Abstract
Fabry disease is a rare X-linked lysosomal storage disorder of glycosphingolipid catabolism caused by deficient activity of the lysosomal hydrolase alpha-galactosidase A (ɑ-Gal A). A 20-year-old woman was referred to our hospital because of proteinuria and persistent macroscopic hematuria. Based on the typical renal pathological findings, deficient activity of the ɑ-Gal A, and heterozygous mutation in the ɑ-Gal A gene, she was diagnosed with Fabry disease. After 1 year of enzyme replacement therapy with agalsidase alfa at 0.2 mg/kg every other week, the patient’s proteinuria and hematuria were disappeared. In our patient, enzyme replacement therapy with agalsidase alfa was observed to be safe and well-tolerated during her pregnancy, with no significant negative effects on her or her child. Here, we report clinical and pathological evaluations of a patient through repeat kidney biopsy after 6 years of enzyme replacement therapy. Furthermore, we discussed the appropriate enzyme replacement therapy and its safety in pregnant women with Fabry disease.
Keywords: Heterozygous Fabry disease, Enzyme replacement therapy, Agalsidase alfa, Agalsidase beta, Kidney biopsy, Pregnancy
Introduction
Fabry disease (FD: OMIM 301500) is a rare X-linked lysosomal storage disorder of glycosphingolipid catabolism caused by deficient activity of the lysosomal hydrolase alpha-galactosidase A (ɑ-Gal A). α-Gal A gene (GLA) mutations cause the enzymatic deficiency, which further results in the systemic accumulation of glycolipids, primarily globotriaosylceramide (Gb3), in the vascular endothelium and other tissues. Gb3 deposits in various kidney cells. Morbidity and mortality resulting from FD, caused by renal failure, cardiac disease, and early onset stroke, increase with age. More than half of the male patients and more than 20% of female patients eventually develop advanced renal disease [1].
Enzyme replacement therapy (ERT) with agalsidase alfa or beta has been developed for FD treatment. Agalsidase alfa is authorized at a dose of 0.2 mg/kg (Shire, Lexington, MA, USA) and agalsidase beta is authorized at a dose of 0.3–1.0 mg/kg (Genzyme, a Sanofi company, Cambridge, MA, USA) [2]. It is recommended that ERT should be initiated at the early stage of the disease, that is, before the appearance of proteinuria, which is the first renal clinical sign of FD [2–4]. However, few studies have reported histopathological evaluations of the efficacy of ERT in heterozygous FD. In addition, pregnancy and delivery of patients with heterozygous FD during ERT are rare. Here, we report on a patient of heterozygous Fabry nephropathy, histopathological evaluations by repeat kidney biopsy after 6 years of ERT with agalsidase alfa, and the patient’s successful pregnancy outcome during ERT.
Case history
A 20-year-old woman was referred to our hospital because of proteinuria and persistent macroscopic hematuria. She had no family history of kidney diseases. Two years ago, her urinalysis on school examination was normal. After a 2-year follow-up at our outpatient clinic, she was admitted for a kidney biopsy. On admission, her height was 158.4 cm, weight 66.5 kg, body mass index 26.5, blood pressure 114/70 mmHg, pulse 80/min, and temperature 36.9 °C. She was neither pale nor icteric. Physical examination of the chest and abdomen was unremarkable. She exhibited no edema or lymphadenopathy, and no skin lesions were observed. She was not deaf.
Her urinary protein level was 0.2–0.3 g/day. The urinary sediment showed > 100 erythrocytes and < 1 leukocytes per high-power field. The hematocrit was 36.7%, hemoglobin concentration 11.9 g/dl, platelet count 383,000/mm3, and leukocyte count 5,400/mm3. The serum urea nitrogen level was 8.9 mg/dl, creatinine 0.55 mg/dl, uric acid 3.8 mg/dl, total cholesterol 191 mg/day, total protein 7.4 g/dl, and albumin 4.8 g/dl. The C reactive protein level was < 0.05 mg/dl, Immunoglobulin (Ig) G 1,106 mg/dl (normal range: 870–1,700 mg/dl), IgA 315 mg/dl (80–410 mg/dl), and IgM 146 mg/dl (34–220 mg/dl). Total complement level was 38 IU/l (30–45 IU/l), C3 112 mg/dl (80–140 mg/dl), C4 23.0 mg/dl (11–30 mg/dl), and C1q < 1.5 μg/ml. Representative antigens and antibodies that cause kidney diseases were negative. All other laboratory tests were within normal limits. A chest X-ray and an electrocardiogram were normal. Renal ultrasound and computed tomography showed normal kidneys. From these imaging findings, there was no possibility of nutcracker syndrome.
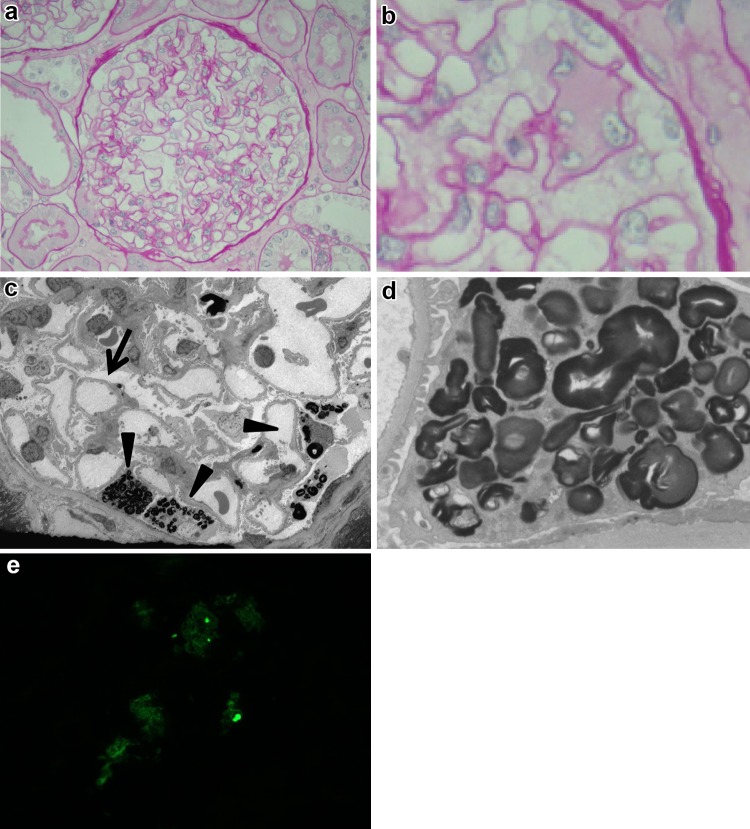
On the 2nd hospital day, a kidney biopsy was performed to investigate the cause of mild proteinuria and persistent macroscopic hematuria. The biopsied sample contained 26 glomeruli, one of which was sclerosed. Light microscopy demonstrated mild mesangial cell proliferation. The expanded podocytes had a patchy distribution and appeared foamy with pale cytoplasm (Fig. 1a, b). Mild focal tubular atrophy accompanied by thickening of the tubular basement membrane and mild interstitial inflammation was observed. Immunofluorescent examination revealed no significant deposits of immunoglobulins or complement components. An electron microscope examination revealed partial foot process effacement and lamellar inclusions in podocytes (Fig. 1c, d) consistent with Fabry nephropathy. Gb3 accumulation in podocytes was confirmed by immunostaining for Gb3 (Fig. 1e).
Fig. 1.
a Light microscopy showed only mild segmental mesangial cell proliferation (periodic acid-Schiff stain, original magnification ×400). b Several glomerular podocytes were swollen and mildly vacuolated (periodic acid-Schiff stain, original magnification ×400). c Electron microscopic examination revealed no electron dense deposits along the GBM, but showed foot partial process effacement (arrow) and lipid inclusions in podocytes (arrow head) (electron microscopy, original magnification ×600). d Higher magnification showing several whorled myeloid bodies in podocytes (electron microscopy, original magnification ×2500). e Immunostaining of globotriaosylceramide was partially positive in podocytes
Analysis of the leukocytes showed an ɑ-Gal A activity level of 35 (normal range: 52–86) nmol/h/mg. After careful consideration of the findings, the patient lacked any other pathognomonic signs of FD, such as acroparesthesias, dyshidrosis, corneal opacities, or cutaneous angiokeratomas. After obtaining informed consent, we collected DNA from her and her mother. Gene analysis revealed a heterozygous mutation, c.1124G>A, p.G375E, in the ɑ-Gal A (HUGO GLA) gene in both DNA samples.
At the age of 22 years, ERT was scheduled; however, before it began, the patient discovered she was pregnant. The patient and her family accepted responsibility for the outcome and requested the initiation of ERT with agalsidase alfa [Replagal; 0.2 mg/kg infused every other week (eow)] at 8 weeks of gestation. The dose and frequency of the intravenous enzyme substitution remained unchanged during the pregnancy. ERT during pregnancy seemed to be well-tolerated, with no negative effects, including infusion-related reactions, on the mother or child. At 40 weeks of gestation and an uneventful pregnancy, the patient delivered a healthy girl. At birth, the baby’s length and weight were 49 cm and 2,734 g, respectively. APGAR scores were 8/9. A mutation analysis of the baby’s GLA gene was performed, and a diagnosis of FD was made based on the results (c.1124G>A, p.G375E). ERT continued for approximately 6 years after her delivery. After the initiation of ERT, the patient’s proteinuria and hematuria gradually improved, and after 1 year of ERT, her proteinuria and hematuria were disappeared.
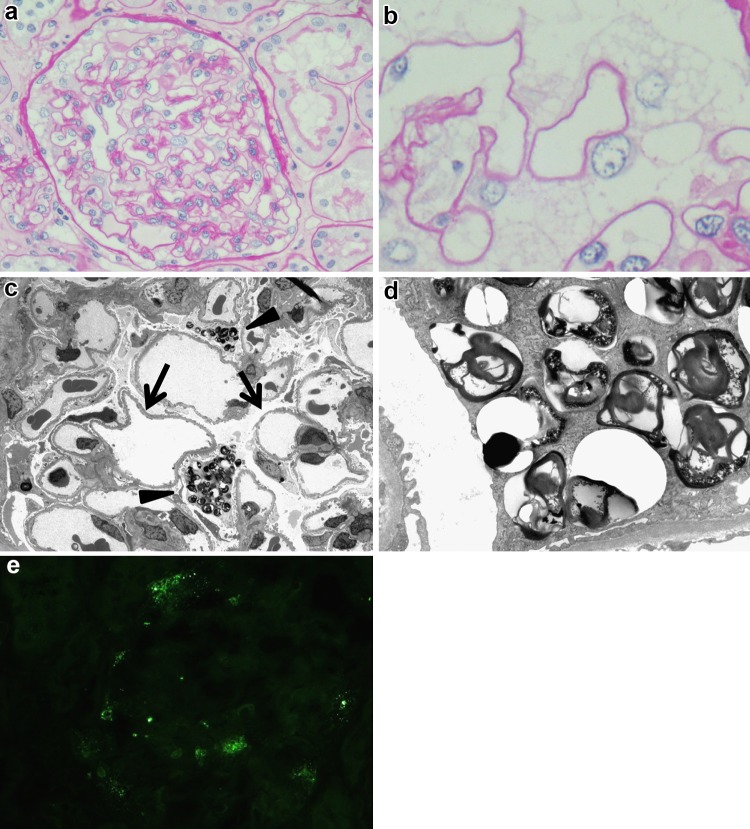
At the age of 28 years, we performed a repeat kidney biopsy to evaluate the effect of ERT. The biopsied sample contained 18 glomeruli, and no glomerulus was obsolete or sclerosed. Using light microscopy, no obvious changes were observed in glomeruli and tubules compared with the first biopsy (Fig. 2a, b). Electron microscopic examination revealed partial foot process effacement and lamellated lipid inclusion in podocytes similar to that observed in the first biopsy, and the structure of the lamellar lipid inclusions was more variegated than that in the first biopsy (Fig. 2c, d). Immunostaining for Gb3 was partially positive in the podocytes, as observed in the first biopsy, and the ratio of Gb3-positive cells was similar to that in the first biopsy (Fig. 2e).
Fig. 2.
a Compared with the first biopsy, no obvious changes of glomeruli or tubules were observed with light microscopy (periodic acid-Schiff stain, original magnification ×400). b Vacuolar changes of podocytes were similar to that of the first biopsy (periodic acid-Schiff stain, original magnification ×400). c Electron microscopic examination revealed partial process effacement and lipid inclusions in podocytes, as in the first biopsy (electron microscopy, original magnification ×600). d Higher magnification showing several whorled myeloid bodies in podocytes. The structure of the lamellated lipid inclusion was highly variegated and seemed looser than in the first kidney biopsy. In some vacuoles, whorled myeloid bodies were decreased. (electron microscopy, original magnification ×2500). e Immunostaining of globotriaosylceramide was partially positive in podocytes, as in the first biopsy, and the ratio of globotriaosylceramide positive cells was similar to that in the first biopsy
We observed the patient as she continued ERT with agalsidase alfa in our outpatient clinic. When the patient was 28 years, her, her mother’s and daughter’s deacylated soluble derivative globotriaosylsphingosine (lyso-Gb3) were within the normal range (1.5, 0.8, and 1.1 ng/ml, respectively; normal range < 2 ng/m). Currently, the patient is 30 years, and she is free from proteinuria, hematuria, renal insufficiency (serum creatinine levels 0.60 mg/dl), or any FD-related symptoms.
Discussion
We report on a case of a young female with heterozygous FD who underwent repeat kidney biopsy after 6 years of ERT with agalsidase alfa. In our patient, ERT with agalsidase alfa was observed to be safe and well-tolerated during her pregnancy, with no significant negative effects on her or her child.
The renal histopathologic hallmark of FD is accumulation of Gb3 within different renal cells. Short-term morphologic studies demonstrated clearance of Gb3 from different renal cells treated with agalsidase beta [5], and treatment with agalsidase alfa resulted in decreased mesangial widening [6]. Long-term ERT in young patients can result in complete clearance of Gb3 from mesangial and glomerular endothelial cells across all dosage regimens, and clearance of inclusions from podocytes is dose dependent [7]. In that study, complete clearance of Gb3 from epithelial cells in the distal tubuli was found in only 2 (1 male and 1 female) of 12 patients. The podocytes were almost completely cleared in one young male patient, and three patients showed substantial clearance after 5 years. However, these patients were relatively young (7–18 years); hence, the histopathologic effectiveness of long-term ERT in patients with heterozygous FD, particularly in adolescents, is obscure.
Genetic analysis revealed that the patient, her mother, and her daughter had a heterozygous mutation c.1124G>A, p.G375E in the GLA gene, which have been previously reported [8]. Based on the mutant enzyme activity in vitro and lyso-Gb3 measurement study, the clinical phenotype of this mutation is relatively benign [8]; however, it is difficult to predict the phenotype severity based on the genetic mutation patterns, including the mutation in this patient, and to decide which patients require ERT for heterozygous FD. Lyso-Gb3 levels are useful marker for diagnosis, initial phenotypic assignment, and therapeutic monitoring in patients with Fabry disease [9]. Until now, we did not plan to initiate ERT to her mother and her daughter; however, we would like to measure serum lyso-Gb3 levels of them when the level of lyso-Gb3 rise and clinical symptoms appear, such as albuminuria; we will consider initiating ERT to them.
In our patient, after 6 years of ERT with agalsidase alfa at 0.2 mg/kg eow, obvious changes in light microscopic findings or immunostaining of Gb3 were not observed; however, lamellar lipid inclusions in some vacuoles of the podocytes were decreased and a slight structural change in lamellar lipid inclusions was observed on the electron microscopic examination. Transition from Gb3 accumulation in the podocytes to the earliest phases of injury denoted by podocyte foot process effacement occurs before there are clinically evident increases in urinary albumin or protein excretion [10]. Identification of patients in the early disease stage of FD and rapid ERT initiation is currently recommended by several studies [2–4, 11]. However, the issue of dosage has been confounded by the fact that the label-recommended dose of the two available agalsidase preparations has a five-fold difference. In our patient, abnormal urinalysis was the only initial symptom and the initiation of ERT was relatively early; however, the effectiveness of ERT was different from that observed by Tøndel [8], which showed an obvious clearance of Gb3 from renal cells. In Tøndel’s study, complete clearance of inclusions from renal cells was observed only using 1.0 mg/kg eow of agalsidase beta, which was not observed using 0.2 mg/kg eow of agalsidase alfa. Recently, Ito et al. reported the case of a child with a classical phenotype (10-year-old boy) that showed complete Gb3 clearance of inclusions and resolution of foot process effacement in renal cells by ERT with 1.0 mg/kg eow of agalsidase beta. They speculated that prompt diagnosis and early intervention with high-dose ERT (agalsidase beta 1.0 mg/kg eow) could improve the quality of life of patients and prevent renal disease [12]. However, head-to-head comparative studies would be needed to demonstrate conclusive evidence for the superiority of either enzyme.
In our patient, ERT was safe and well-tolerated, even during her pregnancy, with no significant negative effects on her or her child. First, Wendt et al. [13] mentioned that pregnancy should not be a contraindication for ERT. Until now, at least nine females with FD have had successful pregnancy outcomes during ERT [14]. Gb3 deposits have been described in many renal cell types as early as 17 weeks of gestation [15] and have been described in the placental tissue of patients with FD [16]. There are still not enough studies to clarify whether the enzyme (product) passes the placental barrier [16]. FD is a genetic, chronic disorder; thus, the patient and his/her family experience considerable associated stress. ERT can reduce this stress, which may be particularly important during pregnancy. Although more research is needed, this and other reports support the use of ERT in patients with FD during pregnancy.
In conclusion, we have reported on a young female with heterozygous FD who underwent a repeat kidney biopsy after 6 years of ERT with agalsidase alfa. In our patient, although complete clearance of Gb3 was not observed after 6 years of ERT with agalsidase alfa, the treatment was safe and well-tolerated, even during pregnancy, without any disease progression or significant negative effects. In adolescents, even in patients with heterozygous FD, it may be necessary to use a higher dose of agalsidase beta to achieve complete clearance of Gb3. Further reports should be accumulated to determine which enzymes and doses are appropriate for this treatment and to determine the safety and efficacy of ERT for patients with FD during pregnancy.
Acknowledgements
The authors are grateful to Mr. N. Sakamoto, Ms. S. Tsuchida, Ms. M. Yoshinuma, and Ms. M. Igashima (Department of Pathology, Shinrakuen Hospital) for their technical assistance.
Compliance with ethical standards
Conflict of interest
H. M. has received speaker fees, research support from Sanofi. I. N. has received donations for research from Sanofi and Sumitomo Dainippon Pharm.
Human and animal rights statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Desnick RJ, Ioannou YA, Eng CM. Alpha-galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited diseases. 8. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- 2.Biegstraaten M, Arngrímsson R, Barbey F, Boks L, Cecchi F, Deegan PB, Feldt-Rasmussen U, Geberhiwot T, Germain DP, Hendriksz C, Hughes DA, Kantola I, Karabul N, Lavery C, Linthorst GE, Mehta A, van de Mheen E, Oliveira JP, Parini R, Ramaswami U, Rudnicki M, Serra A, Sommer C, Sunder-Plassmann G, Svarstad E, Sweeb A, Terryn W, Tylki-Szymanska A, Tøndel C, Vujkovac B, Weidemann F, Wijburg FA, Woolfson P, Hollak CE. Recommendations for initiation and cessation of enzyme replacement therapy in patients with Fabry disease: the European Fabry Working Group consensus document. Orphanet J Rare Dis. 2015;10:36. doi: 10.1186/s13023-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiffmann R, Hughes DA, Linthorst GE, Ortiz A, Svarstad E, Warnock DG, West ML, Wanner C. Conference participants. screening, diagnosis, and management of patients with Fabry disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91:284–293. doi: 10.1016/j.kint.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Warnock DG, Ortiz A, Mauer M, Linthorst GE, Oliveira JP, Serra AL, Maródi L, Mignani R, Vujkovac B, Beitner-Johnson D, Lemay R, Cole JA, Svarstad E, Waldek S, Germain DP, Wanner C, Fabry Registry Renal outcomes of agalsidase beta treatment for Fabry disease: role of proteinuria and timing of treatment initiation. Nephrol Dial Transpl. 2012;27:1042–1049. doi: 10.1093/ndt/gfr420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thurberg BL, Rennke H, Colvin RB, Dikman S, Gordon RE, Collins AB, Desnick RJ, O’Callaghan M. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933–1946. doi: 10.1046/j.1523-1755.2002.00675.x. [DOI] [PubMed] [Google Scholar]
- 6.Schiffmann R, Kopp JB, Austin HA, 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 7.Tøndel C, Bostad L, Larsen KK, Hirth A, Vikse BE, Houge G, Svarstad E. Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol. 2013;24:137–48. doi: 10.1681/ASN.2012030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukas J, Scalia S, Eichler S, Pockrandt AM, Dehn N, Cozma C, Giese AK, Rolfs A. Functional and clinical consequences of novel α-galactosidase A mutations in Fabry disease. Hum Mutat. 2016;37:43–51. doi: 10.1002/humu.22910. [DOI] [PubMed] [Google Scholar]
- 9.Nowak A, Mechtler T, Kasper DC, Desnick RJ. Correlation of Lyso-Gb3 levels in dried blood spots and sera from patients with classic and Later-Onset Fabry disease. Mol Genet Metab. 2017;121:320–324. doi: 10.1016/j.ymgme.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Tøndel C, Kanai T, Larsen KK, Ito S, Politei JM, Warnock DG. Foot process effacement is an early marker of nephropathy in young classic Fabry patients without albuminuria. Nephron. 2015;129:16–21. doi: 10.1159/000369309. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann B. Fabry disease: recent advances in pathology, diagnosis, treatment and monitoring. Orphanet J Rare Dis. 2009;4:21. doi: 10.1186/1750-1172-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito S, Ogura M, Kamei K, Matsuoka K, Warnock DG. Significant improvement in Fabry disease podocytopathy after 3 years of treatment with agalsidase beta. Pediatr Nephrol. 2016;31:1369–1373. doi: 10.1007/s00467-016-3387-4. [DOI] [PubMed] [Google Scholar]
- 13.Wendt S, Whybra C, Kampmann C, Teichmann E, Beck M. Successful pregnancy outcome in a patient with Fabry disease receiving enzyme replacement therapy with agalsidase alfa. J Inherit Metab Dis. 2005;28:787–788. doi: 10.1007/s10545-005-0018-9. [DOI] [PubMed] [Google Scholar]
- 14.Senocak Tasci E, Bicik Z. Safe and successful treatment with agalsidase beta during pregnancy in Fabry disease. Iran J Kidney Dis. 2015;9:406–408. [PubMed] [Google Scholar]
- 15.Brady RO, Uhlendorf BW, Jacobson CB. Fabry’s disease: antenatal detection. Science. 1971;172:174–175. doi: 10.1126/science.172.3979.174. [DOI] [PubMed] [Google Scholar]
- 16.Vedder AC, Strijland A, vd Bergh Weerman MA, Florquin S, Aerts JM, Hollak CE. Manifestations of Fabry disease in placental tissue. J Inherit Metab Dis. 2006;29:106–11. doi: 10.1007/s10545-006-0196-0. [DOI] [PubMed] [Google Scholar]