Abstract
Aims
Periodontitis and psoriatic disease, including psoriasis (PS) and psoriatic arthritis (PsA), share the common risk factors and co-morbidities. However, the risk of periodontitis in patients with psoriatic disease still needs further investigation. This study was a nationwide population-based retrospective cohort study assessing the risk of periodontitis from psoriatic disease exposure.
Materials and Methods
Patients with newly diagnosed psoriatic disease from 2003 to 2012 were identified from the Taiwanese National Health Insurance Research Database. The 1:4 ratio propensity score matched controls were selected from no psoriatic disease participations. The subsequent risk of periodontitis was evaluated in exposure and comparison groups. Multiple Cox proportional hazard models were used for the estimation.
Results
A total of 3,487 psoriatic disease patients and 13,948 controls were identified. Incidence rate of periodontitis was higher in patients with PsA. The adjusted hazard ratio (aHRs) for moderate/severe periodontitis were 0.85 (95% CI [0.65–1.11]) in PS group and 1.66 (95% CI [0.99–2.78]) in PsA group. The aHRs of PsA were increased over time, aHRs was changed from 0.65 (0–11 months from index date) to 1.34 (≥12 months from index date) in all types of periodontitis and from 1.09 to 1.79 in moderate/severe periodontitis group, respectively.
Conclusions
The increased risk of periodontitis was observed, especially the association between PsA and moderate/severe periodontitis. The patients with psoriatic disease should receive regular periodontal evaluation.
Keywords: Psoriasis, Periodontitis, Psoriatic arthritis, Nationwide population, Cohort study
Introduction
Psoriatic disease is an immune-mediated disease characterized by inflammation of the dermis and epidermis caused by atypical keratinocyte differentiation and hyperproliferation with predominantly skin and joint manifestations. The psoriasis (PS) patients may also have psoriatic arthritis (PsA) which cutaneous lesions indicative of PS precede development of arthritic signs and symptoms (Parisi et al., 2013; Wang, Hsieh & Tsai, 2016). The comorbidities reported to be associated with psoriatic disease include diabetes, metabolic syndrome, chronic obstructive pulmonary disease and cardiovascular disease (Tsai et al., 2011; Yang, Keller & Lin, 2011). It has been suggested that bacteria play an important role in the immunopathogenesis of psoriatic disease (Fry et al., 2013; Fry et al., 2015).
Periodontitis is a chronic inflammatory disease triggered by microbial biofilm and characterized by an immunologically moderated destruction of dental supporting tissues of the teeth, progressive attachment loss, and bone resorption, as well as characterized by pocket formation (Kinane & Bartold, 2007). The etiology of periodontitis relates to microbial biofilm infection and subsequent host-pathogen interactions. The association between periodontitis and immune-mediated inflammatory systemic diseases has been increasingly recognized (Ebersole et al., 2017).
Although psoriatic disease and periodontitis have same pathogenic mechanisms and associated conditions in common, few studies have shown that patients with periodontitis have a significantly elevated risk of PS (Keller & Lin, 2012; Nakib et al., 2013; Lazaridou et al., 2013; Skudutyte-Rysstad et al., 2014; Sharma, Raman & Pradeep, 2015; Egeberg et al., 2017). Contrarily, a few reports have shown no relationship (Fadel et al., 2013; Sezer et al., 2016) but not all studies have had negative findings in this regard.
However, the risk of periodontitis in patients with psoriatic disease still needs further investigation. The Taiwanese National Health Insurance Research Database (NHIRD) has facilitated many population-based longitudinal studies in Taiwan (Chen, Wu & Chang, 2017a; Chen, Wu & Chang, 2017b; Yang et al., 2017; Yang et al., in press). Therefore, this study assessed the risk of periodontitis in a large, nationally representative, population-based cohort of patients with psoriatic disease in Taiwan.
Materials and Methods
Database and ethical consideration
The NHIRD is a nationwide, single-payer, obligatory health insurance program launched on March 1, 1995, covering over 99% of the Taiwanese population in 2010 in Taiwan. The NHIRD is released for research purposes and consists of comprehensive claims data, including dental services, hospitalization services, outpatient services, traditional Chinese medical services, and detailed drug prescription records. To improve data accuracy, the Bureau of NHI routinely performs random checks of patient charts (Cheng, 2003). The Longitudinal Health Insurance Research Database 2010 (LHIRD 2010), a subset of NHIRD, was used for this cohort study. This LHIRD 2010 dataset included health care information from a randomly selected sample of one million beneficiaries in 2010. All subject information was anonymized and de-identified to protect privacy. On the basis of this detailed information, NHIRD can provide sufficient sample size, generalizability, and statistical power to assess epidemiological profiles of the entire Taiwanese population.
This study employed a retrospective cohort study design. The Ethics Review Board at the Chung Shan Medical University Hospital approved this study (CS2-15071). Informed consent was not required because this was a secondary data analysis. This report also complies with STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for observational studies.
Exposure of psoriatic disease
The exposure group included patients who were newly diagnosed with psoriatic disease by the International Classification of Diseases, Ninth Clinical Modification (ICD-9-CM) codes 696.0 and 696.1 from 2003 to 2012. In order to avoid reverse causality, we excluded the prevalent cases with psoriatic diseases before 2003. Furthermore, the new psoriasis patients diagnosed in 2013 were excluded from analysis, because these patients were followed for less than one year. We excluded psoriatic disease patients without any treatment including the use of biologic drugs, corticosteroids, cyclosporine, psoralens, retinoids, methotrexate, or phototherapeutics within one year. The index date was identified as the date of initial psoriatic disease diagnosis. The psoriatic disease cases were classified into two groups, included PsA (ICD-9 code of 696.0) and PS (ICD-9 code of 696.1). If the case had both disease codes, we identified the case as PsA.
Comparison group and propensity score match (PSM)
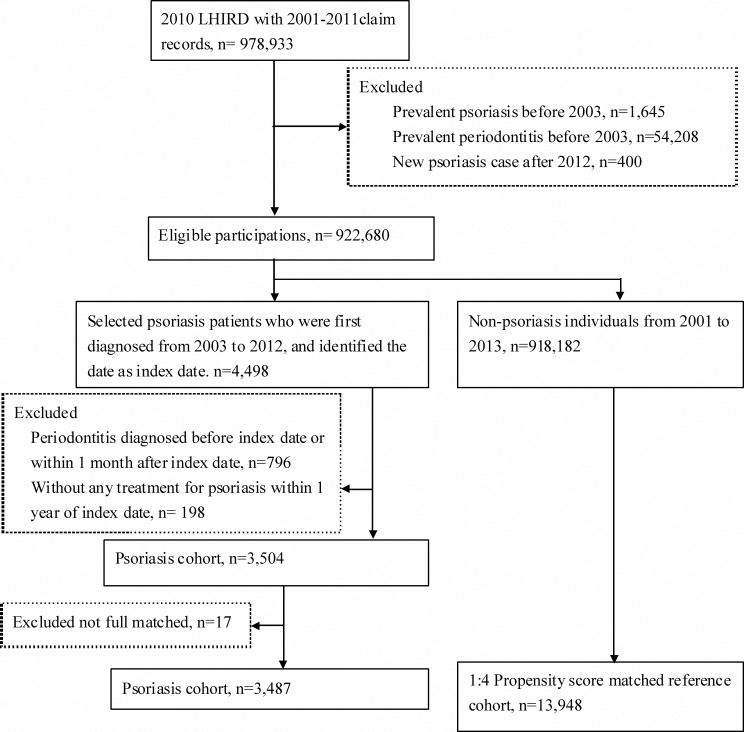
As shown in Fig. 1, the comparison group included participations who were never diagnosed with psoriatic disease from 2001 to 2013. In order to reduce the confounding bias, we used propensity score matching to select controls. Propensity score of participants which predicted the probability of psoriatic disease exposure for participants was estimated by logistic regression modeling. The predictors involved birth year, sex, and co-morbidities at baseline. The 1:4 matched comparisons were selected with the same propensity score (stepwise selected from 8-digit exact matching to 1-digit exact matching) in as exposure subject. Furthermore, the controls were still at risk on index date.
Figure 1. Flowchart of study design for propensity score matched retrospective cohort study.
Periodontitis event
This study determined the occurrence of new cases of periodontitis by ICD-9 codes 523.3–523.5, which received ≥2 times dental scaling in 90 days or underwent periodontitis surgery. The severity of periodontal disease was classified into three levels included mild periodontitis (only dental scaling, ICD-9 procedure code 96.54), moderate periodontitis (subgingival curettage, ICD-9 procedure code 24.31), and severe periodontitis (periodontal flap operation, ICD-9 procedure code 24.39). The grade of the highest disease severity was identified within 90 days after periodontitis diagnosis.
Co-morbidities
Potential confounders in this study were co-morbidities such as chronic obstructive obstructive pulmonary disease, COPD (ICD-9: 490, 491, 492, 494, and 496), diabetes (ICD-9: 250), hypertension (ICD-9: 401–405), and hyperlipidemia (ICD-9: 272). To improve the validity of the co-morbidities, these co-morbidities were identified as ≧2 outpatient visits or at least one admission within one year before index date.
The frequency of dental visit before index date was included in the analysis as the utilization of dental care also affected the probability of diagnosis with dental disease. Therefore, we used this variable as covariate in multiple regressions, or performed the stratified analysis by frequency of dental visit.
Statistical analysis
This was a retrospective cohort study design with PSM, and we conducted a time to event analysis. All participations were followed from index date to event occurred or censored (death, withdraw, or the end of study on December, 31, 2013). The two tail t test and chi-square were used to test the difference of continuous and categorical variables, respectively. The generalized linear model with Poisson response and log function was applied to calculate the crude incidence rate of periodontitis and 95% confidence interval. The Cox proportional hazard models were applied to estimate the hazard ratios of periodontitis, and the multiple Cox models were conducted using age, sex and co-morbidities as covariates. All analyses were performed by SAS software (Version 9.4; SAS Institute, Inc., Cary, NC, USA), and the significant level was 0.05.
Results
A total of 3,487 psoriatic patients diagnosed between 2003 and 2010 were included in the exposure cohort. The non-psoriatic disease group comprised 13,948 controls with 1:4 propensity score matched (Table 1). Patients with psoriatic disease were predominantly males (60.6% vs 39.4%) with a mean age of 45.28 years (standard deviation = 19.52). All baseline characteristics including the frequency of dental visit within 365 days prior to index date were not significantly different between psoriatic disease cohort and comparison. There were 415 (11.90%) PsA and 3,072 (88.10%) PS patients in psoriatic disease group.
Table 1. Demographic characteristics of study population.
| Comparison n = 13, 948 | Psoriatic disease n = 3, 487 | p value | |
|---|---|---|---|
| Age | 45.43 ± 19.62 | 45.28 ± 19.52 | 0.6782 |
| Sex | 0.4145 | ||
| Female | 5,391 (38.65%) | 1,374 (39.40%) | |
| Male | 8,557 (61.35%) | 2,113 (60.60%) | |
| Co-morbidities | |||
| COPD | 486 (3.48%) | 143 (4.10%) | 0.0808 |
| Diabetes | 1,369 (9.82%) | 336 (9.64%) | 0.7499 |
| Hypertension | 2,658 (19.06%) | 651 (18.67%) | 0.6020 |
| Hyperlipidemia | 1,270 (9.11%) | 311 (8.92%) | 0.7317 |
| Frequency of dental visit within 365 days prior to index date | 0.2996 | ||
| 0 | 9,481 (67.97%) | 2,313 (66.33%) | |
| 1 | 1,707 (12.24%) | 457 (13.11%) | |
| 2 | 988 (7.08%) | 259 (7.43%) | |
| ≧3 | 1,772 (12.7%) | 458 (13.13%) | |
| Type of psoriatic disease | |||
| Psoriasis | – | 3,072 (88.10%) | |
| Psoriatic arthritis | – | 415 (11.90%) |
A total of 3,103 individuals with periodontitis were selected in this study. Patients (n = 345) were recognized as moderate/severe periodontitis among the periodontitis group. As shown in Table 2, crude incidence rates (per 103 person months) of periodontitis were 3.579 (95% CI [3.455–3.707]), 4.425 (95% CI [3.641–5.378]), and 3.652 (95% CI [3.393–3.932]) in comparison group, PsA, and PS group, respectively. For moderate/severe periodontitis, crude incidence rate (per 1,000 person months) were 0.398 (95% CI [0.358–0.442]), 0.657 (95% CI [0.396–1.090]), and 0.341 (95% CI [0.268-0.435]) in comparison group, PsA, and PS group, respectively.
Table 2. Incidence of periodontitis.
| Comparison | Psoriatic disease | ||
|---|---|---|---|
| Psoriasis arthritis | Psoriasis | ||
| n = 13, 948 | n = 415 | n = 3, 072 | |
| Follow up person months | 867,106 | 22,826 | 193,303 |
| Periodontitis case | |||
| All types | 3,103 | 101 | 706 |
| Moderate/severe | 345 | 15 | 66 |
| Incidence rate (per 103 person months) | |||
| All types | 3.579 (3.455–3.707) | 4.425 (3.641–5.378) | 3.652 (3.393–3.932) |
| Moderate/severe | 0.398 (0.358–0.442) | 0.657 (0.396–1.090) | 0.341 (0.268–0.435) |
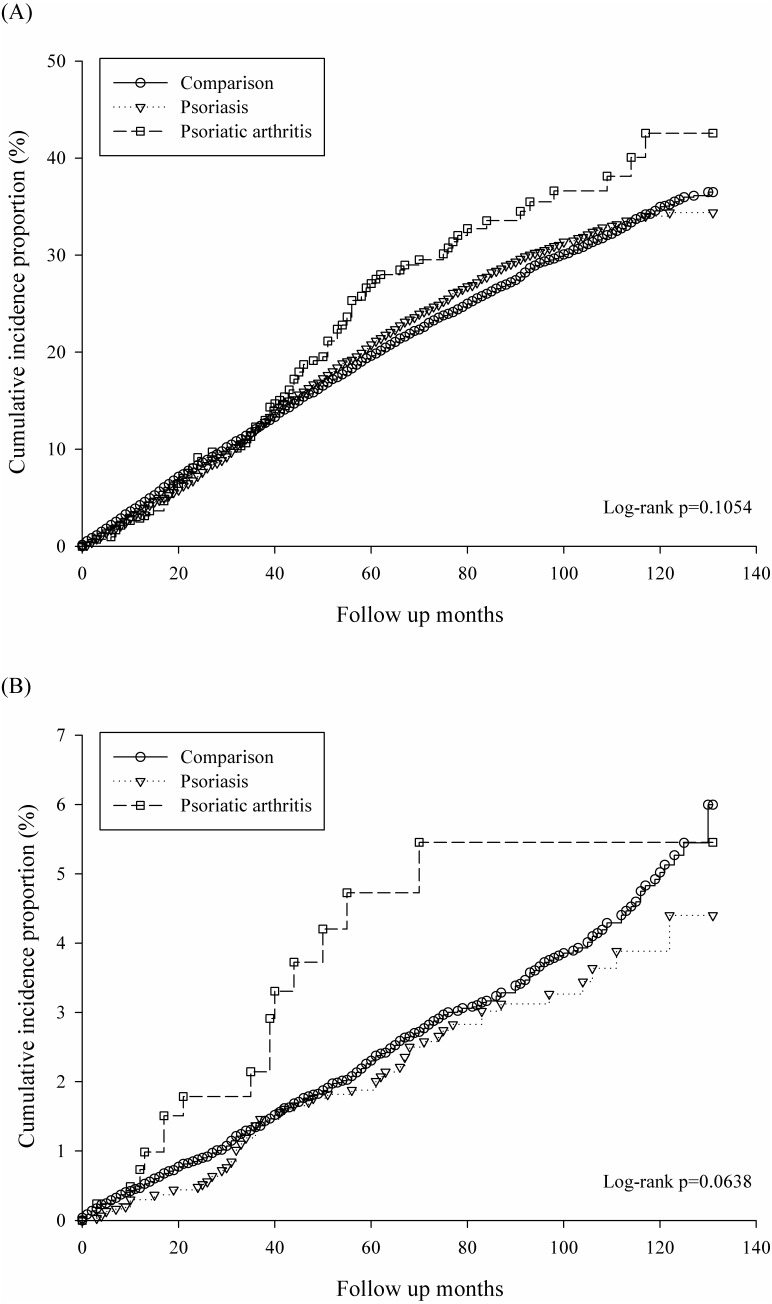
Figure 2A revealed the un-adjusted Kaplan–Meier curves of cumulative incidence proportion in all types of periodontitis for psoriatic disease exposure. PsA group had higher risk of periodontitis, but the log rank test did not reach significance (p = 0.1054).In addition, Figure 2B demonstrated cumulative incidence proportion of moderate/severe periodontitis and PsA group had highest incidence risk of periodontitis with borderline significance (log rank p = 0.06).
Figure 2. Kaplan–Meier curves of cumulative incidence proportion of periodontitis: (A) for all types of periodontitis, (B) for moderate/severe periodontitis.
The result of Multivariate Cox modeling is shown in Table 3. For all types of periodontitis, PsA had borderline significance for higher risk of periodontitis (adjusted HR, aHR = 1.20, 95% CI [0.99–1.47], p = 0.07) compared with comparison group. The risk of periodontitis was not significantly different between the PS group and the comparison group (aHR = 1.01, 95% CI [0.93–1.09], p = 0.84). For moderate/severe periodontitis, the aHRs were borderline significantly increased in the PsA group (aHR = 1.66, 95% CI [0.99–2.78], p = 0.06) and not significant in the PS group (95% CI [0.65–1.11], p = 0.22), respectively. Moreover, the pre-existence of COPD, hyperlipidemia and frequency of dental visit within 365 days before index date was significantly associated with periodontal treatment.
Table 3. Hazard ratios of periodontitis by multivariate Cox proportional model.
| All types of periodontitis | Moderate/severe periodontitis | |||
|---|---|---|---|---|
| aHRa (95% CI) | p value | aHRa (95% CI) | p value | |
| Exposure group | ||||
| Comparison | Reference | Reference | ||
| Psoriasis | 1.01 (0.93–1.09) | 0.8415 | 0.85 (0.65–1.11) | 0.2233 |
| Psoriatic arthritis | 1.20 (0.99–1.47) | 0.0681 | 1.66 (0.99–2.78) | 0.0566 |
| Age (per 1 year) | 1.00 (1.00–1.00) | 0.0531 | 1.01 (1.00–1.02) | 0.0004 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.91 (0.85–0.97) | 0.0033 | 0.98 (0.81–1.20) | 0.8697 |
| Co-morbidities | ||||
| COPD | 1.25 (1.06–1.48) | 0.0072 | 0.77 (0.44–1.34) | 0.3555 |
| Diabetes | 0.95 (0.84–1.08) | 0.4330 | 0.95 (0.68–1.33) | 0.7583 |
| Hypertension | 0.98 (0.89–1.08) | 0.7035 | 0.96 (0.73–1.26) | 0.7819 |
| Hyperlipidemia | 1.22 (1.08–1.37) | 0.0013 | 1.69 (1.24–2.29) | 0.0008 |
| Frequency of dental visit within 365 days prior to index date | ||||
| 0 | Reference | Reference | ||
| 1 | 1.57 (1.44–1.72) | <.0001 | 1.87 (1.44–2.42) | <.0001 |
| 2 | 1.69 (1.51–1.88) | <.0001 | 1.47 (1.03–2.09) | 0.0350 |
| ≧3 | 1.83 (1.68–1.99) | <.0001 | 1.68 (1.29–2.19) | 0.0001 |
Notes.
Adjusted hazard ratio: this was adjusted for psoriasis exposure, age, sex, co-morbidities, and frequency of dental visit within 365 days prior to index date.
The results of further stratification by frequency of dental visit within 365 days prior to index date is shown in Table 4. The aHRs of moderate/severe periodontitis for PsA exposure were 1.87 (95% CI [0.96–3.66], p = 0.07) and 2.72 (95% CI [1.08–6.83], p = 0.03) in subgroup 1 (without dental visit) and 3 (received ≧ 3 times), respectively. However, there was no significnt association with periodontitis for exposure of PS in any subgroup stratified by dental care visits.
Table 4. The stratified analysis by the frequency of dental visit within 365 days prior to index date.
| All types of periodontitis | Moderate/severe periodontitis | |||
|---|---|---|---|---|
| aHRa (95% CI) | p value | aHRa (95% CI) | p value | |
| Subgroup 1: without dental visit within 365 days prior to index date | ||||
| Comparison | Reference | Reference | ||
| Psoriasis | 1.08 (0.97–1.20) | 0.1907 | 0.82 (0.58–1.17) | 0.2782 |
| Psoriatic arthritis | 1.19 (0.90–1.57) | 0.2334 | 1.87 (0.96–3.66) | 0.0656 |
| Subgroup 2: dental visit 1∼2 times within 365 days prior to index date | ||||
| Comparison | Reference | Reference | ||
| Psoriasis | 0.91 (0.77–1.08) | 0.2728 | 1.05 (0.66–1.68) | 0.8348 |
| Psoriatic arthritis | 1.19 (0.81–1.74) | 0.3841 | 0.42 (0.06–2.98) | 0.3818 |
| Subgroup 3: dental visit ≧3 times within 365 days prior to index date | ||||
| Comparison | Reference | Reference | ||
| Psoriasis | 0.96 (0.79–1.17) | 0.6910 | 0.58 (0.28–1.22) | 0.1524 |
| Psoriatic arthritis | 1.29 (0.85–1.96) | 0.2354 | 2.72 (1.08–6.83) | 0.0334 |
Notes.
Adjusted hazard ratio: this was adjusted for psoriasis exposure, age, sex, and co-morbidities.
Table 5 presented the time varied HRs of periodontitis for psoriatic disease exposure. For the early period (0–11 months from psoriatic disease diagnosed) of PS exposure, there was no significant association between PS and periodontitis. For the later period (≧12 months from psoriatic disease diagnosed), the results demonstrated at least one year exposure of PsA significantly increased the risk for all types of periodontitis (aHR = 1.34: 95% CI [1.09–1.65]) and moderate/severe periodontitis (aHR = 1.79: 95% CI [1.03–3.13]).
Table 5. Hazard ratios of periodontitis stratified analysis by follow up period.a.
| Hazard ratio (95% CI) | ||||
|---|---|---|---|---|
| All types of periodontitis | Moderate/severe periodontitis | |||
| 0–11 months from index date | ≧12 months from index date | 0–11 months from index date | ≧12 months from index date | |
| Exposure group | ||||
| Comparison | Reference | Reference | Reference | Reference |
| Psoriasis | 0.80 (0.65–1.00) | 1.05 (0.96–1.15) | 0.66 (0.33–1.32) | 0.89 (0.67–1.18) |
| Psoriatic arthritis | 0.65 (0.36–1.19) | 1.34 (1.09–1.65) | 1.09 (0.27–4.47) | 1.79 (1.03–3.13) |
Notes.
All models were adjusted for age, sex, co-morbidities, and frequency of dental visit within 365 days prior to index date.
Discussion
The relationship between psoriatic disease and periodontitis was first reported by Keller & Lin (2012) who stated the increased risk for PS among patients with chronic periodontitis in Taiwan. In this large-scale population-based longitudinal study, we first found that in Taiwanese patients with psoriatic disease, risk of periodontitis was increased compared to the general population and the incidence was highest for PsA. Similar results were found in a Danish nationwide cohort that the risk of periodontitis was highest in patients with severe PS and PsA (Egeberg et al., 2017). Moreover, we further classified the periodontitis based on the periodontal treatment records in this study. The patients with PsA had higher moderate/severe periodontitis probability than comparison group or PS group. This implies that the link between periodontitis and psoriatic disease depends on the disease severity.
In an age- and gender-balanced study of Turkish patients, demonstrated that periodontitis severity was higher in the PsA group (Üstün et al., 2013). Another study from a Norway University, in the propensity score (age, gender and education) matched sample (n = 100) psoriasis remained significantly associated with moderate/severe periodontitis (Skudutyte-Rysstad et al., 2014). Recently, a case control study showed that the periodontal status was associated with severity of PS in India (Sharma, Raman & Pradeep, 2015). However, these studies were all case-controlled in design and had limited sample sizes making it difficult to estimate the temporal frequency of periodontitis development in patients with psoriatic disease and generalize the study results.
To the best of our knowledge, our study is the first to evaluate the risk of periodontitis stratified by follow-up years in multivariable Cox proportional hazard regression. The risk of periodontitis was significantly higher in the PsA as compared to the PS group. This maybe because PsA is an advanced form of PS. Taken together, these findings indicate that intensive regular oral check-up for periodontal status is necessary for individuals with PS, especial for PsA patients.
The pathophysiology underlying the association between periodontitis and psoriatic disease remains hypothetical. The possible causes related to periodontitis in patients with psoriatic disease may include the underlying inflammatory and immunological processes of the diseases. It is well known that interleukin-17 plays a central role in innate immunity, inflammation and osteoclastogenesis all of which have been recognized in the pathogenesis of periodontitis as well as psoriasis (Zenobia & Hajishengalli, 2015). In addition, higher variety and concentrations of oral bacterial DNAs have been detected in synovial fluid compared to serum of PsA patients. In contrast, the predominant periodontal pathogens such as T. forsythensis, P. gingivalis, and Prevotella species were not detected in synovial fluids from patients with PsA (Moen et al., 2006). Bacteria originating from oral cavity might be important factors for the initiation and perpetuation of joint inflammation by oral microorganisms in PsA. However, this speculation still needs further investigation.
The Danish cohort study (Egeberg et al., 2017) used the Health Improvement Network which collected data only about hospital procedures (including hospital-based pharmacological treatment, e.g., with biological therapy) which are coded as treatment procedure codes (Andersen et al., 1999). The data source of current study was the NHIRD which collected data from both general practices and hospitals, allowing a more comprehensive data base and ultimately better generalizability of the findings.
This study still has potential limitations which should be considered. First, the data source of this study was the NHIRD which lacked relevant clinical variables such as laboratory data and pathology findings. In addition, the severity of periodontitis based on (ICD-9-CM) codes without correlated periodontal clinical parameters may not truly reflect the periodontal status. The codes for those patients who had osseous surgery, guided tissue regeneration, or implant due to severe periodontal disease, or extraction due to periodontal disease, could let these patients missed under “patient with periodontitis”. The operator variability in the treatment plan, the potential differences in patient acceptance of recommended treatment, patients’ compliance would also influence the data and hence the results of the study. There may be some patients with undiagnosed periodontitis in the control group. However, propensity score matching was performed in this study to reduce the selection bias and avoid the confounding variates. In addition, we identified the severity of periodontitis through the procedure codes for grading the specific periodontal condition. Third, the data on some potential factors for periodontitis and psoriatic disease, including tobacco use, physical activity, and body-mass index, were not available in the NHIRD dataset. Fourth, since this data is a claim-based of all medical treatments records among beneficiaries, the individuals served with more dental care had higher chance to be diagnosed with periodontitis. In this study, we added the frequency of dental visit within 365 days prior to index date to reduce the misclassification from information bias.
The results of this large-scale epidemiological study indicate that periodontitis is associated with increased risk of psoriatic diseases which was highest in patients with PsA. The mechanisms underlying this association require to be further clarified. Dentists should pay more attention to periodontitis patients with psoriatic disease.
Conclusions
In this study, we found that the incidence rate of periodontitis were higher in patients with PsA. The aHRs of PsA were increased over time in periodontitis patients. As per the result section, majority of the associations noted were borderline and not statistically significant. Taken together, patients with psoriatic disease may benefit regular periodontal evaluation.
Supplemental Information
Observational raw data.
Descriptions of variables in the raw dataset.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ni-Yu Su conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, reviewed drafts of the paper.
Jing-Yang Huang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Chien-Jen Hu and Hui-Chieh Yu conceived and designed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Yu-Chao Chang conceived and designed the experiments, wrote the paper.
Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The Ethics Review Board at the Chung Shan Medical University Hospital approved this study (CS2-15071).
Data Availability
The following information was supplied regarding data availability:
The raw data has been provided in the Supplemental Files.
References
- Andersen et al. (1999).Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The danish national hospital register. A valuable source of data for modern health sciences. Danish Medical Bulletin. 1999;46:263–268. [PubMed] [Google Scholar]
- Chen, Wu & Chang (2017a).Chen CK, Wu YT, Chang YC. Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based matched-cohort study. Alzheimer’s Research & Therapy. 2017a;9:56. doi: 10.1186/s13195-017-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Wu & Chang (2017b).Chen CK, Wu YT, Chang YC. Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: a population-based retrospective matched-cohort study. PeerJ. 2017b;5:e3647. doi: 10.7717/peerj.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng (2003).Cheng TM. Taiwan’s new national health insurance program: genesis and experience so far. Health Affairs. 2003;22:61–76. doi: 10.1377/hlthaff.22.3.61. [DOI] [PubMed] [Google Scholar]
- Ebersole et al. (2017).Ebersole JL, Dawson 3rd D, Emecen-Huja P, Nagarajan R, Howard K, Grady ME, Thompson K, Peyyala R, Al-Attar A, Lethbridge K, Kirakodu S, Gonzalez OA. The periodontal war: microbes and immunity. Periodontology 2000. 2017;75:52–115. doi: 10.1111/prd.12222. [DOI] [PubMed] [Google Scholar]
- Egeberg et al. (2017).Egeberg A, Mallbris L, Gislason G, Hansen PR, Mrowietz U. Risk of periodontitis in patients with psoriasis and psoriatic arthritis. Journal of European Academy of Dermatology and Venereology. 2017;31:288–293. doi: 10.1111/jdv.13814. [DOI] [PubMed] [Google Scholar]
- Fadel et al. (2013).Fadel HT, Flytström I, Calander AM, Bergbrant IM, Heijl L, Birkhed D. Profiles of dental caries and periodontal disease in individuals with or without psoriasis. Journal of Periodontology. 2013;84:477–485. doi: 10.1902/jop.2012.120119. [DOI] [PubMed] [Google Scholar]
- Fry et al. (2015).Fry L, Baker BS, Powles AV, Engstrand L. Psoriasis is not an autoimmune disease? Experimental Dermatology. 2015;24:241–244. doi: 10.1111/exd.12572. [DOI] [PubMed] [Google Scholar]
- Fry et al. (2013).Fry L, Baker BS, Powles AV, Fahlen A, Engstrand L. Is chronic plaque psoriasis triggered by microbiota in the skin? British Journal Dermatology. 2013;169:47–52. doi: 10.1111/bjd.12322. [DOI] [PubMed] [Google Scholar]
- Keller & Lin (2012).Keller JJ, Lin HC. The effects of chronic periodontitis and its treatment on the subsequent risk of psoriasis. British Journal of Dermatology. 2012;167:1338–1344. doi: 10.1111/j.1365-2133.2012.11126.x. [DOI] [PubMed] [Google Scholar]
- Kinane & Bartold (2007).Kinane DF, Bartold PM. Clinical relevance of the host responses of periodontitis. Periodontology 2000. 2007;43:278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Lazaridou et al. (2013).Lazaridou E, Tsikrikoni A, Fotiadou C, Kyrmanidou E, Vakirlis E, Giannopoulou C, Apalla Z, Ioannides D. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. Journal of European Academy of Dermatology and Venereology. 2013;27:967–972. doi: 10.1111/j.1468-3083.2012.04615.x. [DOI] [PubMed] [Google Scholar]
- Moen et al. (2006).Moen K, Brun JG, Valen M, Skartveit L, Eribe EK, Olsen I, Jonsson R. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clinical and Experimental Rheumatology. 2006;24:656–663. [PubMed] [Google Scholar]
- Nakib et al. (2013).Nakib S, Han J, Li T, Joshipura K, Qureshi AA. Periodontal disease and risk of psoriasis among nurses in the United States. Acta Odontologica Scandinavica. 2013;71:1423–1429. doi: 10.3109/00016357.2013.766360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi et al. (2013).Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Identification and Management of Psoriasis and Associated Comorbidity (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. The Journal of Investigative Dermatology. 2013;133:377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- Sezer et al. (2016).Sezer U, Şenyurt SZ, Gündoğar H, Erciyas K, Üstün K, Kimyon G, Kırtak N, Taysı S, Onat AM. Effect of chronic periodontitis on oxidative status in patients with psoriasis and psoriatic arthritis. Journal of Periodontology. 2016;87:557–565. doi: 10.1902/jop.2015.150337. [DOI] [PubMed] [Google Scholar]
- Sharma, Raman & Pradeep (2015).Sharma A, Raman A, Pradeep AR. Association of chronic periodontitis and psoriasis: periodontal status with severity of psoriasis. Oral Diseases. 2015;21:314–319. doi: 10.1111/odi.12271. [DOI] [PubMed] [Google Scholar]
- Skudutyte-Rysstad et al. (2014).Skudutyte-Rysstad R, Slevolden EM, Hansen BF, Sandvik L, Preus HR. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139. doi: 10.1186/1472-6831-14-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai et al. (2011).Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M, Tang CH. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. Journal of Dermatological Sciences. 2011;63:40–46. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Üstün et al. (2013).Üstün K, Sezer U, Kısacık B, Şenyurt SZ, Özdemir EÇ, Kimyon G, Pehlivan Y, Erciyas K, Onat AM. Periodontal disease in patients with psoriatic arthritis. Inflammation. 2013;36(3):665–669. doi: 10.1007/s10753-012-9590-y. [DOI] [PubMed] [Google Scholar]
- Wang, Hsieh & Tsai (2016).Wang TS, Hsieh CF, Tsai TF. Epidemiology of psoriatic disease and current treatment patterns from 2003 to 2013: a nationwide, population-based observational study in Taiwan. Journal of Dermatological Science. 2016;84:340–345. doi: 10.1016/j.jdermsci.2016.08.535. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang PY, Chen YT, Wang YH, Su NY, Yu HC, Chang YC. Malignant transformation of oral submucous fibrosis in Taiwan: a nationwide population-based retrospective cohort study. Journal of Oral Pathology and Medicine. 2017;46:1040–1045. doi: 10.1111/jop.12570. [DOI] [PubMed] [Google Scholar]
- Yang, Keller & Lin (2011).Yang YW, Keller JJ, Lin HC. Medical comorbidity associated with psoriasis in adults: a population-based study. British Journal of Dermatology. 2011;165:1037–1043. doi: 10.1111/j.1365-2133.2011.10494.x. [DOI] [PubMed] [Google Scholar]
- Yang et al. (in press).Yang SF, Wang YH, Su NY, Yu HC, Wei C, Yu CH, Chang YC. Changes in prevalence of pre-cancerous oral submucous fibrosis from 1996–2013 in Taiwan: a nationwide population-based retrospective study. Journal of the Formosan Medical Association. doi: 10.1016/j.jfma.2017.01.012. In Press. [DOI] [PubMed] [Google Scholar]
- Zenobia & Hajishengalli (2015).Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000. 2015;69:142–159. doi: 10.1111/prd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Observational raw data.
Descriptions of variables in the raw dataset.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been provided in the Supplemental Files.