Abstract
Study Design
Economic evaluation alongside a randomized trial of cognitive-behavioral therapy (CBT) and mindfulness-based stress reduction (MBSR) versus usual care alone (UC) for chronic low-back pain (CLBP).
Objective
Determine the one-year cost-effectiveness of CBT and MBSR compared to UC.
Summary of Background Data
CLBP is expensive in terms of healthcare costs and lost productivity. Mind-body interventions have been found effective for back pain, but their cost-effectiveness is unexplored.
Methods
342 adults in an integrated healthcare system with CLBP were randomized to receive MBSR (n = 116), CBT (n = 113), or UC (n = 113). CBT and MBSR were offered in 8 weekly 2-hour group sessions. Cost-effectiveness from the societal perspective was calculated as the incremental sum of healthcare costs and productivity losses over change in quality-adjusted life-years (QALYs). The payer perspective only included healthcare costs. This economic evaluation was limited to the 301 health plan members enrolled >=180 days in the years pre-and post-randomization.
Results
Compared to UC, the mean incremental cost per participant to society of CBT was $125 (95% CI: −4103, 4307) and of MBSR was −$724 (CI: −4386, 2778)—i.e., a net saving of $724. Incremental costs per participant to the health plan were $495 for CBT over UC and −$982 for MBSR, and incremental back-related costs per participant were $984 for CBT over UC and −$127 for MBSR. These costs (and cost savings) were associated with statistically significant gains in QALYs over UC: 0.041 (0.015, 0.067) for CBT and 0.034 (0.008, 0.060) for MBSR.
Conclusions
In this setting CBT and MBSR have high probabilities of being cost-effective, and MBSR may be cost saving, as compared to UC for adults with CLBP. These findings suggest that MBSR, and to a lesser extent CBT, may provide cost-effective treatment for CLBP for payers and society.
Keywords: Mindfulness-based stress reduction, cognitive-behavioral therapy, chronic low-back pain, mind-body interventions, mindfulness meditation, yoga, cost-effectiveness analysis, cost-utility analysis, economic evaluation, cost savings
Introduction
Low back pain is expensive both in terms of healthcare costs and lost employee productivity. Annual healthcare expenditures for those with back pain are estimated to be $90 billion higher than for those without,1 and lost productivity costs are even higher.2 Cognitive behavioral therapy (CBT) has been found effective3 and is recommended for treatment of persistent back pain.4 Mindfulness-based stress reduction5 (MBSR) has also been found effective for back pain.6–8 However, little is known about the economic impacts of these interventions.9,10
The objective of this study was to perform an economic evaluation alongside an already-published randomized controlled trial (RCT) comparing MBSR with CBT and usual care alone (UC) for individuals with chronic low-back pain (CLBP)6,11 in order to determine their one-year cost-effectiveness to society and payers.
Methods
The data for this analysis come from the Mind-Body Approaches to Pain (MAP) trial, a RCT of adults with back pain in Group Health Cooperative (GHC), a large health plan in Washington State. Details on the design of the trial, including its interventions and outcomes were previously published.6,11 The trial compared the effectiveness of two mind-body interventions (MBSR and CBT) to UC in individuals with chronic (>3 months) nonspecific low-back pain. The study enrolled participants 20-to-70 years of age from September 2012 through April 2014. Individuals with back pain associated with a specific diagnosis (e.g., spinal stenosis), litigation, self-rated pain bothersomeness <4 or pain interference with activities <3 on 0-to-10-point scales, or who faced language or other barriers to participation were excluded.
MBSR and CBT were both manualized and provided in groups (10–12 participants per group) 2 hours per week for 8 weeks. The MBSR program also included an optional 6-hour retreat. MBSR was provided by experienced MBSR instructors and was modeled after the original MBSR program.5 CBT was provided by licensed PhD-level psychologists experienced in group CBT and chronic pain, and included techniques most commonly applied for CLBP.12–16 All participants received UC and were compensated $20 per assessment; those randomized to UC received an additional $50 for their participation. The trial is registered; clinicaltrials.gov identifier: NCT01467843.
Effectiveness
Clinical outcomes were assessed by telephone interviewers, blinded to treatment group, at baseline and 4, 8, 26 and 52 weeks post-randomization. The primary effectiveness outcome for the cost-effectiveness analysis was change in quality-adjusted life-years (QALYs), which was calculated using preference-weighted utility (SF-6D17) scores calculated from self-reported health-related quality of life (HRQoL; SF-1218) data.17
Costs
All costs are reported in 2013 US dollars. Healthcare costs were adjusted to 2013 dollars using the monthly non-seasonally-adjusted medical care consumer price index (CPI).19
Healthcare costs
The cost per participant for the MBSR and CBT interventions was based on instructor hours (including preparation and actual session time), their hourly earnings plus fringe benefits, materials costs (e.g., copies of the manual), and the number of participants per group. The higher hourly cost of the PhD psychologists (CBT) balanced out the longer hours for the MBSR instructors (6-hour retreat) resulting in our use of the same estimated cost per participant for each (Table 1).
Table 1.
Unit costs and sources
| Cost per person per event in 2013 US$ | |
|---|---|
| Cost of intervention | |
| CBT (per 16 hours of group sessions)1 | $150 |
| MBSR (per 16 hours of group sessions plus 6-hour retreat; 22 hours in total)1 | $150 |
| Health care cost per visit, mean (SD) | |
| All office-based and outpatient care2 | $79.97 (196.52) |
| Emergency department2 | $306.86 (238.22) |
| Hospital inpatient2 | $4,242.35 (9,150.44) |
| Pharmacy2 | $60.82 (129.79) |
| Imaging2 | $112.18 (84.03) |
| Copays | |
| Outpatient visits (per visit)3 | $20.00 |
| Emergency department (per visit)3 | $125.00 |
| Hospital inpatient (per stay)3 | $65.00 |
| Pharmacy (per prescription)3 | $15.00 |
| Lost productivity costs | |
| Employer Cost (per hour)4 | $31.21 |
CBT = cognitive behavioral therapy; MBSR = mindfulness-based stress reduction. The cost per participant for each was estimated based on the numbers of hours worked by the instructors per class (16 hours for offering the CBT sessions and 22 hours for MBSR, plus approximately 4 and 5 hours, respectively, for preparation) divided by the mean number of participants per class for each treatment (11.3 for CBT and 11.6 for MBSR); their hourly earnings plus fringe benefits ($77.00 per hour for PhD psychologists and $63.14 per hour for MBSR instructors), and materials costs ($5 per participant in each intervention). Since the two estimated amounts were so similar, we used $150 per participant for each.
Mean (and standard deviation) of the actual costs of each type of healthcare for the patients in this sample.
These are roughly the typical amounts patients paid out-of-pocket for visits and hospitalizations during the study.
Mean 2013 national total employer costs of employee compensation for civilian workers from the Bureau of Labor Statistics.
GHC’s electronic databases record healthcare utilization and costs for services delivered at GHC facilities and at non-GHC facilities covered by the health plan.20 Services provided at GHC were assigned actual costs, including the cost of facilities, payroll, overhead, and supplies. The cost of services performed by external providers is the amount reimbursed by the health plan. We obtained healthcare utilization and cost data for one year before and one year after randomization for all participants with ≥180 days GHC enrollment in both years. The pre-randomization utilization was used to adjust participants’ post-randomization usage for pre-study differences across participants. We also isolated back pain-related utilization and costs by flagging healthcare events associated with at least one back pain-related diagnosis code,21 and identifying common back-pain medications: narcotic analgesics, anti-inflammatories, muscle relaxants. In order to assist readers who face other costs, the average cost and member out-of-pocket copayment used for each type of healthcare event are shown in Table 1.
Productivity losses
Absenteeism and presenteeism (lowered productivity while working) were captured from participants using the Work Productivity and Activity Impairment Questionnaire22 with the term “low-back pain” inserted as the specific health problem. This questionnaire is short (6 items), used frequently in economic studies, has adequate test-retest reliability and construct validity, and generates scores that are monetizable.23–25 Absentee hours during the past seven days due to low-back pain were elicited directly. Presenteeism lost hours were calculated by multiplying the reported proportion of time low-back pain affected productivity while working by the hours worked. Respondents reporting that they were not currently working for pay were not asked this question, but everyone was asked how much their low-back pain affected their regular non-job daily activities. The hours of work lost due to absenteeism and presenteeism across the year were adjusted for baseline using regression.26 Lost productivity hours were valued at $31.21, the average hourly 2013 national total employer costs of employee compensation from the Bureau of Labor Statistics.27
Analysis
Cost-effectiveness was calculated from the societal and health plan (payer) perspectives. For both, effectiveness was measured as QALY gains over the 1-year study period. QALYs were calculated as the area under the SF-6D score curve over the year, regression-adjusted for baseline SF-6D values.26 Costs for the societal perspective include participant co-payments for healthcare, employer productivity losses, and overall healthcare costs to the health plan. Costs for the payer perspective included only overall healthcare costs. Back pain-related healthcare costs were also calculated. Healthcare utilization and costs for participants with less than 365 days enrollment in the health plan in either the pre- or post-randomization year were adjusted proportionally up to 365 day-equivalent. Because of the 1-year timeframe of the study, neither costs nor effects were discounted. We used intent-to-treat principles. Missing self-report data were handled using multiple imputation methods.28,29 Because cost data tend to be highly skewed, bias-corrected and accelerated bootstrap estimates (1000 replications) were used to determine confidence intervals for utilization and costs.30,31 The bootstrapped societal cost–QALY pairs were also shown on a cost-effectiveness plane.32 Sensitivity analyses examined the effects of proportionally adjusting healthcare costs to 365-day equivalents for participants with less than 365-days enrollment, and several versions of productivity losses. Baseline between-group differences were analyzed using t tests (continuous variables) and chi-squared tests (frequencies). All calculations used Excel 2010 (Microsoft Corporation, Redmond, WA) or Intercooled Stata 8 (Stata Corporation, College Station, TX).
Results
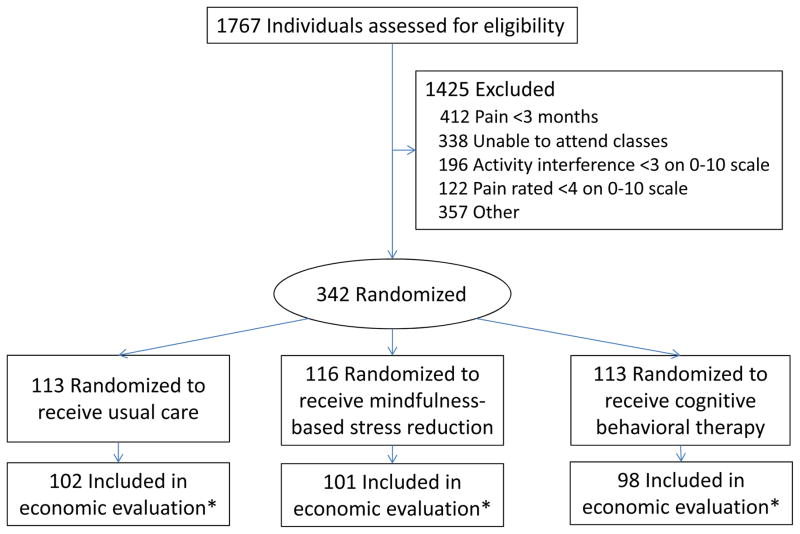
Figure 1 shows the participant flow. This economic evaluation was limited to the 301 of the 342 study participants (102 or 90.3% of those randomized to UC, 98 or 86.7% for CBT, and 101 or 87.1% for MBSR) enrolled in the health plan for ≥180 days in the years pre-and post-randomization. When baseline characteristics for those who had this level of enrollment (n=301) were compared to those who did not (n=41), only two comparisons had P values less than .05. Those with sufficient health plan enrollment were more likely to be employed (79% versus 63%, P = .02) and more likely to report annual household income >$55,000 (67% versus 33%, P = .0001). Table 2 shows baseline characteristics for the three groups included in this study.
Figure 1.
Flow of Participants Through Trial Comparing Mindfulness-Based Stress Reduction With Cognitive Behavioral Therapy and Usual Care for Chronic Low-Back Pain
Table 2.
Baseline characteristics of participants in economic evaluation
| Characteristic | Usual Care (n=102) | CBT (n=98) | MBSR (n=101) | p value |
|---|---|---|---|---|
| Age, mean (SD), y | 49.3 (12.2) | 49.3 (12.7) | 50.2 (11.3) | 0.836 |
| Women | 77 (76%) | 60 (61%) | 63 (62%) | 0.058 |
| Education | ||||
| <High school | 5 (5%) | 4 (4%) | 14 (14%) | 0.056 |
| Some college or vocational school | 34 (33%) | 34 (35%) | 36 (36%) | |
| College graduate | 63 (62%) | 60 (61%) | 51 (51%) | |
| Race | ||||
| White | 79 (80%) | 82 (84%) | 85 (85%) | 0.444 |
| Asian | 2 (2%) | 6 (6%) | 4 (4%) | |
| African American | 3 (3%) | 3 (3%) | 3 (2%) | |
| Other | 15 (15%) | 7 (7%) | 9 (9%) | |
| Hispanic ethnicity | 8 (8%) | 9 (9%) | 5 (5%) | 0.499 |
| Married or living as married | 72 (71%) | 73 (75%) | 74 (73%) | 0.817 |
| Annual family income > US $55,000 | 67 (68%) | 65 (69%) | 61 (62%) | 0.536 |
| Employed | 81 (79%) | 78 (80%) | 79 (78%) | 0.967 |
| Back Pain History and Expectations | ||||
| >1 Year since 1 week without LBP | 77 (76%) | 78 (80%) | 81 (80%) | 0.675 |
| Had spinal injection for LBP | 2 (2%) | 2 (2%) | 3 (4%) | 0.859 |
| Currently reporting “a lot of pain” in site other than back | 34 (33%) | 28 (29%) | 28 (28%) | 0.643 |
| Duration of back pain, mean days (SD) | 321.7 (433) | 412.0 (516) | 369.3 (437) | 0.397 |
| Baseline Measures of Primary Outcome Scores | ||||
| RDQ (modified), mean (SD) | 10.9 (5.0) | 11.4 (5.0) | 11.9 (4.6) | 0.371 |
| Pain bothersomeness rating, mean (SD) | 5.9 (1.6) | 5.9 (1.6) | 6.0 (1.6) | 0.906 |
| Baseline Measures of Secondary Outcome Scores | ||||
| Characteristic pain intensity, mean (SD) | 5.8 (1.3) | 5.8 (1.2) | 6.0 (1.3) | 0.613 |
| PHQ-8, mean (SD) | 5.5 (4.0) | 5.8 (4.5) | 5.7 (4.1) | 0.880 |
| GAD-2, mean (SD) | 1.5 (1.4) | 1.5 (1.5) | 1.3 (1.4) | 0.424 |
| SF-12 Physical, mean (SD) | 39.8 (7.1) | 39.9 (8.5) | 38.0 (7.5) | 0.136 |
| SF-12 Mental, mean (SD) | 39.8 (7.4) | 39.0 (8.4) | 41.0 (8.0) | 0.214 |
| Health-related quality of life (SF-6D) | 70.8 (12.2) | 69.5 (12.4) | 68.0 (13.3) | 0.438 |
| Health-related quality of life (EQ-5D) | 72.0 (15.2) | 73.8 (11.7) | 72.7 (14.4) | 0.677 |
| Any medication use for LBP in past week | 76 (75%) | 75 (77%) | 73 (72%) | 0.789 |
| Opioids use for LBP in past week | 10 (10%) | 12 (12%) | 14 (14%) | 0.669 |
| Back-specific exercise >3 days in past week | 38 (37%) | 39 (40%) | 38 (38%) | 0.924 |
| General exercise >3 days in past week | 47 (46%) | 51 (52%) | 49 (49%) | 0.699 |
| Productivity measures, mean (SD) | ||||
| Hours per week absent because of LBP – measured for those employed only | 1.0 (2.4) | 1.0 (3.3) | 1.2 (4.0) | 0.862 |
| How much LBP affects work 0=no effect, 10=completely prevents work – measured for those employed only | 2.9 (2.2) | 2.1 (2.1) | 2.8 (2.2) | 0.043 |
| How much LBP affects regular activities 0=no effect, 10=completely prevents activities - measured on all | 3.5 (2.2) | 3.4 (2.1) | 3.9 (2.2) | 0.337 |
Changes in resource use by various healthcare cost categories and productivity loss hours over the study year for each group, and health-related quality-of-life (SF-6D) scores across the data collection timepoints are shown in Table 3. No clear trends are apparent in the changes in resource use. Both CBT and MBSR reported more absentee hours and fewer lost presentee hours than UC, and both seem to improve health-related quality of life compared to UC across data collection points.
Table 3.
Average Resource Use, Productivity Loss, and Health-Related Quality of Life
| UC | CBT | MBSR | CBT-UC | MBSR-UC | MBSR-CBT | |
|---|---|---|---|---|---|---|
| Healthcare utilization (all) over 12 months of the study net of previous year’s use (bootstrap BCa 95% CI)* | ||||||
| All office-based and outpatient care (visits) | −1.6 (−11.1, 3.2) | 1.5 (−3.8, 8.1) | −1.1 (−5.7, 3.7) | 3.1 (−4.3, 13.7) | 0.5 (−6.4, 9.6) | −2.6 (−11.0, 3.9) |
| Emergency department (visits) | −0.1 (−0.8, 0.8) | 0.0 (−1.1, 0.9) | 0.5 (0.1, 1.3) | 0.0 (−1.2, 1.3) | 0.6 (−0.4, 1.5) | 0.5 (−0.5, 1.7) |
| Hospital inpatient (stays) | 0.2 (0.0, 1.1) | 0.7 (0.1, 2.7) | 0.0 (0.0, 0.1) | 0.5 (−0.2, 2.2) | −0.2 (−1.1, 0.0) | −0.7 (−2.9, −0.1) |
| Pharmacy (prescriptions) | 1.0 (−0.2, 2.0) | 0.4 (−1.6, 2.1) | 0.2 (−1.1, 2.0) | −0.7 (−2.9, 1.6) | −0.8 (−2.6, 1.2) | −0.1 (−2.5, 2.6) |
| Imaging (visits) | 0.3 (−0.4, 1.0) | 0.7 (0.0, 1.6) | 0.2 (−0.6, 1.0) | 0.4 (−0.7, 1.4) | −0.1 (−1.3, 0.9) | −0.5 (−1.7, 0.5) |
| Productivity loss (calculated as area under the curve net of baseline) due to LBP as reported by employed participants (bootstrap BCa 95% CI)* | ||||||
| Absenteeism lost hours | 25.7 (12, 49) | 32.2 (13, 64) | 35.0 (17, 62) | 6.5 (−20, 40) | 9.3 (−16, 41) | 2.8 (−30, 34) |
| Presenteeism lost hours | 104.3 (63, 153) | 83.4 (40, 127) | 101.0 (62, 142) | −20.9 (−83, 43) | −3.2 (−65, 56) | 17.6 (−40, 80) |
| Health-related quality of life (SF-6D, score out of 100) mean (95% CI)† | ||||||
| Baseline | 70.3 (46, 94) | 69.5 (45, 94) | 68.7 (46, 91) | −0.8 (−4.1, 2.5) | −1.6 (−4.9, 1.7) | −0.8 (−4.1, 2.5) |
| 4 weeks | 70.8 (48, 94) | 72.5 (48, 97) | 71.0 (46, 96) | 1.7 (−1.9, 5.3) | 0.2 (−3.3, 3.7) | −1.5 (−5.2, 2.2) |
| 8 weeks | 72.3 (47, 98) | 76.3 (53, 100) | 75.7 (50, 101) | 3.9 (0.3, 7.6) | 3.4 (−0.4, 7.1) | −0.6 (−4.5, 3.4) |
| 26 weeks | 72.6 (47, 98) | 77.3 (50, 104) | 75.8 (49, 103) | 4.7 (0.6, 8.8) | 3.2 (−0.7, 7.1) | −1.5 (−5.8, 2.8) |
| 52 weeks | 74.6 (50, 99) | 77.6 (54, 101) | 76.6 (50, 103) | 3.0 (−0.7, 6.7) | 2.0 (−1.9, 5.8) | −1.0 (−5.0, 3.0) |
Bias-corrected and accelerated bootstrap 95% confidence interval.
Standard error-based 95% confidence interval adjusted for missing data imputation estimate variance.
CI = confidence interval.
The mean incremental cost per participant to society of CBT versus UC was $125 and the mean incremental cost per participant of MBSR was −$724 (ie, a net saving of $724 compared to UC; Table 4). Most of the cost savings for MBSR were attributable to reduced payer healthcare costs – an average savings to the health plan of $982 per participant. These cost savings were associated with statistically significant QALY gains. The incremental cost-effectiveness ratio for CBT was $3049/QALY; well below the $50,000/QALY threshold used to determine cost-effectiveness.33
Table 4.
Costs (Net of Baseline; 2013 US dollars) and Changes in Quality-Adjusted Life Years*
| Usual Care (UC) | Cognitive Behavioral Therapy (CBT) | Mindfulness-Based Stress Reduction (MBSR) | CBT-UC | MBSR-UC | MBSR-CBT | |
|---|---|---|---|---|---|---|
| Cost of the intervention | $0 | $150 | $150 | $150 | $150 | $0 |
| All office-based and outpatient care | $991 ($196, $2519) | $753 (−$45, $1834) | $540 (−$290, $1533) | −$237 (−$1876, $1019) | −$451 (−$2033, $743) | −$214 (−$1590, $1177) |
| Emergency department | $152 (−$92, $686) | $232 (−$140, $883) | $93 (−$30, $302) | $80 (−$462, $769) | −$59 (−$556, $231) | −$139 (−$742, $270) |
| Hospital inpatient | $902 ($280, $2973) | $1527 ($279, $3528) | $366 (−$858, $1509) | $625 (−$1211, $2490) | −$536 (−$2548, $891) | −$1160 (−$3688, $587) |
| Pharmacy | $141 ($58, $271) | $43 (−$92, $263) | $34 (−$67, $158) | −$98 (−$279, $134) | −$107 (−$262, $54) | −$9 (−$227, $169) |
| Imaging | $80 (−$11, $185) | $55 (−$32, $184) | $100 (−$34, $279) | −$24 (−$167, $129) | $20 (−$158, $207) | $45 (−$137, $253) |
|
| ||||||
| Total overall healthcare (payer) costs | $2265 ($778, $5542) | $2760 ($896, $5613) | $1283 (−$263, $3011) | $495 (−$2741, $3550) | −$982 (−$4108, $1301) | −$1477 (−$4956, $1017) |
|
| ||||||
| All office-based and outpatient care | $219 (−$31, $973) | $305 (−$31, $911) | −$17 (−$208, $303) | $86 (−$490, $652) | −$236 (−$1022, $103) | −$322 (−$942, $116) |
| Emergency department | −$14 (−$61, $0) | $3 (−$42, $100) | $12 ($0, $36) | $17 (−$37, $115) | $26 ($0, $94) | $9 (−$91, $63) |
| Hospital inpatient | $463 ($0, $2693) | $1195 ($304, $3231) | $407 ($81, $1778) | $733 (−$898, $2401) | −$55 (−$1921, $728) | −$788 (−$2573, $392) |
| Pharmacy | $10 (−$4, $27) | $3 (−$18, $35) | −$9 (−$28, $11) | −$7 (−$33, $28) | −$19 (−$43, $6) | −$12 (−$50, $15) |
| Imaging | $22 (−$16, $78) | $26 (−$11, $124) | $29 (−$11, $97) | $5 (−$58, $98) | $7 (−$56, $79) | $3 (−$108, $63) |
|
| ||||||
| Total back-related healthcare (payer) costs, including cost of intervention | $699 ($4, $3763) | $1683 ($436, $4380) | $572 ($66, $2250) | $984 (−$1075, $3385) | −$127 (−$2670, $942) | −$1111 (−$3662, $488) |
|
| ||||||
| Patient copay amounts | −$19 (−$191, $141) | $61 (−$112, $293) | $52 (−$58, $175) | $80 (−$161, $352) | $71 (−$124, $277) | −$9 (−$254, $194) |
| Lost productivity from absenteeism | $803 ($377, $1528) | $1004 ($411, $2008) | $1092 ($535, $1945) | $201 (−$632, $1237) | $289 (−$488, $1284) | $88 (−$942, $1053) |
| Lost productivity from presenteeism | $3254 ($4768, $4768) | $2603 ($3961, $3961) | $3153 ($4441, $4441) | −$651 ($1349, $1349) | −$101 ($1748, $1748) | $550 ($2487, $2487) |
|
| ||||||
| Total Societal costs** | $6304 ($4193, $9805) | $6428 ($3676, $10262) | $5580 ($3465, $8343) | $125 (−$4103, $4347) | −$724 (−$4386, $2778) | −$849 (−$5338, $2662) |
|
| ||||||
| Quality-adjusted life-years (QALYs) | 0.728 (0.521, 0.936) | 0.765 (0.551, 0.978) | 0.753 (0.532, 0.975) | 0.041 (0.015, 0.067) | 0.034 (0.008, 0.060) | −0.007 (−0.035, 0.021) |
All confidence intervals for costs are bias-corrected and accelerated bootstrap generated 95% confidence intervals. All quality-adjusted life-year (QALY) confidence intervals are standard error-based 95% confidence intervals. Both types of confidence intervals are adjusted for missing data imputation estimate variance.
Total Societal costs = Total overall healthcare costs + Patient copay amounts + Lost productivity from absenteeism + Lost productivity from presenteeism
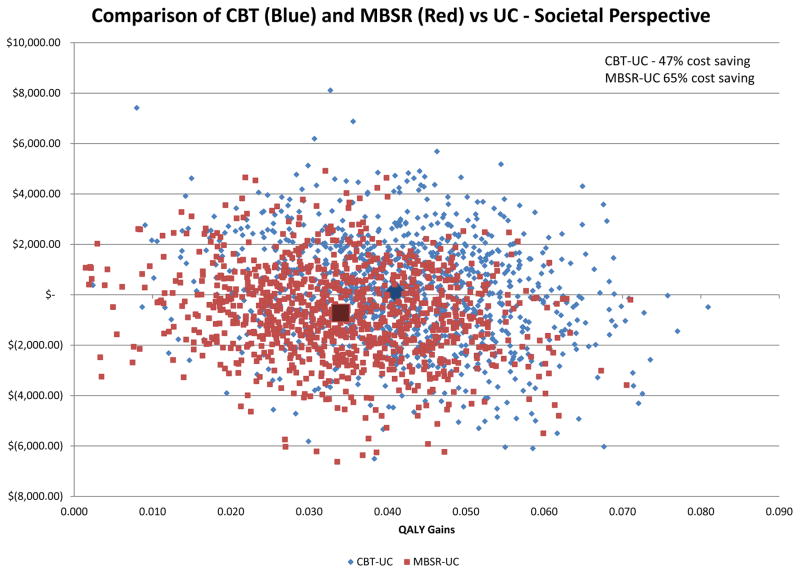
Figure 2 shows the societal cost–QALY plane for both MBSR and CBT, each compared to UC (the 0, $0 point). Across the 1000 bootstrapped societal cost–QALY estimate pairs for both interventions, all show a gain in QALYs and 65% of the MBSR results and 47% of the CBT results show cost savings. From a payer perspective 76% of the MBSR results and 37% of the CBT results show cost savings (data not shown). Looking at these data another way, MBSR has a 90% probability of being less than $50,000/QALY (a common assumed threshold for society’s willingness to pay for an additional QALY33) and CBT has a 81% probability of being less than $50,000/QALY (data not shown).
Figure 2.
Cost-Effectiveness Plane Showing Total Societal Cost and Quality-Adjusted Life-Year (QALY) Gain Pairs Over 1000 Bootstrapped Replications for Mindfulness-Based Stress Reduction Versus Usual Care (Red Squares) And Cognitive Behavioral Therapy Versus Usual Care (Blue Diamonds) for Chronic Low-Back Pain Over One Year
Back pain-related healthcare costs show the same pattern as seen in overall healthcare costs: an increase in healthcare costs over UC for CBT and a reduction for MBSR (Table 4).
Table 5 shows sensitivity analysis results. The top section shows the impact of estimating full-year costs pre- and post-randomization for those with less than full-year enrollment in those years--18% of our sample needed at least one year adjusted—22% in UC, 14% in CBT and 17% in MBSR, and this adjustment had little effect on total and incremental costs. The lower portion of Table 5 shows the impact of various assumptions regarding estimates of absenteeism and presenteeism. The base case societal costs (Table 4) include absenteeism and presenteeism as reported by employed respondents. Rows labeled (1) and (2) also include estimates for employed respondents, but now assuming everyone works 40-hours/week, which is more than the average reported. The row labeled (3) shows the results for presenteeism assuming that all participants were employed and worked 40-hours/week. The presenteeism estimates for non-employed respondents used their reports of how much their low-back pain affected their regular daily activities. This assumption increased the presenteeism losses in each group but did not result in much change in the differences between groups.
Table 5.
Sensitivity Analyses (2013 US Dollars)*
| Usual Care (UC) | Cognitive Behavioral Therapy (CBT) | Mindfulness-Based Stress Reduction (MBSR) | CBT-UC | MBSR-UC | MBSR-CBT | |
|---|---|---|---|---|---|---|
| Total overall healthcare (payer) costs (Base case) | $2265 ($778, $5542) | $2760 ($896, $5613) | $1283 (−$263, $3011) | $495 (−$2741, $3550) | −$982 (−$4108, $1301) | −$1477 (−$4956, $1017) |
| Total healthcare costs without adjustment for enrollment <1 year | $2199 ($727, $5534) | $2560 ($745, $5197) | $1217 (−$327, $2948) | $361 (−$2838, $3328) | −$982 (−$4101, $1313) | −$1343 (−$4498, $1152) |
|
| ||||||
| Total Societal costs (Base case) | $6304 ($4193, $9805) | $6428 ($3676, $10262) | $5580 ($3465, $8343) | $125 (−$4103, $4347) | −$724 (−$4386, $2778) | −$849 (−$5338, $2662) |
| Lost productivity from absenteeism assuming all employed work 40 hours per week (1) | $1028 ($561, $1802) | $1451 ($709, $2713) | $1171 ($610, $2136) | $423 (−$667, $1751) | $143 (−$748, $1206) | −$280 (−$1719, $831) |
| Lost productivity from presenteeism assuming all employed work 40 hours per week (2) | $4302 ($2994, $5726) | $2865 ($1449, $4165) | $2976 ($1845, $4311) | −$1437 (−$3524, $393) | −$1326 (−$3237, $552) | $111 (−$1582, $2038) |
| Lost productivity from presenteeism assuming all are employed (3) | $5250 ($3538, $7105) | $3916 ($2361, $5529) | $3982 ($2505, $5768) | −$1335 (−$3773, $1250) | −$1268 (−$3748, $1050) | $67 (−$2193, $2327) |
| Total Societal costs including (1) and (2) | $7575 ($5425, $10729) | $7135 ($4223, $10971) | $5482 ($3314, $8084) | −$440 (−$4829, $3680) | −$2093 (−$5890, $1359) | −$1653 (−$6213, $1990) |
| Total Societal costs including (1) and (3) | $8524 ($5808, $11596) | $8186 ($5197, $12078) | $6489 ($4130, $9183) | −$338 (−$4713, $4250) | −$2035 (−$5814, $2008) | −$1697 (−$6520, $1899) |
All confidence intervals for costs are bias-corrected and accelerated bootstrap generated 95% confidence intervals.
Discussion
MBSR reduced total societal costs by $724 per participant across one year versus UC, and reduced healthcare costs to the payer by $982 per participant. These cost savings came with a gain in QALYs of 0.034—an increase in HRQoL of approximately 5 percent for the year. CBT was not found to be cost saving compared to UC, but was relatively inexpensive ($125 per participant to society and $495 to the payer) with slightly larger QALY gains (0.041).
We used overall healthcare costs as our base case because CBT and MBSR could have health (and healthcare utilization) benefits beyond those associated with low-back pain. Our estimates of back-related healthcare costs show that CBT did not reduce back-related healthcare costs when compared to UC (these increased by $984 per participant). However, both CBT and MBSR reduced non-back related healthcare costs compared to UC. CBT reduced these costs by an average of $489 ($984 minus $495) per participant, and MBSR reduced these costs by an average of $855 (−$127 minus −$982) during the study year. Given the potentially wide-ranging health impacts of these interventions, this might not be surprising. However, these savings would have been missed if only back-related healthcare costs were measured.
Lamb et al14 also performed an economic evaluation alongside a RCT of primary care-based group CBT versus UC. This UK trial found a gain in QALYs of 0.099 per participant and an increase in back-related healthcare costs of £196.87 per participant in 2008£ (approximately $425.82 in 2013USD using the 2008 exchange rate and adjusting across years using the medical care CPI). Johnson et al34 also performed an economic evaluation alongside a RCT of primary care group CBT-based exercise compared to UC in the UK. Their QALY estimate was not specified, but they reported an even smaller healthcare cost increase for CBT of £27 per participant in 2003/04£ (approximately $65.81 in 2013USD adjusted by their reported exchange rate and the medical care CPI).
One problem comparing the costs in these studies to our estimates is that the simple application of an exchange rate does not adequately capture the different healthcare cost structures between the UK and the US.35 Norton et al36 used the outcomes and resource use data from Lamb et al14 in a decision-analytic model and applied US costs for each resource. Their estimate of one-year back-related healthcare costs was $793 per participant in 2008USD ($926 in 2013USD), which is remarkably close to our estimate of $984.
We were unable to identify other trial-based economic evaluations of MBSR, but we did find two of group yoga, a component of MBSR, for back pain. Chuang et al37 performed an economic evaluation alongside a RCT comparing group yoga to UC in the UK. They found that yoga resulted in an adjusted gain of 0.037 QALYs and an increase in healthcare costs of £124.3 ((£529.7+£439.3)-(£762+£331.3)) in 2008/2009£. Aboagye et al38 compared yoga to exercise therapy and self-care advice in a RCT in Sweden. They found that yoga resulted in a gain of 0.036 QALYs and a €150 (2011/2012€) increase in costs. Our study showed a remarkably similar gain in QALYs for MBSR (0.034), but with a reduction in healthcare costs rather than these small increases. Both the Chuang and Aboagye studies also showed reductions in absentee productivity losses from yoga, whereas our study showed an increase in these productivity losses.
Two other studies used claims data and matched controls to examine changes in healthcare utilization from mindfulness-based interventions and found substantial reductions in healthcare utilization. Stahl et al39 performed a retrospective controlled-cohort study comparing individuals with a variety of conditions who followed a Relaxation Response Resiliency Program (an integrated program including meditation and mindfulness exercises) to a propensity score-matched control group.39 Clinical encounters decreased by 41.9%, imaging by 50.3%, lab encounters by 43.5%, procedures by 21.4%, and emergency department visits by 52.8% in the 3RP group. The authors did not capture the actual cost savings associated with these reductions, but did calculate an expected range of cost savings based on “median values for visits at these treatment sites” of $640 to $25,500/person/year. This range includes our estimate of reduced overall healthcare costs of $982.
Klatt et al40 retrospectively compared participants in a worksite-based intervention using mindfulness meditation to a propensity score-matched control group. Five years of healthcare utilization were captured for members of the university health plan. The study showed a reduction in healthcare costs of $6,196 (19,592 vs 25,788) in 2009USD over five years for the mindfulness intervention. No one-year estimates were given, but their published graph indicates that the cost savings for mindfulness start in the first year. The study is also of interest in that it hints that healthcare costs may continue to decrease over time.
Although most (72%) of the originally randomized sample had a full year of healthcare utilization data available both pre- and post-randomization, and using a cutoff of ≥180 days of health plan enrollment we were able to include almost 90 percent of original participants in this study, one limitation of this study is that excluded participants may have had employment status and income levels which could make these results more applicable to a slightly more employed and affluent population. However, our estimation of full years’ of healthcare utilization for those who had less seems to have little impact on outcomes. Also, the availability of these healthcare utilization data stands in contrast to the other RCT-based studies discussed above, all of which used cost data based on individual self-report. Finally, as is true of any economic evaluation, these results are not, without adjustment, generalizable beyond this healthcare setting.41 To assist readers in estimating the potential impacts of these interventions in other settings, as recommended, we reported unit costs and changes resource use so that the impact of different cost structures can be determined.35
Conclusions
CBT and MBSR were cost-effective, and MBSR may be cost saving, as compared to UC for adults with CLBP in this large integrated healthcare system in Washington State. These findings suggest that MBSR, and to a lesser extent CBT, may provide cost-effective treatment for CLBP for payers and society.
Acknowledgments
The National Center for Complementary and Integrative Health (NICCIH) of the National Institutes of Health (NIH) (award number R01AT006226) funds were received in support of this work.
Relevant financial activities outside the submitted work: grants.
The authors gratefully acknowledge Kristin Delaney for her work assembling the healthcare utilization data for this project.
References
- 1.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 2.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. The Cochrane Library. 2010 doi: 10.1002/14651858.CD002014.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Annal Intern Med. 2007;147(7):478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, NY: Random House; 2005. [Google Scholar]
- 6.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: A randomized clinical trial. JAMA. 2016;315(12):1240–1249. doi: 10.1001/jama.2016.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer H, Haller H, Lauche R, Dobos G. Mindfulness-based stress reduction for low back pain. A systematic review. BMC Complement Altern Med. 2012;12(1):1. doi: 10.1186/1472-6882-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morone NE, Greco CM, Moore CG, et al. A mind-body program for older adults with chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2016;176(3):329–337. doi: 10.1001/jamainternmed.2015.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman PM, Poindexter BL, Witt CM, Eisenberg DM. Are complementary therapies and integrative care cost-effective? A systematic review of economic evaluations. BMJ Open. 2012;2(5):e001046. doi: 10.1136/bmjopen-2012-001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henschke N, Ostelo RW, van Tulder MW, et al. Behavioural treatment for chronic low-back pain. The Cochrane Library. 2010;(7) doi: 10.1002/14651858.CD002014.pub3. Art. No.: CD002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherkin DC, Sherman KJ, Balderson BH, et al. Comparison of complementary and alternative medicine with conventional mind–body therapies for chronic back pain: protocol for the Mind–body Approaches to Pain (MAP) randomized controlled trial. Trials. 2014;15:211. doi: 10.1186/1745-6215-15-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol. 2014;69(2):153. doi: 10.1037/a0035747. [DOI] [PubMed] [Google Scholar]
- 13.Turner JA, Romano JM. Cognitive-behavioral therapy for chronic pain. In: Loeser JD, Butler SH, Chapman CR, Turk DC, editors. Bonica’s Management of Pain. Philadelphia, PA: Lippincott, Williams & Wilkins; 2001. pp. 1751–1758. [Google Scholar]
- 14.Lamb SE, Hansen Z, Lall R, et al. Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. The Lancet. 2010;375(9718):916–923. doi: 10.1016/S0140-6736(09)62164-4. [DOI] [PubMed] [Google Scholar]
- 15.Turk DC, Winter F. The Pain Survival Guide: How to Reclaim Your Life. Washington, DC: American Psychological Association; 2005. [Google Scholar]
- 16.Otis JD. Managing Chronic Pain: A Cognitive-Behavioral Therapy Approach (Therapist Guide) New York, NY: Oxford University Press; 2007. [Google Scholar]
- 17.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42(9):851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Consumer Price Index, CUUR0000SAM, Not Seasonally Adjusted, US City Average, Medical Care. Bureau of Labor Statistics; 2016. [Accessed October 24, 2016]. http://data.bls.gov/pdq/SurveyOutputServlet. [Google Scholar]
- 20.Saunders KW, Stergachis A, Von Korff M. Group Health Cooperative of Puget Sound. In: Strom BL, editor. Pharmacoepidemiology. 2. New York, NY: Wiley; 1994. pp. 171–185. [Google Scholar]
- 21.Cherkin DC, Deyo RA, Volinn E, Loeser JD. Use of the International Classification of Diseases (ICD-9-CM) to identify hospitalizations for mechanical low back problems in administrative databases. Spine. 1992;17(7):817–825. doi: 10.1097/00007632-199207000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 23.Lofland JH, Pizzi L, Frick KD. A review of health-related workplace productivity loss instruments. Pharmacoeconomics. 2004;22(3):165–184. doi: 10.2165/00019053-200422030-00003. [DOI] [PubMed] [Google Scholar]
- 24.Mattke S, Balakrishnan A, Bergamo G, Newberry SJ. A review of methods to measure health-related productivity loss. Am J Managed Care. 2007;13:211–217. [PubMed] [Google Scholar]
- 25.Prasad M, Wahlqvist P, Shikiar R, Shih Y-CT. A review of self-report instruments measuring health-related work productivity. Pharmacoeconomics. 2004;22(4):225–244. doi: 10.2165/00019053-200422040-00002. [DOI] [PubMed] [Google Scholar]
- 26.Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ. 2005;14(5):487–496. doi: 10.1002/hec.944. [DOI] [PubMed] [Google Scholar]
- 27.Bureau of Labor Statistics. Employer Costs for Employee Compensation Historical Listing, National Compensation Survey. Washington, DC: US Department of Labor; 2016. [Google Scholar]
- 28.Briggs A, Clark T, Wolstenholme J, Clarke P. Missing.... presumed at random: cost-analysis of incomplete data. Health Econ. 2003;12(5):377–392. doi: 10.1002/hec.766. [DOI] [PubMed] [Google Scholar]
- 29.McKnight PE, McKnight KM, Sidani S, Figueredo AJ. Missing Data: A Gentle Introduction. New York, NY: The Guilford Press; 2007. [Google Scholar]
- 30.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19(23):3219–3236. doi: 10.1002/1097-0258(20001215)19:23<3219::aid-sim623>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1994. [Google Scholar]
- 32.Drummond MF, Sculpher MJ, Torrance GW, O’Brien B, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005. [Google Scholar]
- 33.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 34.Johnson RE, Jones GT, Wiles NJ, et al. Active exercise, education, and cognitive behavioral therapy for persistent disabling low back pain: a randomized controlled trial. Spine. 2007;32(15):1578–1585. doi: 10.1097/BRS.0b013e318074f890. [DOI] [PubMed] [Google Scholar]
- 35.Drummond M, Manca A, Sculpher M. Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care. 2005;21(02):165–171. [PubMed] [Google Scholar]
- 36.Norton G, McDonough CM, Cabral H, Shwartz M, Burgess JF. Cost-Utility of Cognitive Behavioral Therapy for Low Back Pain From the Commercial Payer Perspective. Spine. 2015;40(10):725–733. doi: 10.1097/BRS.0000000000000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuang L-H, Soares MO, Tilbrook H, et al. A pragmatic multicentered randomized controlled trial of yoga for chronic low back pain: economic evaluation. Spine. 2012;37(18):1593–1601. doi: 10.1097/BRS.0b013e3182545937. [DOI] [PubMed] [Google Scholar]
- 38.Aboagye E, Karlsson ML, Hagberg J, Jensen I. Cost-effectiveness of early interventions for non-specific low back pain: A randomized controlled study investigating medical yoga, exercise therapy and self-care advice. J Rehab Med. 2015;47(2):167–173. doi: 10.2340/16501977-1910. [DOI] [PubMed] [Google Scholar]
- 39.Stahl JE, Dossett ML, LaJoie AS, et al. Relaxation response and resiliency training and its effect on healthcare resource utilization. PloS One. 2015;10(10):e0140212. doi: 10.1371/journal.pone.0140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klatt MD, Sieck C, Gascon G, Malarkey W, Huerta T. A healthcare utilization cost comparison between employees receiving a worksite mindfulness or a diet/exercise lifestyle intervention to matched controls 5 years post intervention. Complement Ther Med. 2016;27:139–144. doi: 10.1016/j.ctim.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 41.Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health. 2009;12(4):409–418. doi: 10.1111/j.1524-4733.2008.00489.x. [DOI] [PubMed] [Google Scholar]