Abstract
Objective
We aimed to investigate the effect of liraglutide treatment on heart function in type 2 diabetes (T2D) patients with subclinical heart failure.
Methods
Randomized open parallel-group trial. 62 T2D patients (45 male) with subclinical heart failure were randomized to either once daily liraglutide 1.8 mg, or glimepiride 4 mg, both add on to metformin 1 g twice a day. Mitral annular systolic (s′) and early diastolic (e′) velocities were measured at rest and during bicycle ergometer exercise, using tissue Doppler echocardiography. The primary endpoint was 18-week treatment changes in longitudinal functional reserve index (LFRIdiastolic/systolic).
Results
Clinical characteristics between groups (liraglutide = 33 vs. glimepiride = 29) were well matched. At baseline left ventricle ejection fraction (53.7 vs. 53.6%) and global longitudinal strain (−15.3 vs. −16.5%) did not differ between groups. There were no significant differences in mitral flow velocities between groups. For the primary endpoint, there was no treatment change [95% confidence interval] for: LFRIdiastolic (−0.18 vs. −0.53 [−0.28, 2.59; p = 0.19]), or LFRIsystolic (−0.10 vs. −0.18 [−1.0, 1.7; p = 0.54]); for the secondary endpoints, there was a significant treatment change in respect of body weight (−3.7 vs. −0.2 kg [−5.5, −1.4; p = 0.001]), waist circumference (−3.1 vs. −0.8 cm [−4.2, −0.4; p = 0.019]), and heart rate (HR) (6.3 vs. −2.3 bpm [−3.0, 14.2; p = 0.003]), with no such treatment change in hemoglobin A1c levels (−11.0 vs. −9.2 mmol/mol [−7.0, 2.6; p = 0.37]), between groups.
Conclusion
18-week treatment of liraglutide compared with glimepiride did not improve LFRIdiastolic/systolic, but however increased HR. There was a significant treatment change in body weight reduction in favor for liraglutide treatment.
Keywords: longitudinal functional reserve index, liraglutide, subclinical heart failure, tissue Doppler echocardiography, type 2 diabetes
Introduction
Liraglutide is a glucagon-like peptide receptor agonist (GLP-1RA) approved for the treatment of type 2 diabetes (T2D). Besides lowering, glucose GLP-1RA have other beneficial effects such as weight reduction and low risk of hypoglycemia. Other than glycemic actions also have gained increasing attention, in which potential beneficial role on cardiovascular (CV) function is of high interest (1).
In the Liraglutide Effect and Action in Diabetes trial, which was launched to assess CV safety in T2D patients with high CV risk, treatment with liraglutide was compared with placebo (2). The time-to-event analysis for the composite endpoint, i.e., the rate of the first occurrence of CV death, nonfatal myocardial infarction, or nonfatal stroke was significantly lower among T2D patients treated with liraglutide, compared with placebo. This effect was mainly driven by a significantly lower rate of CV death. The rates of nonfatal myocardial infarction, nonfatal stroke, and hospitalization of heart failure also were, however, nonsignificantly lower in the liraglutide group than in the placebo group (2).
In T2D, diastolic heart failure is common and precedes overt heart failure (3). The detection of diastolic heart failure may provide an approach both for identifying and to treat high-risk individuals who may benefit from earlier and more active intervention to prevent overt heart failure (3). Several small clinical studies have shown beneficial action on systolic heart function after GLP-1 treatment (4, 5); however, recent studies have not been conclusive (6, 7). Notwithstanding this, studies on the treatment of GLP-1RA in T2D patients with subclinical heart failure are scarce (8, 9).
We aimed to investigate whether 18-week treatment of liraglutide, compared with glimepiride, could improve diastolic and or systolic longitudinal functional reserve index (LFRI), using stress exercise tissue Doppler echocardiography (TDE), in T2D patients with subclinical heart failure.
Materials and Methods
Trial Design
This is an open, assessor-blinded, randomized, controlled, parallel-group trial. Patients with T2D and subclinical heart failure was block randomized (randomly permuted blocks), with sealed envelope, to receive liraglutide, or glimepiride during 18-week treatment. Patients were recruited from the Endocrinology and Cardiology units of two hospitals (Central Hospital, Karlstad and Södersjukhuset, Stockholm) in Sweden. The trial protocol was reviewed and approved by the regional ethics committee at both participating centers and the study has been carried out following the International Conference on Harmonization-Good Clinical Practice guidelines. All patients provided written informed consent before enrollment. Clinical Trial Registration Information URL: http://www.clinicaltrials.gov. Unique identifier: NCT01425580.
Subjects
Type 2 diabetes patients who had a glycated hemoglobin A1c (HbA1c) between 45 and 97 mmol/mol were eligible if they had not been previously treated with GLP-1RA, dipeptidyl peptidase-4 inhibitors, or glimepiride. Patients who met these criteria were invited for echocardiographic screening, in which one of the following criteria had to be fulfilled: left ventricle ejection fraction (LVEF) ≤50%, or evidence of diastolic dysfunction as shown by E/e′ ratio > 15, or with signs of increased left atrial size (>49 ml/m2), or decreased systolic velocity (s′) ≥ 20% in at least two of four segments compared to normal population (10).
The major exclusion criteria were: type 1 diabetes, treatment with glitazones during the last 6 months, treatment with sulfonylureas the last 3 months or insulin treatment within the last month, heart failure classified according to the New York Heart Association classification (NYHA) 3–4, past history of atrial fibrillation or flutter, presence of acute myocarditis or significant valvulopathies, uncontrolled hypertension, severe heart conduction disturbances or ventricular tachyarrhythmia within the last 3 months, unstable angina or myocardial infarction the previous 8-week, estimated glomerular filtration rate <30 ml/min, hemoglobin <90 g/l, BMI >40 kg/m2, severe gastrointestinal disease, history of acute or chronic pancreatitis, malign neoplasia within the last 5 years, current drug or alcohol abuse, and pregnancy.
Outcomes
The primary endpoint was treatment change in either LFRIdiastolic or LFRIsystolic. LFRI have previously been used to detect subclinical LV dysfunction in patients with diabetes compared to non-diabetic controls (11). Briefly, LFRI was constructed by collecting e′ and s′ at rest (e′rest, s′rest), and during bicycle exercise stress test (e′exercise, s′exercise) using the formula: LFRIdiastolic = Δe′ × [1 − (1/e′rest)], and LFRIsystolic = Δs′ × [1 − (1/s′rest)], respectively. Where Δe′ is the difference between e′exercise and e′rest and Δs′ is the difference between s′exercise and s′rest (Note that LFRI has no magnitude).
Secondary endpoints were treatment changes in: LVEF, global longitudinal strain (GLS), E/e′ ratio, body weight, waist circumference, systolic and diastolic blood pressure, heart rate (HR), glycemic control measured with HbA1c, and metabolic markers of: lipids, C-reactive protein (CRP), and N-terminal-pro brain natriuretic protein (NT-proBNP).
Procedures and Treatment
Patients attended a screening visit (visit 1) to assess their eligibility. Patients, if found eligible patients, were scheduled within the next 4 weeks to up-titrate metformin, up to 1 g twice daily, or the maximal tolerated dosage (run-in period). At visit 2, following data were collected: clinical assessment of heart failure according to NYHA classification, anthropometric assessment, blood testing for metabolic parameters, rest TDE, and bicycle ergometer stress test with TDE assessment. Thereafter, patients were randomized to receive the study drug liraglutide, or the comparator treatment glimepiride. For the liraglutide group, the initial dose of liraglutide was 0.6 mg (s.c.) with an up-titration of 0.6 mg every week achieving a final dose of 1.8 mg per day. In the comparator group, 2 mg glimepiride was initially administrated with an up-titration of 1 mg every week reaching a final dose of 4 mg per day. All patients were supplied with a glucometer device (Abbot Contour®). All measurements were performed with drawn capillary blood with test strips calibrated to plasma values. The participants were asked to monitor a 7-point profile glucose curve three days before visit 3 and visit 4, which were telephone-visits, and at the end of treatment (visit 5). At visit 5, the patients were re-tested as for visit 2. All patients were offered a final visit (visit 6) in which the anti-diabetic treatment to be followed the trial was decided. Monitoring drug accountability assessed patients’ treatment compliance.
Clinical Data and Metabolic Parameters
Height, body weight, waist circumference, and clinical data of: diabetes duration, the presence of diabetes-related complications, previous history of CV disease, treatment of hypertension, or dyslipidemia and smoking were collected at visit 2. After 15 min of rest, in a sitting position, measurements of systolic and diastolic blood pressure and HR (systolic and diastolic blood pressure was recorded three times in both arms, simultaneously with measurements of HR), and finally a venipuncture for blood test, were carried out.
Echocardiographic Parameters and Bicycle Ergometer Stress Test
Transthoracic TDE was performed with a Vivid E9 system (GE, Vingmed Ultrasound, Horten, Norway) with a 3.5 MHz phased-array transducer as well as a triplane 3.5 MHz phased-array transducer. Patients were lying in the left lateral decubitus position in quiet respiration. Cine-loops including at least three consecutive heartbeats were saved and transmitted to a work station (EchoPAC-PC version 60; GE Medical systems, Horten, Norway) for off-line analysis. All off-line analyses were made by one investigator (JW) to reduce variability. Measurements of cardiac dimensions were made in accordance with recommendations of the American Society of Echocardiography Committee (10). Left ventricular ejection fraction was assessed by biplane Simpson’s rule, and GLS were calculated for the entire U-shaped length of LV myocardium (10). Diastolic mitral inflow velocities were obtained from apical 4-chamber view. TDE imaging loops were recorded from apical 2- and 4-chamber views. Pulsed TDE imaging loops, and color-coded TDE imaging recordings were saved. Sector width was optimized for adequate myocardial visualization and frame-rate of far more than 100/s was obtained. Long axes myocardial velocities from the septal, lateral, anterior, and inferior basal parts of the left ventricle were extracted from color-coded TDE imaging recordings. The sampling volume was placed close to the mitral annulus. A mean value from three heart cycles was calculated from each site. A graded bicycle ergometer (Monark 839E, Varberg, Sweden) submaximal test with a stepwise increase of 20 W every other minute, until Borg scale of 15 was reached, was used (12). This was performed using a Case 5 system (GE Medical Systems, WI, USA). Immediately after the submaximal bicycle ergometer stress test, patients were re-investigated with TDE in a lying position.
Adverse Events
All adverse effects regardless of relationship to study drug, or protocol procedure were registered, reported and monitored. Glycemic reports and adverse effects were evaluated in every visit and when clinically needed. Regarding hypoglycemia episodes, subjects were asked to record all plasma glucose values ≤3.9 mmol/l when hypoglycemic symptoms had occurred.
Power Calculation
The primary endpoint was an improvement in LFRIdiastolic or LFRIsystolic measured with TDE. The study was designed to detect an absolute increase in LFRI of 0.7 (i.e., a 15% relative increase in e′ or s′, cm/s) from an assumed mean LFRIdiastolic or LFRIsystolic of 4 (11). We needed to investigate 42 patients to demonstrate a mean absolute difference in LFRI of 0.7 with an alpha error of 5% and a beta error of 80%. This calculation was done with a SD for the method assumed to be 0.8 cm/s for e′ or s′, respectively.
Statistical Analysis
Normality of continuous data was checked using Shapiro–Wilk W test and homogeneity of variances was checked using Levene’s test. Normally distributed continuous data were summarized as mean ± SD, continuous data with skewed distribution were summarized as median [first quartile (Q1), third quartile (Q3)], and categorical data are presented as percentage. We used Student’s t-test, Mann–Whitney U test, and Fisher’s exact test to analyze baseline characteristics and treatment change, between groups, for the primary and secondary endpoints. Also, an intention-to-treat analysis was performed after multiple imputations by using iterative Markov chain Monte Carlo method for missing values in multiple continuous variables (13). Five imputed data set were generated and difference between groups was examined using general linear regression model. The estimates from the five data set were combined according to Rubin’s rules. A two-sided p value of less than 0.05 was considered statistically significant. Data has been analyzed using Stata 14.2 (StataCorp LLC, College Station, TX, USA).
Results
Clinical Characteristics and Echocardiographic Parameters
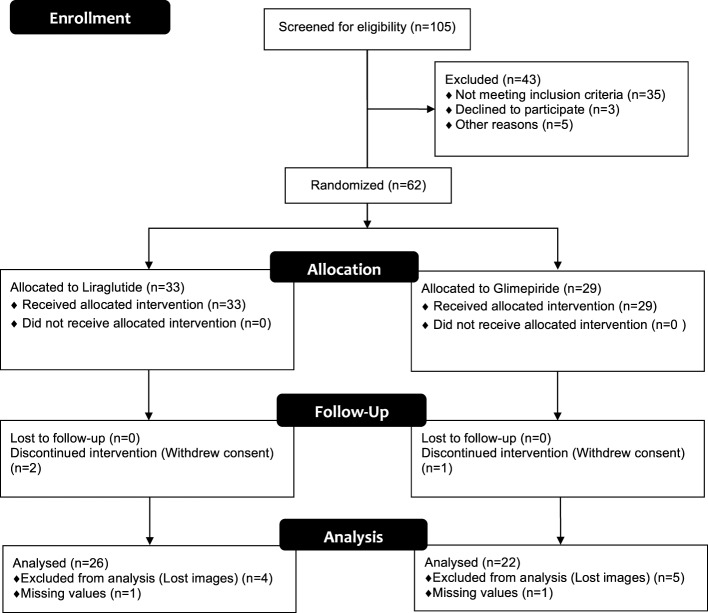
A flow chart of the study is given in Figure 1. In total, 105 patients were screened for eligibility, in which 62 met the criteria and were randomized to liraglutide or glimepiride. Due to technical hitches; i.e., TDE images of 9 patients were not correctly spared, and for 2 more patients there were missing TDE data for s′ and e′ at exercise, respectively, and to dropouts; i.e., 3 patients withdrew their informed consent; 2 due to nausea (liraglutide group), and 1 due to lack of time (glimepiride group) 26 in the liraglutide, and 22 in the glimepiride fulfilled the protocol and analyzed per-protocol (Figure 1). After multiple imputations, an intention-to-treat analysis (n = 62 patients) was also performed. All clinical baseline characteristics and echocardiographic parameters are given in Table 1. All data, except triglycerides, were normally distributed. Groups were well matched without any significant differences between them (Table 1). According to NYHA classification, there were three patients classified as NYHA II, all other patients were classified as NYHA I. This was further reflected by the mean of EF, which was of 54%, both groups (Table 2).
Figure 1.
Flow chart for the study groups. 105 patients were screened, whereas 62 patients were eligible for the study, of these 33 vs. 29 were randomized to liraglutide vs. glimepiride, respectively. Due to technical hitches and dropouts, there were 26 in the liraglutide vs. 22 in glimepiride group, who were analyzed per-protocol, i.e., full data set.
Table 1.
Basal clinical characteristics and echocardiographic parameters of the studied T2DM patients.
| Basal clinical characteristics | Liraglutide (n = 33) | Glimepiride (n = 29) | p Value |
|---|---|---|---|
| Age, years | 61 (7.6) | 63 (6.8) | 0.240a |
| Male sex | 24 (72.7) | 21 (72.4) | 1.000b |
| Diabetes duration, years | 5 (1, 10) | 3 (1, 7) | 0.368c |
| Smoking | 3 (9.1) | 4 (13.8) | 0.852b |
| BMI, kg/m2 | 30.5 (4.4) | 29.0 (3.2) | 0.152a |
| Body weight, kg | 91.8 (15.9) | 89.0 (9.9) | 0.411a |
| Waist circumference, cm | 109.0 (13.0) | 106.3 (9.7) | 0.366a |
| Mean systolic BP, mmHg | 139 (17) | 137 (12) | 0.541a |
| Mean diastolic BP, mmHg | 83 (9) | 83 (8) | 0.719a |
| eGFR, ml/min/1.72 m2 | 88.3 (15.0) | 87.4 (13.1) | 0.654 |
| Complications | |||
| Hypertension | 29 (87.9) | 21 (72.4) | 0.224b |
| Hyperlipidemia | 25 (75.8) | 23 (79.3) | 0.980b |
| Coronary artery disease | 10 (30.3) | 11 (37.9) | 0.714b |
| Stroke | 1 (3) | 2 (6.9) | 0.902b |
| Proliferative retinopathy | 1 (3) | 1 (3.4) | 1.000b |
| Treatment | |||
| Antiplatelet therapy | 11 (33.3) | 12 (41.4) | 0.696b |
| Anticoagulant treatment | 3 (9.1) | 1 (3.4) | 0.714b |
| ACE inhibitors/ARB blockers | 25 (75.8) | 20 (69.0) | 0.754b |
| Beta-blockers | 14 (42.4) | 13 (44.8) | 1.000b |
| Calcium inhibitors | 13 (39.4) | 10 (34.5) | 0.894b |
| Diuretics | 11 (33.3) | 6 (20.7) | 0.408b |
| Statins | 22 (66.7) | 24 (82.8) | 0.248b |
| Biochemical parameters | |||
| HbA1c, mmol/mol | 54 (50, 60) | 50 (49, 54) | 0.036c |
| Triglycerides, mmol/l | 2.0 (1.4, 2.6) | 1.5 (1.0, 2.2) | 0.029c |
| Total cholesterol, mmol/l | 4.4 (4.0, 6.0) | 4.5 (3.7, 4.8) | 0.370c |
| LDL-cholesterol, mmol/l | 2.8 (1.2) | 2.5 (1.0) | 0.440a |
| HDL-cholesterol, mmol/l | 1.1 (0.3) | 1.2 (0.3) | 0.417a |
| CRP, mg/l | 2.0 (1.0, 4.0) | 1.0 (0.9, 2.4) | 0.016c |
| NT-proBNP, mg/l | 63 (35, 104) | 50 (35, 146) | 0.621c |
| Echocardiographic parameters | Liraglutide (n = 26) | Glimepiride (n = 22) | p Value |
| LV and atrial dimensions | |||
| LVSV, ml | 80.2 (17.1) | 76.4 (13.4) | 0.376a |
| LVED diameter, mm | 51.0 (6.5) | 48.0 (6.3) | 0.096a |
| LVES diameter, mm | 37.8 (8.7) | 33.8 (8.1) | 0.097a |
| Left atrial volume, ml | 73.8 (23.9) | 54.3 (16.6) | 0.013a |
| Systolic function | |||
| LVEF at rest, % | 53.6 (11.5) | 53.7 (9.2) | 0.994 |
| GLS at rest, % | −15.3 (4.3) | −16.5 (3.7) | 0.319a |
| s′ at rest, cm/s | 5.7 (1.1) | 5.7 (1.0) | 0.918a |
| s′ during exercise, cm/s | 8.6 (2.1) | 9.4 (1.8) | 0.132a |
| LFRIsystolicd | 2.4 (1.1) | 3.0 (1.3) | 0.058a |
| Diastolic function | |||
| E-wave at rest, cm/s | 66.9 (14.1) | 66.8 (14.1) | 0.987 |
| e′ at rest, cm/s | 5.5 (1.1) | 5.5 (1.2) | 0.929a |
| E/e′ at rest | 12.5 (3.4) | 12.3 (2.7) | 0.880a |
| e′ during exercise, cm/s | 8.0 (1.7) | 8.7 (1.7) | 0.127a |
| LFRIdiastolicd | 2.0 (1.1) | 2.6 (1.1) | 0.625a |
Quantitative data are mean (SD) or median (Q1, Q3), and categorical data are n (%).
aStudent’s t-test was used.
bDoubled one-sided p value of Fisher’ exact test was used.
cMann–Whitney U test was used.
dNote that LFRI has no magnitude.
ARB, angiotensin receptor blockers; BP, blood pressure; CRP, C-reactive protein; E, early diastolic peak velocity; e′, early diastolic mitral annulus velocity; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HbA1c, hemoglobin A1c; HR, heart rate; NT-proBNP, N-terminal pro b-type natriuretic peptide; LFRI, longitudinal function reserve index; LVEF, left ventricular ejection fraction; LVSV, left ventricular stroke volume; LVED, left ventricular end-diastolic; LVES, left ventricular end-systolic; s′, peak systolic annular velocity.
Table 2.
Treatment change of secondary outcomes during 18-week treatment.
| Clinical characteristics | Liraglutide (n = 31) | Glimepiride (n = 28) | p Value |
|---|---|---|---|
| Body weight, kg | −3.5 (−6,0, −1.1) | 0.5 (−1.2, 2.1) | 0.001a |
| Waist circumference, cm | −3.1 (2.8) | −0.8 (4.4) | 0.019b |
| Mean systolic BP, mmHg | −3.0 (−13.0, 3.0) | −0.5 (−8.5, 8.0) | 0.176a |
| Mean diastolic BP, mmHg | −0.3 (8.0) | −0.9 (7.9) | 0.761b |
| HR at rest, bpm | 6.3 (9.6) | −2.3 (9.4) | 0.004b |
| HR during exercise, bpm | −1.0 (−6.0, 8.0) | −4.5 (−12.0, 1.0) | 0.150a |
| HbA1c, mmol/mol | −10.0 (−18.0, −4.0) | −5.5 (−12.5, −3.0) | 0.112a |
| Triglycerides, mmol/l | −0.2 (0.4) | −0.1 (0.8) | 0.492b |
| LDL, mmol/l | −0.1 (−0.5, 0.1) | −0.2 (−0.5, 0.1) | 0.994a |
| HDL, mmol/l | 0.1 (0.0, 0.2) | 0.0 (−0.1, 0.1) | 0.386a |
| CRP, mmol/l | 0.0 (−1.0, 0.1) | 0.0 (0.0, 0.2) | 0.351a |
| NT-proBNP, mg/l | −0.0 (−25.0, 11.0) | 0.0 (−15.5, 24.5) | 0.616a |
| Echocardiographic parameters | Liraglutide (n = 26) | Glimepiride (n = 22) | p Value |
| LVEF at rest, % | −2.1 (4.6) | −0.6 (5.9) | 0.367 |
| GLS at rest, % | 0.0 (2.4) | 0.6 (1.8) | 0.450b |
| E/e′ at rest | −0.5 (3.1) | −0.4 (2.4) | 0.867b |
Data are mean (SD) or median (Q1, Q3).
aMann–Whitney U test was used.
bStudent’s t-test was used.
BP, blood pressure; CRP, C-reactive protein; E, early diastolic peak velocity; E, early diastolic peak velocity; e′, early diastolic mitral annulus velocity; GLS, global longitudinal strain; HbA1c, hemoglobin A1c; HR, heart rate; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro b-type natriuretic peptide.
Bicycle Ergometer Stress Echocardiography
The perceived exertion was set to 15 according to the Borg scale (12). The workload capacity between visit 2 and visit 5 did not differ within groups (liraglutide 115.9 ± 29.4 vs. 115.2 ± 30.4 W, ns; and glimepiride 124.4 ± 29.5 vs. 123.1 ± 29.1 W, ns).
Primary Outcome
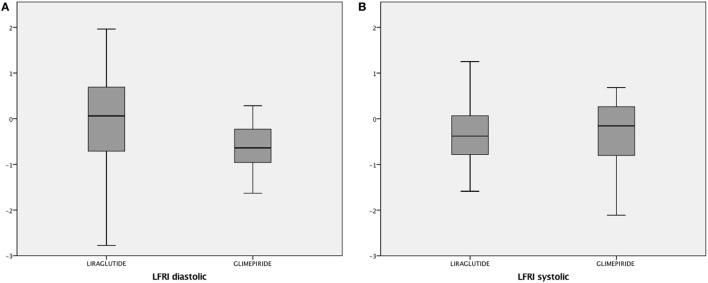
There was no treatment change between groups for LFRIdiastolic or LFRIsystolic (Figure 2). After imputations (intention-to-treat analysis), results did not change from the per-protocol analysis (data not shown).
Figure 2.
Boxplot representing treatment change, during 18-week treatment of liraglutide vs. glimepiride, for the primary endpoint of diastolic and systolic longitudinal functional reserve index (LFRIdiastolic and LFRIsystolic). Note that LFRI has no magnitude. Shapiro–Wilk W test for normality; change in diastolic LFRI liraglutide group, p = 0.733; change in diastolic LFRI glimepiride group, p = 0.273; change in systolic LFRI liraglutide group, p = 0.830; change in systolic LFRI glimepiride group, p = 0.182.
Secondary Outcome
All predefined secondary outcomes are given in Table 2. There was a significant treatment change in body weight reduction, and a decrease in waist circumferences in favor of liraglutide treated group. In both groups, there was a robust decrease in HbA1c, however, nonsignificant treatment change for the same (Table 2). There was an increased HR (at rest) in patients treated with liraglutide, compared with glimepiride, without such difference at exercise (Table 2). There was no treatment change for LVEF, GLS, or E/e′, between groups (Table 2). Nor were there any treatment change in CRP levels, or blood lipids, between groups (Table 2). After multiple imputation (intention-to-treat analysis), results did not change from per-protocol analysis (data not shown).
Adverse Events
We observed the following non-serious gastrointestinal side effects with liraglutide: nausea 8, diarrhea 3, vomiting 1, constipation 1, dizziness 1, urinary tract infection 1, headache 1, and orthostatic reaction 1; and with glimepiride: headache 2, hematuria 1, diarrhea 1, vomiting 1, itching 1, and gingival hyperplasia 1. All adverse events were transient and mild but for two subjects with nausea leading to discontinuation of the study. One severe adverse event (arm fracture leading to surgery) occurred in the liraglutide group. Severe hypoglycemia did not occur in any of the treatment groups. Hypoglycemia occurred in 6 subjects (n = 33, 18%) of the patients treated with liraglutide, and in 17 subjects (n = 29, 59%) of the patients treated with glimepiride. All the events were mild and documented by self-measured blood glucose.
Discussion
In the present study, we found that 18-week treatment of liraglutide, compared with glimepiride, did not improve LFRIdiastolic or LFRIsystolic in T2D patients with subclinical heart failure. There was a robust decrease in HbA1c, in both treated groups, with no treatment change between groups. Liraglutide significantly reduced body weight and waist circumferences, and significantly increased HR, compared with glimepiride.
One novelty in our study was that it was conducted on T2D patients with diastolic dysfunction. The spectrum of diabetic heart disease involves a progression from the normal heart to subclinical diastolic dysfunction followed by clinically overt symptomatic heart failure (3). In our study, patients had no overt symptoms of heart failure, and their mean LVEF was above 50%; although patients had signs of diastolic dysfunction and therefore the development of heart failure with preserved EF (HF-PEF) (13). While a recent published placebo-controlled crossover study failed to show any improvement of systolic function in newly diagnosed T2D patients treated with liraglutide (14), there is some evidence that this drug may have a role in cardiac remodeling diastolic heart function (15, 16). It also was recently demonstrated, in a prospective observational study in patients with T2D, that 6 months liraglutide treatment was associated with a significant improvement in diastolic function concomitant with body weight reduction, although with no correlation between the improvement of the diastolic function and body weight loss (17). The lack of an adequate comparable parallel group in the above study was a major weakness and therefore not easy to interpret (17).
Current study was an open randomized parallel-group study where glimepiride was used as the comparator. This was chosen due to its equal glycemic reduction to liraglutide (18), and that glimepiride may be neutral for heart function (19). In the present study, groups were well matched showing no difference at baseline for the studied parameters; however, there was no treatment change between groups for the primary outcome, except for body weight and waist circumference, which mostly was expected (18). There was an increase in HR in the liraglutide treated group; which also has been observed for the treatment of T2D with GLP-1RA (20), although with some mixed results between types of GLP-1RA (21–23). Our study results contrast with a recent study where 33 T2D patients were treated with liraglutide for 16 weeks compared with placebo add on to supervised exercise. In that study, there was no change in HR between groups; however diastolic function upon supervised exercise was improved in the placebo group and blunted in the liraglutide treated T2D patients (8). In our study, we used bicycle ergometer exercise to sharp the sensitivity of our method, and not for the treatment of diastolic function, therefore studies are not easy to compare. Moreover, in our study, it was an increase in HR (at rest) in the liraglutide treated group that may have influenced our primary outcome. Despite, this there are yet no large clinical studies demonstrating any negative action with GLP-1RA on CV risks (2, 24, 25), or hospitalization for heart failure (26). Since GLP-1 receptors only are located near the sinus atrial node (27), the action of increased HR from GLP-1RA may be of direct nature, although a sympathetic influence from GLP-1RA cannot be ruled out.
There are several indirect actions reported on heart function evoked by GLP-1RA (28). These actions may include alterations in the substrate of fatty acids and glucose delivered to the heart, decreased low-grade inflammation in the myocardium, and a reduction in systolic blood pressure (28). T2D commonly associates with the metabolic syndrome in which obesity, elevated levels of triglycerides, low levels of high-density lipoprotein cholesterol, hypertension, and low-grade inflammation (reflected by an increase in CRP levels) are involved. These are all risk factors that may affect myocardial structure and heart function and subsequent development of HF-PEF in subjects with T2D (29). In the current study, T2D patients had the metabolic syndrome, although treated groups were well matched in terms of clinical characteristics and parameters in the metabolic syndrome. Hyperglycemia, reflected by elevated HbA1c, is also an important risk factor for heart failure (30). In the present study, HbA1c was robustly decreased in both groups, with no treatment change between groups; however, treatment change was observed in body weight reduction, in favor for liraglutide, which might have biased our results on heart function (31), in which it should rather have improved diastolic function in the liraglutide treated group (32).
Although mechanisms behind the CV benefits of the LEADER study remains elusive (2), it is suggested related to the modified atherosclerotic process rather than prompt hemodynamic actions, as suggested for the EMPA-REG OUTCOME trial (33). Recent large well-conducted randomized studies have demonstrated neutral effect with GLP-1RA on stabile heart failure (7), or advanced heart failure (34), in patients with and without diabetes. Among patients hospitalized with heart failure, liraglutide treatment did not lead to greater clinical stability (6), a finding concordant with some other small clinical studies showing no beneficial effects from GLP-1RA on heart failure (8, 35). A recent double-blind placebo-controlled crossover study, which used dobutamine stress TDE, to investigate changes in systolic heart function in newly diagnosed T2D patients with coronary artery disease, also failed to show any beneficial action from liraglutide treatment compared with placebo treatment (14). There are, however, some differences between our and recent studies (8, 14, 17, 35) for the investigation of GLP-1RA treatment in T2D patients with heart failure which has to be discussed. First, we investigated T2D patients with HF-PEF with expectation to restore early signs of heart failure; this is in contrast to studies using GLP-1RA treatment into patients with more advanced heart failure (6, 7, 35, 36), or solely systolic heart function (14). Second, we investigated the assessments of LFRI to investigate whether treatment with liraglutide may restore HF-PEF in T2D patients (11). Third, we used sulfonylurea, instead of placebo, to rule out difference in glycemic control as a confounding factor for the primary outcome (32).
In the present study, there are several limitations that have to be discussed. Due to technical hitches and dropouts, 80% (50 out of 62) of patients had full data and was subsequently analyzed per-protocol. Despite this, we believe that current study was powered for our primary endpoint. After multiple imputations (intention-to-treat analysis), the results were not changed supporting the per-protocol analysis. Factors such as transducer position, echocardiography operators (two different sites) may have contributed to some difference. However, due to our pre-specified protocol for the TDE measurements (which also was reflected by the well-matched workload in stress exercise echocardiography between visit 2 and visit 5) and with one blinded off-line investigator make these differences of less concern. Even though the groups were well matched, and well controlled during the study, they might simply have been to healthy for the detection of treatment change, e.g., short diabetes duration, low prevalence of smoking, normal eGFR, and properly controlled hypertension. Also, one major limitation might be too short follow-up study period to detect any treatment change between groups.
In conclusion, 18-week liraglutide treatment did not improve diastolic or systolic LFRI, however increase HR, in T2D patients with subclinical heart failure. There was a robust decrease in HbA1c with no treatment change between groups, and a significant treatment change in body weight reduction in favor for liraglutide treatment. Evidence of GLP-1RA treatment on heart failure are mixed. Since LEADER trial show beneficial effect on CV outcome further clinical studies should be launched to investigate more in detail what mechanisms are behind these results.
Ethics Statement
All patients provided written informed consent to participate in the study, which was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The research project was approved by the Ethics Committee of Stockholm Sweden, and the Swedish Medical Products Agent. Moreover, the study was registered in http://www.clinicaltrials.gov. Unique identifier: NCT01425580.
Author Contributions
All authors contributed to the study conception and design. TN and IS analyzed data and wrote the first draft of the paper. All authors commented and took part of the revision of the paper. TN is the guarantor of the manuscript.
Conflict of Interest Statement
TN has received unrestricted grants from AstraZenca and consultancy fees from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Merck, and Sanofi-Aventis. JJ has received consultancy fees from Boehringer Ingelheim, Eli Lilly, NovoNordisk, Medtronic, Menarini Diagnostics, MSD, and Roche. No other disclosures were reported. The reviewer JB and handling editor declared their shared affiliation.
Acknowledgments
TN thanks William Heinessen and Sara Rönnås for great housing hospitality while writing the draft of the manuscript. We thank Dr. Hans Pettersson for excellent statistical advice.
Footnotes
Funding. This study was investigator-initiated and investigator-designed. The investigators received an unrestricted grant from Novo Nordisk A/S, but the company was not involved in data collection, study management, analysis, or interpretation of data. Nor was the company involved in the decisions regarding the submission of the manuscript. Financial support was provided through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institutet, and by the Swedish Society for Medical Research, the Swedish Society of Medicine, the Swedish Heart and Lung Foundation, and NovoNordisk. None of the funding sources had any involvement in the study design; collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the paper.
References
- 1.Nystrom T. The potential beneficial role of glucagon-like peptide-1 in endothelial dysfunction and heart failure associated with insulin resistance. Horm Metab Res (2008) 40:593–606. 10.1055/s-0028-1082326 [DOI] [PubMed] [Google Scholar]
- 2.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med (2016) 375:311–22. 10.1056/NEJMoa1607141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol (2010) 55:300–5. 10.1016/j.jacc.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation (2004) 109:962–5. 10.1161/01.CIR.0000120505.91348.58 [DOI] [PubMed] [Google Scholar]
- 5.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail (2006) 12:694–9. 10.1016/j.cardfail.2006.08.211 [DOI] [PubMed] [Google Scholar]
- 6.Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA (2016) 316:500–8. 10.1001/jama.2016.10260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hanselmann A, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail (2017) 19:69–77. 10.1002/ejhf.657 [DOI] [PubMed] [Google Scholar]
- 8.Jorgensen PG, Jensen MT, Mensberg P, Storgaard H, Nyby S, Jensen JS, et al. Effect of exercise combined with glucagon-like peptide-1 receptor agonist treatment on cardiac function: a randomized double-blind placebo-controlled clinical trial. Diabetes Obes Metab (2017) 19:1040–44. 10.1111/dom.12900 [DOI] [PubMed] [Google Scholar]
- 9.Nathanson D, Zethelius B, Berne C, Lind L, Andren B, Ingelsson E, et al. Plasma levels of glucagon like peptide-1 associate with diastolic function in elderly men. Diabet Med (2011) 28:301–5. 10.1111/j.1464-5491.2010.03207.x [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr (2015) 28:1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 11.Ha JW, Lee HC, Kang ES, Ahn CM, Kim JM, Ahn JA, et al. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart (2007) 93:1571–6. 10.1136/hrt.2006.101667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc (1982) 14:377–81. 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 13.Senni M, Paulus WJ, Gavazzi A, Fraser AG, Diez J, Solomon SD, et al. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J (2014) 35:2797–815. 10.1093/eurheartj/ehu204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumarathurai P, Anholm C, Nielsen OW, Kristiansen OP, Molvig J, Madsbad S, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide on systolic function in patients with coronary artery disease and type 2 diabetes: a randomized double-blind placebo-controlled crossover study. Cardiovasc Diabetol (2016) 15:105. 10.1186/s12933-016-0425-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson E, Tate M, Lockhart S, McPeake C, O’Neill KM, Edgar KS, et al. Metabolically-inactive glucagon-like peptide-1(9-36)amide confers selective protective actions against post-myocardial infarction remodelling. Cardiovasc Diabetol (2016) 15:65. 10.1186/s12933-016-0386-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Anderson C, Broyde A, Polizzi C, Fernandez R, Baron A, et al. Glucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol (2010) 9:76. 10.1186/1475-2840-9-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saponaro F, Sonaglioni A, Rossi A, Montefusco L, Lombardo M, Adda G, et al. Improved diastolic function in type 2 diabetes after a six month liraglutide treatment. Diabetes Res Clin Pract (2016) 118:21–8. 10.1016/j.diabres.2016.04.046 [DOI] [PubMed] [Google Scholar]
- 18.Nauck M, Frid A, Hermansen K, Thomsen AB, During M, Shah N, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab (2013) 15:204–12. 10.1111/dom.12012 [DOI] [PubMed] [Google Scholar]
- 19.Hung YC, Lin CC, Wang TY, Chang MP, Sung FC, Chen CC. Oral hypoglycaemic agents and the development of non-fatal cardiovascular events in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev (2013) 29:673–9. 10.1002/dmrr.2444 [DOI] [PubMed] [Google Scholar]
- 20.Lorenz M, Lawson F, Owens D, Raccah D, Roy-Duval C, Lehmann A, et al. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol (2017) 16:6. 10.1186/s12933-016-0490-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol (2010) 9:6. 10.1186/1475-2840-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumarathurai P, Anholm C, Larsen BS, Olsen RH, Madsbad S, Kristiansen O, et al. Effects of liraglutide on heart rate and heart rate variability: a randomized, double-blind, placebo-controlled crossover study. Diabetes Care (2017) 40:117–24. 10.2337/dc16-1580 [DOI] [PubMed] [Google Scholar]
- 23.Dejgaard TF, Johansen NB, Frandsen CS, Asmar A, Tarnow L, Knop FK, et al. Effects of liraglutide on cardiovascular risk factors in patients with type 1 diabetes. Diabetes Obes Metab (2017) 19:734–8. 10.1111/dom.12841 [DOI] [PubMed] [Google Scholar]
- 24.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med (2016) 375:1834–44. 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med (2015) 373:2247–57. 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- 26.Filion KB, Azoulay L, Platt RW, Dahl M, Dormuth CR, Clemens KK, et al. Study of incretin-based drugs and heart failure. N Engl J Med (2016) 374:1145–54. 10.1056/NEJMoa1506115 [DOI] [PubMed] [Google Scholar]
- 27.Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology (2014) 155:1280–90. 10.1210/en.2013-1934 [DOI] [PubMed] [Google Scholar]
- 28.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev (2012) 33:187–215. 10.1210/er.2011-1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Heerebeek L, Somsen A, Paulus WJ. The failing diabetic heart: focus on diastolic left ventricular dysfunction. Curr Diab Rep (2009) 9:79–86. 10.1007/s11892-009-0014-9 [DOI] [PubMed] [Google Scholar]
- 30.Lind M, Olsson M, Rosengren A, Svensson AM, Bounias I, Gudbjornsdottir S. The relationship between glycaemic control and heart failure in 83,021 patients with type 2 diabetes. Diabetologia (2012) 55:2946–53. 10.1007/s00125-012-2681-3 [DOI] [PubMed] [Google Scholar]
- 31.Verges B, Bonnard C, Renard E. Beyond glucose lowering: glucagon-like peptide-1 receptor agonists, body weight and the cardiovascular system. Diabetes Metab (2011) 37:477–88. 10.1016/j.diabet.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 32.Erdogan D, Akcay S, Yucel H, Ersoy IH, Icli A, Kutlucan A, et al. The effects of good glycaemic control on left ventricular and coronary endothelial functions in patients with poorly controlled Type 2 diabetes mellitus. Clin Endocrinol (Oxf) (2015) 82:388–96. 10.1111/cen.12520 [DOI] [PubMed] [Google Scholar]
- 33.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med (2015) 373:2117–28. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 34.Lepore JJ, Olson E, Demopoulos L, Haws T, Fang Z, Barbour AM, et al. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail (2016) 4:559–66. 10.1016/j.jchf.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 35.Halbirk M, Norrelund H, Moller N, Holst JJ, Schmitz O, Nielsen R, et al. Cardiovascular and metabolic effects of 48-h glucagon-like peptide-1 infusion in compensated chronic patients with heart failure. Am J Physiol Heart Circ Physiol (2010) 298:H1096–102. 10.1152/ajpheart.00930.2009 [DOI] [PubMed] [Google Scholar]
- 36.Nathanson D, Ullman B, Lofstrom U, Hedman A, Frick M, Sjoholm A, et al. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia (2012) 55:926–35. 10.1007/s00125-011-2440-x [DOI] [PubMed] [Google Scholar]