Abstract
Objective :
To determine the effects of chitosan-zinc oxide nanocomposite conduit on transected sciatic nerve in animal model of rat.
Methods:
Sixty male White Wistar rats were used in this study. A 10-mm sciatic nerve defect was bridged using a chitosan-zinc oxide nanocomposite conduit (CZON) filled with phosphate buffered saline. In chitosan group (CHIT) the chitosan conduit was filled with phosphate buffered saline solution. In sham-operated group (SHAM), sciatic nerve was exposed and manipulated. In transected group (TC), left sciatic nerve was transected and nerve cut ends were fixed in the adjacent muscle. The regenerated fibers were studied within 12 weeks after surgery.
Results:
The behavioral and functional tests confirmed faster recovery of the regenerated axons in CZON group compared to Chitosan group (p<0.05). The mean ratios of gastrocnemius muscles weight were measured. There was statistically significant difference between the muscle weight ratios of CZON and Chitosan groups (p<0.05). Morphometric indices of regenerated fibers showed number and diameter of the myelinated fibers were significantly higher in CZON than in Chitosan. In immuohistochemistry, the location of reactions to S-100 in CZON was clearly more positive than Chitosan group.
Conclusion:
Chitosan-zinc oxide nanocomposite conduit resulted in acceleration of functional recovery and quantitative morphometric indices of sciatic nerve.
Key Words: Peripheral nerve repair, Sciatic, Chitosan-zinc oxide nanocomposite, Local
Introduction
Various materials and techniques have been used to enhance nerve regeneration [1,2].
Biodegradable nerve guides as a temporary scaffold are better than non-degradable biomaterials because the latter remain in place as a foreign body and ultimately result in limiting recovery of nerve function [3]. Beneficial effects of chitosan as a conduit in promoting nerve regeneration have already been documented and it seems chitosan, a natural polymer, bears excellent properties and might be a suitable functional material for peripheral nerve regeneration [4,5].
Various investigations have introduced toxic effects of pure zinc oxide (ZnO)in diverse forms such as nanoparticles (NPs) and nanowires [6-8]. The appearance and extent of nanostructural ZnO cytotoxicity play an important role in the application of ZnO NPs in the treatment of human diseases. While some studies have shown severe cytotoxicity of ZnO nanostructures, other studies have not claimed remarkable cell toxic effect because of changes in cell density or amount of ZnO NPs [9,10]. Consequently, in order to use ZnO nanostructures in tissue engineering and regenerative medicine, these nanostructures need to be incorporated with a carrier polymer or other type of material. On the other hand, numerous studies have reported that the mechanical and electrical properties of different polymers have improved when ZnO NPs are incorporated into the polymeric matrices [11-13]. Chitosan has been used for many different biomedical applications such as drug delivery and tissue engineering due to its favorable biocompatible and biodegradable properties [14,15].
To the best knowledge of the authors the literature is poor regarding effect of chitosan-zinc oxide nanocomposite conduit on functional recovery of transected peripheral nerves. To accelerate nerve regeneration a chitosan-zinc oxide nanocomposite conduit was designed for the local guidance of injured nerves, which help increase local effect while decrease systemic toxicities.
Materials and Methods
Experimental Design
Sixty male White Wistar rats weighing approximately 280 g were divided into four experimental groups (n = 15), randomly: Transected control group (TC), sham-surgery group (SHAM), a chitosan conduit group (CHIT) and a chitosan-zinc oxide nanocomposite conduit (CZON). Each group was again subdivided into three subgroups of five animals each and surveyed within12 weeks. Two weeks before and during the entire experiments, the animals were housed in individual plastic cages with an ambient temperature of 23 ± 3º C, stable air humidity, and a 12:12 ligth/dark cycle. The rats had free access to standard rodent laboratory food and tap water.
Preparation of chitosan-zinc oxide nanocomposite conduit
Chitosan solution was prepared by dissolving medium molecular weight, crab shell chitosan (~400kDa, 85% deacetylated) (Fluka, Sigma-Aldrich St. Louis, MO, USA) in an aqueous solution (1% v/v) of glacial acetic acid (Merck, Darmstadt, Germany) to a concentration of 2% (w/v) while stirring on a magnetic stirrer-hot plate. The solution was stirred with low heat (at 50oC) for 3hour. The resultant chitosan solution was filtered through a Whatman No. 3 filter paper then vacuum filtration to remove any un-dissolved particles. To overcome the fragility of chitosan, glycerol (Sigma Chemical Co., St. Louis, MO, USA) was added as 30% (w/w) of the total solid weight in solution [16, 17]. Chitosan conduit was made by gentle injection of the prepared solution into a home-made mold. The prepared conduit was 2 mm in external diameter, 1.8 mm in internal diameter and 14 mm in length. This internal diameter complies with optimal function in rat models.
Characterization of zinc oxide nanoparticles
The morphology images of zinc oxide nanoparticles were obtained using a scanning electron microscopy (SEM) (Hitachi S3200) operating with an accelerating voltage of 20 kV under high vacuum. A conventional secondary electron scintillator detector was used with a tungsten filament. The zinc oxide nanoparticles were coated with a gold layer (~5 nm) using a sputter coater (Denton vacuum model Desk II).
Grafting procedure
Animals were anesthetized by intraperitoneal administration of ketamine-xylazine (ketamine hydrochloride 5%, 90mg/kg and xylazine hydrochloride 2%, 5mg/kg). The procedures were carried out based on the guidelines of the Ethics Committee of the International Association for the Study of pain [18]. The University Research Council approved all experiments.
Following surgical preparation in the SHAM group, the left sciatic nerve was exposed through a gluteal muscle incision and after careful homeostasis the muscle was sutured with resorbable 4/0 sutures, and the skin was closed with 3/0 nylon. In TC group, the left sciatic nerve was transected proximal to the tibio-peroneal bifurcation where a 7mm segment was excised, leaving a 10mm gap due to retraction of nerve ends. Proximal and distal stumps were fixed in the adjacent muscle with 10/0 nylon epineurial suture. No graft was interposed between the stumps. In the CHIT group the left sciatic nerve was exposed through a gluteal muscle incision and transected proximal to the tibio-peroneal bifurcation where a 7 mm segment was excised, leaving a gap about 10 mm due to retraction of nerve ends. Proximal and distal stumps were each inserted 2 mm into a chitosan conduit and two 10/0 nylon sutures were placed at each end of the cuff to fix the tube in place and to leave a 10-mm gap between the stumps. The conduit was filled with 20 µL phosphate buffered saline. In the CZON group the chitosan-zinc oxide nanocomposite conduit was filled with phosphate buffered saline. The sterile Vaseline was used to seal the ends of the tubes to avoid leakage. Currently acceptable technique of euthanasia in neurosciences include perfusion fixation. The animals were anesthetized (see above) and euthanized with transcardial perfusion of a fixative containing 2% paraformaldehyde and 1% glutaraldehyde buffer (pH: 7.4) 4, 8 and 12 weeks after surgery.
Behavioral Testing
Functional recovery of the nerve was assessed using the Basso, Beattie, and Bresnahan (BBB) locomotor rating scale for rat hind limb motor function [19]. Although BBB is widely used to assess functional recovery in spinal cord injured animals, however, it has been demonstrated that it could be most useful in assessment of never repair processes in peripheral nerve injuries [20].
The testing was performed in a serene environment. The animals were observed and assessed within a course of a 4-minute exposure to an open area of a mental circular enclosure. BBB scores were recorded once before surgery in order to establish a baseline control and again weekly thereafter to assess functional recovery during 12 weeks.
Functional assessment of reinnervation
Sciatic functional index (SFI)
Walking track analysis was performed 4, 8 and 12 weeks after surgery based on Bain et al., 1989 [21]. The lengths of the third toe to its heel (PL), the first to the fifth toe (TS), and the second toe to the fourth toe (IT) were measured on the experimental side (E) and the contralateral normal side (N) in each rat. The Sciatic Function Index (SFI) in each animal was calculated by the following formula:
SFI= - 38.3 × (EPL-NPL)/NPL + 109.5 × (ETS-NTS)/NTS + 13.3 × (EIT-NIT)/NIT-8.8
Static sciatic index (SSI)
SSI is a time-saving digitized static footprint analysis described by others [22]. A good correlation between the traditional SFI and the newly developed static sciatic index (SSI) and static toe spread factor (TSF), respectively, has been reported by others [22]. The SSI is a time-saving and easy technique for accurate functional assessment of peripheral nerve regeneration in rats and is calculated using the static factors, not considering the print length factor (PL), according to the equation:
SSI = [(108.44 × TSF) + (31.85 × ITSF)] − 5.49
Where:
TSF = (ETS-NTS)/NTS
ITSF = (EIT-NIT)/NIT
Biomechanical testing
The regenerated nerves were harvested and placed in a normal saline bath at room temperature. The samples were then fixed between frozen fixtures in a mechanical apparatus. The TA.XTPlus Texture Analyzer mechanical test device was used for the assessment (Stable Micro Systems, Surrey GU7 1YL, UK). After 5 minutes, the frozen fixtures were tightened to ensure that no slippage occurred during testing. The initial length was set to 10 mm. Each sample was stretched at a constant rate of 1 mm/min. The load and displacement were sampled 5 times per second. Each sample was stretched to complete tensile failure. Samples were kept wet moist during testing using a drop of normal saline solution to the nerve segments.
Muscle mass
Recovery assessment was also indexed using the weight ratio of the gastrocnemius muscles 12 weeks after surgery. Immediately after sacrificing of animals, gastrocnemius muscles were dissected and harvested carefully from intact and injured sides and weighed while still wet, using an electronic balance. All measurements were made by two independent observers unaware of the analyzed group.
Histological preparation and quantitative morphometric studies
Operated nerve was dissected from surrounding tissues and a segment including several millimeters proximal and distal to the graft was harvested. Graft middle cable of SHAM, TC, Chitosan and CHIT/CZON groups were fixed in 2.5 percent glutaraldehyde. The grafts were postfixed in OsO4 (2%, 2 h), dehydrated through an ethanol series and embedded in Epon. Samples were cut in 5 μm, stained with toluidine blue and examined under light microscopy.
Morphometrical analysis was carried out using an image analyzing software (Image-Pro Express, version 6.0.0.319, Media Cybernetics, Silver Springs, MD, USA). Equal opportunity, systematic random sampling and two-dimensional dissector rules were followed in order to cope with sampling-related, fiber-location-related and fiber-size related biases [23].
Immunohistochemical analysis
In this study, anti-S-100 (1:200, DAKO, USA) was used as marker for myelin sheath. Specimens were post fixed with 4% paraformaldehyde for 2h and embedded in paraffin. Prior to immunohistochemistry nerve sections were dewaxed and rehydrated in PBS (pH 7.4). Then the nerve sections were incubated with 0.6% hydrogen peroxide for 30 minutes. To block non-specific immunoreactions the sections were incubated with normal swine serum (1:50, DAKO, USA). Sections were then incubated in S-100 protein antibody solution for 1h at room temperature. They were washed three times with PBS and incubated in biotinylated anti-mouse rabbit IgG solution for 1h. Horseradish peroxidase-labelled secondary antibody was applied for 1 h. After that all sections were incubated with 3,3'- diaminobenzidine tetrahydrochloride chromogene substrate solution (DAB, DAKO, USA) for 10 min. The results of immunohistochemistry were examined under a light microscope.
Statistical Analysis
Experimental results were expressed as means ± SD. Statistical analyses were performed using PASW 18.0 (SPSS Inc., Chicago, IL, USA). Model assumptions were evaluated by examining the residual plot. Results were analyzed using a factorial ANOVA with two between-subjects factors. Bonferroni test for pairwise comparisons was used to examine the effect of time and treatments. The differences were considered significant when P< 0.05.
Results
Morphology of zinc oxide nanoparticles
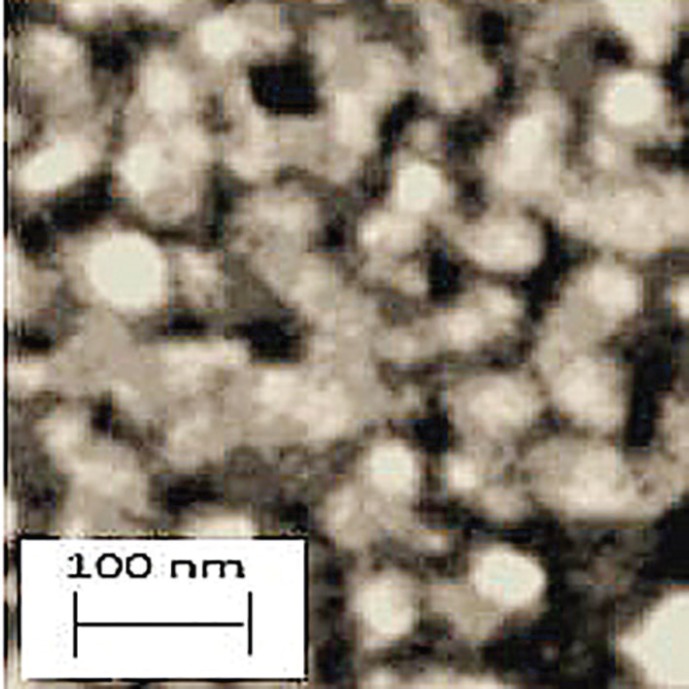
The zinc oxide nanostructures can be in different forms such as fibers, particles, wires and nanorods. Examination of zinc oxide morphology is important to understand its nano-scale properties. Therefore, the morphology of zinc oxide nanostructures was studied using SEM. The average size of zinc oxide nanoparticles was 30 nm (Figure 1).
Fig. 1.
SEM image confirmed that zinoxide nanoparticles were observed almost sphere like in morphology and approximately 30 nm in diameter
BBB recovery
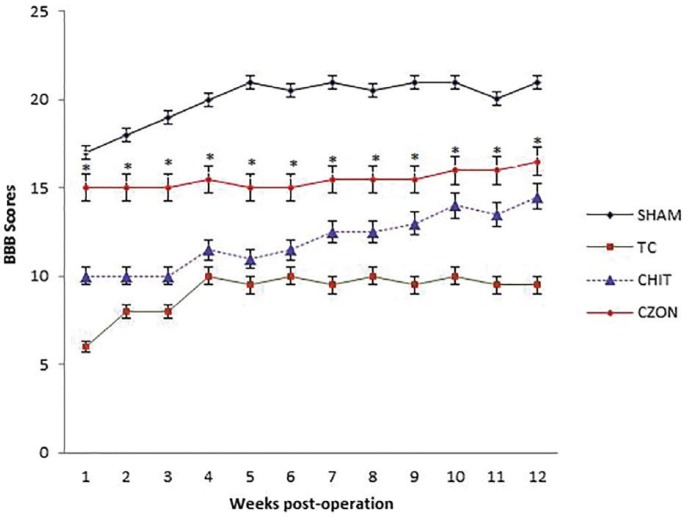
In order to assess hind limb recovery, the open field locomotor was used. Figure 2 shows BBB scores compared to the baseline. All experimental groups, except for SHAM, showed the greatest degree of functional deficit one week after surgery. The CZON group showed significant improvement in locomotion of the operated limb compared to the CHIT group during the study period (p<0.05).
Fig. 2.
BBB score for all experimental groups. Chitosan-zinc oxide nanocomposite conduit grafting gave better scores than in Chitosan group. Standard error at each data point is shown with bars
Recovery of sciatic nerve function and reinnervation
SFI outcome
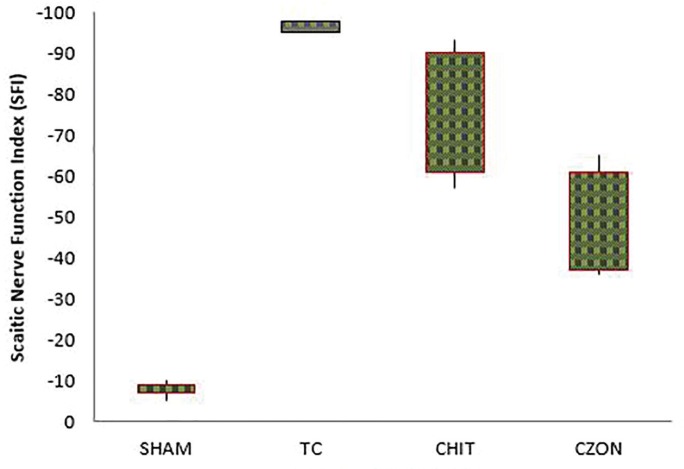
Figure 3 shows sciatic function index (SFI) values in experimental groups. Prior to surgery, SFI values in all groups were near zero. After the nerve transection, the mean SFI decreased to -100 due to the complete loss of sciatic nerve function in all animals. The statistical analyses revealed that the recovery of nerve function was significantly faster in CZON than in Chitosan (p<0.05).
Fig. 3.
Box-and-whisker plots of sciatic nerve function index values in each experimental group 4, 8 and 12 weeks after surgery. chitosan-zinc oxide nanocomposite conduit grafting gave better results in functional recovery of the sciatic nerve than in Chitosan group
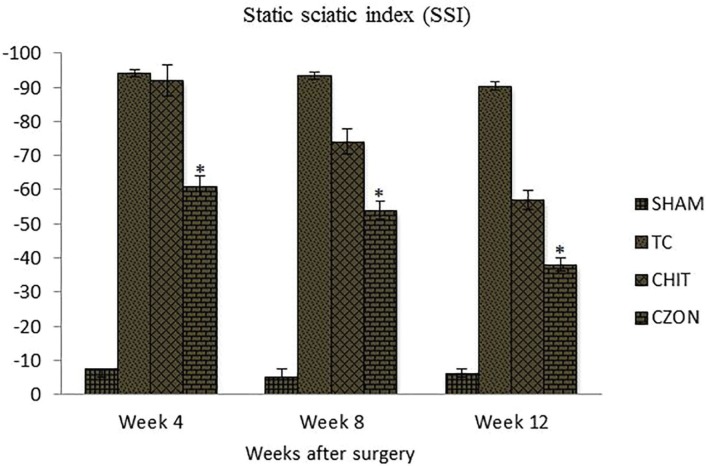
Changes in SSI were similar to those observed in SFI, indicating significant deficit following the sciatic nerve transection (Figure 4). Changes in SSI were significant at weeks 4, 8 and 12 weeks of recovery (p<0.05). The contrasts indicated SSI values at week 12 to differ significantly from those obtained from other experimental groups, a trend also noticed for SFI (p<0.05).
Fig. 4.
Bar graph indicating static sciatic index (SSI) values in each experimental group during the study period. Chitosan-zinc oxide nanocomposite conduit grafting gave better results in functional recovery of the sciatic nerve than in Chitosan group. Data are presented as mean ± SD. * P < 0.05 vs Chitosan group
Biomechanical measurements
Maximum pull force (Fmax) of normal sciatic nerve was found to be 5.46 ± 0.40. Fmax of nerve samples in experimental groups are shown in Table 1. Fmax in CZON group was significantly higher than that in Chitosan group (p<0. 05). Tensile strength, the amount of force per unit of initial cross-sectional area at tensile failure, was measured based on Fmax and nerve cross sectional area. 12 week assessment revealed tensile strength of regenerated nerves treated with CZON was higher than those in Chitosan group (p<0. 05). Ultimate strain, the amount of elongation divided by the initial specimen length achieved at the point of tensile failure, in CZON group was significantly higher than that in Chitosan group (p<0. 05). Toughness, reflecting the properties of anti-deformation and anti-fracture of nerve, was determined by the nerve itself and could reflect “looseness” or “toughness” of nerve. Toughness in CZON group was significantly higher than that in Chitosan group (p<0. 05).
Table 1.
Biomechanical analyses of sciatic nerve in each of the experimental groups: Values are given as mean ± SD
| Groups | Maximum Pull Force (N) | Tensile Strength (MPa) | Ultimate Strain | Toughness (N/mm) |
|---|---|---|---|---|
| SHAM | 5.44 ± 0.42 | 7.33 ± 1.21 | 0.56 ± 0.05 | 1.27 ± 0.53 |
| TC | 1.41 ± 0.17 | 2.31 ± 0.32 | 0.14 ± 0.04 | 0.25 ± 0.07 |
| CHIT | 3.15 ± 0.32 | 3.11 ± 0.12 | 0.22 ± 0.03 | 0.42± 0.21 |
| CZON | 4.33± 0.23a | 4.35 ± 0.17 a | 0.37± 0.06 a | 0.77 ± 0.11 a |
The mean difference is significant at the .05 level vs. CHIT group.
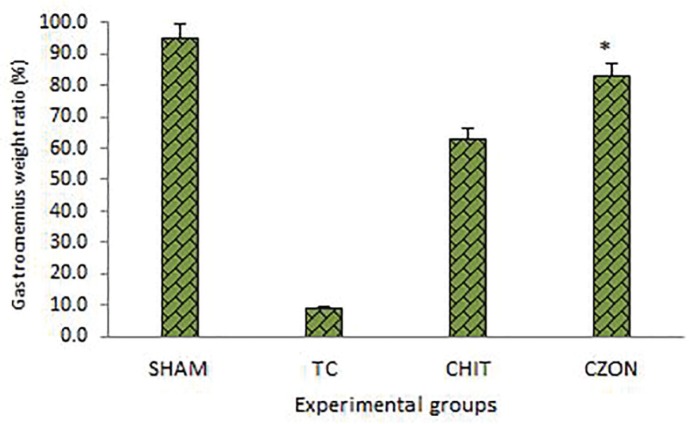
Muscle mass measurement
Gastrocnemius muscles weight of injured and uninjured sides were measured in each group. There was statistically significant difference between percentage of the mean muscle weight ratios of CZON and Chitosan groups (p<0.05). The results showed that in CZON group muscle weight ratio was bigger than in Chitosan group (Figure 5).
Fig. 5.
Gastrocnemius muscle weight measurement. The gastrocnemius muscles of both sides (operated left and unoperated right) were excised and weighed in the experimental groups at 12 weeks after surgery. Data are presented as mean ± SD. * P < 0.05 vs Chitosan group
Morphological findings
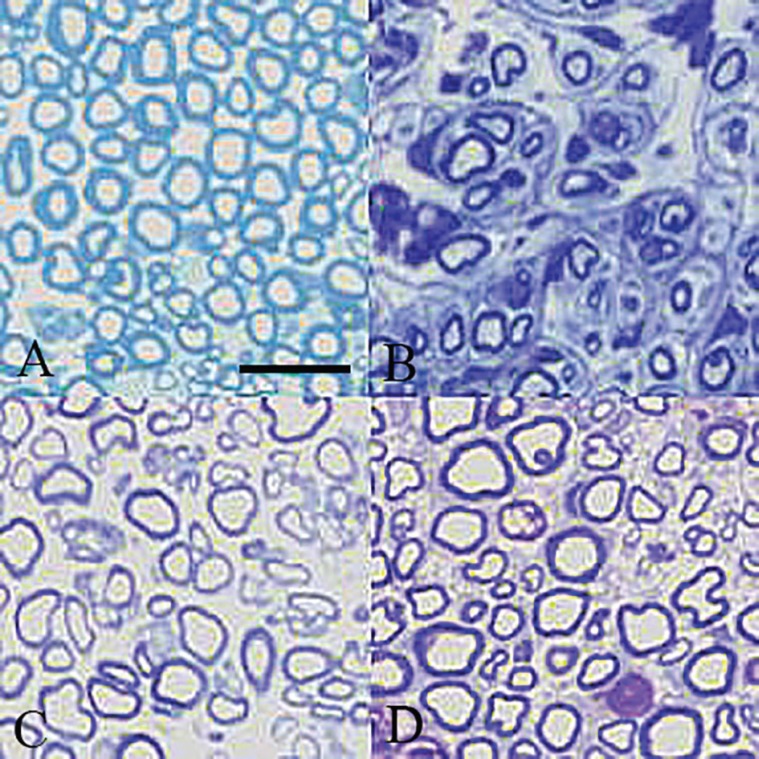
Table 2 shows quantitative morphometric analyses of regenerated nerves for each of the experimental groups. Four weeks after surgery, CZON group presented significantly greater nerve fiber, axon diameter and myelin sheath thickness compared to Chitosan animals (p<0.05) (Figure 6). Although Chitosan presented regeneration patterns, the morphometric indices in CZON group both after 8 and 12 weeks were better than in Chitosan.
Table 2.
Morphometric analyses of sciatic nerve in each of the experimental groups 12 weeks after surgery: Values are given as mean ± SD
| Groups | Axon counts fb/mm2 | Axon diameter (µm) | Myelin sheath thickness(µm) |
|---|---|---|---|
| SHAM | 29517 ± 2175 | 11.31 ± 0.17 | 2.62± 0.05 |
| TC | 4173 ± 2207 | 3.33 ± 0.11 | 1.03± 0.02 |
| CHIT | 21009 ± 2307 | 3.71± 0.10 | 1.03 ± 0.02 |
| CZON | 23703 ± 2137 a | 6.41 ± 0.17 a | 1.03 ± 0.07 |
The mean difference is significant at the .05 level vs. CHIT group
Fig. 6.
Light micrograph of representative cross section taken from (A) midpoint of normal SHAM, (B) TC, (C) CHIT and (D) CZON, 12 weeks after surgery. (Toluidine blue, Scale bar: 25 μm
Using Factorial ANOVA analysis with two between-subjects factors (Group × time); in the CZON group number of nerve fibers and myelin thickness did not show significant difference between 8 and 12 weeks (p>0.05). Increase in mean thickness of myelin sheath did not show statistical difference between 4, 8 and 12 weeks inside each group (p>0.05). Mean thickness of myelin sheath from week 8 onward did not show significant difference between CZON and CHIT group (p>0.05).
Immunohistochemistry
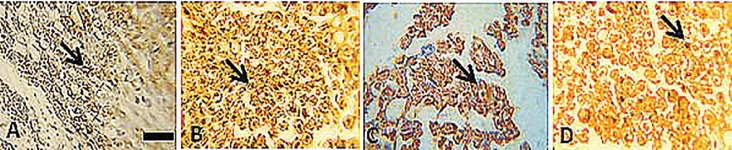
Immunoreactivity to S-100 protein was extensively observed in the cross sections of regenerated nerve segments. The expression of S-100 protein signal was located mainly in the myelin sheath. The axon also showed a weak expression indicating that Schwann cell-like phenotype existed around the myelinated axons (Figure 7). In both CZON and Chitosan groups, the expression of S-100 and the findings resembled those of the histological evaluations.
Fig. 7.
Immunohistochemical analysis of the regenerated nerves 12 weeks after surgeryfrom middle cable (A) TC, (B) SHAM, (C) CHIT and (D) CZON.There is clearly more positive staining of the myelin sheath-associated protein S- 100 (arrows) within the periphery of nerve, indicating well organized structural nerve reconstruction in CZON compared to that of the Chitosan. Scale bar:10μm
Discussion
Restoration of normal neurological function of transected peripheral nerve remains a great challenge in regenerative medicine and surgery. Entubulation neurorrhaphy is an excellent alternative to short interposition nerve grafts [24]. Selection of an appropriate method to evaluate functional recovery of nerve regeneration is extremely influential. Walking is a coordinated activity involving sensory input, motor response and cortical integration [25]. Therefore, walking track analysis (sciatic function index) is a comprehensive test. The results of the present study showed that chitosan when loaded with zinc oxide nano particles ended up a faster and significant improvement of functional recovery of the sciatic nerve throughout the study period.Castaneda et al., [26] suggested that arrival of sprouts from the proximal stump at the distal nerve stump does not necessarily imply recovery of nerve function. Walking track analysis has frequently been used to reliably determine functional recovery following nerve repair in rat models [27,28]. Our results showed that moephometric indices were not significantly different between CZON and Chitosan groups after 8 weeks .In contrary, functional recovery occurred from week 8 to week 12 in CZON supporting again this idea that selection of an appropriate method to evaluate functional recovery is crucial. This study also supported the idea that the walking track analysis (SFI) is more comprehensive and reliable than histomorphometric methods in peripheral nerve repair studies [26,29].
Recording wet muscle weight is a previously utilized alternative for motor target organ reinnervation [30-33]. In vitro evidence suggests that CZON treatment improves the motor neuron activity, possibly acting as a neurotrophic factor [34].
The strongest connective tissue layers in peripheral nerves are the perineurium and, to a lesser extent, the epineurium. Changes in the epineurium and perineurium extracellular matrix composition are likely to have significant effects on the biomechanical properties of acellular nerve [35]. The connective tissue from the epineurium forms a layer of fiber membrane at the 3rd day postoperatively and then forms collagen at the 8th day. The key point influencing functional recovery is the number of axons throughout the suture that enhances the anti-tension capacity of the nerve [36]. CZON treatment in the present study resulted in the enhanced biomechanical indices that were in agreement with functional and morphometric findingsAs the posterior tibial branch of the sciatic nerve regenerates into the gastrocnemius muscle, it will regain its mass proportional to the amount of axonal reinnervation [37,38]. In the present study 12 weeks after surgery the muscle mass was found in both experimental groups. However, CZON group showed significantly greater ratios of the mean gastrocnemius muscle weight than Chitosan group indicating indirect evidence of successful end organ reinnervation.
In the histological studies, quantitative morphometrical indices of regenerated nerve fibers showed significant difference between Chitosan and CZON groups indicating beneficial effect zinc oxide nano particles on the nerve regeneration.
In immunohistochemistry the expression of axon and myelin sheath special proteins was evident in both groups which indicate the normal histological structure. The location of reactions to S-100 in CZON group was clearly more positive than in Chitosan group further implying that both regenerated axon and Schwann cell-like cells existed and were accompanied by the process of myelination and the structural recovery of regenerated nerve fibers.
Numerous biomaterials have provided promising results toward improving the function of injured nervous system tissue, however, significant hurdles, such as delayed or incomplete tissue regeneration, remain toward full functional recovery of nervous system tissue [39]. Because of this continual need for better nervous system biomaterials, more recent approaches to design the next generation of tissue engineering scaffolds for the nervous system have incorporated nanotechnology, or more specifically, nanoscale surface feature dimensions which mimic natural neural tissue [40]. Compared to conventional materials with micron-scale surface dimensions, nanomaterials have exhibited an ability to enhance desirable neural cell activity while minimizing unwanted cell activity, such as reactive astrocyte activity in the central nervous system. The complexity of neural tissue injury and the presence of inhibitory cues as well as the absence of stimulatory cues may require multifaceted treatment approaches with customized biomaterials that nanotechnology can provide [40]. Combinations of stimulatory cues may be used to incorporate nanoscale topographical and chemical or electrical cues in the same scaffold to provide an environment for tissue regeneration that is superior to inert scaffolds. Ongoing research in the field of electrically active nanomaterials includes the fabrication of composite materials with nanoscale, piezoelectric zinc oxide particles embedded into a polymer matrix. Zinc oxide, when mechanically deformed through ultrasound, for example, can theoretically provide an electrical stimulus, a known stimulatory cue for neural tissue regeneration. The combination of nanoscale surface dimensions and electrical activity may provide an enhanced neural tissue regeneration environment; such multifaceted nanotechnology approaches deserve further attention in the neural tissue regeneration field [39].
Even though our study showed the neuroregenerative action of chitosan zinc oxide nanocomposite in peripheral nerve injuries, data regarding the molecular mechanisms leading to the neuroprotective action remain to be investigated in depth. We have not given the histological and molecular evidence for neuroprotective action of zinc oxide nanoparticle. This may be considered as a limitation to our study.
Therefore, the authors stress that the aim of the current investigation was to evaluate effect of chitosan zinc oxide nanocomposite on nerve regeneration. The results of the present study indicated that use of a chitosan zinc oxide nanocomposite seems to have several distinct advantages for the treatment of transected peripheral nerves because it is inert, does not induce extensive scarring or degeneration after implantation, available and easily performed.
In conclusion, this study demonstrated that chitosan zinc oxide nanocomposite conduit improved functional recovery of transected sciatic nerve in rat. Supported by previous findings, the results from our present study would imply that the final outcome of both motor and sensory regeneration and reinnervation following repair of a peripheral nerve by chitosan zinc oxide nanocomposite may be of clinical benefit. There are reasonable grounds to believe that this approach could deliver a superior quality of reinnervation in a shorter period of time, compared to repair without nanoparticle treatment.
Acknowledgments
The authors would like to thank Urmia Pathobiology Center, for their technical expertise.
Conflict of Interests:
There are no conflicts of interests to declare.
References
- 1.Chaiyasate K, Schaffner A, Jackson IT, Mittal V. Comparing FK-506 with basic fibroblast growth factor (b-FGF) on the repair of a peripheral nerve defect using an autogenous vein bridge model. J Invest Surg. 2009;22(6):401–5. doi: 10.3109/08941930903410775. [DOI] [PubMed] [Google Scholar]
- 2.Lago N, Rodriguez FJ, Guzman MS, Jaramillo J, Navarro X. Effects of motor and sensory nerve transplants on amount and specificity of sciatic nerve regeneration. J Neurosci Res. 2007;85(12):2800–12. doi: 10.1002/jnr.21286. [DOI] [PubMed] [Google Scholar]
- 3.Rosales-Cortes M, Peregrina-Sandoval J, Banuelos-Pineda J, Sarabia-Estrada R, Gomez-Rodiles CC, Albarran-Rodriguez E, et al. Immunological study of a chitosan prosthesis in the sciatic nerve regeneration of the axotomized dog. J Biomater Appl. 2003;18(1):15–23. doi: 10.1177/0885328203018001002. [DOI] [PubMed] [Google Scholar]
- 4.Wang A, Ao Q, Wei Y, Gong K, Liu X, Zhao N, et al. Physical properties and biocompatibility of a porous chitosan-based fiber-reinforced conduit for nerve regeneration. Bio Technol Lett. 2007;29(11):1697–702. doi: 10.1007/s10529-007-9460-0. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Lu G, Ao Q, Gong Y, Zhang X. Preparation of cross-linked carboxymethyl chitosan for repairing sciatic nerve injury in rats. Biotechnol Lett. 2010;32(1):59–66. doi: 10.1007/s10529-009-0123-1. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Liu X, Lu H, Zhang X, Ma L, Gao R, et al. Real-time investigation of acute toxicity of ZnO nanoparticles on human lung epithelia with hopping probe ion conductance microscopy. Chem Res Toxicol. 2012;25(2):297–304. doi: 10.1021/tx2004823. [DOI] [PubMed] [Google Scholar]
- 7.Heng BC, Zhao X, Xiong S, Ng KW, Boey FY, Loo JS. Cytotoxicity of zinc oxide (ZnO) nanoparticles is influenced by cell density and culture format. Arch Toxicol. 2011;85(6):695–704. doi: 10.1007/s00204-010-0608-7. [DOI] [PubMed] [Google Scholar]
- 8.Kasemets K, Ivask A, Dubourguier HC, Kahru A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol In Vitro. 2009;23(6):1116–22. doi: 10.1016/j.tiv.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Yin H, Casey PS, McCall MJ. Surface modifications of ZnO nanoparticles and their cytotoxicity. J Nanosci Nanotechnol. 2010;10(11):7565–70. doi: 10.1166/jnn.2010.2833. [DOI] [PubMed] [Google Scholar]
- 10.Yan D, Yin G, Huang Z, Li L, Liao X, Chen X, et al. Cellular compatibility of biomineralized ZnO nanoparticles based on prokaryotic and eukaryotic systems. Langmuir. 2011;27(21):13206–11. doi: 10.1021/la2008107. [DOI] [PubMed] [Google Scholar]
- 11.Han Y, Kiat-amnuay S, Powers JM, Zhao Y. Effect of nano-oxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J Prosthet Dent. 2008;100(6):465–73. doi: 10.1016/S0022-3913(08)60266-8. [DOI] [PubMed] [Google Scholar]
- 12.Zou Y, Huang Z, Wang Y, Liao X, Yin G, Gu J. Synthesis and cellular compatibility of Co-doped ZnO particles in silk-fibroin peptides. Colloids Surf B Biointerfaces. 2013;102:29–36. doi: 10.1016/j.colsurfb.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Balakrishnan B, Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26(18):3941–51. doi: 10.1016/j.biomaterials.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Ramay HR, Hauch KD, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–28. doi: 10.1016/j.biomaterials.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 15.Bhat A, Dreifke MB, Kandimalla Y, Gomez C, Ebraheim NA, Jayasuriya AC. Evaluation of cross-linked chitosan microparticles for bone regeneration. J Tissue Eng Regen Med. 2010;4(7):532–42. doi: 10.1002/term.270. [DOI] [PubMed] [Google Scholar]
- 16.Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chemistry. 2010;122(1):161–6. [Google Scholar]
- 17.Jayasuriya AC, Aryaei A, Jayatissa AH. ZnO nanoparticles induced effects on nanomechanical behavior and cell viability of chitosan films. Mater Sci Eng C Mater Biol Appl. 2013;33(7):3688–96. doi: 10.1016/j.msec.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 19.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 20.Dinh P, Hazel A, Palispis W, Suryadevara S, Gupta R. Functional assessment after sciatic nerve injury in a rat model. Microsurgery. 2009;29(8):644–9. doi: 10.1002/micr.20685. [DOI] [PubMed] [Google Scholar]
- 21.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83(1):129–38. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 22.Bervar M. Video analysis of standing--an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods. 2000;102(2):109–16. doi: 10.1016/s0165-0270(00)00281-8. [DOI] [PubMed] [Google Scholar]
- 23.Geuna S, Gigo-Benato D, Rodrigues Ade C. On sampling and sampling errors in histomorphometry of peripheral nerve fibers. Microsurgery. 2004;24(1):72–6. doi: 10.1002/micr.10199. [DOI] [PubMed] [Google Scholar]
- 24.Wang KK, Costas PD, Bryan DJ, Eby PL, Seckel BR. Inside-out vein graft repair compared with nerve grafting for nerve regeneration in rats. Microsurgery. 1995;16(2):65–70. doi: 10.1002/micr.1920160205. [DOI] [PubMed] [Google Scholar]
- 25.Berry M, Ahmed Z, Douglas MR, Logan A. Epidermal growth factor receptor antagonists and CNS axon regeneration: mechanisms and controversies. Brain Res Bull. 2011;84(4-5):289–99. doi: 10.1016/j.brainresbull.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Castaneda F, Kinne RK. Omental graft improves functional recovery of transected peripheral nerve. Muscle Nerve. 2002;26(4):527–32. doi: 10.1002/mus.10229. [DOI] [PubMed] [Google Scholar]
- 27.de Ruiter GC, Spinner RJ, Alaid AO, Koch AJ, Wang H, Malessy MJ, et al. Two-dimensional digital video ankle motion analysis for assessment of function in the rat sciatic nerve model. J Peripher Nerv Syst. 2007;12(3):216–22. doi: 10.1111/j.1529-8027.2007.00142.x. [DOI] [PubMed] [Google Scholar]
- 28.Sarikcioglu L, Demirel BM, Utuk A. Walking track analysis: an assessment method for functional recovery after sciatic nerve injury in the rat. Folia Morphol (Warsz) 2009;68(1):1–7. [PubMed] [Google Scholar]
- 29.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98(1):16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Menderes A, Yilmaz M, Vayvada H, Ozer E, Barutcu A. Effects of nerve growth factor on the neurotization of denervated muscles. Ann Plast Surg. 2002;48(4):415–22. doi: 10.1097/00000637-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Fitton AR, Berry MS, McGregor AD. Preservation of denervated muscle form and function by clenbuterol in a rat model of peripheral nerve injury. J Hand Surg Br. 2001;26(4):335–46. doi: 10.1054/jhsb.2001.0603. [DOI] [PubMed] [Google Scholar]
- 32.Day CS, Riano F, Tomaino MM, Buranatanitkit B, Somogyi G, Sotereanos D, et al. Growth factor may decrease muscle atrophy secondary to denervation. J Reconstr Microsurg. 2001;17(1):51–7. doi: 10.1055/s-2001-12689. [DOI] [PubMed] [Google Scholar]
- 33.Day CS, Buranapanitkit B, Riano FA, Tomaino MM, Somogyi G, Sotereanos DG, et al. Insulin growth factor-1 decreases muscle atrophy following denervation. Microsurgery. 2002;22(4):144–51. doi: 10.1002/micr.21742. [DOI] [PubMed] [Google Scholar]
- 34.Bigini P, Larini S, Pasquali C, Muzio V, Mennini T. Acetyl-L-carnitine shows neuroprotective and neurotrophic activity in primary culture of rat embryo motoneurons. Neurosci Lett. 2002;329(3):334–8. doi: 10.1016/s0304-3940(02)00667-5. [DOI] [PubMed] [Google Scholar]
- 35.Ma XL, Sun XL, Yang Z, Li XL, Ma JX, Zhang Y, et al. Biomechanical properties of peripheral nerve after acellular treatment. Chin Med J (Engl) 2011;124(23):3925–9. [PubMed] [Google Scholar]
- 36.Jiang B, Zhang P, Yan J, Zhang H. Dynamic observation of biomechanic properties of sciatic nerve at the suture site in rats following repairing. Artif Cells Blood Substit Immobil Biotechnol. 2008;36(1):45–50. doi: 10.1080/10731190701857777. [DOI] [PubMed] [Google Scholar]
- 37.Schmidhammer R, Nogradi A, Szabo A, Redl H, Hausner T, van der Nest DG, et al. Synergistic motor nerve fiber transfer between different nerves through the use of end-to-side coaptation. Exp Neurol. 2009;217(2):388–94. doi: 10.1016/j.expneurol.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Zhao W, He J, Zhao Y, Ding F, Gu X. Nerve conduits based on immobilization of nerve growth factor onto modified chitosan by using genipin as a crosslinking agent. Eur J Pharm Biopharm. 2011;79(3):519–25. doi: 10.1016/j.ejpb.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Seil JT, Webster TJ. Electrically active nanomaterials as improved neural tissue regeneration scaffolds. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(6):635–47. doi: 10.1002/wnan.109. [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, O'Brien C, O'Brien JR, Zhang LG. 3D nano/microfabrication techniques and nanobiomaterials for neural tissue regeneration. Nanomedicine (Lond) 2014;9(6):859–75. doi: 10.2217/nnm.14.36. [DOI] [PubMed] [Google Scholar]