Abstract
Introduction
Muscle biopsy is a minor surgical procedure that has been conducted over several decades in clinical practice. Over the years, the technique to implement this procedure has been modified to make it easier to perform and more tolerable for the patient. This study aimed to assess the feasibility of muscle biopsy as an office based procedure, by using a vacuum assisted biopsy system.
Method
The procedure was successfully carried out on 57 individuals with/without diabetes, currently involved in the African American Diabetes Mellitus Study. One specimen was collected percutaneously from the vastus lateralis, under local anesthesia. A 16-gauge needle was used.
Results
Muscle biopsies were successfully carried out on all study participants. The study participants reported no complications after the procedure.
Conclusion
The findings from our study show that muscle biopsy can be feasibly implemented as an office based procedure, involving minimal muscle invasion, less trauma, hospital stay time, and expenses.
Keywords: Muscle, biopsy, vacora, vacuum, tissue
Introduction
Muscle biopsy is a relatively simple surgical procedure performed as part of standard of care in the management of many neuromuscular and metabolic disorders. The procedure has evolved from open and semi-open biopsies to needle biopsies, which are now increasingly being used. Micro-biopsies, the use of fine-needles to obtain muscle biopsies is slowly gaining ground but its scope is limited by the small amount of tissue obtained and potential for tissue disruption. Muscle biopsies are also occasionally required for biomedical research into various diseases including Chronic Obstructive Pulmonary Disease [1], skeletal tumors [2], muscular dystrophies [3] nervous system diseases [4], diseases of the vascular system [5] and connective tissue [6]. In addition, given the importance of the muscle as a major metabolic tissue, muscle biopsies are also being done in metabolomics and proteomics research.
The type of muscle biopsy procedure depends on the indication, which bears on the cost and logistics involved in the procedure. Open biopsy is performed in the operating theatre through an incision that is about 4 cm long, made over the skin of the muscle to be biopsied. After a biopsy is taken, the incision is closed with stitches. The Bergstrom technique [7] described as a semi-open biopsy [8], has been shown to achieve results similar to those obtained with open biopsy procedures [9,10]. This method combines the advantage of the “open-surgical” method with those of needle biopsy methods [11]. Needle biopsies may be performed in a ward or an office via incisions that may be as long as 1cm [12].
Open biopsies may be complicated by pain, bleeding, infection and muscle weakness. They are also more expensive than needle biopsies. Whereas needle biopsies are relatively safer, cheaper and quicker, it may not be possible to obtain adequate tissue materials from the procedure, particularly for gene expression and metabolic studies. Where open biopsies are done, patients stay in hospital for at least a few hours to allow monitoring. They have to wear dressing over the wound site and comply with instructions to keep the site as dry as possible. Open biopsies occasionally necessitate prolonged hospital stay with increased risk of acquiring nosocomial infections.
The complications of muscle biopsy are particularly troubling when the indication is for nontherapeutic research such as gene expression studies and metabolomics, where participants do not derive any treatment benefit nor is the research likely to directly benefit them in the near future. This necessitated the search for alternative methods that will provide adequate amount of tissue samples while minimizing the risk to the research participant.
Materials and method
Study design
As part of a long-term strategy to understand the genetic basis of type 2 diabetes (T2D) in African ancestry populations, the African American Diabetes Mellitus (AADM) study [13] designed a project to obtain muscle biopsies from patients with T2D at varying levels of glycemic controls and normoglycemic individuals as control subjects. Prospective participants were enrolled at the Diabetes Clinic of the University College Hospital, Ibadan, Nigeria. They were counseled about the study, its aims and objectives and the fact that there is no direct therapeutic benefit for participants. Invited participants were asked to take the consent forms home for review and consideration, and to return to the clinic only if they wished to participate in the study. All individuals approached for muscle biopsy consented to participate in the study, having met the inclusion criteria for the overall AADM study. All participants were asked about history of bleeding disorders, drug allergies and current medication especially anticoagulants and aspirin. Hematological tests to ascertain Prothrombim Time (PT) and Partial Prothromboplastin Time with Kaolin (PTTK) levels were carried out for all participants who consented to participate in the study.
Biopsy device
The biopsy procedure was performed with Vacora™ Vacuum Assisted Biopsy System - a compact, hand held system which is vacuum enabled to obtain large sample size using a minimally invasive technique [14]. It has the capacity to collect samples as large as 170mg in weight. A back pressure system ejects the sample and cleans the device.
Muscle biopsy procedure
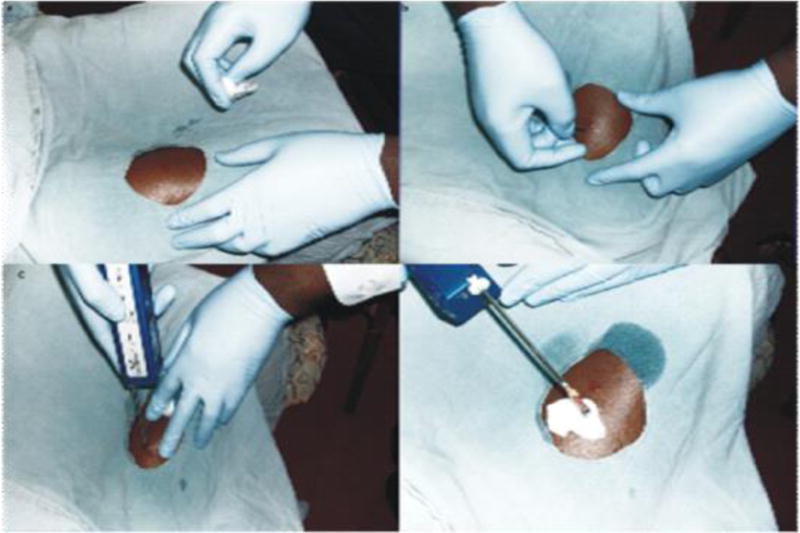
The biopsy site used was the vastus lateralis muscle, in the anterior third of the thigh. This muscle was chosen because of its bulk, distance from major vascular structures, accessibility and redundancy, so that post-biopsy use will not be associated with significant pain. In addition, its ratio of fatty tissue to muscle is favorable, therefore the sample collected will have more muscular than fatty tissue. Throughout the procedure, every effort was made to ensure asepsis. First, we checked that the Vacora™ was in good working order by assembling it and turning on the power switch. We ensured all instruments to be used for the procedure such as a size 15 scalpel, sterile drapes, needles and syringes, methylated spirit, 1% local anesthetic with adrenaline, sterile gauze and skin preparatory agents were in place.
Study personnel educated participants on the biopsy procedure including what to expect during the process of obtaining tissue samples and associated risks such as post-procedure complications and follow up plan for pain assessment and complication management. Participants were required to fast for 8 hours before the procedure. Just before the procedure, the participants were given deep intramuscular injection of a 3rd generation cephalosporin, in order to minimize the risk of infectious complications. For the procedure, they were asked to sit comfortably with knees flexed and thigh to be biopsied exposed. The skin over the biopsy site was prepared with methylated spirit and a sterile fenestrated drape was placed over it. After 30 minutes, the skin, subcutaneous tissue and muscles were infiltrated with about 10 mls of 1% Xylocaine with adrenaline.
After 3 – 5 minutes of administering the anesthetic, the biopsy site was tested for sensitivity to pain by applying deep pressure with a sterile needle to the site. Once we certified the site was insensitive to pain, a size 15 surgical blade was used to lance the skin, to enhance penetration of the biopsy needle. We used a 16G size Vacora™ biopsy needle. The depth of the needle insertion within the body of the muscle varied according to the body size of the study participants’. Subsequently, the Vacora™ system was switched on and held in place for about 30 seconds during which the biopsy was automatically obtained. The obtained sample was placed in a solution of Allprotect®. Gentle pressure was applied over the biopsy site for a few minutes to ensure there was no bleeding; thereafter the site was covered with a sterile band aid. Participants were observed in the office for 30 minutes after the biopsy and were surveyed about their experience of the procedure. We collected information on the level of pain or discomfort felt during and after the procedure. We informed the participants that they could resume normal activities shortly after the procedure and to remove the band aid after a few hours. We called the participants 24 hours and 1 week after the procedure, to ask about pain, bleeding and any other complications they may have experienced, following the procedure.
Results
There were 39 (71%) male and 16 (29%) female participants with mean age of 48 years and mean BMI of 34 kg/m2. All the participants tolerated the procedure well and all rated the immediate and 24 hours post procedure pain as 0. They all complained of mild discomfort at the beginning of the procedure during the infiltration of the skin with local anesthesia. There were no complications attributable to the procedure and no additional analgesic was required by any of the participants. The procedure was well tolerated and lasted an average of 12 minutes from the moment the participant was educated on the study procedure to the time he/she started completing the pain assessment questionnaire. RNA samples were subsequently extracted from all donated tissues and all but 2 samples (4%) were adequate for gene expression studies. A re-biopsy of the inadequate samples was not done. No complication was reported by any of the participants during the follow up period, a week after the procedure.
Discussion
Our study shows that an office based muscle biopsy using a vacuum assisted biopsy needle is capable of obtaining sufficiently large samples for research with minimal risk of pain and other complications to the participant. This is particularly important in situations where the research has no therapeutic intent and does not provide benefit for the participants in any direct manner. In such situations, researchers and ethics committees compare the risk to participants with the potential beneficial knowledge that will be derived from the study - a so-called risk-knowledge calculus [15,16]. The results of the gene expression studies have been presented elsewhere [17].
Prior to the implementation of this study, we carried out extensive discussions with the institutional ethics committee in order to fully explain the rationale of the research, its risks and benefit and potential to contribute to scientific understanding of the metabolic derangements associated with diabetes mellitus. The need to search for and use the best minimally invasive technology available, was considered an ethical imperative, before approval for this study was granted [18]. In addition, we discussed appropriate compensation for participants considering the invasive nature of the research and lack of therapeutic benefit. We found a balance that, in the judgment of the ethics committee was appropriate without constituting undue inducement according to the Nigerian National Code for Health Research Ethics [16].
In conclusion, an office based muscle biopsy technique using vacuum assisted biopsy needle generates adequate tissue sample for metabolic and gene expression studies while being associated with minimal risk of complications to the participants.
Figure.
References
- 1.Hayot M, Michaud A, Koechlin C, et al. Skeletal muscle microbiopsy: a validation study of a minimally invasive technique. Eur Respir J. 2005;25:431–440. doi: 10.1183/09031936.05.00053404. [DOI] [PubMed] [Google Scholar]
- 2.Aboulafia AJ, Malawer MM. Surgical management of pelvic and extremity osteosarcoma. Cancer. 1993;71:3358–3366. doi: 10.1002/1097-0142(19930515)71:10+<3358::aid-cncr2820711738>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Bushby K. Diagnosis and management of the limb girdle muscular dystrophies. Pract Neurol. 2009;9:314–323. doi: 10.1136/jnnp.2009.193938. [DOI] [PubMed] [Google Scholar]
- 4.Finsterer J, Strobl W. Presentation, Etiology, Diagnosis, and Management of Camptocormia. Eur Neurol. 64:1–8. doi: 10.1159/000314897. [DOI] [PubMed] [Google Scholar]
- 5.Faulk WP, Labarrere CA, Pitts D, Halbrook H. Laboratory-clinical correlates of time-associated lesions in the vascular immunopathology of human cardiac allografts. J Heart Lung Transplant. 1993;12:S125–134. [PubMed] [Google Scholar]
- 6.Pestronk A, Schmidt RE, Choksi R. Vascular pathology in dermatomyositis and anatomic relations to myopathology. Muscle Nerve. 42:53–61. doi: 10.1002/mus.21651. [DOI] [PubMed] [Google Scholar]
- 7.Skeletal muscle dysfunction in chronic obstructive pulmo nary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159:S1–40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 8.Maltais F, LeBlanc P, Simard C, et al. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:442–447. doi: 10.1164/ajrccm.154.2.8756820. [DOI] [PubMed] [Google Scholar]
- 9.Barreiro E, Gea J, Corominas JM, Hussain SN. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2003;29:771–778. doi: 10.1165/rcmb.2003-0138OC. [DOI] [PubMed] [Google Scholar]
- 10.Magistris MR, Kohler A, Pizzolato G, et al. Needle muscle biopsy in the investigation of neuromuscular disorders. Muscle Nerve. 1998;21:194–200. doi: 10.1002/(sici)1097-4598(199802)21:2<194::aid-mus6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Henriksson KG. “ Semi-open” muscle biopsy technique. A simple outpatient procedure. Acta Neurol Scand. 1979;59:317–323. [PubMed] [Google Scholar]
- 12.Welker JA, Henshaw RM, Jelinek J, et al. The percutaneous needle biopsy is safe and recommended in the diagnosis of musculoskeletal masses. Cancer. 2000;89:2677–2686. doi: 10.1002/1097-0142(20001215)89:12<2677::aid-cncr22>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Rotimi CN, Dunston GM, Berg K, et al. In search of susceptibility genes for type 2 diabetes in West Africa: the design and results of the first phase of the AADM study. Ann Epidemiol. 2001;11:51–58. doi: 10.1016/s1047-2797(00)00180-0. [DOI] [PubMed] [Google Scholar]
- 14.Bard Biopsy Product. Accessed at http://bardbiopsy.com/products/vacora.php. last accessed, 20 Dec. 2010.
- 15.Deakin CT, Alexander IE, Kerridge I. Accepting risk in clinical research: is the gene therapy field becoming too risk-averse? Mol Ther. 2009;17:1842–1848. doi: 10.1038/mt.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Health Research Ethics Committee of Nigeria. Nigeria NHREC. Abuja, Nigeria: Federal Ministry of Health of Nigeria; 2006. National Code for Health Research Ethics. [Google Scholar]
- 17.Rotimi C. Human Heredity and Health in Africa (H3Africa) Cape Town, South Africa: LOC, H3Africa Initiative; 2011. Type 2 Diabetes. 2011. [Google Scholar]
- 18.Emanuel EJ, Wendler D, Grady C. What makes research ethical. JAMA. 2000;283:2701–2711. [Google Scholar]


