Abstract
In female mammals, up-regulation of Xist triggers X-chromosome inactivation in cis. Up-regulation is inhibited by sequences 3′ to Xist contained within the antisense locus, Tsix. Inhibition could depend on transcription of Tsix and/or on DNA elements therein. Here we test the role of Tsix transcription by augmenting the duration and strength of Tsix expression. We find that Tsix hypertranscription is sufficient to block Xist RNA accumulation in a cis-limited manner. We propose that Tsix transcription is necessary to restrict Xist activity on the future active X and, conversely, that Tsix repression is required for Xist RNA accumulation on the future inactive X. We also find that Tsix hypertranscription does not affect X-chromosome choice. Thus, choice is mediated by elements within Tsix that are independent of promoter activity.
X-inactivation, the transcriptional silencing of a single X chromosome during female embryogenesis, ensures equal dosage of X-linked genes in male (XY) and female (XX) mammals (ref. 1; reviewed in ref. 2). During the process of X-inactivation, a counting mechanism determines X-chromosome number, a choice mechanism randomly selects one active and one inactive X chromosome, and a silencing mechanism operates on the chosen inactive X to shut down expression of nearly all genes in cis. Numerous genetic studies have established the noncoding X-linked gene, Xist, as the trigger for silencing (3–9). At the onset of cellular differentiation, Xist RNA accumulates on all but one randomly selected X in each cell, an event sufficient for X-chromosome silencing (9) and “coating” of the inactive X by Xist transcripts (3, 10).
How Xist RNA accumulation is blocked on the future active X remains poorly understood. With the use of mouse embryonic stem (ES) cells to model X-inactivation in vitro, recent genetic studies have implicated the sequences 3′ to Xist in X-chromosome choice and in regulating Xist accumulation in cis (11, 12). In both studies, female cells heterozygous for 3′ deletions maintain two active X's before differentiation and properly undergo inactivation of a single X upon cell differentiation. However, the mutated X is almost always chosen for Xist accumulation, resulting in severe skewing of X-inactivation in differentiated populations. Thus, elements removed by these deletions function in cis to regulate X-chromosome choice and to prevent Xist RNA accumulation.
The 3.7-kb region common to both deletions spans a CpG-rich domain and the putative promoter of Tsix, a gene antisense to Xist (13). Tsix initiates 15 kb downstream of the Xist 3′ terminus and produces a noncoding transcript extending across the entire Xist locus. The expression pattern of Tsix is consistent with a role in regulating Xist (13). All active X chromosomes express Tsix before differentiation, a time when Xist expression is low. Tsix expression is extinguished before Xist RNA accumulation on the future inactive X. On the future active X, Tsix expression persists until Xist expression is silenced. Thus, the loss of Tsix expression is closely associated with an increased steady-state level of Xist transcripts in cis. Although the consequences of deleting Tsix demonstrate its involvement in X-chromosome choice and in Xist repression, the molecular basis of these two activities has not been established. These activities may depend on DNA sequences within the 3.7-kb region deleted by the TsixΔCpG knockout (12). Alternatively, they may depend on Tsix transcription or on the antisense transcript itself.
In mammals, many antisense genes have been described, especially within domains subject to imprinting (reviewed in ref. 14). In the murine Igf2r locus, a CpG island associated with an oppositely imprinted antisense RNA is required for imprinting of Igf2r (15). In the Prader–Willi/Angelman locus, the maternally expressed UBE3A gene is associated with paternally expressed antisense transcripts (16). As in the case of Xist and Tsix, the functional significance of these antisense transcripts has remained unclear. Specifically, do antisense genes work through DNA elements associated with them, or is their expression necessary for the inhibitory effect on sense genes?
Here we address whether Tsix transcription plays a role in Xist regulation. We augment and extend Tsix expression by inserting a constitutive promoter into one Tsix allele in XX ES cells. This gain of function is sufficient to block Xist accumulation and X-inactivation in cis but does not affect X-chromosome choice. Because this allele removes no sequences, we suggest that altered X-chromosome choice in previous Tsix deletions is caused by the loss of DNA elements that regulate choice independently of antisense transcription. Thus, these results support separate functional roles for Tsix transcription in antagonizing Xist accumulation and for DNA elements in regulating X-chromosome choice.
Materials and Methods
Cell Lines and Culture Conditions.
To generate the targeting vector, a hybrid human EF-1α/HTLV 5′ long terminal repeat promoter (bp 374-1563 of GenBank J04617 fused to bp 373–647 of GenBank J02029) that confers constitutive expression in murine cell lines (F. Randow and B. Seed, personal communication) was coupled to a 1.9-kb BamHI–SalI Tsix fragment and placed in the XhoI site of pLNTK (17). The adjacent 5.8-kb BamHI fragment of Tsix was placed in the SalI site of pLNTK. Tsix sequence was of 129 origin. Linearized targeting vector was electroporated into 16.7 (12), G418- and ganciclovir-resistant colonies were selected (targeting frequency 6/141), and the Neo cassette was excised, all as performed elsewhere (12). ES and embryoid body (EB) cell culture conditions have been described (12). For analysis of differentiation, EBs were allowed to adhere to plates on day 4 to enable cellular outgrowth.
Reverse Transcription–PCR (RT-PCR).
For allele-specific RT-PCR, RNA was isolated with Trizol (GIBCO/BRL), DNase treated (1 unit/5 μg of RNA), and reverse transcribed at 37°C with Moloney murine leukemia virus RT (GIBCO/BRL) and 200 ng of random primer, or at 50°C with Superscript II (GIBCO/BRL) and 3 pmol of strand-specific primer. Single-nucleotide polymorphisms 2.3 kb and 25.3 kb downstream from the Tsix initiation site yield additional MnlI and ScrFI sites respectively, on the Mus musculus castaneus X chromosome. Transcripts containing these polymorphisms were amplified with the use of flanking primers in RT-PCR (30 cycles: 94°C 45 s, 55°C 45 s, 72°C, 1 min). RT-PCR product was diluted 12.5-fold into fresh PCR mixture and cycled once to minimize heteroduplex DNA, digested with MnlI or ScrFI to liberate polymorphic M. m. castaneus and 129 fragments, fractionated on agarose gels, blotted, and detected by hybridization to designated 32P-end-labeled primers. Phosphorimaging (Molecular Dynamics) was used to obtain quantitative measures of relative allelic amounts, as PCR products differ by only a single base substitution and are amplified and detected with primers identical in 129 and M. m. castaneus. Primers A-I shown in Fig. 2A are, respectively, NS30 5′-CCCTGCTTGCTCAACTCTACG-3′, NS31 5′-TTAGCCCGCATCTCACCCAC-3′, NS18 5′-GGTAACAATTTTCCCGCCATGTG-3′, NS22 5′-TGCGATAACTTTCTTTGAGAAGCCTTGGAAGTTGAGACCT-3′, NS19 5′-GGAAATAAACGGAACGCAGTACC-3′, NS33 5′-CAGAGTAGCGAGGACTTGAAGAG-3′, NS67 5′-CCAGAGTCTGATGTAACGGAGG-3′, NS60 5′-CCCGCTGCTGAGTGTTTGATATG-3′, and NS66 5′-GCTGGTTCGTCTATCTTGTGGG-3′. Rrm2 primers are as described (18). For quantitative RT-PCR, Xist and Rpo2–1 were coamplified for 22 cycles with primers NS33, NS66, Rpo2–1a 5′-GGACTAATGGATCCACGGCAG-3′, and Rpo2–1b 5′-GGTCATAGACATGCGTAAGCCG-3′. Oct3/4 was coamplified with Rrm2 for 20 cycles with Rrm2 primers, Oct3a 5′-GGCGTTCTCTTTGGAAAGGTGTTC-3′ and Oct3b 5′-CTCGAACCACATCCTTCTCT-3′. Both reactions were within exponential amplification ranges as determined by pilot experiments. NS66, Oct3a, Rpo2–1a, and Rrm2a were 32P-end-labeled, and RT-PCR products were separated on acrylamide gels.
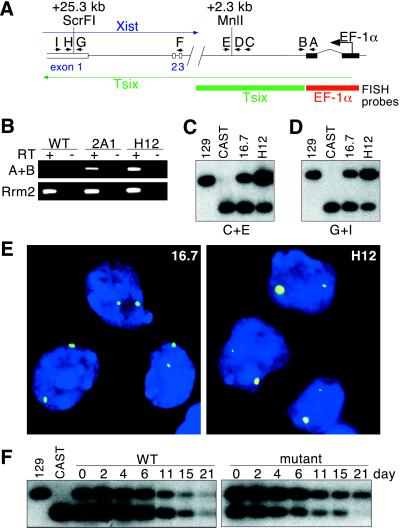
Figure 2.
The EF-1α promoter confers high-level, persistent Tsix expression. (A) Map of the TsixEF-1α locus. Xist exons 1, 2, and 3 are depicted at the left. Locations of primers A–I and distances of M. m. castaneus restriction polymorphisms from the Tsix initiation site are denoted. (B) Transcriptional fusion of the EF-1α leader to TsixEF-1α transcripts. Rrm2 (ribonucleotide reductase M2), positive control. (C) Tsix 5′ allele-specific RT-PCR. cDNA was generated with primer E and amplified with primers C and E. Polymorphic 129 and M. m. castaneus MnlI fragments were detected by hybridization to primer D. No amplification was observed in RT samples (data not shown). (D) Tsix 3′ allele-specific RT-PCR. cDNA was generated with primer I and amplified with primers G and I. Polymorphic ScrFI fragments were detected by hybridization to primer H. (E) RNA-FISH of undifferentiated wild-type (16.7) and mutant (H12) cells, with double-stranded EF-1α (red) and Tsix (green) probes shown in A. (F) Allele-specific RT-PCR analysis of Tsix in wild-type (16.7) and mutant (2B1) cells on different days of differentiation. Random-primed cDNA was amplified with primers C and E, and MnlI fragments were detected with D.
Fluorescence in Situ Hybridization (FISH).
Double-stranded probes for EF-1α, Tsix, and Xist were labeled with tetramethylrhodamine-5-dUTP or fluorescein-12-dUTP and used in RNA-FISH as described (12). Single-stranded Tsix and Xist riboprobe cocktails were generated by in vitro transcription with fluorescein-12-UTP and digoxigenin-11-UTP, respectively, and visualized with rhodamine-conjugated anti-digoxigenin antibody (Roche Molecular Biochemicals). Mecp2 probes were as described (12). Xist probe cocktails were complementary to exons 1 and 7.
Cell Death Analysis.
Sedimented day 2 and 4 EBs were dispersed by trypsinization and stained with trypan blue. Live and dead cells were scored on a hemacytometer, and the percentage of dead cells was calculated. Alternatively, whole cultures (including supernatant and EBs) were counted. The results were similar in the two cases.
Results
Insertion of the EF-1α Promoter Provides High-Level and Persistent Tsix Expression.
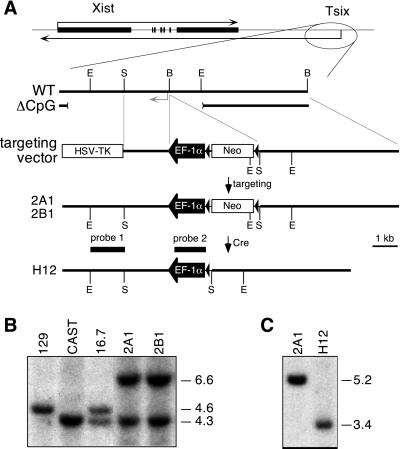
We inserted the constitutive human EF-1α promoter upstream of the major Tsix transcription start sites (TsixEF-1α allele, Fig. 1A) by homologously targeting one allele in XX ES cells. The 16.7 ES line carries polymorphic X chromosomes derived from M. m. castaneus (Xcas) and 129 (X129) strains (12). As determined by an EcoRI polymorphism (19) flanking the targeted region, all insertion events occurred on X129 (Fig. 1B; data not shown). Mutant lines containing (lines 2A1, 2B1; Fig. 1B) and lacking (line H12; Fig. 1C) the PGK-Neo selection marker were analyzed in parallel. All lines behaved similarly.
Figure 1.
Construction of the TsixEF-1α allele. (A) Map of the Xist/Tsix locus and targeting scheme. Gray arrow, Tsix transcription initiation site (ref. 13; RNase protection; N.S., unpublished results). Dark triangles, loxP sites. ΔCpG indicates the region removed by the Tsix knockout (12). B, BamHI; E, EcoRI; S, SalI. (B) Southern blot analysis of controls and two representative mutant lines, 2A1 and 2B1. Genomic DNA was digested with EcoRI and hybridized to probe 1. 129 and M. m. castaneus EcoRI fragments are polymorphic (19). (C) Southern blot analysis of parental 2A1 and Neo-excised subclone H12. H12 was derived from 2A1 by transient expression of Cre recombinase. Genomic DNA was digested with SalI and hybridized to probe 2.
In the undifferentiated state, mutant and wild-type cells grew indistinguishably. RT-PCR analysis indicated that transcription was correctly initiated within the EF-1α promoter and extended into Tsix (Fig. 2 A and B). An allele-specific RT-PCR assay able to distinguish Xcas and X129 Tsix transcripts revealed that TsixEF-1α increased the steady-state level of Tsix RNA in cis (Fig. 2 C and D). In 16.7 cells, 35% of total Tsix RNA was derived from X129. In contrast, 60% was of X129 origin in mutant cells. Assuming that transcription from Xcas was similar in 16.7 and mutant cells, this difference indicated that TsixEF-1α increased steady-state RNA levels by approximately 3-fold in cis. This elevation was observed at 5′ and 3′ positions within Tsix (Fig. 2 A, C, and D), suggesting that transcripts initiated from the EF-1α promoter proceeded through at least 25 kb of Tsix and covered most of the Xist locus. RNA FISH analysis also showed that TsixEF-1α was overexpressed. Like wild-type cells, all (>95%) undifferentiated mutant cells showed two nuclear Tsix RNA foci but differed in that the TsixEF-1α signal was larger and more intense than that of the untargeted allele (91% with X129 > Xcas, n = 277; Fig. 2E). Together, these data showed that insertion of the EF-1α promoter increased the strength of Tsix expression in cis.
Tsix expression is asynchronously down-regulated in female cells during differentiation, with down-regulation occurring first on the future inactive X and subsequently on the future active X after Xist expression is silenced (13). This finding has raised the possibility that persistence of Tsix expression on one X is critical for maintaining its active state. To determine whether insertion of the EF-1α promoter extended the duration of Tsix expression from X129, we cultured ES cells in the absence of LIF for 2 to 21 days to induce differentiation into EBs (20), a state that normally induces down-regulation of Tsix. As in undifferentiated cells, we found that, whereas wild-type 16.7 cells expressed more Tsix RNA from Xcas, mutant cells consistently displayed more TsixEF-1α RNA from X129 throughout differentiation (Fig. 2F). Moreover, the fraction of total Tsix RNA originating from TsixEF-1α increased progressively during differentiation, suggesting not only that TsixEF-1α conferred stronger expression, but that expression persisted beyond that of the unmodified Tsix allele on Xcas.
To test this idea further, we performed FISH on mutant cells that were placed under differentiation conditions for 6 to 13 days. On day 6, Tsix expression from the mutant locus was detectable in nearly all cells. In cells with monoallelic Tsix signals, two-color FISH with EF-1α and Tsix probes indicated that antisense transcripts nearly always originated from the TsixEF-1α allele (99%, n = 108). On day 13, Tsix RNA was still present in >60% of mutant cells, whereas it was seen in <5% of wild-type cells. In mutant cells, Tsix signals originated only from TsixEF-1α (data not shown). Notably, monoallelic Tsix expression was not detected from Xcas on either day 6 or day 13 cultures. These findings provided further evidence that the EF-1α promoter conferred persistent expression under differentiation conditions.
TsixEF-1α Suppresses Xist Accumulation in cis.
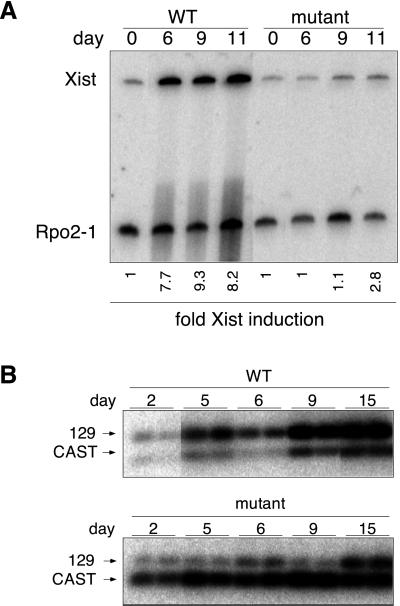
We examined the effects of TsixEF-1α on Xist expression when mutant cells were placed under differentiation conditions to induce X-inactivation. As demonstrated by quantitative RT-PCR, Xist expression in both mutant and wild-type cells was low before the onset of X-inactivation (Fig. 3A). This observation indicated that Tsix overexpression did not affect steady-state Xist levels before initiation of X-inactivation. Upon placement in differentiation conditions, wild-type cells showed an 8.2-fold induction of Xist relative to RNA polymerase II large subunit (Rpo2–1). In contrast, mutant cells exhibited only a 2.8-fold induction (Fig. 3A). This reduced level of Xist induction might reflect compromised Xist induction in all mutant cells or, alternatively, compromised Xist induction in a subset of the population. In the latter case, the overall level as measured by RT-PCR would be the weighed average of Xist expression across different cell populations.
Figure 3.
Effects of TsixEF-1α on Xist expression under differentiation conditions. (A) Quantitative RT-PCR for Xist expression in wild-type (16.7) and mutant (H12) cells placed under differentiation conditions for the number of days shown. Xist levels were normalized to Rpo2–1 levels; fold Xist induction reflects the change from day 0. (B) Allele-specific Xist RT-PCR of wild-type (16.7) and mutant (2B1) cells. Amplification was carried out with primers F and H (spanning Xist introns, as shown in Fig. 2A) and polymorphic ScrFI fragments detected with G.
We next used allele-specific RT-PCR to examine Xist induction from wild-type and mutant X chromosomes. In the parental 16.7 line, Xist from X129 accounted for 85% of total Xist RNA (Fig. 3B). This skewing reflects biased X-chromosome choice found in 129/castaneus hybrid mice and has been attributed to effects of the X-controlling element (Xce) modifier (21). Xcas carries a strong Xcec relative to X129 (Xcea) and is more likely to remain active (22). Analysis of mutant cultures revealed a striking reversal of the 129/castaneus Xist ratio, with X129 providing only 10–15% of total Xist RNA (Fig. 3B). Therefore, despite an intrinsic bias for expressing the 129 allele in the parental cell line, Xist accumulation in mutant cells was chiefly from M. m. castaneus from days 2 to 9. In late day cultures, the expression of the 129 allele increased somewhat, a finding we believe to stem from growing numbers of poorly differentiated cells specific to late-day mutant cultures (see below; these ES-like cells apparently expressed low levels of Xist from both X chromosomes).
The results of these RT-PCR experiments suggested that reduced total Xist expression in mutant cultures resulted from the failure to up-regulate Xist from the mutant X129 chromosome. To address this possibility further, we analyzed single cells with RNA FISH, as described below.
X-Chromosome Choice: Two Distinct Cell Fates in Mutant Cultures.
To perform single-cell analysis, ES cells were placed under differentiation conditions for 9–15 days to obtain EB outgrowths for RNA FISH. In this assay, small groups of ES cells aggregate and grow into EBs for 4 days in suspension culture. On day 4, the cells are allowed to adhere to the culture dish, whereupon differentiating cells migrate from the EB core as a monolayer of mixed cell types (20). Wild-type cultures consistently yielded large, well-differentiated EBs (Fig. 4A) with typical structures such as beating hearts and tubular networks (Fig. 4B). High-level Xist expression was present in >90% of wild-type cells (n > 2,000; Fig. 4C). Analysis of mutant cultures revealed a dramatically different phenotype, with differentiation conditions yielding two distinct EB types.
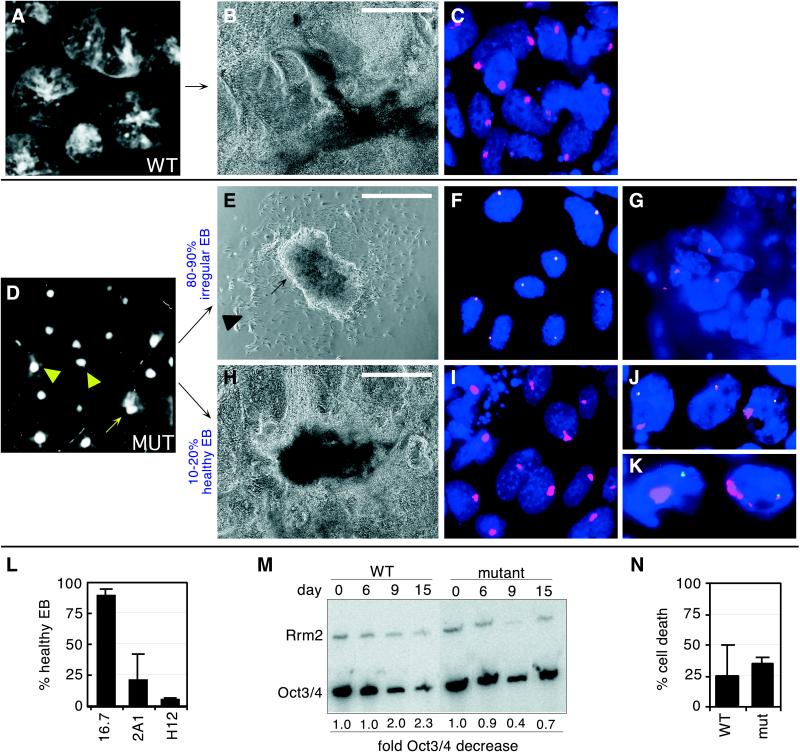
Figure 4.
Differentiation conditions yield two types of mutant cells. (A) Dark-field micrograph of control EBs from 16.7 cells on day 9. (B) A phase-contrast micrograph of day 15 16.7 EBs shows well-differentiated structures. (Scale bar = 100 μm.) (C) RNA FISH with single-stranded Xist probes (red) of day 9 16.7 EB cells. (D) Dark-field micrograph of typical mutant EB cultures on day 9 (H12). Triangles: small, irregular EBs; the EB on the left contains one sector of differentiation. Arrow: medium-sized, healthy EB with many sectors of well-differentiated cells. (E) Phase-contrast micrograph of a typical poorly differentiated day 15 mutant EB (H12). The arrow points to a poorly differentiated ES-like cluster. The triangle points to a partially differentiated outgrowth. (F) RNA FISH of cells in a representative small irregular EB, with EF-1α (green) and double-stranded Tsix probes (red). Experiments with Tsix-specific probes yielded identical results (data not shown). EF-1α signals identify X129. (G) RNA FISH of cells from a representative irregular EB, with single-stranded Xist probes (red), on day 9 mutant cells (H12). (H) A representative healthy mutant EB (H12). (I) RNA FISH of a representative healthy mutant EB on day 9, with single-stranded Xist probes (red). (J) RNA FISH of mutant (H12) cells on day 4, with double-stranded probes to EF-1α (green) and Xist (red). (K) RNA FISH of day 4 mutant (H12) cells, with double-stranded Xist (red) and Mecp2 probes (green). (L) Percentage of EBs showing healthy growth in 16.7 (wild type), 2A1, and H12 (mutant). (M) Quantitative RT-PCR for Oct3/4 expression in wild-type (16.7) and mutant (H12) cells placed under differentiation conditions for the number of days shown. Oct3/4 levels were normalized to Rrm2 levels; the fold Oct3/4 decrease reflects the change from day 0. (N) Cell death analysis of wild-type (16.7) and mutant (H12) EBs. Results indicate the average of day 2 and day 4 samples. Data represent the average of at least four independent experiments. Error bars indicate one standard deviation.
The more prevalent type of EB was strikingly small and accounted for 80–90% of the total (Fig. 4 D and L). These EBs showed irregular growth, with some sectors of the EB growing more robustly than others (Fig. 4D, triangles). The small irregular EBs lacked beating hearts, tube networks, and other obvious differentiated structures. Interestingly, they had large central cell clusters that resembled undifferentiated ES colonies, in that the clusters had smooth borders and bright, refractile centers (Fig. 4E, arrow). These clusters were often surrounded by cells that appeared to be partially differentiated in morphology (Fig. 4E, triangle). In contrast to cells of wild-type EBs, cells in these EBs continued to express Tsix from the mutant X chromosome (Fig. 4F; 85–90% with expression, n > 2,000) and lacked Xist RNA accumulation (85–90% of cells without Xist signals, n > 2,000; Fig. 4G). Thus, small irregular EBs were dominated by cells that failed to undergo X-inactivation. As prior studies have linked cell differentiation and X-inactivation (23, 24), we surmise that the poor overall differentiation in these EBs resulted from the inability of most cells to undergo X-inactivation.
The second type of EB had an intermediate size and accounted for 10–20% of the total (Fig. 4D, arrow, and Fig. 4L). As in wild-type EBs, differentiated structures, including beating hearts and tubular networks, were regularly observed (Fig. 4H). Similarly, high-level Xist expression was seen in ≈90% of differentiating outgrowths (Fig. 4I, n > 2,000). TsixEF-1α RNA signals could still be detected in 50–60% of cells within healthy EBs and, importantly, did not colocalize with Xist RNA accumulation. The remaining 40% of cells could not be scored because EF-1α RNA signals were not visible, and attempts at EF-1α DNA FISH were unsuccessful because of the small size (1.4 kb) of the EF-1α probe. To circumvent this problem, we examined earlier day cultures where TsixEF-1α RNA was expressed in >95% of all cells. On day 4 (Fig. 4J), two-color RNA FISH revealed that 95% (n = 127) of EF-1α signals were spatially distinct from high-level Xist signals. Thus, Xist accumulation occurred almost exclusively from Xcas. This pattern of nonrandom X-inactivation was consistent with the results of allele-specific RT-PCR (Fig. 3B) and suggested that transcription from TsixEF-1α is sufficient to block Xist RNA accumulation in cis. On Xcas, accumulation of Xist RNA led to silencing of Mecp2 (87%, n = 281; Fig. 4K), indicating that the silencing step of X-inactivation was not affected by the TsixEF-1α allele expressed in trans. Unlike in the small irregular EBs, the abundance of cells that underwent successful, albeit skewed, X-inactivation in the healthy EBs probably permitted the formation of well-differentiated structures.
The poor overall differentiation of mutant cultures was consistent with higher levels of the ES-specific marker Oct3/4 (25) (Fig. 4M). Because failure to compensate for X-linked gene dosage is toxic to development (7, 26), the in vitro fate of cells that failed to up-regulate Xist was of some interest. Cell death was not elevated in mutant cultures (Fig. 4N). Instead, there was an increase in cell number within the poorly differentiated ES-like clusters during late-day culture (Fig. 4E and data not shown). This result suggested that cells that failed to up-regulate Xist were able to remain viable in a poorly differentiated state.
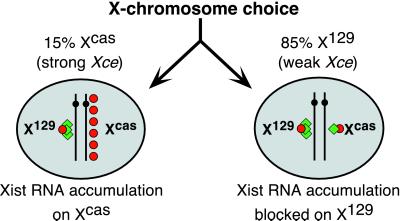
Thus, placing the mutant cells in differentiation conditions yielded two cell fates. In one, Xist could not be up-regulated and cells remained poorly differentiated in appearance. In the other, Xist RNA could accumulate to high levels, but did so primarily from Xcas. These cells showed healthy differentiation. In both classes of cells, expression from TsixEF-1α was observed. We propose that the two fates reflected an epigenetic decision made before the initiation of Xist expression. Specifically, we suggest that the decision regarding X-chromosome choice was not affected by TsixEF-1α. In this model (Fig. 5), mutant cells would retain the ability to count and randomly choose one active and one inactive X. In the parental cell line (16.7), X129 was inactivated 85% of the time. We suggest that, likewise, the mutant cell lines chose X129 for inactivation 85% of the time, but that high-level and persistent antisense transcription from TsixEF-1α precluded accumulation of Xist RNA in cis. In this model, mutant cells would choose Xcas for inactivation 15% of the time. Indeed, we found that an average of 10–20% of cells from all mutant EBs showed a high level of Xist expression. The random distribution of Xist-expressing and -nonexpressing cells among different EB colonies would then determine the degree of differentiation within individual EBs and thereby determine whether they appeared irregular or healthy.
Figure 5.
Model: X-chromosome choice is not disrupted by the TsixEF-1α allele. Xist RNA, red circles; Tsix RNA, green diamonds. See text for details.
Although we favor this model, we also considered alternative explanations for the two cell fates. One possibility was that the EF-1α insertion affected X-chromosome counting and prevented X-inactivation as a result. This possible outcome appeared less likely to us, however, because 10–20% of the cells properly inactivated one X chromosome, with inactivation highly specific for Xcas. Another possibility was that the EF-1α insertion nonspecifically affected cell differentiation. This explanation was also not favored, because a reproducible subpopulation of mutant cells could differentiate and form healthy EBs. Furthermore, the extreme bias toward inactivating Xcas cannot be explained by a defect in cell differentiation alone. For these reasons, our data support the explanation that expression from TsixEF-1α provides a secondary block to Xist accumulation and X-inactivation without affecting X-chromosome choice.
Discussion
We have addressed the question of whether transcription plays a role in the inhibitory effect of Tsix on Xist by creating a gain of function in Tsix transcription. Insertion of the constitutive human EF-1α promoter enabled us to distinguish the effect of transcription from that of specific DNA elements. Although the TsixEF-1α allele did not delete any sequence within Tsix, it is possible that the disruption of endogenous sequence by the EF-1α insertion could have contributed to the phenotype. We consider this outcome unlikely, however, given that a disruption would have more likely yielded a loss-of-function than a gain-of-function phenotype. Importantly, the TsixEF-1α allele produced a pattern of nonrandom inactivation that is the opposite of that in the TsixΔCpG loss-of-function mutation, a mutation that led to nearly exclusive inactivation of the mutant X. In the gain-of-function mutant, we showed that augmentation of Tsix transcription was sufficient to block Xist accumulation on the mutant X chromosome. This result demonstrates that Tsix transcription is indeed functional.
Our results directly support the hypothesis that silencing of Tsix expression is a prerequisite for Xist up-regulation on the future inactive X. Consistent with this hypothesis, prior study showed that Tsix is repressed before or around the same time that Xist RNA accumulates in cis (13). Furthermore, Xist transgenes lacking the 5′ end of Tsix and therefore presumably lacking antisense expression are all permissive for high-level Xist expression (8, 9). We also suggest that, conversely, Tsix transcription on the future active X is necessary to maintain X-chromosome activity in female cells. Indeed, Tsix persists transiently on the presumptive active X even after Xist RNA has accumulated on the newly inactivated X (13). Inhibition of Xist may require either increased levels of Tsix expression or a mere prolongation of Tsix transcription. The means by which Tsix transcription inhibits Xist accumulation is not yet clear; the act of Tsix transcription could interfere with Xist accumulation indirectly, or, alternatively, inhibition could depend on the antisense RNA product.
Finally, our findings uncover a role for Tsix that is independent of transcription. The disruption of X-chromosome choice by TsixΔCpG, but not by TsixEF-1α, implies that a DNA element within the 3.7 kb removed by TsixΔCpG mediates the act of choice. Classic models of X-inactivation have postulated the existence of a “blocking factor,” a trans-acting factor that mediates choice by binding to one X and preventing Xist from initiating chromosome-wide silencing (reviewed in ref. 27). It is therefore possible that the DNA element implied by our results acts as a binding site for the putative blocking factor. Binding of the factor could elevate or prolong Tsix transcription and thereby block Xist up-regulation. In this model, Tsix transcription is epistatic to choice and is itself sufficient to prevent the initiation of long-range silencing of the X chromosome.
The abundance of noncoding and antisense genes within imprinted regions has suggested that they are critical to the mechanism of allelic exclusion. The present work establishes the importance of their transcription. Our findings contrast with the report that H19 and its transcription are dispensable for imprinting Igf2 (28, 29). Antisense transcripts have also been reported for Igf2 (30), but their significance remains unknown at this time. The antisense gene, Air, within the Igf2r locus contains necessary information for Igf2r imprinting (15), but whether its transcription is important also remains unclear. Within the Prader–Willi/Angelman syndrome region, many imprinted genes have associated antisense transcription or are themselves genes for noncoding RNAs (reviewed in ref. 31). Given the diverse nature of these transcripts and their associated imprinted domains, it is likely that many mechanisms are involved in the regulation of imprinting. Antisense transcription may be only one of many mechanisms.
Acknowledgments
We thank B. Seed and F. Randow for the EF-1α promoter; S. Dymecki for Cre reagents; J. Urbach and D. Wilson for discussion of heteroduplex formation; G. Lazar for aid with photography; and W. Chao, D. Cohen, J. Conaty, K. Huynh, C. Kaplan, R. Spencer, F. Winston, and T. Wu for critical reading of the manuscript and discussions. This work was supported by Howard Hughes Medical Institute Predoctoral and Ryan fellowships (to N.S.) and grants from Hoechst, the March of Dimes (Basil O'Connor Scholarship), and the National Institutes of Health (to J.T.L.). J.T.L. is also a Pew Scholar and an assistant investigator for the Howard Hughes Medical Institute.
Abbreviations
- ES
embryonic stem
- EBs
embryoid bodies
- RT-PCR
reverse transcription–PCR
- FISH
fluorescence in situ hybridization
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 10025.
References
- 1.Lyon M F. Nature (London) 1961;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- 2.Heard E, Clerc P, Avner P. Annu Rev Genet. 1997;31:571–610. doi: 10.1146/annurev.genet.31.1.571. [DOI] [PubMed] [Google Scholar]
- 3.Brown C J, Hendrich B D, Rupert J L, Lafreniere R G, Xing Y, Lawrence J, Willard H F. Cell. 1992;71:527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- 4.Brockdorff N, Ashworth A, Kay G F, McCabe V M, Norris D P, Cooper P J, Swift S, Rastan S. Cell. 1992;71:515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- 5.Penny G D, Kay G F, Sheardown S A, Rastan S, Brockdorff N. Nature (London) 1996;379:131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- 6.Lee J T, Strauss W M, Dausman J A, Jaenisch R. Cell. 1996;86:83–94. doi: 10.1016/s0092-8674(00)80079-3. [DOI] [PubMed] [Google Scholar]
- 7.Marahrens Y, Panning B, Dausman J, Strauss W, Jaenisch R. Genes Dev. 1997;11:156–166. doi: 10.1101/gad.11.2.156. [DOI] [PubMed] [Google Scholar]
- 8.Herzing L B, Romer J T, Horn J M, Ashworth A. Nature (London) 1997;386:272–275. doi: 10.1038/386272a0. [DOI] [PubMed] [Google Scholar]
- 9.Wutz A, Jaenisch R. Mol Cell. 2000;5:695–705. doi: 10.1016/s1097-2765(00)80248-8. [DOI] [PubMed] [Google Scholar]
- 10.Clemson C M, McNeil J A, Willard H F, Lawrence J B. J Cell Biol. 1996;132:259–275. doi: 10.1083/jcb.132.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerc P, Avner P. Nat Genet. 1998;19:249–253. doi: 10.1038/924. [DOI] [PubMed] [Google Scholar]
- 12.Lee J T, Lu N. Cell. 1999;99:47–57. doi: 10.1016/s0092-8674(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 13.Lee J T, Davidow L S, Warshawsky D. Nat Genet. 1999;21:400–404. doi: 10.1038/7734. [DOI] [PubMed] [Google Scholar]
- 14.Tilghman S M. Cell. 1999;96:185–193. doi: 10.1016/s0092-8674(00)80559-0. [DOI] [PubMed] [Google Scholar]
- 15.Wutz A, Smrzka O W, Schweifer N, Schellander K, Wagner E F, Barlow D P. Nature (London) 1997;389:745–749. doi: 10.1038/39631. [DOI] [PubMed] [Google Scholar]
- 16.Rougeulle C, Cardoso C, Fontes M, Colleaux L, Lalande M. Nat Genet. 1998;19:15–16. doi: 10.1038/ng0598-15. [DOI] [PubMed] [Google Scholar]
- 17.Gorman J R, van der Stoep N, Monroe R, Cogne M, Davidson L, Alt F W. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]
- 18.Lee J T, Jaenisch R. Nature (London) 1997;386:275–279. doi: 10.1038/386275a0. [DOI] [PubMed] [Google Scholar]
- 19.Debrand E, Chureau C, Arnaud D, Avner P, Heard E. Mol Cell Biol. 1999;19:8513–8525. doi: 10.1128/mcb.19.12.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin G R, Evans M J. Proc Natl Acad Sci USA. 1975;72:1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattanach B M, Isaacson J H. Genetics. 1967;57:331–346. doi: 10.1093/genetics/57.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cattanach B M, Rasberry C. Mouse Genome. 1994;92:114. [Google Scholar]
- 23.Monk M, Harper M I. Nature (London) 1979;281:311–313. doi: 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- 24.Martin G R, Epstein C J, Travis B, Tucker G, Yatziv S, Martin D W, Jr, Clift S, Cohen S. Nature (London) 1978;271:329–333. doi: 10.1038/271329a0. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H. Cell. 1990;60:461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- 26.Takagi N, Abe K. Development (Cambridge, UK) 1990;109:189–201. doi: 10.1242/dev.109.1.189. [DOI] [PubMed] [Google Scholar]
- 27.Lyon M F. Nature (London) 1996;379:116–117. doi: 10.1038/379116a0. [DOI] [PubMed] [Google Scholar]
- 28.Jones B K, Levorse J M, Tilghman S M. Genes Dev. 1998;12:2200–2207. doi: 10.1101/gad.12.14.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt J V, Levorse J M, Tilghman S M. Proc Natl Acad Sci USA. 1999;96:9733–9738. doi: 10.1073/pnas.96.17.9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore T, Constancia M, Zubair M, Bailleul B, Feil R, Sasaki H, Reik W. Proc Natl Acad Sci USA. 1997;94:12509–12514. doi: 10.1073/pnas.94.23.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann M R W, Bartolomei M S. Hum Mol Genet. 1999;8:1867–1873. doi: 10.1093/hmg/8.10.1867. [DOI] [PubMed] [Google Scholar]