Abstract
Immune-checkpoint inhibitor nivolumab (anti-PD-1 antibody) blocks T cell inhibition and stimulate immunologic response toward cancer cells. It was also revealed that PD-1/PD-L1 interaction crucially controls the effector differentiation of auto-reactive T cells to maintain self-tolerance. Therefore, potential autoimmunological side-effect can occur in any organ. Here, we report a case of 67-year-old Japanese male with lung adenocarcinoma treated with nivolumab who developed acute tubulointerstitial nephritis after the third infusion of nivolumab. Kidney biopsy showed distinct histological findings: Proliferation of CD38 positive and IgG positive plasma cells, and affluent infiltration of FoxP3+ regulatory T cells. Herein, we do pathological discussion concerning acute tubulointerstitial nephritis occurred in this case based on these histological findings.
Keywords: Immune-checkpoint inhibitor, Anti-programmed cell death-1, Nivolumab, Tubulointerstitial nephritis, FoxP3
Introduction
Anti-programmed cell death-1 (PD-1) antibodies are expanding and important in the treatment of non-small cell lung cancer (NSCLC). The medication is currently also approved for patient with renal cell carcinoma. PD-1 ligand 1 (PD-L1) is up-regulated on various tumors and its binding to PD-1 on activated T cells results in T cell exhaustion and anergy [1, 2]. This mechanism makes tumor cells enable to evade immune surveillance. Nivolumab is a human immunoglobulin (Ig) G4 anti PD-1 monoclonal antibody, and which can affect immune-related adverse events caused by self-reactive T cells following immune system stimulation of this treatment [3, 4]. Clinical trials report an incidence of grade 3/4 nephrotoxicity ranging from 0.4 to 3% without identifying clear cause [5, 6].
In this report, we present a case of nivolumab-induced tubulointerstitial nephritis.
Case report
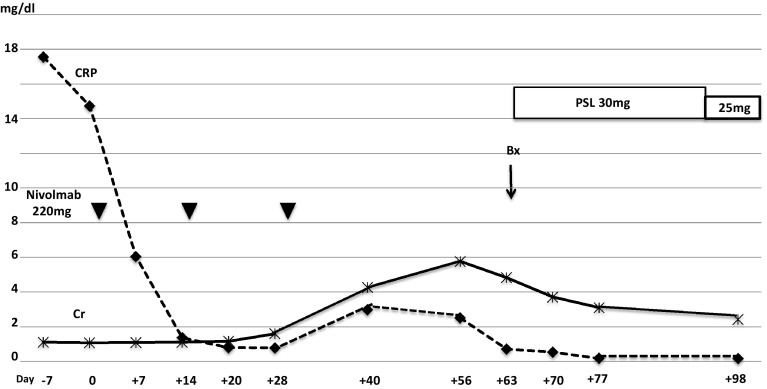
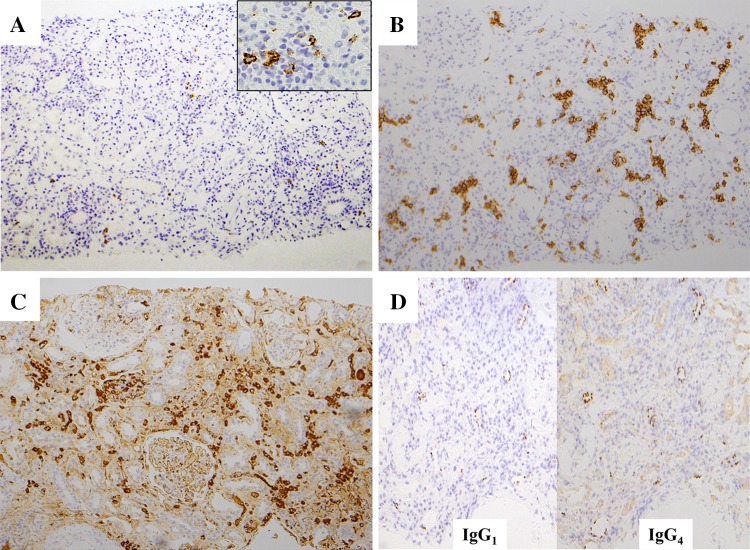
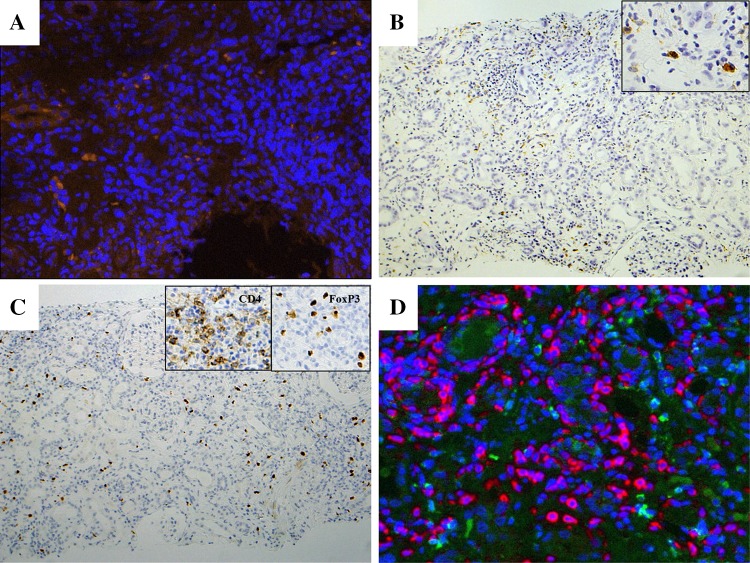
The patient was 67-year-old Japanese male with lung adenocarcinoma (T4N0M1a, stage IV, EML4-ALK fusion gene negative, EGFR gene wild). Immunohistological examination revealed PD-1 negative, and PD-L1 positive. He presented with a serum creatinine (SCr) level increased to 4.2 mg/dL at 2 weeks after the third infusion of nivolumab 220 mg (Fig. 1). BP was 120–64 mmHg, pulse rate was 89 beat/min, and physical examination findings were unremarkable. Upon admission (day 56 since the 1st nivolumab infusion), basic metabolic panel revealed sodium 137 mM/L, potassium 6.1 mM/L, chloride 106 mM/L, BUN 59 mg/dL and creatinine 5.0 mg/dL. Urinalysis showed 1+ protein and 1+ occult blood. In urine microscopic exam there were 1–4 RBC /HPF. The concentrations of urinary β2MG and NAG were 59.4 mg/dL (normal range <0.29 mg/L) and 15.6 U/L (normal range 0.3–11.5 U/L), respectively. Hepatitis B surface antigen, hepatitis C antibody, antinuclear antibody, glomerular basement membrane antibody, myeloperoxidase–anti-neutrophil cytoplasmic antibody and proteinase-3 anti-neutrophil cytoplasmic antibody were all negative. The serum levels of C3 and C4 were 139 mg/dL (normal range 86–160 mg/dL), 29 mg/dL (normal range 17–45 mg/dL), respectively (Table 1). Renal biopsy of right lower pole of kidney was performed on day 63. Sections stained with hematoxylin–eosin (HE), periodic acid and Schiff (PAS), Masson-Trichrome (MT) and periodic acid-methenamine silver (PAM) were observed by light microscopy. It demonstrated active tubulointerstitial nephritis on chronic interstitial nephritis. Acute tubular necrosis in recovery stage was also observed (Fig. 2a). Additionally minor glomerular abnormalities with sclerosing glomeruli and severe arteriosclerotic changes were recognized. Immunohistochemical studies by avidin–biotin method were performed used the antibodies for following molecules: PD-1 (Abcam, Cambridge, UK), PD-L1 (ProteoTech Inc, Kirkland, WA, USA), IgG (EMD Millipore Co, Billerica, MA, USA), IgG1, IgG4, CD3, CD4, CD8, CD20 (Dako, Glostrup, Denmark), CD38 (Leica, Wetzlar, Germany), CD68, CD1a, and Foxp3 (eBioscience Inc., San Diego, CA, USA). Immunofluorescent study was performed with OPAL-7-plex reagents, imaged at 20× on the Mantra Quantitative Pathology Imaging System, and analyzed using inForm software (all from Perkin-Elmer, Waltham, MA, USA). Immunostaining indicated PD-L1 expression in renal tubular cells was absent (data not shown). CD3 positive lymphocytes expansively infiltrated (Fig. 2b), and CD8 positive cells predominated over CD4 positive cells in the interstitium (Fig. 2c, d). Also CD20 positive cells were certainly found (Fig. 3a), and CD38 positive and IgG positive plasma cells were abundantly recognized (Fig. 3b, c). IgG4 producing cells did not proliferate abundantly compared with IgG1 producing cells (Fig. 3d). Antigen-presenting cells (APCs), such as dendritic cells (DCs) and macrophages (Mϕ) were predominantly infiltrated in injured area (Fig. 4a, b). In addition FoxP3 positive cell also infiltrated and most of them were also CD4 positive cells (Fig. 4c), and were distributed near CD4 positive cells, not CD8 positive cells (Fig. 4d).
Fig. 1.
Clinical course of the patient. The change of the value of serum creatinine (line) and CRP (dotted line) are indicated. Timing of nivolumab administration (black down-pointing triangle), kidney biopsy procedure (Bx) and prednisolone treatment (PSL) are shown. Time course is described as days after first nivolumab administration
Table 1.
Table laboratory data on admission day 56
| Urinary exam | Hematology | Biochem | |||
| Specific gravity | 1.013 | WBC | 6000/µL | TP | 8.5 g/dL |
| pH | 6 | Neu | 60% | Alb | 4.0 g/dL |
| Protein | 1+ | Eo | 2% | BUN | 59 mg/dL |
| OB | 1+ | Lymp | 30% | Cr | 5.0 mg/dL |
| Sed | RBC 1–4/HPF | Mo | 8% | AST | 13 IU/L |
| WBC <1/HPF | RBC | 285 × 104/µL | ALT | 9 IU/L | |
| β2MG | 59.4 mg/dL | Hb | 9.0 g/dL | LDH | 223 IU/L |
| NAG | 15.6 IU/L | Ht | 27% | Alp | 230 IU/L |
| MCV | 94.7 | gGTP | 30 mg/dL | ||
| Immunology | MCH | 31.6 | T-Bil | 0.3 mg/dL | |
| CRP | 1.10 mg/dL | MCHC | 33.3 | T chol | 206 mg/dL |
| IgG | 2548 mg/dL | Plt | 21 × 104/µL | TG | 304 mg/dL |
| IgA | 525 mg/dL | CK | 184 IU/L | ||
| IgM | 85 mg/dL | Na | 137 mEq/L | ||
| ANA | Negative | K | 6.1 mEq/L | ||
| C3 | 139 mg/dL | Cl | 106 mEq/L | ||
| C4 | 29 mg/dL | ||||
Fig. 2.
Light microscopy of the kidney biopsy: PAS staining showed severe tubulointerstitial nephritis (a). Immunohistochemical study revealed abundant CD3 positive cells (b), CD8 positive cells (c), and CD4 positive cells (d)
Fig. 3.
Light microscopy of the kidney biopsy: immunohistochemical study revealed proliferated CD20 positive cells (a), CD38 positive cells (b), and which produced IgG (c). IgG4 producing cells did not proliferate abundantly compared with IgG1 producing cells (d)
Fig. 4.
Immunofluorescent study showed several CD1a positive DCs (orange) within massive cell infiltration (blue) (magnification, ×200) (a). While immunohistochemical study revealed CD68 positive Mϕ showed similar distribution (magnification, ×100). Boxed area showed positive cells in high power field (magnification, ×400) (b). Immunohistochemical study revealed FoxP3 positive cells also proliferated. (magnification, ×100). Boxed area constituted CD4 and FoxP3 positive cells images, respectively (magnification, ×400) (c). Immunofluorescent study by Mantra Quantitative Pathology Imaging System showed the distribution of CD8 positive cells (red), CD4 positive cells (green), FoxP3 positive cells (cyan), and all nuclei (blue) in the interstitium (d)
These findings established a diagnosis of Nivolumab-induced tubulointerstitial nephritis. Nivolumab was discontinued and oral prednisolone 30 mg was initiated. Oral prednisone has been tapering, and at day 98 the values of serum creatinine, urinary β2MG and NAG were 2.42, 9.38 mg/dL and 9.4 U/L, respectively. While recovering from nivolumab-induced tubulointerstitial nephritis, patient did not receive any treatment for lung carcinoma, but anti-tumor activity seemed to continue on day 400.
Discussion
Nivolumab, monoclonal anti PD-1 antibody, blocks T cell inhibition and stimulate immunologic response toward cancer cells. However, eliminating PD-1 signaling unleashed accumulation of high-affinity CD8+ T cells with tissue specificity. Precisely, it was revealed that PD-1/PD-L1 interaction crucially controls the effector differentiation of auto-reactive T cells to maintain self-tolerance [7, 8]. Therefore, potential autoimmunological side effect can occur in any organ of the body, but it is known to predominate in gastrointestinal tract, lung, liver and endocrine system [9]. In clinical trials of nivolumab for patients with non-small cell lung cancer (NSCLC), the number of patients who developed renal side effect was not as large as those who did other organ side effects: 5 out of 287 patients (2%) developed elevated serum creatinine, 2 of them (0.7%) had renal failure [10].
The present case developed acute tubulointerstitial nephritis with CD8 positive lymphocytes predominant infiltration (Fig. 2c). The ability of renal proximal tubular epithelial cells (TEC) to promote T cell activation is attributable to their expression of major histocompatibility complex (MHC) class I and class II molecules and co-stimulatory molecules. Renal TECs act as non-professional antigen-presenting cells (APC) to trigger specific T cell responses, and while PD-1/PD-L1 signaling pathway plays principal role to regulate immune reaction. In fact renal TECs are the main target of infiltrating alloreactive CD4+ and CD8+ T cells causing tubulointerstitial injury in allograft rejection [11], and also PD-L1 is strongly up-regulated by TECs in rejected kidney transplants as well as in inflamed kidneys [12–14]. While in the present case PD-L1 expressions in renal tubular cells were not recognized in spite of interstitial massive CD3+ infiltration. More similar case studies are required to clarify whether it is common finding in acute tubulointerstitial nephritis caused by anti-PD-1 antibody.
Distinct histological finding of this case was proliferation of CD38 positive and IgG positive plasma cells (Fig. 3b, c). Based on this finding we speculated the cause why this patient developed tubulointerstitial nephritis as adverse effect of nivolumab as following. The present patient had taken NSAIDs to relieve cancer pain, and PPIs for prevention of peptic ulcer. Shirali AC et al described 6 cases of acute kidney injury secondary to biopsy-proven acute interstitial nephritis associated nivolumab or pembrolizumab use in patients with advanced NSCLC, and that all of these 6 patients received NSAIDs or PPIs [15]. These nephritogenic drugs might have induced to exhibit various self-antigen of tubulointerstitial cells for APC such as DCs, Mϕ and TEC. The constitutive presentation of tissue antigens had resulted in tolerance of auto-reactive T cells using PD-1/PD-L1 pathway. However, nivolumab disrupted this tolerance; thereafter these APCs might stimulate auto-reactive CD8+ T cells and also CD4+ T cells. Actually in the injured lesion, not intact area, tubular structure was destroyed and various CD1a positive DCs were infiltrated (Fig. 4a). This distribution pattern was more apparent for CD68 positive Mϕ (Fig. 4b). It is suggested that this interstitial environment might induce abundant proliferation of IgG producing plasma cells.
Another distinct histological finding was affluent infiltration of FoxP3+ regulatory T cells (Treg) (Fig. 4c), which was comparable with that of IgG4-related kidney disease [16]. Both FoxP3 expression and Treg function require T cell receptor (TCR) recognition of self-antigen [17, 18]. It is suggested PD-1 and FoxP3 work together for maintenance of peripheral tolerance, and PD-1 signaling maintains homeostasis of the Treg cells and the fine-tuned balance between the regulatory and effector T cell compartment. PD-1 signaling attenuates TCR downstream signaling, and inhibition of PD-1 pathway may enhance TCR signaling in Treg cell, which induce their proliferation and in turn strengthens their regulatory function [19]. In this case PD-1 was inhibited by nivolumab, and Treg might be forced to work substantially for maintenance of peripheral tolerance, and proliferate and distribute nearby CD4+ effector cells (Fig. 4d).
In summary, we experienced a case of nivolumab-induced tubulointerstitial nephritis. Immunohistochemical studies for biopsied kidney tissue presented proliferation of IgG producing plasma cells and FoxP3 positive cells. We speculated nivolumab disrupted immunological peripheral tolerance against cellular damage by antecedent nephritogenic drugs. Steroid therapy for this case was effective for recovery of renal function.
Acknowledgements
We thank Drs. Mamoru Tanaka and Yusuke Kashiwado (Kyushu University) for their generous help on immunofluorescent study.
Compliance with ethical standards
Ethics approval and consent to participate
This study was exempted from institutional review board approval because it was a case report.
Conflict of interest
All the authors have declared no competing interests.
Informed consent
Informed consent was obtained from the patient in this report.
References
- 1.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 2.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luke JJ, Ott PA. PD-1 pathway inhibitors: the next generation of immunotherapy for advanced melanoma. Oncotarget. 2015;6(6):3479–3492. doi: 10.18632/oncotarget.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbee MS, Ogunniyi A, Horvat TZ, Dang TO. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49(8):907–937. doi: 10.1177/1060028015586218. [DOI] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting Edge: programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol. 2006;177(12):8291–8295. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 8.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6(3):280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 9.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR, Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornell LD, Smith RN, Colvin RB. Kidney transplantation: mechanisms of rejection and acceptance. Annu Rev Pathol. 2008;3:189–220. doi: 10.1146/annurev.pathmechdis.3.121806.151508. [DOI] [PubMed] [Google Scholar]
- 12.Schoop R, Wahl P, Le Hir M, Heemann U, Wang M, Wuthrich RP. Suppressed T-cell activation by IFN-gamma-induced expression of PD-L1 on renal tubular epithelial cells. Nephrol Dial Transplant. 2004;19(11):2713–2720. doi: 10.1093/ndt/gfh423. [DOI] [PubMed] [Google Scholar]
- 13.de Haij S, Woltman AM, Trouw LA, Bakker AC, Kamerling SW, van der Kooij SW, Chen L, Kroczek RA, Daha MR, van Kooten C. Renal tubular epithelial cells modulate T-cell responses via ICOS-L and B7-H1. Kidney Int. 2005;68(5):2091–2102. doi: 10.1111/j.1523-1755.2005.00665.x. [DOI] [PubMed] [Google Scholar]
- 14.Ding H, Wu X, Gao W. PD-L1 is expressed by human renal tubular epithelial cells and suppresses T cell cytokine synthesis. Clin Immunol. 2005;115(2):184–191. doi: 10.1016/j.clim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68(2):287–291. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura E, Hisano S, Nakashima H, Takeshita M, Saito T. Immunohistological analysis for immunological response and mechanism of interstitial fibrosis in IgG4-related kidney disease. Mod Rheumatol. 2015;25(4):571–578. doi: 10.3109/14397595.2014.1001474. [DOI] [PubMed] [Google Scholar]
- 17.Killebrew JR, Perdue N, Kwan A, Thornton AM, Shevach EM, Campbell DJ. A self-reactive TCR drives the development of Foxp3+ regulatory T cells that prevent autoimmune disease. J Immunol. 2011;187(2):861–869. doi: 10.4049/jimmunol.1004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15(11):1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Chikuma S, Hori S, Fagarasan S, Honjo T. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci USA. 2016;113(30):8490–8495. doi: 10.1073/pnas.1608873113. [DOI] [PMC free article] [PubMed] [Google Scholar]