Abstract
Few data exist on direct current cardioversion (DCCV) in adult patients with congenital heart disease (CHD). This is a retrospective case-control study of 279 adults with CHD and 279 adults without CHD (control group) who had elective DCCV for atrial arrhythmias at Mayo Clinic, 2001 to 2013. Control patients were matched by gender and arrhythmia type. The objective was to compare DCCV procedural failure (failure to terminate the presenting arrhythmia) and arrhythmia recurrence (AR). In the CHD group (mean age 55 – 20 years; men 166 [59%]), the most common diagnosis was Fontan palliation (61; 22%). Trans-esophageal echocardiography was performed before DCCV in 216 patients (77%); 162 (58%) had atrial flutter, and 117 (42%) had atrial fibrillation. Procedural failure and AR between the case and the control groups were more common in the CHD group (14% vs 7%, p = 0.01) and (83% vs 66% at 60 months, p = 0.001) respectively. There were no deaths or thromboembolic complications. The multivariable risk factors for procedural failure were Fontan palliation and spontaneous echocardiographic contrast; the risk factors for AR were Fontan palliation and atrial fibrillation. When patients with Fontan palliation were excluded from the analysis, the outcome of DCCV (failure and recurrence rates) was similar for the CHD and non-CHD groups despite the age difference between the cohorts. In conclusion, the present study showed that DCCV outcomes were similar for CHD and non-CHD patients, with the exception of patients with Fontan palliation.
Atrial arrhythmia is common in adult patients with congenital heart disease (CHD), and the prevalence varies with lesion complexity and age of the patient.1–3 It is associated with a higher risk of heart failure, ventricular dysfunction, and thromboembolic complications.1–4 Restoration and maintenance of the sinus rhythm is therefore important to prevent these complications.5 There are limited data on the outcomes of elective direct current cardioversion (DCCV) in adults with CHD.6,7 A previous study6 from our institution, involving a small cohort with limited follow-up, showed that DCCV is safe and effective in terminating atrial arrhythmia in this population. The present study involved a large cohort with a longer follow-up and compared outcomes after DCCV in patients with CHD and patients without CHD in the same period.
Methods
For this retrospective review of data from the Mayo Clinic Cardioversion Unit in Rochester, Minnesota, all adults (age >18 years) with CHD who had DCCV for atrial arrhythmia from January 2001 through December 2013 were identified. A control group was selected from patients without a history of CHD who had DCCV within the same period. The cases (CHD patients) and the controls (“non-CHD” patients) were matched 1:1 by gender, type of atrial arrhythmia at the time of DCCV, and date of DCCV (within 3 months). The groups were not matched by age because the CHD patients were much younger than the non-CHD patients. The Mayo Clinic Institutional Review Board approved this study.
Clinical notes, electrocardiograms before and after DCCV, Holter monitor records, and echocardiograms were reviewed. Atrial arrhythmias were defined as atrial flutter and atrial fibrillation. All electrocardiograms were reviewed manually. Ectopic atrial tachycardia was grouped with atrial flutter because of the difficulty in differentiating between them with surface electrocardiography.
The primary study outcomes were the rates of procedural failure, procedural complications, and arrhythmia recurrence for the 2 groups. The secondary outcome was identification of the predictors of procedural failure and arrhythmia recurrence in the CHD group.
Our definitions were similar to those used previously.8 Procedural failure was defined as failure to terminate the presenting atrial arrhythmia or recurrence of the same arrhythmia while the patient was still in the cardioversion suite. Procedural complications were a tachyarrhythmia that began immediately after DCCV, esophageal injury related to intubation for transesophageal echocardiography, death, or thromboembolism (stroke, transient ischemic attack, or pulmonary embolism) within 30 days after DCCV. Arrhythmia recurrence was defined as a documented atrial arrhythmia (confirmed with electrocardiography, Holter monitoring, or device interrogation) that occurred after the patient left the cardioversion suite. Arrhythmia recurrence was identified through chart review.
All patients received anticoagulation with unfractionated heparin or warfarin, and the therapeutic adequacy of anticoagulation was determined from the international normalized ratio (≥2.0) or partial thromboplastin time assay (≥55 seconds) performed within 24 hours before DCCV. As in a previous study,6 TEE was performed immediately before DCCV in the setting of complex CHD or if any international normalized ratio within 4 weeks before DCCV was less than 2.0 for patients receiving long-term warfarin therapy.
All DCCVs were performed electively in the Mayo Clinic Cardioversion Unit as previously described.6 The standard DCCV protocol, described previously,8 included the use of defibrillator pads to the right of the sternum and over the left scapula. Synchronized DCCV began at 75 J and was increased to 150, 300, and 360 J as needed. Successful DCCV was confirmed with a 12-lead electrocardiogram. For atrial arrhythmia that recurred within 5 minutes, the previously successful level of joules was delivered in another shock.
All statistical analyses were performed with JMP version 10.0 software (SAS Institute Inc., Cary, North Carolina). Categorical variables were expressed as number of patients (percentage of sample), and continuous variables were expressed as mean ± SD or median (interquartile range) for skewed data. Categorical variables were compared with the chi-square 2-sided test or Fisher’s exact test; continuous variables were compared with a 2-sided unpaired Student t test or Wilcoxon rank sum test, as appropriate. Predictors of procedural failure and arrhythmia recurrence were identified with univariable and multivariable Cox proportional hazards models. Hazard ratio (HR) and 95% confidence interval (CI) were used to express the risk associated with each variable. The Kaplan-Meier method was used to calculate arrhythmia recurrence rates, which were compared with the log-rank test. p Values were 2-sided, and p values less than 0.05 were considered significant.
Results
During the study period, 281 patients with CHD were scheduled for DCCV. Left atrial thrombi were identified from 2 of 216 TEEs (0.9%), necessitating cancellation of the DCCV. A total of 279 patients with CHD underwent DCCV (mean age 55 ± 20 years; 166 [59%] were men). Table 1 shows a comparison of the cohort characteristics of the case and control groups, and Table 2 shows DCCV outcomes for the individual CHD lesions.
Table 1.
Baseline characteristics of case and control groups
| Characteristics | Congenital Heart Disease
|
||
|---|---|---|---|
| Yes | No | P | |
| Age (years) | 55±20 | 72±14 | <.001 |
| Male | 166 (59%) | 166 (59%) | .99 |
| Follow-up after DCCV (months) | 49±23 | 46±19 | .63 |
| Time since initial arrhythmia (years) | 11.5±4.3 | 6.6±2.1 | <.001 |
| Prior catheter ablation | 51 (18%) | 22 (8%) | .02 |
| Prior maze procedure | 36 (13%) | 7 (3%) | .04 |
| Prior DCCV | 84 (30%) | 50 (18%) | .02 |
| Atrial arrhythmia | |||
| Atrial flutter | 162 (58%) | 162 (58%) | .99 |
| Atrial fibrillation | 117 (42%) | 117 (42%) | .99 |
| AAD class use at time of DCCV | |||
| Class I | 26 (9%) | 43 (15%) | .03 |
| Class II | 91 (33%) | 139 (50%) | <.001 |
| Class III | 106 (38%) | 63 (23%) | <.001 |
| Class IV | 65 (23%) | 71 (25%) | .14 |
| Digoxin | 31 (11%) | 12 (4%) | .06 |
| DCCV | |||
| Patients requiring >1 shock | 41 (15%) | 38 (14%) | .16 |
| Energy, median (IQR), Joules | 88 (75–150) | 81 (75–150) | .28 |
| Arrhythmia onset within 48 h | 125 (45%) | 116 (42%) | .09 |
| Inpatient | 238 (85%) | 127 (46%) | <.001 |
| TEE guidance | 216 (77%) | 145 (52%) | .02 |
| Failed cardioversion | 38 (14%) | 20 (7%) | .01 |
| Procedural complications | 5 (2%) | 7 (3%) | .36 |
| Anticoagulation or antiplatelet therapy | |||
| Warfarin | 97 (35%) | 103 (37%) | .08 |
| Antiplatelet agent | 123 (44%) | 201 (72%) | <.001 |
| Unfractionated heparin | 138 (49%) | 141 (51%) | .24 |
| Low-molecular-weight heparin | 21 (8%) | 23 (8%) | .11 |
| NOAC | 3 (1%) | 31 (11%) | .001 |
| Echocardiography | |||
| LV (systemic) ejection fraction <35% | 50 (18%) | 35 (13%) | .03 |
| Spontaneous echocardiographic contrast | 70 (25%) | 83 (30%) | .22 |
AAD = antiarrhythmic drug; DCCV = direct current cardioversion; IQR = interquartile range; LV = left ventricle; NOAC = novel oral anticoagulants; TEE = transesophageal echocardiogram.
Table 2.
Outcomes among patients with congenital heart disease
| Diagnosis | All (N=279) | Procedural Failure (n=38) | Arrhythmia Recurrence
|
|
|---|---|---|---|---|
| Present (n=118) | Absent (n=123) | |||
| Fontan physiology | 61 | 18 (30%) | 39 (64%) | 4 (7%) |
| Eisenmenger syndrome | 9 | 2 (22%) | 5 (56%) | 2 (22%) |
| Non-Fontan single-ventricle physiology | 8 | 3 (38%) | 3 (38%) | 2 (25%) |
| Secundum atrial septal defect | 27 | 0 | 9 (33%) | 18 (67%) |
| Sinus venosus atrial septal defect | 12 | 0 | 4 (33%) | 8 (67%) |
| Atrial septal defect—unrepaired | 4 | 0 | 4 (100%) | 0 |
| Ventricular septal defect | 6 | 0 | 1 (17%) | 5 (83%) |
| Partial atrioventricular canal | 13 | 1 (8%) | 7 (54%) | 5 (38%) |
| Complete atrioventricular canal | 4 | 0 | 3 (75%) | 1 (25%) |
| TOF/DORV | 43 | 5 (12%) | 14 (33%) | 24 (56%) |
| Pulmonary atresia | 10 | 2 (20%) | 3 (30%) | 5 (50%) |
| Ebstein anomaly | 32 | 5 (16%) | 11 (34%) | 16 (50%) |
| Systemic outflow obstruction | 14 | 0 | 3 (21%) | 11 (79%) |
| Prior atrial switch operation* | 12 | 2 (17%) | 6 (50%) | 4 (33%) |
| L- transposition of great arteries | 19 | 0 | 5 (26%) | 14 (74%) |
| Anomalous pulmonary venous return | 3 | 0 | 0 | 3 (100%) |
| Truncus arteriosus | 2 | 0 | 1 (50%) | 1 (50%) |
DCCV = direct current cardioversion; DORV = double outlet right ventricle; TOF = tetralogy of Fallot.
Patients with complete transposition of great arteries.
Cardioversion was successful in 241 patients with CHD (86%). The average number of DCCV shocks was 1.8 per attempt (range 1 to 5). Compared with the non-CHD group, the CHD group had a significantly higher procedural failure rate (14% vs 7%, p = 0.01). The rate of procedural failure varied by CHD diagnosis; the highest rates were for patients with non-Fontan single ventricle (38%) or Fontan physiology (30%; Table 2). However, if patients with Fontan palliation were excluded, the procedural failure rate among patients with the other CHD diagnoses was comparable with that of the non-CHD group (9% vs 7%, p = 0.26).
The multivariable predictors of procedural failure were Fontan palliation (HR, 4.24; 95% CI, 1.22 to 8.23; p = 0.001) and the presence of spontaneous echocardiographic contrast in the atria (HR, 2.41; 95% CI, 1.25 to 4.32; p = 0.03; Table 3).
Table 3.
Predictors of procedural failure
| Variable | Univariable
|
Multivariable
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per decade increase) | 1.24 (0.67–3.26) | .41 | … | … |
| Class I agents* | 0.87 (0.34–3.13) | .39 | … | … |
| Class III agents | 0.63 (0.27–0.91) | .01 | 0.82 (0.45–1.49) | .11 |
| Digoxin | 1.32 (0.61–4.23) | .47 | … | … |
| Cardioversion within 48 h after arrhythmia onset | 1.22 (0.65–3.25) | .17 | … | … |
| Prior cardioversion | 1.83 (0.243–2.86) | .21 | … | … |
| Fontan physiology | 4.33 (2.22–7.24) | <.001 | 4.24 (1.22–8.23) | .001 |
| Non–Fontan single-ventricle physiology | 6.34 (0.28–12.06) | <.001 | 2.42 (0.92–4.11) | .06 |
| Prior Mustard procedure | 1.75 (0.53–4.74) | .41 | … | … |
| Functional class III or IV | 3.43 (0.89–15.32) | .29 | … | … |
| Atrial fibrillation | 4.37 (2.65–6.29) | .01 | 1.88 (0.42–2.87) | .09 |
| Spontaneous echocardiographic contrast | 2.64 (1.12–3.98) | .01 | 2.41 (1.25–4.32) | .03 |
| Moderate-severe right atrial enlargement | 2.06 (0.31–5.63) | .29 | … | … |
| Moderate-severe left atrial enlargement | 1.75 (0.53–4.74) | .41 | … | … |
| Moderate-severe systemic ventricular dysfunction | 1.34 (0.28–4.12) | .39 | … | … |
| Moderate-severe nonsystemic ventricular dysfunction | 2.43 (0.30–4.63) | .31 | … | … |
Agents* = antiarrhythmic drug; HR = hazard ratio.
Procedural complications occurred in 5 patients (2%): minor skin burn in 4 and ventricular fibrillation requiring defibrillation in 1. No patient died or had thromboembolic complications within 30 days after DCCV. The occurrence of procedural complications between the CHD group and the control group was not significantly different (2% vs 3%, p = 0.27). Postcardioversion bradycardia occurred in 21 patients in the CHD group, and 6 of those patients required temporary pacing; 16 of the 21 (76%) had Fontan palliation.
In the CHD group, 51 patients died (18% all-cause mortality) during follow-up; the causes of death were peri-operative in 21 patients, endocarditis in 4, heart failure in 2, sepsis in 3, malignancy in 8, and unknown causes in 13.
In the analysis for arrhythmia recurrence, we considered the time of last electrocardiogram or Holter monitor recording documented in the medical records as the last follow-up to avoid the confounding effect of underreporting for patients lost to follow-up. Patients with unsuccessful DCCV were excluded from the analysis of arrhythmia recurrence.
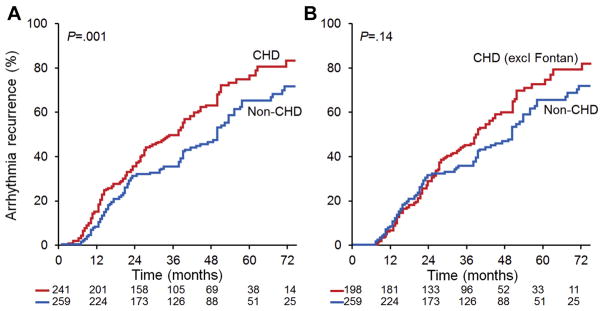
Arrhythmia recurred in 184 of 241 patients with CHD (76%) during a follow-up of 49 ± 23 months, and 129 of those 184 patients (70%) required a second DCCV. By comparison, arrhythmia recurred in 136 of 259 non-CHD patients (53%) during a follow-up of 46 ± 19 months, and 61 of those 136 patients (45%) required a second DCCV. The arrhythmia recurrence rate was higher in the CHD group than in the non-CHD group (83% vs 66% at 60 months, p = 0.001). When patients with Fontan palliation were excluded, the arrhythmia recurrence rate was not significantly different between the CHD and non-CHD groups (75% vs 66% at 60 months, p = 0.14; Figure 1). The multivariable predictors of arrhythmia recurrence were the presence of Fontan physiology (HR, 2.16; 95% CI, 1.24 to 4.35; p <0.001) and history of atrial fibrillation (HR, 1.71; 95% CI, 1.18 to 3.61; p = 0.03; Table 4).
Figure 1.
Kaplan-Meier curves for arrhythmia recurrence. The risk of arrhythmia recurrence at 5 years was significantly higher for patients with CHD than for patients without CHD (A). When patients with Fontan palliation were excluded (excl Fontan), the risk of arrhythmia recurrence was not different between patients with CHD and patients without CHD (B).
Table 4.
Predictors of arrhythmia recurrence
| Variable | Univariable
|
Multivariable
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per decade increase) | 2.43 (0.24–3.4) | .16 | … | … |
| Class I agents* (>3 months after cardioversion) | 0.73 (0.64–1.29) | .39 | … | … |
| Class III agents (>3 months after cardioversion) | 0.96 (0.31–3.63) | .43 | … | … |
| Digoxin (>3 months after cardioversion) | 1.75 (0.53–3.71) | .19 | … | … |
| Fontan physiology | 3.86 (1.32–6.88) | <.001 | 2.16 (1.24–4.35) | <.001 |
| Non-Fontan single-ventricle physiology | 1.44 (0.73–3.65) | .19 | … | … |
| Prior Mustard procedure | 2.16 (1.18–4.54) | .02 | 1.76 (0.64–2.19) | .17 |
| Functional class III or IV | 2.43 (0.89–6.11) | .48 | … | … |
| Atrial fibrillation | 3.12 (1.65–5.32) | .01 | 1.71 (1.18–3.61) | .03 |
| Spontaneous echocardiographic contrast | 1.31 (0.72–1.98) | .15 | … | … |
| Moderate-severe right atrial enlargement | 1.42 (0.63–2.44) | .15 | … | … |
| Moderate-severe left atrial enlargement | 1.01 (0.22–4.12) | .71 | … | … |
| Moderate-severe systemic ventricular dysfunction | 2.66 (1.53–3.34) | .001 | 1.96 (0.84–3.44) | .42 |
| Moderate-severe nonsystemic ventricular dysfunction | 1.75 (0.53–4.74) | .41 | … | … |
Agents* = antiarrhythmic drug; HR = hazard ratio.
Discussion
The main finding was that the rate of DCCV failure was higher among CHD patients than among non-CHD patients. DCCV was unsuccessful in terminating the presenting arrhythmia in 14% of the CHD group compared with 7% of the non-CHD group. Some of the patients included in the present study have been previously described.6,8
Variation in the rate of DCCV failure within the CHD group was based on a specific CHD diagnosis: patients with the highest failure rates had Fontan physiology (failure rate 30%) or non-Fontan single-ventricle physiology (failure rate 38%). A previous study by Ammash et al6 reviewed DCCV outcomes among 63 patients with CHD and reported a procedural failure rate of 6%. The failure rate of 14% in the present study is higher, and we speculate that the observed difference in outcome might be due to differences in the spectrum of CHD diagnoses in the 2 studies. The cardioversion protocol was similar in both study periods.
Fontan physiology was one of the multivariable risk factors for DCCV failure. The procedural failure rate among the 61 patients with Fontan palliation in this cohort was 30%. In a study of 86 patients with Fontan palliation who underwent 152 DCCVs, Egbe et al8 reported a 27% DCCV failure rate, a finding that is concordant with the results of the present study. These studies suggest that atrial arrhythmias in patients with Fontan physiology respond differently to DCCV than atrial arrhythmias in patients with other CHD diagnoses and biventricular circulation. In support of this postulation, we observed that if patients with Fontan palliation were excluded from the CHD cohort, there was no difference in the DCCV failure rate between the CHD and the non-CHD groups (9% vs 7%, p = 0.26).
The other multivariable predictor of DCCV failure was the presence of spontaneous echocardiographic contrast. Previous studies have identified spontaneous echocardiographic contrast as a marker of electromechanical atrial dysfunction and a risk factor for arrhythmia recurrence after DCCV.9–11 We speculate that spontaneous echocardiographic contrast may identify patients with more advanced atrial dysfunction and hence a poor response to DCCV.
Antiarrhythmic drug (AAD) therapy is an alternative or adjunct to DCCV for restoring sinus rhythm in patients with atrial arrhythmia. In a multicenter study of 92 adults with CHD, 35 patients underwent DCCV and 34 received oral AAD without DCCV.7 Sinus rhythm was restored in 89% of the DCCV group and in 88% of the oral AAD group. The success rate of AAD without DCCV for terminating atrial arrhythmia has been reported as 70% for sotalol and dofetilide in several case series.12–14 The present study suggests that DCCV is comparable with and may be superior to AAD conversion rates reported for acute treatment of atrial arrhythmia.
Thromboembolic complication is a main concern after DCCV for atrial arrhythmia.15 The absence of thromboembolic complications in the present study may be attributed to appropriate anticoagulation at DCCV and the liberal use of TEE (77%) for excluding thrombi before DCCV. Although postcardioversion bradycardia was not considered a procedural complication in this study, patients with Fontan palliation accounted for 76% of all cases of post-cardioversion bradycardia. Providers performing cardioversion in patients with Fontan palliation must be aware of this and must be prepared to initiate pacing if required.
The arrhythmia recurrence rate was higher among CHD patients than among non-CHD patients (83% vs 66% at 60 months, p = 0.001), but this difference was eliminated when patients with Fontan palliation were excluded from analysis (75% vs 66% at 60 months, p = 0.14). Fontan physiology and atrial fibrillation were risk factors for recurrence on multivariable analysis. Catheter ablation is an effective therapy for arrhythmia recurrent in patients with CHD.16
Ammash et al6 reported that 60% of the CHD group had arrhythmia recurrence within 18 months after DCCV, a recurrence rate comparable with that for the non-CHD group in that study. The risk factors for arrhythmia recurrence were atrial fibrillation and spontaneous echocardiographic contrast. A study by Koyak et al7 reported a 55% arrhythmia recurrence rate within 3 years after DCCV and that the use of class III AADs was protective against arrhythmia recurrence. In contrast, the use of AADs was not a risk factor for procedural failure or arrhythmia recurrence in the present study.
Fontan physiology was associated with the highest rates of procedural failure and arrhythmia recurrence. With the exclusion of patients with Fontan palliation from the analysis, the outcomes of DCCV (failure and recurrence rates) were similar between the CHD and non-CHD groups. This highlights the uniqueness of Fontan physiology and the heterogeneity of the CHD population.
This retrospective review of outcomes among patients who underwent DCCV at a single referral center was prone to selection bias. Because we relied on electrocardiographic and Holter monitor documentation in the medical records to determine arrhythmia recurrence outcome, we may have underestimated the recurrence rate.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;11:534–545. doi: 10.1161/CIRCULATIONAHA.105.592410. [DOI] [PubMed] [Google Scholar]
- 2.Triedman JK. Arrhythmias in adults with congenital heart disease. Heart. 2002;87:383–389. doi: 10.1136/heart.87.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchardy J, Therrien J, Pilote L, Ionescu-Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319. [DOI] [PubMed] [Google Scholar]
- 4.Ghai A, Harris L, Harrison DA, Webb GD, Siu SC. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol. 2001;37:585–592. doi: 10.1016/s0735-1097(00)01141-4. [DOI] [PubMed] [Google Scholar]
- 5.Poterucha JT, Egbe AC, Johnson JN, Niaz T, Wackel PL, Cannon BC, Eidem BW, Cetta F. Improved ventricular function after TEE-guided cardioversion of atrial arrhythmias in patients after the Fontan operation. Congenit Heart Dis. 2016;11:578–583. doi: 10.1111/chd.12339. [DOI] [PubMed] [Google Scholar]
- 6.Ammash NM, Phillips SD, Hodge DO, Connolly HM, Grogan MA, Friedman PA, Warnes CA, Asirvatham SJ. Outcome of direct current cardioversion for atrial arrhythmias in adults with congenital heart disease. Int J Cardiol. 2012;154:270–274. doi: 10.1016/j.ijcard.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Koyak Z, Kroon B, de Groot JR, Wagenaar LJ, van Dijk AP, Mulder BA, Van Gelder IC, Post MC, Mulder BJ, Bouma BJ. Efficacy of antiarrhythmic drugs in adults with congenital heart disease and supraventricular tachycardias. Am J Cardiol. 2013;112:1461–1467. doi: 10.1016/j.amjcard.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 8.Egbe AC, Connolly HM, Niaz T, McLeod CJ. Outcome of direct current cardioversion for atrial arrhythmia in adult Fontan patients. Int J Cardiol. 2016;208:115–119. doi: 10.1016/j.ijcard.2016.01.209. [DOI] [PubMed] [Google Scholar]
- 9.Sadanandan S, Sherrid MV. Clinical and echocardiographic characteristics of left atrial spontaneous echo contrast in sinus rhythm. J Am Coll Cardiol. 2000;35:1932–1938. doi: 10.1016/s0735-1097(00)00643-4. [DOI] [PubMed] [Google Scholar]
- 10.Bollmann A, Binias KH, Grothues F, Schwerdtfeger A, Klein HU. Left atrial appendage function and pulmonary venous flow in patients with nonrheumatic atrial fibrillation and their relation to spontaneous echo contrast. Echocardiography. 2002;19:37–43. doi: 10.1046/j.1540-8175.2002.00037.x. [DOI] [PubMed] [Google Scholar]
- 11.Okcun B, Yigit Z, Kucukoglu MS, Mutlu H, Sansoy V, Guzelsoy D, Uner S. Predictors for maintenance of sinus rhythm after cardioversion in patients with nonvalvular atrial fibrillation. Echocardiography. 2002;19:351–357. doi: 10.1046/j.1540-8175.2002.00351.x. [DOI] [PubMed] [Google Scholar]
- 12.Banchs JE, Baquero GA, Nickolaus MJ, Wolbrette DL, Kelleman JJ, Samii S, Grando-Ting J, Penny-Peterson E, Davidson WR, Jr, Young SK, Naccarelli GV, Gonzalez MD. Clinical efficacy of dofetilide for the treatment of atrial tachyarrhythmias in adults with congenital heart disease. Congenit Heart Dis. 2014;9:221–227. doi: 10.1111/chd.12129. [DOI] [PubMed] [Google Scholar]
- 13.Beaufort-Krol GC, Bink-Boelkens MT. Sotalol for atrial tachycardias after surgery for congenital heart disease. Pacing Clin Electrophysiol. 1997;20:2125–2129. doi: 10.1111/j.1540-8159.1997.tb03642.x. [DOI] [PubMed] [Google Scholar]
- 14.Beaufort-Krol GC, Bink-Boelkens MT. Effectiveness of sotalol for atrial flutter in children after surgery for congenital heart disease. Am J Cardiol. 1997;79:92–94. doi: 10.1016/s0002-9149(96)00687-x. [DOI] [PubMed] [Google Scholar]
- 15.Hansen ML, Jepsen RM, Olesen JB, Ruwald MH, Karasoy D, Gislason GH, Hansen J, Kober L, Husted S, Torp-Pedersen C. Thromboembolic risk in 16 274 atrial fibrillation patients undergoing direct current cardioversion with and without oral anticoagulant therapy. Europace. 2015;17:18–23. doi: 10.1093/europace/euu189. [DOI] [PubMed] [Google Scholar]
- 16.Yap SC, Harris L, Silversides CK, Downar E, Chauhan VS. Outcome of intra-atrial re-entrant tachycardia catheter ablation in adults with congenital heart disease: negative impact of age and complex atrial surgery. J Am Coll Cardiol. 2010;56:1589–1596. doi: 10.1016/j.jacc.2010.04.061. [DOI] [PubMed] [Google Scholar]