20467. [DOI] [PubMed] [Google Scholar]
- Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: Part I-all cancers and lung cancer and part II-colorectal, prostate, breast, and cervical cancers. Journal of Cancer Epidemiology. 2011;2011:107497. doi: 10.1155/2011/107497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: A review of current American cancer society guidelines and issues in cancer screening. CA: A Cancer Journal for Clinicians. 2010;60(2):99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- Snedeker SM. Pesticides and breast cancer risk: A review of DDT, DDE, and dieldrin. Environmental Health Perspectives. 2001;109(Suppl 1):35–47. doi: 10.1289/ehp.01109s135. doi:sc271_5_1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillance Research Program, National Cancer Institute. SEER*Stat software ( seer.cancer.gov/seerstat) version 8.3.2.
- U. S. Department of Health and Human Services, & Health Resources and Services Administration, Bureau of Health Workforce. Area health resources files (AHRF), 2000. 2016 Retrieved from http://ahrf.hrsa.gov/
- U. S. Preventive Services Task Force. Final update summary: Cervical cancer: Screening. 2016 Retrieved from https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/cervical-cancer-screening.
- U.S. Census Bureau. American FactFinder. 2014 Retrieved from http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml.
- U.S. Census Bureau. American national standards institute (ANSI) codes. 2015 Retrieved from https://www.census.gov/geo/reference/ansi_statetables.html.
- U.S. Preventive Services Task Force. Final update summary: Breast cancer: Screening. 2016 Retrieved from https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/breast-cancer-screening1.
- United States Department of Agriculture. Rural-urban continuum codes: Overview. 2013 Retrieved from http://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx.
- Wells BL, Horm JW. Targeting the underserved for breast and cervical cancer screening: The utility of ecological analysis using the national health interview survey. American Journal of Public Health. 1998;88(10):1484–1489. doi: 10.2105/ajph.88.10.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabroff KR, Lawrence WF, King JC, Mangan P, Washington KS, Yi B, Mandelblatt JS. Geographic disparities in cervical cancer mortality: What are the roles of risk factor prevalence, screening, and use of recommended treatment? The Journal of Rural Health: Official Journal of the American Rural Health Association and the National Rural Health Care Association. 2005;21(2):149–157. doi: 10.1111/j.1748-0361.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes & Control: CCC. 2001;12(8):703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes & Control: CCC. 2014;25(1):81–92. doi: 10.1007/s10552-013-0310-1. [DOI] [PubMed] [Google Scholar]
- Zahnd WE, Scaife SL, Francis ML. Health literacy skills in rural and urban populations. American Journal of Health Behavior. 2009;33(5):550–557. doi: 10.5993/ajhb.33.5.8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.