Abstract
Background
Real-world evidence of statin side effects is potentially biased because statin use is neither randomized nor unblinded. An innovative study design can mitigate these biases. For example, in the recent ASCOT-LLA trial, patient-reported adverse events such as muscle pain and weakness were higher in the non-randomized and non-blinded setting than in the randomized, blinded setting. Less optimally, secondary re-analysis of clinical trials in which statin use is recorded and in which serious adverse events (SAEs) are adjudicated may be conducted.
Objective
The objective of this study was to evaluate SAEs by statin use at baseline among participants in the SPRINT blood pressure (BP) management trial.
Methods
Unadjusted overall SAE and treatment-related SAE rates by statin use as well as adjusted hazard ratios for statin use were computed in four cohorts [by baseline clinical cardiovascular disease (CVD), by intervention arm].
Results
Statin use at baseline was not associated with higher overall or treatment-related SAE rates among (1) those without pre-existing CVD, regardless of BP arm, nor among (2) those randomized to standard BP management, regardless of pre-existing CVD. Among higher risk patients with existing clinical CVD randomized to intensive BP management, a small but significant increase in overall SAE rate was found among those taking statin at baseline.
Conclusions
In SPRINT, generally statin use was not associated with increased risk of reporting SAEs. Only statin use by higher risk patients was associated with more overall SAEs. Confounding by clinical CVD and the polytherapy of intensive BP management may explain this.
Key Points
| Secondary re-analysis of the SPRINT trial data suggests that statin use is generally not associated with increases in serious adverse events except among higher risk patients. |
| Secondary re-analyses of randomized clinical trials are a useful adjunct to primary analysis of randomized clinical trials, although concerns about power and data completeness remain. |
Introduction
Despite consensus guidelines [1], debate continues about the potential harms from statin use [2]. Much of the case for harms rests on real-world reported evidence of adverse events [1–4], although meta-analyses of randomized clinical trials do not support significant increases in serious adverse events (SAEs) with statin use [5].
However, it is well known that observational reports are susceptible to two well-known important sources of bias [6–8]. First, since real-world use of statins is generally not randomized, unmeasured confounding may bias the rate of reported side effects. This is particularly the case where confounding by indication exists: the indications for statin prescription are independently associated with side effects, beyond any potential causeway through statin use. Such bias is difficult and may be impossible to adjust for [9].
Second, since real-world use of statins is unblinded, an ascertainment bias may impact the reporting of side effects [10]. Without the masking of treatment assignments, it is possible that patients or their physicians wrongly attribute a side effect to the use of statins [11]. Such an effect is suggested by the recent Odyssey Alternative trial: one in 16 patients with documented statin intolerance were screened out during a 4-week placebo run-in period, thereby “suggesting that some patients may experience muscle symptoms because of negative expectations surrounding the potential for statin treatment or for reasons unrelated to statin therapy” [12].
To tease apart the impact of such biases on reported statin adverse events, the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA) recently compared events in the non-randomized and non-blinded follow-on phase with events reported in the earlier randomized, blinded phase [13]. This demonstrated that patient-reported adverse events such as muscle pain and weakness were higher in the non-randomized and non-blinded setting than in the randomized, blinded setting.
In this current study, inspired by the ASCOT-LLA approach, a secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) blood pressure (BP) management trial is conducted. The objective is to understand whether adjudicated SAEs, either deemed related to BP treatment or not, differed by baseline statin use. The premise of this study is simply that any such association between statin use and SAEs is observable in the data. This study is silent as to possible mechanisms for such effects, which could be due to statin therapy alone or the interaction between statin therapy and BP management with one or more classes of BP-lowering medicines.
For example, while short-term imbalances in sodium and potassium levels are well known to be associated with diuretic-based BP treatments, one large observational study of statin use in the UK found significant associations between statin use and liver dysfunction [12]. Similarly, while declines in kidney function may occur with the beta blockers or renin-angiotension system blockers used in BP management in either arm of SPRINT, the same study showed higher rates of acute kidney failure among statin users [12]. Again, while the thiazide diuretics [14, 15] and beta blockers [16] used in SPRINT are known to increase diabetes risk, this risk was also found to be associated with statin use in the Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial [17]. In all these examples, therefore, whether the SAE was deemed related to the BP treatment or not, it will be ascertained as an SAE. Moreover, if an interaction occurs between statin therapy and BP management pharmacological therapy, this would also be ascertained as an SAE as well.
In another example, far less likely to be due to BP treatment, cognitive status and glucose tests were explicitly assessed in all SPRINT participants and reported as an SAE if abnormal. Cognitive functioning abnormalities and diabetes have previously been of concern to some as a side effect of statins [2, 5]; if present, such potential SAEs would likely have been ascertained. Finally, some possible rare effects of statins such as rhabdomyolysis or more common effects such as muscle weaknesses and myalgia, if unexpected and sufficiently serious, would also have been reported as an SAE.
Accordingly, I hypothesized that if statins are associated with SAEs, then comparing overall SAE and treatment-related SAE rates by statin use would reveal this signal. To reduce confounding by statin indication, adjustment was made for baseline characteristics and the main analysis was restricted to those without baseline cardiovascular disease (CVD). To reduce confounding from the effect of the BP intervention, the main analysis was further restricted to only those randomized to standard BP management.
Materials and Methods
Data Source
The SPRINT BP trial dataset [18] was obtained from the National Heart, Lung and Blood Institute under an institutional data use agreement with the Pennsylvania State University College of Medicine. This study was reviewed and approved by the Institutional Review Board of the Penn State College of Medicine, which determined that this research was not human research, was Health Insurance Portability and Accountability Act compliant and was exempt from informed consent requirements.
Cohort Construction
Patients’ baseline status of documented CVD was ascertained based on the SPRINT criteria of one or more of previous myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, carotid endarterectomy or carotid stenting; peripheral artery disease with revascularization; acute coronary syndrome or positive cardiac imaging study; at least 50% diameter stenosis of a coronary, carotid or lower extremity artery; or abdominal aortic aneurysm more than 5 cm in diameter.
Patients were randomized in SPRINT to standard or intensive BP management. Patients randomized to the standard arm had a systolic target BP of less than 140 mmHg; intensive-arm patients had a target of 120 mmHg. Of the total 9361 participants in SPRINT, the following four cohorts were created (Table 1). A total of 3746 participants had not been diagnosed with CVD at baseline and had been randomized to the standard BP management arm and thus formed the main analytic set.
Table 1.
Cohorts constructed from SPRINT trial participants
| Clinical cardiovascular disease at baseline | Subtotals | |||
|---|---|---|---|---|
| No | Yes | |||
| Randomized to BP target | ||||
| Standard (systolic < 140 mmHg) | 3746 (main) | 937 (sensitivity) | 4683 | |
| Intensive (< 120 mmHg) | 3738 (sensitivity) | 940 (sensitivity) | 4678 | |
| Subtotals | 7484 | 1877 | 9361 | |
BP blood pressure
Outcome of Interest
The pre-specified main outcomes were overall SAEs and SAEs classified as possibly or definitely related to the BP intervention by the SPRINT investigators. In SPRINT, only some selected SAEs were described separately. These included hypotension, syncope, bradycardia, electrolyte abnormalities, injurious fall, and acute kidney injury or acute renal failure. The overwhelming majority of SAEs were not separately described except as events that were fatal or life threatening, resulted in significant or persistent disability, required or prolonged a hospitalization, or were an important medical event that the investigator judged to be a significant hazard or harm to the participant that may have required medical or surgical intervention to prevent one of the other SAEs.
Statistical Analysis
Outcomes were calculated as overall unadjusted event rates over the median 3.3 years of follow-up by cohort. The hazard rate for outcomes was also modeled using adjusted Cox proportional hazard models within each of the four cohorts. In these Cox models, the effect of statins was adjusted for 18 baseline characteristics.
All baseline characteristics were ascertained by SPRINT at baseline: age, gender, body mass index, African-American race, systolic and diastolic BP, current smoker status, chronic kidney disease, serum creatinine, estimated glomerular filtration rate, ratio of urinary albumin to creatinine, cholesterol, high-density lipoprotein cholesterol, glucose, triglycerides, number of BP agents used, whether no agents were used, and aspirin use.
Estimated 10-year atherosclerotic cardiovascular disease (ASCVD) risk was calculated in this paper following published methods [19]. This calculation comprised a non-linear function of BP treatment status at baseline, race, gender, and baseline systolic BP and cholesterol, and also included current smoking status as a baseline risk adjuster. All data management and analyses were performed on Stata 14.2 (Stata, Plano, TX, USA).
Results
In the main cohort of patients randomized to the standard BP arm without pre-existing clinical CVD, 38.5% were taking statins at baseline. Among patients in the standard BP arm with pre-existing clinical CVD, 69.8% were taking statins, while among patients in the intensive arm without or with pre-existing clinical CVD, statin use at baseline was 35.8 and 69.3%, respectively.
Unadjusted Results
Without regard to classification as related to treatment, unadjusted SAE rates in the main cohort were significantly higher among those taking statins at baseline compared to those not (37.4 vs 31.9%, p = 0.001). In sensitivity analyses, unadjusted overall SAE rates were also significantly higher among statin users in the other three cohorts compared to those not taking statins at baseline (Table 2).
Table 2.
Unadjusted overall serious adverse event rates
| Overall serious adverse events (% over trial) | ||||
|---|---|---|---|---|
| Statin use at baseline | No statin use at baseline | Unadjusted difference | p value | |
| Main cohort: standard arm, no clinical CVD | 37.4 | 31.9 | + 5.5 | 0.0006 |
| Standard arm, clinical CVD | 52.5 | 41.6 | + 10.9 | 0.0022 |
| Intensive arm, no clinical CVD | 38.1 | 33.8 | + 4.3 | 0.008 |
| Intensive arm, clinical CVD | 54.3 | 41.5 | + 12.8 | 0.0003 |
CVD cardiovascular disease
The subset of SAEs deemed related to treatment was small relative to overall SAEs. Unadjusted treatment-related SAE rates over the trial were slightly but not statistically significantly lower among those taking statins at baseline compared to those not (2.2 vs 2.4%, p = 0.64). In sensitivity analyses, statin use at baseline was similarly not associated with statistically significantly higher treatment-related SAE rates in the other cohorts examined (Table 3).
Table 3.
Unadjusted treatment-related serious adverse event rates
| Treatment-related serious adverse events (% over trial) | ||||
|---|---|---|---|---|
| Statin use at baseline | No statin use at baseline | Unadjusted difference | p value | |
| Main cohort: standard arm, no clinical CVD | 2.17 | 2.41 | − 0.25 | 0.64 |
| Standard arm, clinical CVD | 3.69 | 2.49 | + 1.20 | 0.35 |
| Intensive arm, no clinical CVD | 4.96 | 3.91 | + 1.05 | 0.13 |
| Intensive arm, clinical CVD | 6.79 | 4.87 | + 1.92 | 0.26 |
CVD cardiovascular disease
Adjusted Results
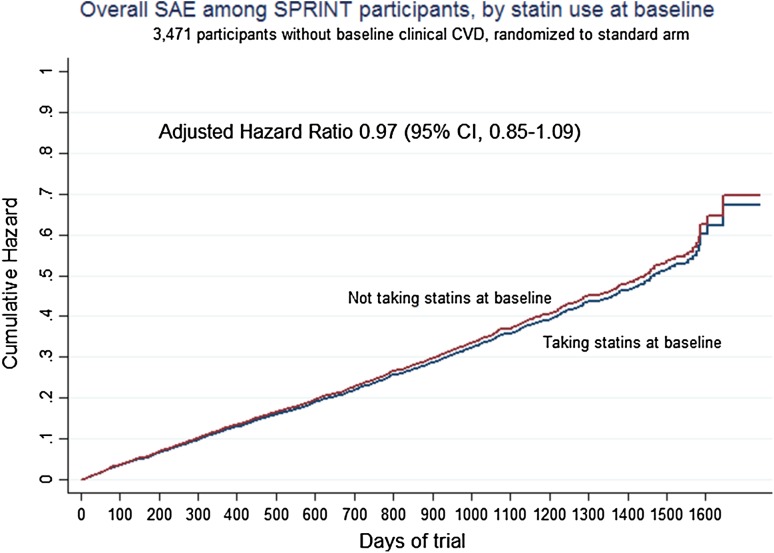
Adjusting for 18 baseline patient characteristics, the adjusted impact of statin use at baseline on overall SAEs was not statistically significant in the main analysis cohort of patients randomized to standard BP management without pre-existing clinical CVD (Table 4 and Fig. 1).
Table 4.
Adjusted hazard ratio for overall serious adverse events
| Overall serious adverse events | |||
|---|---|---|---|
| Hazard ratio associated with statin use at baseline | 95% confidence interval | p value | |
| Main cohort: standard arm, no clinical CVD | 0.97 | 0.85–1.09 | 0.58 |
| Standard arm, clinical CVD | 1.21 | 0.95–1.54 | 0.12 |
| Intensive arm, no clinical CVD | 1.00 | 0.89–1.13 | 0.96 |
| Intensive arm, clinical CVD | 1.41 | 1.10–1.79 | 0.006 |
CVD cardiovascular disease
Fig. 1.
Overall SAEs by statin use, among SPRINT participants without baseline clinical CVD and randomized to standard BP care. BP blood pressure, CI confidence interval, CVD cardiovascular disease, SAE serious adverse event
However, among the SPRINT participants that were randomized to intensive BP management and who had documented clinical CVD at baseline (Table 4), a precisely estimated elevated hazard ratio was observed for statin use (1.41, 95% confidence interval 1.10–1.79, p = 0.006).
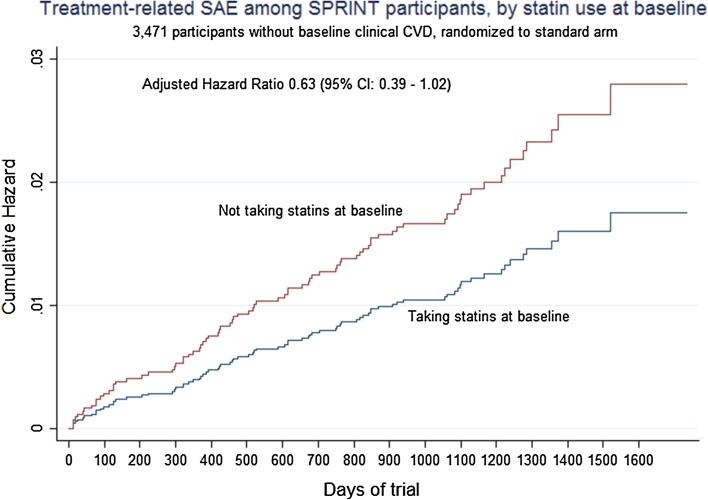
Turning to treatment-related SAEs (Table 5), in the main cohort, the unadjusted and insignificant point estimate of lower treatment-related SAEs among statin users almost reached conventional levels of statistical significance in the adjusted Cox model (adjusted hazard ratio 0.63, 95% confidence interval 0.39–1.02). Examination of the cumulative hazard graph (Fig. 2) in the main cohort shows a clear separation from the beginning of the trial.
Table 5.
Adjusted hazard ratio for treatment-related serious adverse events
| Treatment-related serious adverse events | |||
|---|---|---|---|
| Hazard ratio associated with statin use at baseline | 95% confidence interval | p value | |
| Main cohort: standard arm, no clinical CVD | 0.63 | 0.39–1.02 | 0.06 |
| Standard arm, clinical CVD | 1.28 | 0.50–3.24 | 0.61 |
| Intensive arm, no clinical CVD | 1.20 | 0.85–1.70 | 0.29 |
| Intensive arm, clinical CVD | 1.29 | 0.65–2.58 | 0.47 |
CVD cardiovascular disease
Fig. 2.
Treatment-related SAEs by statin use, among SPRINT participants without baseline clinical CVD and randomized to standard BP care. BP blood pressure, CI confidence interval, CVD cardiovascular disease, SAE serious adverse event
In sensitivity analyses, none of the other cohorts examined showed statistically significant hazard ratios different from 1 (Table 5).
Discussion
This small secondary re-analysis sought to understand whether SAEs among SPRINT participants differed by statin use at baseline. To reduce confounding by indication in the non-randomized use of statins, adjustment was made for observed baseline characteristics. To further reduce confounding of SAEs by differences in pharmacological treatment in the two BP management arms, the main analysis considered only patients without known baseline CVD randomized to standard BP management, while sensitivity analyses also considered homogenous cohorts of other patients with the same CVD status at baseline and the same randomization BP management arm.
The main results do not show any significant increase in SAEs associated with statin use among a homogenous cohort of patients without documented CVD at baseline and randomized to the standard BP management arm. Instead a small risk difference in favor of statin use at baseline was found for treatment-related SAEs in this main cohort.
When incorporating the sensitivity analyses in the other cohorts, this study finds that statin use at baseline was not associated with either treatment-related or overall SAEs either (1) among those without pre-existing CVD, regardless of randomization to BP arm or (2) among those randomized to standard BP management, regardless of pre-existing CVD.
Only in the cohort comprising higher risk patients with clinical CVD randomized to intensive BP management, with more intense pharmaceutical management with either higher doses and/or multiple classes, was a small but significant increase in overall SAE found among those taking statin at baseline, but not in the subset risk of SAE deemed definitely or possibly related to treatment.
It is not known whether this latter finding is through the play of chance given the multiple comparisons made, or is a true positive finding. There is substantial evidence that statins have the potential for drug interactions [20], suggesting that this finding could represent SAEs caused by true interaction effects between statin therapy and the larger number of medicines in different classes that characterized intensive BP management.
Taken together, these results are unexpected for two reasons. First, the non-randomized and unblinded use of statins was expected to lead to higher rates of SAEs in all cohorts studied. This could be due to both confounding by indication and ascertainment biases, as ASCOT-LLA recently showed [14]. The findings of generally lower SAE risk therefore strengthen the conclusion that statin use is generally not associated with major or lasting side effects.
Second, the results in the highest risk cohort are unexpected and raise the question to what extent polypharmacy and interactions among statins and other cardiovascular system medicines may jointly lead to increased SAEs. The findings here suggest the need for further investigation of the interaction between SAE risk and ASCVD risk among those taking statins.
This study is greatly limited by two separate weaknesses: the data origin from a clinical trial, and a maintained hypothesis that any SAE caused directly or indirectly by statin use would be identifiable in the data.
In terms of data origin, this study is weakened by its reliance on a secondary analysis of a randomized clinical trial. To the extent to which this trial is not representative of the real-world population taking statin medication, so too are the results circumscribed. For example, diabetics were excluded from the SPRINT trial, and this secondary analysis cannot comment on this important subgroup. Additionally, this secondary analysis, as many others do, relies on an original trial that was not powered for this re-analysis, and in turn on a re-analysis with uncorrected multiple testing [21, 22]. It is therefore possible that the significantly higher hazard rate for SAEs among statin-using, higher risk patients represents the play of chance rather than a true positive finding.
Data analyzed here are also incomplete in important ways. While the proportion of patients reporting taking statins at baseline was meaningful at between 38 and 69% depending on cohort examined, there were no data recorded in the trial on type of statin taken or dosage, nor was adherence to statin use or crossover to use recorded.
Finally, the reliable attribution of an SAE to a statin medicine taken at baseline as opposed to a particular BP intervention or another cause is not possible. Nevertheless, somewhat mitigating these concerns is the widespread availability of clinical trial data for secondary analyses [23–25].
In terms of the maintained hypothesis, a key premise of this analysis is that reported side effects are temporally related to statin use. However, it is generally believed that statin side effects occur relatively early (weeks to months) on initiating or re-starting statin therapy [26]. If patients had systematically started statin therapy closer to the trial date, or changed statin medications or doses during the trial thereby exposing themselves to greater SAE risk, then this premise would not be threatened. If on the other hand, participants in the SPRINT trial had been stable on statins for some period of time before the trial, most SAEs would have occurred and resolved, and this would lead to a bias towards the null of finding no association between statin use and SAE rates. Assuming the opposite, that this bias was widespread, it could explain the insignificant differences in the lower risk cohorts. However, it would then also strengthen polypharmacy and statin-drug interactions as the explanation for this study’s finding in the highest risk cohort.
Conclusions
Within the important limitations of this clinical trial secondary re-analysis and the other acknowledged design weaknesses, these results support the findings of the original statin randomized clinical trials that statin side effects may be limited in nature. These results also support the concerns that real-world evidence of statin side effects may be biased by unobserved confounding, confounding by indication and ascertainment bias [3, 4]. This study therefore cautiously supports the view among many clinicians that in general and on average, the benefits of statins likely outweigh concerns about statin-related harms [1].
Compliance with Ethical Standards
Funding
No direct funding; unrestricted research allowance.
Conflict of interest
Marco D. Huesch has no conflicts of interest that are directly relevant to the content of this study.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of our institutional research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study used data which were completely de-identified and aggregated and accordingly did not represent human subjects research and did not require an institutional review board (IRB) determination or approval in my institution. Despite this, the study was submitted for IRB determination at my institution, and a formal determination was made that this was not human research.
Consent to participate
Because it was a retrospective secondary analysis of de-identified clinical trial data determined to be not human research, a formal consent was not required in my institution.
References
- 1.Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316(19):2008–2014. doi: 10.1001/jama.2015.15629. [DOI] [PubMed] [Google Scholar]
- 2.Redberg RF, Katz MH. Healthy men should not take statins. JAMA. 2012;307(14):1491–1492. doi: 10.1001/jama.2012.423. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316(19):1997–2007. doi: 10.1001/jama.2016.15450. [DOI] [PubMed] [Google Scholar]
- 4.Redberg RF, Katz MH. Statins for primary prevention: the debate is intense, but the data are weak. JAMA. 2016;316(19):1979–1981. doi: 10.1001/jama.2016.15085. [DOI] [PubMed] [Google Scholar]
- 5.McClure DL, Valuck RJ, Glanz M, Hokanson JE. Systematic review and meta-analysis of clinically relevant adverse events from HMG CoA reductase inhibitor trials worldwide from 1982 to present. Pharmacoepidemiol Drug Saf. 2007;16:132–143. doi: 10.1002/pds.1341. [DOI] [PubMed] [Google Scholar]
- 6.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 7.Byar DP. Problems with using observational databases to compare treatments. Stat Med. 1991;10:663–666. doi: 10.1002/sim.4780100417. [DOI] [PubMed] [Google Scholar]
- 8.Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48(6):S114–S120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosco JL, Silliman RA, Thwin SS, et al. A most stubborn bias: no adjustment method fully resolves confounding by indication in observational studies. J Clin Epidemiol. 2010;63:64–74. doi: 10.1016/j.jclinepi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 11.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ. 2010;340:c2197. doi: 10.1136/bmj.c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: The ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758–769. doi: 10.1016/j.jacl.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Thompson D, Whitehouse A, on behalf of the ASCOT Investigators et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase (published online May 2) Lancet. 2017;389(10088):2473–2481. doi: 10.1016/S0140-6736(17)31075-9. [DOI] [PubMed] [Google Scholar]
- 14.Stears AJ, et al. A double-blind, placebo-controlled, crossover trial comparing the effects of amiloride and hydrochlorothiazide on glucose tolerance in patients with essential hypertension. Hypertension. 2012;59:934. doi: 10.1161/HYPERTENSIONAHA.111.189381. [DOI] [PubMed] [Google Scholar]
- 15.Elliott WJ. Effects of potassium-sparing versus thiazide diuretics on glucose tolerance: new data on an old topic. Hypertension. 2012;59:911. doi: 10.1161/HYPERTENSIONAHA.112.192542. [DOI] [PubMed] [Google Scholar]
- 16.Sarafidis PA, Bakris GL. Do the metabolic effects of β blockers make them leading or supporting antihypertensive agents in the treatment of hypertension? J Clin Hypertens. 2006;8:351–356. doi: 10.1111/j.1524-6175.2005.04679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Culver AL, Ockene IS, Balasubramanian R, et al. Statin use and risk of diabetes mellitus in postmenopausal women in the Women’s Health Initiative. Arch Intern Med. 2012;172(2):144–152. doi: 10.1001/archinternmed.2011.625. [DOI] [PubMed] [Google Scholar]
- 18.The SPRINT Research Group A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013. http://circ.ahajournals.org/content/circulationaha/early/2013/11/11/01.cir.0000437741.48606.98.full.pdf. Accessed 1 Sept 2017.
- 20.Bottorff M. Statin safety and drug interactions: clinical implications. Am J Cardiol. 2006;97(suppl):27C–31C. doi: 10.1016/j.amjcard.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Hayward RA, Kent DM, Vijan S, et al. Reporting clinical trial results to inform providers, payers, and consumers. Health Aff (Millwood) 2005;24:1571–1581. doi: 10.1377/hlthaff.24.6.1571. [DOI] [PubMed] [Google Scholar]
- 22.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 23.Bauchner H, Golub RM, Fontanarosa PB. Data sharing: an ethical and scientific imperative. JAMA. 2016;315(12):1238–1240. doi: 10.1001/jama.2016.2420. [DOI] [PubMed] [Google Scholar]
- 24.National Heart Lung and Blood Institute. Biologic Specimen and Data Repository Information Coordinating Center. Action to Control Cardiovascular Risk in Diabetes (ACCORD). https://biolincc.nhlbi.nih.gov/studies/accord/?q=ACCORD. Accessed 1 Sept 2017.
- 25.New England Journal of Medicine. Call for Entries: SPRINT Data Analysis Challenge. https://challenge.nejm.org/pages/home?emp=marcom&utm_source=nejmlist&utm_medium=email&utm_campaign=challenge_awareness&utm_content=activesub. Accessed 1 Sept 2017.
- 26.Thompson PD. What to believe and do about statin-associated adverse effects. JAMA. 2016;316(19):1969–1970. doi: 10.1001/jama.2016.16557. [DOI] [PubMed] [Google Scholar]