Abstract
Introduction
Obesity is associated with many pathophysiological changes that may result in altered drug metabolism. The aim of this study is to investigate the influence of obesity on the pharmacokinetics of morphine, morphine-3-glucuronide (M3G), and morphine-6-glucuronide (M6G) through a combined analysis in morbidly obese patients and non-obese healthy volunteers.
Methods
In this analysis, data from 20 morbidly obese patients [mean body mass index 49.9 kg/m2 (range 37.6–78.6 kg/m2) and weight 151.3 kg (range 112–251.9 kg)] and 20 healthy volunteers [mean weight 70.6 kg (range 58–85 kg)] were included. Morbidly obese patients received 10 mg of intravenous (I.V.) morphine after gastric bypass surgery, with additional morphine I.V. doses as needed. Healthy volunteers received an I.V. bolus of morphine of 0.1 mg/kg followed by an infusion of 0.030 mg kg−1 h−1 for 1 h. Population pharmacokinetic modeling was performed using NONMEM 7.2.
Results
In morbidly obese patients, elimination clearance of M3G and M6G was decreased substantially compared with healthy volunteers (p < 0.001). Regarding glucuronidation, only a slight decrease in the formation of M6G and a delay in the formation of M3G was found (both p < 0.001). Obesity was also identified as a covariate for the peripheral volume of distribution of morphine (p < 0.001).
Conclusion
Metabolism of morphine is not altered in morbidly obese patients. However, decreased elimination of both M3G and M6G is evident, resulting in a substantial increase in exposure to these two metabolites. A rational explanation of this finding is that it results from alterations in membrane transporter function and/or expression in the liver.
ClinicalTrials.gov identifier: NCT01097148.
Electronic supplementary material
The online version of this article (doi:10.1007/s40262-017-0544-2) contains supplementary material, which is available to authorized users.
Key Points
| Morphine concentrations proved similar between the morbidly obese patients and non-obese patients, indicating that no weight-based dosing adjustments are necessary. |
| However, decreased elimination clearance of morphine-3-glucuronide and morphine-6-glucuronide in morbidly obese patients may result in increased exposure to the metabolites. |
| It seems that the increased exposure to the two metabolites may result from obesity-related alterations in membrane transporter function and/or expression in the liver. |
Introduction
The prevalence of obesity [body mass index (BMI) > 30 kg/m2] and morbid obesity (BMI > 40 kg/m2) is increasing, with around 600 million obese people worldwide [1]. Obesity is associated with an increase in morbidity and mortality and numerous chronic diseases such as diabetes mellitus, cardiovascular diseases, and cancer.
There are several (patho)physiological changes associated with morbid obesity that may impact the pharmacokinetics of drugs. Obesity has been associated with changes in the expression and function of metabolic processes such as cytochrome P450 and conjugation enzymes, fatty liver infiltration, non-alcoholic steatohepatitis (NASH), and altered transporters [2]. These changes have been shown to impact the metabolism of certain drugs, with for instance, increased glucuronidation of paracetamol in morbidly obese patients [3], whereas the metabolism of midazolam is unaltered in morbidly obese patients undergoing bariatric surgery compared with non-obese control patients [4], but was found to increase after gastric bypass-induced weight loss 1 year after surgery [5]. Data on liver blood flow, glomerular filtration and/or tubular-mediated mechanisms in morbidly obese patients are more inconclusive with, for example, data of unchanged cefazolin clearance in morbidly obese patients and unchanged or increased liver blood flow [2, 6].
Morphine is primarily metabolized by the liver uridine diphosphate glucuronosyltransferase (UGT) 2B7 to pharmacologically active metabolites morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). M3G has potential antagonistic or hyperalgesic properties [7], while M6G appears to contribute to analgesia and sedation [8]. Despite the extensive use of morphine, data on the PK of morphine and glucuronide metabolites in morbidly obese patients are limited. A previous study reported an increased ratio between morphine metabolites and morphine after oral administration of morphine in gastric bypass patients when comparing their results with data in the literature [9–11]. In another study, intravenous morphine was administered to 14 healthy volunteers and the results compared with seven obese patients with biopsy-confirmed NASH. This study also suggested a higher area under the curve (AUC) of morphine glucuronides in NASH patients compared with healthy volunteers [12].
In view of the higher susceptibility for pain and the increased use of opioids in obese individuals [13], and the fact that the adverse effects of opioids are feared in obese populations because of the increased risk for respiratory depression, respiratory failure, and other opioid adverse effects [14, 15], knowledge on the pharmacokinetics of morphine and its metabolites in morbidly obese patients is necessary. This study investigates the pharmacokinetics of morphine and its pharmacologically active glucuronides in morbidly obese patients using a population approach on the basis of a combined dataset of morbidly obese patients together with a historic cohort of healthy volunteers [16, 17].
Methods
Patients
The data obtained in the morbidly obese patients were collected as part of a study in which the pharmacokinetics of multiple drugs was investigated [18–20]. Anesthesia was standardized with induction of anesthesia with propofol, atracurium, and fentanyl, after which anesthesia was maintained with continuous infusions of propofol and remifentanil. For this original study, 20 morbidly obese patients (BMI > 40 kg/m2) were included who were scheduled to undergo laparoscopic gastric banding, gastric sleeve, or gastric bypass surgery (Table 1). Inclusion criteria were age between 18 and 60 years, BMI > 40 kg/m2, American Society of Anesthesiologists (ASA) physical status classification of II or III, and a normal renal and liver function as assessed by routine laboratory testing. Exclusion criteria were pregnancy, breastfeeding, and a known allergy to morphine. This study was approved by the local Human Research and Ethics Committee of St. Antonius Ziekenhuis (VCMO, NL35861.100.11) and conducted in accordance with the principles of the Declaration of Helsinki and the Medical Research Involving Human Subjects Act (WMO) of The Netherlands. Before participation, all patients gave written informed consent.
Table 1.
Summary of patients characteristics
| Morbidly obese patients (n = 20) | Healthy volunteers (n = 20) | P value | |
|---|---|---|---|
| Male/female | 9/11 | 10/10 | 0.752 |
| Age (years) | 44.1 ± 10.6 (22–59) | 25.5 ± 4.1 (20–36) | <0.001 |
| Body weight (kg) | 150.5 ± 33.3 (112.0–251.9) | 70.6 ± 8.82 (56.0–85.0) | <0.001 |
| Body mass index (kg/m2) | 49.9 ± 10.2 (37.9–78.6) | ||
| Type of surgery (n, %) | |||
| Gastric bypass | 10 (50.0) | N/A | N/A |
| Gastric banding | 7 (35.0) | ||
| Gastric sleeve | 3 (15.0) | ||
| No. of samples per patient | <0.001 | ||
| Morphine, median (IQR) | 10 (10–10) | 15 (14–15) | |
| M3G | 10 (10–10) | 15 (14–15) | |
| M6G | 10 (10–10) | 15 (13–15) | |
| Total amount of morphine (mg) | 15.7 (4.0) | 9.2 (1.2) | <0.001 |
| Serum creatinine, median (IQR) (µmol/L) | 63 (60–81)a | 80 (–) | 0.014 |
Values are expressed as mean ± standard deviation (range) unless specified otherwise
IQR interquartile range, M3G morphine-3-glucuronide, M6G morphine-6-glucuronide, N/A not applicable
aOne value missing
For the control group, data were available from 20 healthy volunteers, 10 of each sex, who were enrolled as part of two other studies of which detailed information can be found in the references [16, 17]. The subjects were healthy and did not have a history of illicit substance abuse. Approval was obtained from the Human Ethics Committee (Commissie Medisch Ethiek, Leids Universitair Medisch Centrum, Leiden, The Netherlands: protocol No. P00.034). Written and oral informed consent was given.
Study Design
In the prospective observational study (ClinicalTrials.gov: NCT01097148), 20 morbidly patients were studied on the day of gastric bypass surgery and afterwards. According to standard care, all patients received a bolus injection of 10 mg of intravenous morphine at the end of the procedure for the prevention and/or treatment of postoperative pain. If needed based on the local postoperative pain protocol (Numerical Rating Scale ≥ 4), patients received additional intravenous boluses of morphine. Blood samples were drawn before induction of anesthesia (t = 0) and after 5, 15, 30, 45, 75, 90, 120, 150, 250, and 420 min after the first dose of intravenous morphine. Samples were immediately stored on ice, and within 1 h, samples were centrifuged for 10 min at 4 °C to obtain plasma samples and stored immediately at −80 °C until analysis.
The healthy volunteers received an intravenous bolus of 0.10 mg/kg of morphine followed by an infusion of 0.03 mg/kg/h for 1 h. Blood samples were collected at fixed times (t = 5, 10, 20, 30, 40, 50, 60, 65, 70, 80, 100, 130, 180, 300, and 420 min) after the morphine bolus dose.
Analysis
Samples from both studies were analyzed in the same laboratory using a solid-phase extraction and reverse-phase high-performance liquid chromatography, which has been published previously [16]. The lower limit of quantification (LLOQ) for the obese population was 1 µg/L for morphine, 2 µg/L for M3G, and 1 µg/L for M6G. For the analytic method of the healthy volunteer study, the LLOQ values for morphine and M3G were 2 and 30 µg/L. For M6G, the LLOQ values were 2, 5, and 6 µg/L.
Population Pharmacokinetic Analysis and Internal Model Validation
Morphine and metabolite data of both datasets were analyzed using non-linear mixed-effects modeling with NONMEM Version 7.2 software (Icon Development Solutions, Hanover, MD, USA) [21]. Pirana Version 2.9.1 [22], R Version 3.0.1 [23], Xpose Version 4.5.0 [22], and Psn Version 3.6.2 [22] software were used to evaluate and visualize the data. Identifiability of the model was verified using the COMBOS (UCLA Biocybernetics Laboratory Los Angeles, CA, USA) software application (see Electronic Supplementary Material 1) [24].
Concentrations were expressed in nanomoles per liter, using the molecular weights of morphine, M3G and M6G (285.33 and 461.46 g/mol, respectively). The amount of administrated morphine was corrected for morphine hydrochloride (molecular weight 321.8 g/mol).
In the obese population, no data were below the LLOQ. In the healthy volunteer study, 5% (n = 16 of 311) of the morphine concentrations, 4.5% (n = 14 of 311) of the M3G concentrations, and 9.6% (n = 30 of 311) of the M6G concentrations were below the LLOQ. The first below quantification observations were replaced with LLOQ/2 and the rest were discarded, according to the M6 method for handling data below the limit of quantification in population pharmacokinetic studies [25].
Discrimination between different models was made by the likelihood ratio test using the objective function value (OFV, i.e., −2 log likelihood [-2LL]). A p-value of <0.05, representing a decrease of 3.84 in the OFV value between nested models with one degree of freedom, was considered statistically significant. In addition, goodness-of-fit plots for morphine, M3G, and M6G [observed vs. individual-predicted concentrations, observed vs. population-predicted concentrations, conditional weighted residuals (CWRES) vs. time, and CWRES vs. population-predicted concentrations plots] were used for diagnostic purposes. Residual variability was tested using proportional, additive, or combined proportional and additive error models. Furthermore, the confidence interval of the parameter estimates, the correlation matrix, and visual improvement of the individual plots were used to evaluate the model. The delay in formation of morphine metabolites was captured by testing a varying number of transit compartments. Mean transit time (MTT) was calculated from the transit compartment rate constant (Ktr) with n/Ktr, where n is the number of transit compartments.
The non-glucuronide clearance (direct unchanged urinary clearance and non-glucuronide metabolic clearance) was assumed to be 35% of total clearance of a 70-kg healthy subject, based on previous reports [26]. Total clearance (CLtotal) was calculated as M3G clearance (CLM3G) + M6G clearance (CLM6G) + non-glucuronide clearance (CLnonglucuronide). The volume of distribution of the two metabolites M3G and M6G was assumed to be equal (V M3G = V M6G), owing to their comparable molecular structure and weight. Bootstrap procedure using 200 replicates was used to obtain non-parametric confidence intervals and to assess model robustness [27]. Predictability was evaluated with the normalized prediction distribution error method (2000 samples). Results of the normalized prediction distribution error are incorporated in the goodness-of-fit plots, as a replacement of CWRES vs. time and CWRES vs. population-predicted concentrations.
Covariate Analysis
Covariates were plotted independently against the individual estimates of pharmacokinetic parameters to visualize potential relations. Total body weight (TBW) was the main covariate of interest in this study. Age and sex were tested in preliminary models but were further explored in the final model. BMI was not tested because no individual height was available of the healthy volunteers. Continuous covariates were tested using both power and linear equations:
| 1 |
| 2 |
where P i and P p represent individual and population parameter estimates, COV represents the covariate, COVmedian represents the median of the value of the covariate for the population, Y represents a correlation factor between the population parameter and the change in covariate value for a linear function, and X represents the exponential scaling factor for a power function. The categorical covariate (sex) was examined by calculating a separate parameter for each category of the covariate.
Potential covariates were separately entered into the model and statistically tested by use of the likelihood ratio test. In addition, if applicable, it was evaluated whether the inter-individual variability (eta) in the parameter concerned decreased upon inclusion of the covariate on the parameter and whether the plot of the eta vs. covariate was improved. Finally, using forward inclusion (p < 0.05, OFV decrease >3.8) and backward deletion (p < 0.001, OFV decrease 10.8), it was justified to include the covariate.
Simulations
The final population pharmacokinetic model was used to simulate concentration–time curves. An intravenous bolus of 10 mg of morphine HCL was simulated in four patients; two extremes of dataset (respectively, 56 and 251.9 kg) and two patients in-between. Morphine as well as M3G and M6G concentrations were plotted vs. time.
Statistical Analysis
Continuous data are presented as median (interquartile range (IQR)) and analyzed using the Mann–Whitney test, or as mean ± standard deviation and analyzed using the Student’s t test, where appropriate.
Results
Patients
Twenty morbidly obese patients and 20 healthy volunteers were available for analysis. In total, in the obese group, 196 morphine, 196 M3G, and 196 M6G plasma samples were included for analysis. In the healthy volunteers, a total of 290 plasma samples of morphine, 289 plasma samples of M3G, and 285 plasma samples of M6G were included. Differences were the result of the samples below the LLOQ. A summary of patient characteristics is presented in Table 1. Morbidly obese patients received a higher morphine dose compared with the healthy volunteers (15.7 ± 4.0 mg vs. 9.2 ± 1.2 mg, p < 0.05).
Population Pharmacokinetic Model and Internal Model Evaluation
A three-compartment model for morphine, and a one-compartment model for M3G and M6G, with equalized volumes of distribution best fitted the data (Fig. 1). The introduction of multiple transit compartments in the formation of the glucuronides (for M3G n = 5, mean transit time = 3.05 min; for M6G n = 2, mean transit time = 12.7 min) improved the model significantly (p < 0.001). Residual variability was best described by proportional error models, one for each compound, and calculated separately for each group. Table 2 shows the parameter estimates of the simple model without covariates.
Fig. 1.
Schematic of the population pharmacokinetic model of morphine and morphine glucuronides. CL F formation clearance, CL E elimination clearance, Ktr transit rate constant, M3G morphine-3-glucuronide, M6G morphine-6-glucuronide, Q inter-compartmental clearance from the central compartment of morphine to the peripheral compartments of morphine, V1 central volume of distribution, V4 M ,V5 M peripheral compartments of morphine, V3M6G = V2M3G central volumes of morpine glucuronides, CLnon-glucuronide = 35% of Cltotal (70 kg), CLtotal = Clnon-glucuronide + CLF M3G + CLF M6G
Table 2.
Population pharmacokinetic parameters of the base and final pharmacokinetic model for morphine and glucuronides in healthy volunteers and morbidly obese patients and results of the bootstrap analysis
| Parameter | Base model (RSE%) | Final model (RSE%) | Bootstrap (95% confidence interval) |
|---|---|---|---|
| Morphine a | |||
| CLF M3G (L/min) | 0.725 (4.0) | 0.748 (3.0) | 0.748 (0.706–0.797) |
| CLF M6G (L/min) | 0.128 (6.0) | ||
| CLF M6G = CLF M6G, 98.5 kg · (TBW/98.5)K | |||
| CLF M6G, 98.5 kg (L/min) | 0.129 (5.0) | 0.130 (0.119–0.140) | |
| K | −0.329 (36.0) | −0.310 (−0.534 to −0.125) | |
| V1M (L) | 3.96 (5.0) | 4.62 (9.0) | 4.66 (3.95–5.59) |
| V4M (L) | 5.76 (18.0) | 9.52 (33.0) | 9.91 (6.10–15.7) |
| V5M (L) | 101 (5.0) | ||
| V5M = V 98.5 kg · (TBW/98.5)L | |||
| V 98.5 kg (L) | 118 (9.0) | 117.5 (103.7–136.6) | |
| L | 0.483 (48.0) | 0.453 (0.112–0.859) | |
| Q2 (L/min) | 0.625 (7.0) | 0.814 (20.0) | 0.834 (0.598–1.16) |
| Q3 (L/min) | 1.27 (5.0) | 1.29 (5.0) | 1.28 (1.15–1.41) |
| Ktr (min−1) | 1.58 (9.0) | ||
| Ktr = Ktr98.5 kg · (TBW/98.5)M | |||
| Ktr98.5 kg (min-1) | 1.68 (9.0) | 1.71 (1.51–1.98) | |
| M | −0.701 (30.0) | −0.71 (−0.106 to 0.375) | |
| Ktr2 (min−1) | 0.151 (5.0) | 0.159 (7.0) | 0.158 (0.146–0.172) |
| Metabolites (M3G, M6G) | |||
| V M3G = V M6G (L) | 6.47 (7.0) | 5.29 (13.0) | 5.33 (4.28–6.52) |
| CLE M3G (L/min) | 0.131 (14.0) | ||
| CLE M3G = CLE M3G, 98.5 kg · (TBW/98.5)N | |||
| CLE M3G, 98.5 kg (L/min) | 0.134 (10.0) | 0.134 (0.110–0.155) | |
| N | –1.08 (22.0) | –1.06 (–1.53 to -0.60) | |
| CLE M6G (L/min) | 0.171 (15.0) | ||
| CLE M6G = CLE M6G, 98.5 kg · (TBW/98.5)O | |||
| CLE M6G, 98.5 kg (L/min) | 0.149 (10.0) | 0.154 (0.125–0.186) | |
| O | –1.03 (31.0) | –1.06 (–1.64 to –0.56) | |
| Inter-individual variability (%) | |||
| CLF M3G | 24.3 (12.0) | 20.8 (10.0) | 20.3 (16.8–23.4) |
| CLE M3G | 89.0 (19.0) | 65.9 (20.0) | 62.9 (41.9–86.1) |
| V M3G = V M6G | 32.3 (12.0) | 29.7 (12.0) | 29.2 (22.6–35.8) |
| Ktr2 | 37.7 (13.0) | 36.8 (13.0) | 35.9 (27.1–43.6) |
| Residual variability (%) | |||
| Healthy volunteers | |||
| Proportional error for morphine | 15.1 (16.0) | 14.0 (7.0) | 13.8 (12.0–15.7) |
| Proportional error for M3G | 18.0 (25.0) | 17.9 (12.0) | 18.0 (14.3–21.5) |
| Proportional error for M6G | 30.4 (19.0) | 29.5 (8.0) | 29.3 (24.2–32.8) |
| Morbidly obese patients | |||
| Proportional error for morphine | 37.3 (22.0) | 37.9 (11.0) | 37.1 (29.2–44.7) |
| Proportional error for M3G | 18.4 (17.0) | 17.1 (8.0) | 17.1 (14.9–19.1) |
| Proportional error for M6G | 32.8 (37.0) | 28.1 (9.0) | 26.5 (21.5–30.7) |
| OFV (−2LL) | 10,311.38 | 10,116.1 | 10,038.1 (9774.8–10,306.3) |
CL F formation clearance, CL E elimination clearance, Ktr transit rate constant, LL log likelihood, M3G morphine-3-glucuronide, M6G morphine-6-glucuronide, OFV objective function variable, Q inter-compartmental clearance from the central compartment of morphine to the peripheral compartments of morphine, RSE relative standard error, TBW total body weight, V volume of distribution (see also Fig. 1)
aFormation clearances are reported as absolute values, with CLF M3G and CLF M6G being 65% of total morphine clearance (see also Fig. 1)
In the covariate analysis, no substantial influence of TBW on the clearance of morphine was found. Significant influence of TBW was found on several other parameters, all in a non-linear manner. Elimination clearance of both metabolites decreased with TBW (CLE M3G p < 0.001, −16 OFV, CLE M6G p < 0.001, −92 OFV), and the peripheral volume of morphine increased significantly with increasing TBW (p < 0.001, −34 OFV). Formation clearance of M6G decreased with increasing TBW (CLF M6G p < 0.001, −26 OFV). Formation of M3G was delayed with increasing body weight because the mean transit time was increased with TBW [Ktr (p < 0.001, −28 OFV)]. Imputing these functions resulted in a reduction in inter-individual variability (CLF M3G 24.3–20.8%, CLE M3G 89.0–65.9%, V M3G = VM6G 32.3–29.7%, and Ktr2 37.7–36.8%) (see Table 2). Goodness-of-fit plots of the final covariate model are shown in Fig. 2. The empirical Bayes estimates (EBEs) after adding the covariate functions are shown in Fig. 3. This figure shows the population-predicted outcomes of the final covariate model and the influence of TBW on the parameters, where adding TBW improved the model significantly. Final model parameters are summarized in Table 2. The bootstrap analysis was successful in 98.5% of the runs and the obtained parameter confidence intervals were highly similar to the confidence intervals obtained from the standard errors (Table 2).
Fig. 2.
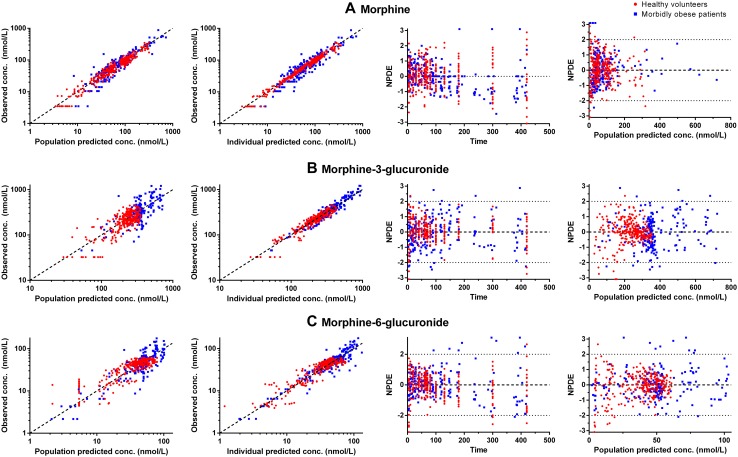
Goodness-of-fit plots of morbidly obese individuals (n = 20, blue squares) and healthy volunteers (n = 20, red rounds). On the first row morphine (a), second row morphine-3-glucuronide (b), and third row morphine-6-glucuronide (c). Please note the scale differences in the y-axis. conc. concentration, NPDE normalized prediction distribution error
Fig. 3.
Post-hoc parameters estimates of morbidly obese individuals (n = 20, blue squares) and healthy volunteers (n = 20, red rounds) from the final model vs. total body weight, including morphine-3-glucuronide elimination clearance (CLE M3G) vs. total body weight (a), morphine-6-glucuronide elimination clearance (CLE M6G) vs. total body weight (b), morphine-3-glucuronide transit rate constant (Ktr) vs. total body weight (c), morphine-6-glucuronide transit rate constant (Ktr2) vs. total body weight (d), peripheral volume of distribution of morphine (V1M) vs. total body weight (e), and formation clearance of morphine-6-glucuronide (CLF M6G) vs. total body weight (f)
Simulations
Figure 4 shows the model-predicted concentration–time profiles of morphine and its metabolites after an intravenous bolus dose of 10 mg of morphine and a 48-h continuous infusion of 2 mg h−1 in four representative individuals from this study with a TBW of 56, 75, 125, and 253 kg. The figure shows that the pharmacokinetic profile of morphine (panels A, D) in this weight range is comparable. However, more pronounced differences are shown in the morphine glucuronides. Here, when a bolus of morphine is given, the maximum concentration of M3G is higher in obese patients (panel B). In addition, as a result of decreased elimination clearance, the AUC is also increased in these patients. For M6G (panel C), an effect of TBW on formation clearance and elimination clearance results in lower peak concentrations, but an increased AUC in obese patients. After a continuous infusion of 48 h of infusion, the 253-kg patient has approximately a five times higher concentration of M3G and a three times higher concentration of M6G compared with the 56-kg healthy volunteer (panels D, E).
Fig. 4.
Population predicted morphine, morphine-3-glucuronide (M3G), and morphine-6-glucuronide (M6G) concentrations over time in four typical study patients (56, 75, 125, and 253 kg) after a 10-mg intravenous bolus dose of morphine hydrochloride (a–c) and a 2-mg/h continuous infusion of morphine hydrochloride for 48 h (d–f)
Discussion
As limited data are available on the pharmacokinetics of morphine in morbidly obese patients, this study aimed to evaluate the influence of obesity on the metabolism of intravenously administered morphine and its pharmacologically active glucuronides (M3G and M6G). The results of this study show that, besides a slight decrease in the formation of M6G, the formation clearance of the main metabolite M3G is similar between the groups, although the formation was delayed. It has been reported before, that UGT-mediated drug metabolism is potentially increased in obese patients in comparison with non-obese patients [28]; for example, paracetamol glucuronidation (and sulfation) is increased in obese patients [3]. The lack of influence of obesity on morphine glucuronidation in the present study may be explained by the fact that morphine is a medium-to-high extraction ratio drug, assuming liver blood flow remains unchanged in morbidly obese patients [2]. Such drugs are rapidly metabolized depending on hepatic blood flow and are relatively insensitive to changes in enzyme activity [14].
The most important finding of the current study is the decrease in elimination clearance of both morphine glucuronides, and the resulting increased exposure to these metabolites that may therefore be expected in the obese patients (Fig. 4). Increased AUC ratios of glucuronides:morphine in obese patients when compared with the metabolic ratios reported for healthy adults in the literature have been reported before [10]. However, from a physiological perspective, these results are somewhat unexpected because the elimination of morphine glucuronides in animals is mainly through renal excretion; i.e., only about 20% of the morphine glucuronides is excreted through bile [29–31]. Therefore, we did not expect such a dramatic reduction in glucuronide clearance in the obese patients, as the routine blood tests of renal function around surgery show no indication that our obese patients had an impaired renal function. A more likely explanation is that the elimination of the morphine glucuronides in the bile plays a much larger role in special patient populations than previously thought, implying a significant role for hepatic transporters.
Multidrug resistance proteins MRP2 (ABCC2) and MRP3 (ABCC3) are known to be involved in the transport of morphine and metabolites. MRP2 is mainly involved in the efflux of molecules from hepatocytes to the bile, while MRP3 is involved in the efflux from hepatocytes to plasma [32]. A decrease in MRP2 activity could therefore lead to a decrease in morphine glucuronide elimination. It is also likely that obese individuals could have decreased MRP2 activity as a result of NASH. This condition is associated with alterations in the expression and function of metabolizing enzymes and transporters [33, 34]. In a NASH model in the rat, impaired function of MRP2 resulted in significantly reduced biliary excretion of M3G [32]. Furthermore, there is genetic evidence in humans that the activity of MRP2 is critical for biliary excretion of substrates. In an inherited medical condition known as Dubin–Johnson syndrome, dysfunctional mutations in the MRP2 gene cause impairment in biliary excretion of bilirubin, such as bilirubin glucuronides. Together with upregulation of MRP3, this results in jaundice in patients with Dubin–Johnson syndrome [35].
A recent clinical study measured bile acids as a surrogate parameter for the activity of protein expression of the hepatic basolateral efflux transporter Mrp-3 [12]. Seven obese patients (mean BMI of 32 kg m−2) with confirmed NASH were included in a non-compartmental analysis and no differences in the pharmacokinetics of morphine compared with healthy subjects were found. Healthy volunteers had no liver biopsy to confirm the absence of NASH. However, an increase of around 50% in the AUC of the glucuronides in the patients with NASH was reported [12]. Upregulated MRP3 could increase the efflux from the hepatocytes to plasma, thereby reducing the concentrations available to be excreted to bile by MRP2 and thus increasing the residence time of M3G in plasma. The question is whether a combination of upregulated MRP3 and a decreased functional MRP2 can account completely for the increased exposure to morphine glucuronides in obese patients. This study of Ferslew et al. shows that increasing severity of NASH correlates with increasing bile acids, meaning that increasing NASH severity may further increase MRP3-mediated efflux clearance [12]. Taking into consideration that our patients have a far greater BMI index (mean 49.9 kg m−2) compared with this study, the impact of the MRP2/MRP3 transporters is potentially even greater.
Remarkably, accumulation of the morphine glucuronides is also seen in other patient populations. The study of Ahlers et al. compared patients in an intensive care unit (ICU) (i.e., cardiac surgery patients and critically ill patients) with healthy volunteers and found that M3G elimination clearance was decreased independently of the creatinine levels [36]. Because these patients had a BMI of around 28 kg m−2, it is possible that obesity-related factors may have caused these results. Moreover, another study found increased expression of MRP3 protein in post-mortem biopsy samples of critically ill patients in an ICU [37]. Similar results on the accumulation of morphine glucuronides have been reported in children undergoing cardiac surgery compared with children not undergoing cardiac surgery [38]. Whether induction or inactivation of transporters in the acute setting such as surgery can play a role in the metabolism of drugs is an area for future research. For example, a rat model of acute sepsis showed upregulation of MRP3 mRNA levels [39].
The time–concentration simulations in Fig. 4 illustrate the large increase in exposure to M3G and M6G that may be expected in individuals of varying body weights. Although the structure of the metabolites is quite similar, the effect of TBW on their profiles is different. This is the result of the different covariate functions on the M6G compared with M3G, and possibly of the lower fraction of morphine that is converted to M6G and the different UGT enzymes responsible for glucuronidation of the metabolites [8]. The clinical relevance of increased concentrations of M3G and M6G is however not clear. The general assumption is that M3G, although showing higher plasma concentrations, has lower opioid receptor binding affinity compared with morphine and lacks opioid activity, although some studies have reported anti-analgesic effects [40–42]. However, M6G binds with high affinity to the opioid receptor and contributes to the analgesic properties of morphine [8]. There is a slow equilibration of the glucuronides between plasma and effect sites in the central nervous system, which is why the contribution of the glucuronides can become more important in prolonged exposure or decreased clearance for example in renal failure [43]. Recently, it has become clear that morbidly obese patients 6 months after gastric bypass surgery had an increase in morphine exposure after oral administration [9]. The exposure of morphine increased probably because of an increase in absorption, while the exposure of glucuronides remained the same compared to the pre-surgery state. This suggests a pathophysiological change after weight loss such as a decrease in glucuronidation capacity, an increase in elimination clearance, or altered liver blood flow and/or liver membrane transporters. There were some limitations in this study. First, even though the impact is expected to be small because morphine is administered at the end of surgery, the effects of anesthesia and surgery on the pharmacokinetics of morphine and its metabolites cannot be assessed. Second, morbidly obese patients were not screened for the presence of NASH because no liver biopsy was taken. Third, TBW was the only body size descriptor available to investigate in this study. Last, no urinary samples were available to measure the concentrations of morphine and its metabolites. In this study, the measurements of morphine, M3G and M6G concentrations came from the same blood samples. The measurements can therefore be assumed to be correlated. While it would have been technically possible to estimate the intra-sample correlations between the concentrations, this was not considered relevant for the estimation of the pharmacokinetic parameters, their variances, and their covariates.
Future studies evaluating the influence of hepatic transporters and bile acid homeostasis in morbidly obese patients and after bariatric surgery are needed to understand more of the pathophysiological changes associated with obesity. In addition, studies should evaluate the clinical effects of increased morphine glucuronides in terms of efficacy and safety.
Conclusion
In morbidly obese patients, the pharmacokinetics of morphine are comparable to healthy volunteers, thus no weight-based dosing adjustments are necessary for pharmacokinetic purposes. However, the elimination clearances of both M3G and M6G are significantly decreased, resulting in increased exposure to the metabolites, especially with prolonged administration of morphine. A suggested underlying mechanism is a change in membrane transporters that are associated with patients with NASH, a hepatic condition common in obese individuals. Additional mechanisms of increased glucuronide concentrations is an area for future research, together with the pharmacodynamic and clinical consequences of increased M3G and M6G concentrations, especially.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Sabine Ahlers is acknowledged for her contributions to this work. Part of this work was carried out on the Dutch national e-infrastructure with the support of SURF Foundation.
Compliance with Ethical Standards
Funding
No sources of funding were used in the preparation of this article.
Conflict of Interest
Sjoerd de Hoogd, Pyry A. J. Välitalo, Albert Dahan, Simone van Kralingen, Michael M. W. Coughtrie, Eric P. A. van Dongen, Bert van Ramshorst, and Catherijne A. J. Knibbe have no conflicts of interest directly relevant to the content of this article.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s40262-017-0544-2) contains supplementary material, which is available to authorized users.
Contributor Information
Sjoerd de Hoogd, Email: s.de.hoogd@antoniusziekenhuis.nl.
Catherijne A. J. Knibbe, Phone: +3188 320 72 01, Email: c.knibbe@antoniusziekenhuis.nl
References
- 1.World Health Organization. Fact sheet on obesity and overweight. January 2015. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 3 Feb 2016
- 2.Knibbe CA, Brill MJ, van Rongen A, et al. Drug disposition in obesity: toward evidence-based dosing. Annu Rev Pharmacol Toxicol. 2015;55:149–167. doi: 10.1146/annurev-pharmtox-010814-124354. [DOI] [PubMed] [Google Scholar]
- 3.van Rongen A, Valitalo PA, Peeters MY, et al. Morbidly obese patients exhibit increased CYP2E1-mediated oxidation of acetaminophen. Clin Pharmacokinet. 2016;55(7):833–847. doi: 10.1007/s40262-015-0357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brill MJ, van Rongen A, Houwink AP, et al. Midazolam pharmacokinetics in morbidly obese patients following semi-simultaneous oral and intravenous administration: a comparison with healthy volunteers. Clin Pharmacokinet. 2014;53(10):931–941. doi: 10.1007/s40262-014-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brill MJ, van Rongen A, van Dongen EP, et al. The pharmacokinetics of the CYP3A substrate midazolam in morbidly obese patients before and one year after bariatric surgery. Pharm Res. 2015;32(12):3927–3936. doi: 10.1007/s11095-015-1752-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brill MJ, Houwink AP, Schmidt S, et al. Reduced subcutaneous tissue distribution of cefazolin in morbidly obese versus non-obese patients determined using clinical microdialysis. J Antimicrob Chemother. 2014;69(3):715–723. doi: 10.1093/jac/dkt444. [DOI] [PubMed] [Google Scholar]
- 7.Martini C, Olofsen E, Yassen A, et al. Pharmacokinetic-pharmacodynamic modeling in acute and chronic pain: an overview of the recent literature. Expert Rev Clin Pharmacol. 2011;4(6):719–728. doi: 10.1586/ecp.11.59. [DOI] [PubMed] [Google Scholar]
- 8.Sverrisdottir E, Lund TM, Olesen AE, et al. A review of morphine and morphine-6-glucuronide’s pharmacokinetic-pharmacodynamic relationships in experimental and clinical pain. Eur J Pharm Sci. 2015;74:45–62. doi: 10.1016/j.ejps.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Lloret-Linares C, Luo H, Rouquette A, et al. The effect of morbid obesity on morphine glucuronidation. Pharmacol Res. 2017;118:64–70. doi: 10.1016/j.phrs.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Lloret-Linares C, Miyauchi E, Luo H, et al. Oral morphine pharmacokinetic in obesity: the role of P-glycoprotein, MRP2, MRP3, UGT2B7, and CYP3A4 jejunal contents and obesity-associated biomarkers. Mol Pharm. 2016;13(3):766–773. doi: 10.1021/acs.molpharmaceut.5b00656. [DOI] [PubMed] [Google Scholar]
- 11.Lloret-Linares C, Hirt D, Bardin C, et al. Effect of a Roux-en-Y gastric bypass on the pharmacokinetics of oral morphine using a population approach. Clin Pharmacokinet. 2014;53(10):919–930. doi: 10.1007/s40262-014-0163-0. [DOI] [PubMed] [Google Scholar]
- 12.Ferslew BC, Johnston CK, Tsakalozou E, et al. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther. 2015;97(4):419–427. doi: 10.1002/cpt.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hitt HC, McMillen RC, Thornton-Neaves T, et al. Comorbidity of obesity and pain in a general population: results from the Southern Pain Prevalence Study. J Pain. 2007;8(5):430–436. doi: 10.1016/j.jpain.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Lloret Linares C, Decleves X, Oppert JM, et al. Pharmacology of morphine in obese patients: clinical implications. Clin Pharmacokinet. 2009;48(10):635–651. doi: 10.2165/11317150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Rose DK, Cohen MM, Wigglesworth DF, DeBoer DP. Critical respiratory events in the postanesthesia care unit: patient, surgical, and anesthetic factors. Anesthesiology. 1994;81(2):410–418. doi: 10.1097/00000542-199408000-00020. [DOI] [PubMed] [Google Scholar]
- 16.Sarton E, Olofsen E, Romberg R, et al. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93(5):1245–1254. doi: 10.1097/00000542-200011000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Romberg R, Olofsen E, Sarton E, et al. Pharmacokinetic-pharmacodynamic modeling of morphine-6-glucuronide-induced analgesia in healthy volunteers: absence of sex differences. Anesthesiology. 2004;100(1):120–133. doi: 10.1097/00000542-200401000-00021. [DOI] [PubMed] [Google Scholar]
- 18.van Kralingen S, Taks M, Diepstraten J, et al. Pharmacokinetics and protein binding of cefazolin in morbidly obese patients. Eur J Clin Pharmacol. 2011;67(10):985–992. doi: 10.1007/s00228-011-1048-x. [DOI] [PubMed] [Google Scholar]
- 19.van Kralingen S, van de Garde EM, Knibbe CA, et al. Comparative evaluation of atracurium dosed on ideal body weight vs. total body weight in morbidly obese patients. Br J Clin Pharmacol. 2011;71(1):34–40. doi: 10.1111/j.1365-2125.2010.03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diepstraten J, Janssen EJ, Hackeng CM, et al. Population pharmacodynamic model for low molecular weight heparin nadroparin in morbidly obese and non-obese patients using anti-Xa levels as endpoint. Eur J Clin Pharmacol. 2015;71(1):25–34. doi: 10.1007/s00228-014-1760-4. [DOI] [PubMed] [Google Scholar]
- 21.Beal S, Sheiner L, Boeckmann A, Bauer R. NONMEM user’s guide (1989–2009) Ellicott City: Icon Development Solutions; 2009. [Google Scholar]
- 22.Keizer RJ, Karlsson MO, Hooker A. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacomet Syst Pharmacol. 2013;2:e50. doi: 10.1038/psp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Development Core Team . R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 24.Meshkat N, Kuo CE, DiStefano J., 3rd On finding and using identifiable parameter combinations in nonlinear dynamic systems biology models and COMBOS: a novel web implementation. PLoS One. 2014;9(10):e110261. doi: 10.1371/journal.pone.0110261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504. doi: 10.1023/A:1012299115260. [DOI] [PubMed] [Google Scholar]
- 26.Andersen G, Christrup L, Sjogren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manag. 2003;25(1):74–91. doi: 10.1016/S0885-3924(02)00531-6. [DOI] [PubMed] [Google Scholar]
- 27.Yafune A, Ishiguro M. Bootstrap approach for constructing confidence intervals for population pharmacokinetic parameters. I: a use of bootstrap standard error. Stat Med. 1999;18(5):581–599. doi: 10.1002/(SICI)1097-0258(19990315)18:5<581::AID-SIM47>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Brill MJ, Diepstraten J, van Rongen A, et al. Impact of obesity on drug metabolism and elimination in adults and children. Clin Pharmacokinet. 2012;51(5):277–304. doi: 10.2165/11599410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Christrup LL. Morphine metabolites. Acta Anaesthesiol Scand. 1997;41(1 Pt 2):116–122. doi: 10.1111/j.1399-6576.1997.tb04625.x. [DOI] [PubMed] [Google Scholar]
- 30.Ouellet DM, Pollack GM. Biliary excretion and enterohepatic recirculation of morphine-3-glucuronide in rats. Drug Metab Dispos. 1995;23(4):478–484. [PubMed] [Google Scholar]
- 31.Garrett ER, Jackson AJ. Pharmacokinetics of morphine and its surrogates. III: morphine and morphine 3-monoglucuronide pharmacokinetics in the dog as a function of dose. J Pharm Sci. 1979;68(6):753–771. doi: 10.1002/jps.2600680627. [DOI] [PubMed] [Google Scholar]
- 32.Dzierlenga AL, Clarke JD, Hargraves TL, et al. Mechanistic basis of altered morphine disposition in nonalcoholic steatohepatitis. J Pharmacol Exp Ther. 2015;352(3):462–470. doi: 10.1124/jpet.114.220764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher CD, Lickteig AJ, Augustine LM, et al. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37(10):2087–2094. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardwick RN, Ferreira DW, More VR, et al. Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2013;41(3):554–561. doi: 10.1124/dmd.112.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konig J, Rost D, Cui Y, Keppler D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology. 1999;29(4):1156–1163. doi: 10.1002/hep.510290404. [DOI] [PubMed] [Google Scholar]
- 36.Ahlers SJ, Valitalo PA, Peeters MY, et al. Morphine glucuronidation and elimination in intensive care patients: a comparison with healthy volunteers. Anesth Analg. 2015;121(5):1261–1273. doi: 10.1213/ANE.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 37.Vanwijngaerden YM, Wauters J, Langouche L, et al. Critical illness evokes elevated circulating bile acids related to altered hepatic transporter and nuclear receptor expression. Hepatology. 2011;54(5):1741–1752. doi: 10.1002/hep.24582. [DOI] [PubMed] [Google Scholar]
- 38.Valkenburg AJ, Calvier EA, van Dijk M, et al. Pharmacodynamics and pharmacokinetics of orphine after cardiac surgery in children with and without Down syndrome. Pediatr Crit Care Med. 2016;17(10):930–938. doi: 10.1097/PCC.0000000000000904. [DOI] [PubMed] [Google Scholar]
- 39.Cherrington NJ, Slitt AL, Li N, Klaassen CD. Lipopolysaccharide-mediated regulation of hepatic transporter mRNA levels in rats. Drug Metab Dispos. 2004;32(7):734–741. doi: 10.1124/dmd.32.7.734. [DOI] [PubMed] [Google Scholar]
- 40.Smith MT, Watt JA, Cramond T. Morphine-3-glucuronide: a potent antagonist of morphine analgesia. Life Sci. 1990;47(6):579–585. doi: 10.1016/0024-3205(90)90619-3. [DOI] [PubMed] [Google Scholar]
- 41.Gong QL, Hedner T, Hedner J, et al. Antinociceptive and ventilatory effects of the morphine metabolites: morphine-6-glucuronide and morphine-3-glucuronide. Eur J Pharmacol. 1991;193(1):47–56. doi: 10.1016/0014-2999(91)90199-Z. [DOI] [PubMed] [Google Scholar]
- 42.Mercadante S. The role of morphine glucuronides in cancer pain. Palliat Med. 1999;13(2):95–104. doi: 10.1191/026921699678158579. [DOI] [PubMed] [Google Scholar]
- 43.Dahan A, Lotsch J. Morphine is not a prodrug. Br J Anaesth. 2015;114(6):1005–1006. doi: 10.1093/bja/aev125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.