Abstract
Until the law in the United Kingdom (UK) changed in May 2016 so called “legal highs” or “new psychoactive substances” were freely available in high street shops across the UK. Following prohibition these drugs are still easily purchased illegally via the internet. We report a case of a patient who self-administered 3-fluorophenmetrazine intravenously with catastrophic consequences. Adverse effects were almost immediate with symptoms of malaise and tachycardia. Two days post administration he was transferred to the intensive therapy unit with acute kidney injury and irreversible four limb ischaemia. He required a period of renal replacement therapy and bilateral lower limb amputation. This case highlights the fact that new psychoactive substances have many unintended adverse effect which have not been previously described. Multiple routes of administration are used by people taking these agents including intravenously. Medical practitioners should always consider ingestion of new psychoactive substances in the differential diagnosis of acutely ill patients.
Keywords: Acute kidney injury, New psychoactive substance, Limb ischaemia
Introduction
Drugs previously known as ‘legal highs’ also referred to as ‘research chemicals’, ‘new psychoactive substances’ (NPS) or ‘club drugs’ are chemicals that have similar effects to illegal drugs, but until very recently remained legal to purchase in the UK. Many drugs had been made illegal under the misuse of Drugs Act 1971 [1]. This provision was insufficiently broad to legislate for the actions of some modern chemists who are intent on circumventing the law. The problem was that as soon as a new drug was identified, the Home Office placed a temporary ban on the chemical while it decided whether the drug should be permanently banned. By the time agents are banned chemists had responded by slightly altering the molecular structure making a new subtly different drug with similar effects. Crucially the fact that these drugs were legal does not mean that they are safe or approved for human use, many vendors labeled them as ‘not fit human consumption’. Psychoactive substances were made illegal in the UK in 2016 [2].
3-Fluorophenmetrazine (3-FPM) also known as PAL-593 was introduced onto the market via the internet in around 2014, since that time it has become increasingly popular. The drug won the VICE Netherlands Designer Drug Awards 2014 “Newest Drug of 2014 Award” [3]. It was legal in the UK until May 2016, it had already been made an illegal substance in Switzerland and Sweden. 3-FPM is one of many phenylmorpholines designed to treat obesity or ameliorate drug dependence [4]. It is suggested that it has properties similar to amphetamines associated with monoamine release [5]. There are many unofficial reports of effects available on the internet, supplied by users. It seems that the majority of reported effects are as a stimulant, however, there are few reports of its precise action [6]. We report a case of dialysis dependent acute kidney injury, four limb ischaemia resulting in bilateral lower limb amputation and loss of digits on his left hand, this occurred as a result of injection of 3-FPM intravenously. This was the first use of this drug by the patient; it was purchased as an alternative to Methiopropamine (MPA).
Case report
A 52-year-old man was brought into hospital by ambulance after he was found in bed confused and incontinent of urine, he had a short history of worsening symptoms over 2 days. The patient was a known intravenous drug user with a previous history of infective endocarditis. He had a bovine mitral valve replacement performed at central London hospital the previous year. He was reported to have injected a drug called 3-FPM, the drug was purchased online 3 days prior to presentation. He denied any recent or co-use of other non-prescription drugs he had discontinued MPA some days prior to administration because he had been unable to obtain it following prohibition. He had purchased this experimental drug as an alternative to MPA which he had been using regularly. The drug was bought in powdered/crystalline form (Fig. 1). He completely dissolved in water without any residue and then injected into his arm. On the same day, after injecting the drug he started to develop flu-like symptoms feeling feverish with general malaise and tachycardia. Over the next 2 days the symptoms worsened, he started to develop symptoms of shortness of breath, a productive cough of white sputum, central chest pain, fever with rigors and multiple episodes of diarrhea and vomiting. He also complained of cold lower limbs with reduced sensation in both legs.
Fig. 1.
Packaging and powder labeled as 3-fluorophenmetrazine. Packaging and presentation of 3-fluorophenmetrazine as a drug for reference use only and not for human consumption sold prior to Psychoactive Substances Act 2016
He lived alone and was previously completely self-caring with a full time job. He had a long term partner of 10 years. He was a lifelong smoker until November 2014. There was no history of alcohol ingestion.
He had previously been diagnosed with attention deficit hyperactivity disorder (ADHD) and depression. In 2014 he developed cord compression at C1/C2 secondary to an abscess at the same time he was diagnosed with endocarditis. He had made a full recovery following treatment for endocarditis which included a long course of antibiotics and a bovine valve replacement. There was no history of chronic kidney disease, diabetes or hypertension.
On admission the patient was treated for suspected sepsis and acute kidney injury (AKI) with intravenous fluids and broad spectrum antibiotics.
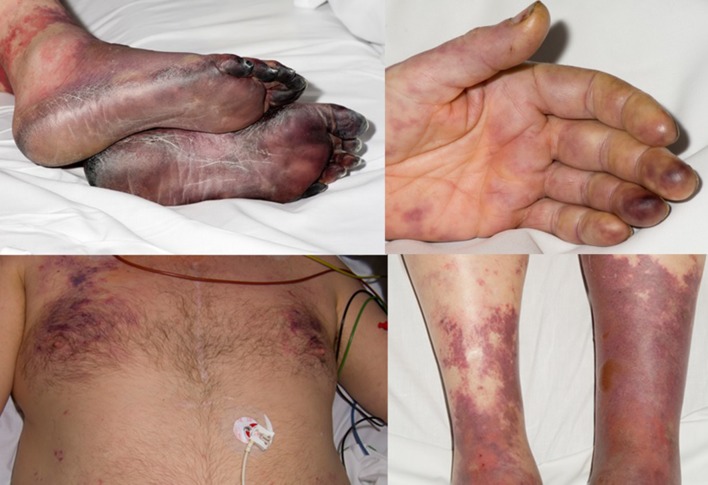
He subsequently developed atrial fibrillation with a rapid ventricular rate and was treated with amiodarone. After 1 day his condition deteriorated and he was transferred into Intensive Therapy Unit, he was hypotensive (BP 95/50 mmHg), respiratory rate 31, pulse rate 97 bpm and temperature 35.2 °C. All four limbs became rapidly ischaemic (Fig. 2) he also had widespread Livedo reticularis.
Fig. 2.
Ischaemic Limbs, Livedo Reticularis. Photographs taken on day 3 post admission with the consent of the patient demonstrating widespread livedo reticularis on the trunk and limbs and critical ischaemia of the lower limbs and fingers
Urinalysis was not performed on admission because the patient was anuric, a urethral catheter was passed. His urine output started to improve after 20 days at that time he did not have significant proteinuria, he had invisible haematuria as expected due to urinary tract instrumentation. Transthoracic echocardiography was performed, there was no evidence of vegetation or significant change in valvular function compared to the previous studies. Bloods showed C3 0.67 g/L (0.75–1.45), C4 0.11 g/L (0.14–0.54), C-reactive protein 188 mg/L, creatinine kinase was 187 U/L. Repeated blood cultures showed no significant bacterial growth, ANA, ANCA and DS-DNA were all negative. Clotting studies, including fibrinogen and thrombin time were normal. Analysis of the drug demonstrated 3-FPM only (City Hospital Birmingham), at the time of the analysis it was noted that the package was mislabelled as containing 1-propionyl-lysergic acid diethylamide which it did not.
The patient was started on continuous veno-venous haemofiltration to manage his acute kidney injury he required renal replacement therapy for 26 days. He received broad spectrum antimicrobials to cover Gram positive, and negative organisms as well as antifungals. In addition, he was treated with epoprostenol sodium intravenously to try and improve peripheral blood flow, he did not receive glucocorticoids during the course of his illness. His respiratory function was supported with hi-flow oxygen, he did not require mechanical ventilation.
He was reviewed regularly by the vascular surgical team. Initially he was too unstable to undergo surgery, however, after 2 weeks he was taken to theater for a below knee amputation of his right leg, a week later he underwent a left below knee amputation. Four weeks following admission his renal function started to recover and he no longer required renal replacement therapy. His ischaemic hands remained a problem on the ward; however, his right hand appeared to have completely resolved with only severe necrosis in his left fourth metatarsal and tips of his other digits on his left hand.
Discussion
The combination of limb ischaemia, livedo reticularis, fever and raised inflammatory markers clearly raises the possibility of systemic inflammatory response syndrome secondary to sepsis, with or without disseminated intravascular coagulation (DIC), his CRP and marginally low complement levels would have supported this, and there was no evidence of DIC based on his laboratory tests. His blood cultures were repeatedly negative although culture negative sepsis occurs in up to 40% of cases [7]. The differential diagnosis also includes cryoglobulinemia, intra-arterial injection, cholesterol embolization, Purpura fulminans and anti-phospholipid syndrome. His symptoms may have been a manifestation of systemic vasculitis secondary to systemic lupus erythematosus, rheumatoid arthritis or dermatomyositis; however, clinical and serological evidence for these disorders was lacking. There have been some unconfirmed reports of vasculitis associated with the use of 3-FPM. Intra-arterial injection remains a possibility, but would not explain four limb ischaemia and widespread livedo. Cholesterol embolization is a possible explanation for the development of critical limb ischaemia, livedo and acute kidney injury, against this differential is a normal eosinophil count and the recovery of renal function. Patients with cholesterol emboli may recover renal function, but in the context of such catastrophic limb effects the mortality rate is reported as approximately 90%. Purpura fulminans has a similar presentation to this case, it is possible that the patient developed this as a direct result of the chemical agent, against this differential is the fact that the patient did not develop laboratory features of DIC. The differential diagnosis also includes infective endocarditis leading to AKI, sepsis and limb ischaemia, transthoracic echocardiography does not exclude a vegetation particularly in the presence of a prosthetic heart valve. The course of his illness including the stability of cardiac function and full recovery of renal function (recovery GFR 60 ml/min/1.73 m2) are against this as the underlying cause. Finally Buerger’s disease (Thromboangiitis obliterans) may have been an underlying cause of his four limb ischaemia; however, in this case the clinical course was very short which is not in keeping with the usual presentation of this disorder.
In this case the patient developed irreversible lower limb ischaemia resulting in the need for amputation in association with acute kidney injury, which recovered. This suggests that the underlying aetiological factor resulted in limb ischaemia with secondary acute tubular necrosis. Another possibility is that the mechanism of injury was different, it is possible that the administration of the drug resulted in a direct nephrotoxic effect or tubulointerstitial nephritis.
We suggest that the injected agent which is said to be amphetamine like in its action caused significant widespread vasoconstriction with or without concomitant infection resulting in widespread ischaemia out of proportion to the clinical feature of sepsis. The underlying cause of both critical limb ischaemia and acute kidney injury are likely to be the same namely ischaemia, however, recovery from acute tubular necrosis is known to occur even in the context of a significant period of ischaemia. Websites reporting adverse effects of this agent when taken orally for recreational purposes describe cold peripheries as a common adverse effect, this supports the hypothesis that the drug when injected resulted in four limb ischaemia. The clinical features are in keeping with adverse events associated with the use of this drug in the STRIDA project in Sweden. Backberg et al. reported 19 cases of people testing positive for 3-FPM, 5 of which were reported as having severe intoxication, prominent clinical signs were tachycardia (47%), depressed consciousness (42%), agitation/anxiety (37%), delirium (37%), dilated pupils (26%), and seizures (16%). The authors did note that all of the patients tested positive for other psychoactive compounds thus making it difficult definitely attribute adverse effects to 3-FPM alone [8]. Our patient administered 3-FPM intravenously, there are no studies on the relative bioavailability of oral and intravenous administration. The metabolism of 3-FPM is poorly understood, it has a chemical structure similar to phenmetrazine which was marketed as an anorectic, but is no longer available or legal. Phenmetrazine has a half-life of approximately 8 h after oral administration. Metabolism of the drug is primarily hepatic (via CYP3A and CYP2D6), it is resistant to metabolism by monoamine oxidase. Metabolism involves deamination to para-hydroxyamphetamine and phenylacetone. It is likely that the effects would be enhanced when administered intravenously due to avoidance of first pass metabolism and poor absorption via the GI tract.
This report serves to provide further evidence to support measures which may reduce the use of drugs which were previously termed as “legal Highs” in the UK.
Author contributions
All authors prepared and reviewed the completed manuscript.
Compliance with ethical standards
Conflict of interest
All authors have declared no conflict of interest.
Ethics committee approval
Forma ethical approval was not required, the patient signed consent to allow publication of this case report and images.
References
- 1.Misuse of Drugs Act 1971, London Stationary Office 2016. http://www.legislation.gov.uk/ukpga/1971/38. Accessed 10 Jan 2017.
- 2.Psychoactive Substances Act 2016, London Stationary Office 2016. https://www.gov.uk/government/collections/psychoactive-substances-bill-2015. Accessed 31 May 2016.
- 3.http://www.vice.com/en_uk/read/designer-drugs-2014-876. Accessed 3rd Aug 2016.
- 4.Poindexter A. Appetite suppressant drugs: a controlled clinical comparison of benzphetamine, phenmetrazine, d-amphetamine and placebo. Curr Therapeut Res Clin Exp. 1960;2:354–363. [PubMed] [Google Scholar]
- 5.McLaughlin G, Morris N, Kavanagh PV, Dowling G, Power JD, Twamley B, O’Brien J, Talbot B, Sitte HH, Brandt SD. Test purchase, synthesis and characterization of 3-fluorophenmetrazine (3-FPM) and differentiation from its ortho- and para-substituted isomers. Drug Test Anal. 2016 doi: 10.1002/dta.1945. [DOI] [PubMed] [Google Scholar]
- 6.Westerbergh J, Backberg M, Beck O, Helander A. Intoxications involving 3-fluorophenmetrazine (3-FPM): results from the STRIDA project. Clin Toxicol. 2016;54(4):378–379. doi: 10.3109/15563650.2016.1139715. [DOI] [PubMed] [Google Scholar]
- 7.Phua Jason, Ngerng Wang, See Kay, Tay Chee, Kiong Timothy, Lim Hui, Chew Mei, Yip Hwee, Tan Adeline, Khalizah Haji, Capistrano Rolando, Lee Kang, Mukhopadhyay Amartya. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Critical Care. 2013;17(5):R202. doi: 10.1186/cc12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bäckberg Matilda, Westerbergh Jenny, Beck Olof, Helander Anders. Adverse events related to the new psychoactive substance 3-fluorophenmetrazine – results from the Swedish STRIDA project. Clinical Toxicology. 2016;54(9):819–825. doi: 10.1080/15563650.2016.1211288. [DOI] [PubMed] [Google Scholar]