Abstract
Anthocyanins are effective antioxidants but they have also been proposed to have other biological activities independent of their antioxidant capacities that produce health benefits. Examples range from inhibition of cancer cell growth in vitro, induction of insulin production in isolated pancreatic cells, reduction of starch digestion through inhibition of a-glucosidase activity, suppression of inflammatory responses as well as protection against age-related declines in cognitive behavior and neuronal dysfunction in the central nervous system. However, to achieve any biological effect in a specific tissue or organ, anthocyanins must be bioavailable; i.e. effectively absorbed from the gastrointestinal tract (GIT) into the circulation and delivered to the appropriate location within the body. In this study, we assess the stability of anthocyanins from commercial Black currant (Ribes nigrum L.) juice using an in vitro digestion procedure that mimics the physiochemical and biochemical conditions encountered in the gastrointestinal tract (GIT). The main objective of this work was the evaluation of stability of anthocyanins during in vitro digestion in gastric and intestinal fluid regarding whether appropriate enzyme (pepsin or pancreatin) was added or not. Anthocyanins present in commercial black currant juice remain stable during in vitro digestion in gastric fluid regardless whether pepsin was added into the medium or not. Also, they remain stable during in vitro digestion in simulated intestinal fluid without pancreatin. The stability studies of anthocyanins in the intestinal fluid containing pancreatin indicated reduced stability, which also mainly contribute to slight reduction of total anthocyanins content (1,83%-) in commercial black currant juice.
Keywords: anthocyanins, stability, black currant, gastrointestinal digestion
INTRODUCTION
Anthocyanins belong to the flavonoid group of polyphenolic compounds and are responsible for the red, purple and blue hues present in plant organs such as fruits, flowers, grains and leaves as well as in products made from those sources (1). Pelargonidin, cyanidin, peonidin, delphinidin, petunidin, and malvidin are the six common anthocyanidins found in nature. Their structures may be altered by glycosidic substitution (glucose, galactose, rhamnose, xylose, and arabinose) at the 3 and 5 positions in the A and C rings. They are industrially important natural food colorings (2). Additional variations occur by acylation of the sugar groups with acids. Acetic acid, p-coumaric acid, caffeic acid, malonic acid, sinapic acid, ferulic acid, oxalic acid, and succinic acid are some of the commonly found acylating groups (3, 4).
Anthocyanins are effective antioxidants (2, 5) but they have also been proposed to have other biological activities that are independent of their antioxidative capacities and produce health benefits. Examples range from inhibition of cancer cell growth in vitro (6), induction of insulin production in isolated pancreatic cells (7), reduction of starch digestion through inhibition of a-glucosidase activity (8), suppression of inflammatory responses (9) as well as protection against age-related declines in cognitive behavior and neuronal dysfunction in the central nervous system (10).
However, to achieve any biological effect in a specific tissue or organ, anthocyanins must be bioavailable; i.e. effectively absorbed from the gastrointestinal tract (GIT) into the circulation and delivered to the appropriate location within the body. Oral intake of anthocyaninrich fruits, extracts or pure anthocyanins has proved to have beneficial effects in preventing or suppressing ailments in vivo (11, 12). Studies of oral administration of anthocyanins have confirmed increased antioxidant status of the serum (11, 13, 14) but this is usually accompanied by very low uptake of anthocyanins into the serum (<1% of dose) (15, 16, 17)) and correspondingly low levels of urinary excretion as intact or conjugated forms. The apparent low bioavailability of anthocyanins seems to cast doubt on their ability to exert their proposed beneficial effects throughout the body.
Assessment of true bioavailability of any class of phytochemicals requires data concerning their absorption, metabolism, tissue and organ distribution and excretion (18). Such studies carried out in animals or human subjects are complex, expensive and raise moral and ethical questions. The relative stability of the anthocyanins under GIT conditions is crucial for the bioavailability of these compounds, as it will determine the pool size for whatever active mechanisms are present in the stomach (19) or the small intestine (20).
In this study, we assess the stability of anthocyanins from Black currant (Ribes nigrum L.) juice using an in vitro digestion procedure that mimics the physiochemical and biochemical conditions encountered in the gastrointestinal tract (GIT). The main objective of this work was the evaluation of stability of anthocyanins during in vitro digestion in gastric and intestinal fluid regarding whether appropriate enzyme (pepsin or pancreatin) was added or not.
MATERIALS AND METHODS
Samples
Juice sample, that was not blend of different fruits, and that represented an array of anthocyanin containing products available at a local market was purchased, brought to the laboratory, aliquoted and stored at -20°C until analyzed.
Chemicals and Reagents
The used reagents were all of analytical grade, unless otherwise stated. Glacial acetic acid (98%), orthophosphoric acid (85%) and sodium chloride were provided by Carlo Erba; monobasic potassium phosphate, concentrated hydrochloric acid (37%) and sodium hydroxide were provided by Merck. Acetonitrile was HPLC grade (Sigma-Aldrich), pepsin from hog stomach (Fluka), pancreatin from porcine pancreas (Sigma).
In vitro digestion procedure
The diluted black currant juice was subjected to successive in vitro gastric and pancreatic digestion, following the procedure:
TEST 1: An aliquot of juice was diluted with hydrochloric acid solution, supplemented with sodium chloride and adjusted to pH 1,2 (100 ml of juice + 400 ml hydrochloric acid solution at pH 1,2 + NaCl); chromatograms were analyzed before and after dissolution procedure (1 hour at 370C, paddle method; stirring speed 100 rpm)
EST 2: An aliquot of juice was diluted with hydrochloric acid solution, supplemented with sodium chloride and pepsin and adjusted to pH 1,2 (100 ml of juice + 400 ml of hydrochloric acid solution at pH 1,2 + NaCl+ pepsin); chromatograms were analyzed before and after dissolution procedure (1 hour at 370C, paddle method; stirring speed 100 rpm)
TEST 3: An aliquot of juice was diluted with phosphate buffer at pH 7,5 (100 ml of juice + 400 ml of phosphate buffer at pH 7,5); chromatograms were analyzed before and after dissolution procedure (2 hours at 370C, paddle method; stirring speed 100 rpm)
TEST 4: An aliquot of juice was diluted with phosphate buffer at pH 7,5 and pancreatin (100 ml of juice + 400 ml of phosphate buffer at pH 7,5 + pancreatin); chromatograms were analyzed before and after dissolution procedure (2 hours at 370C, paddle method; stirring speed 100 rpm)
The tests were performed using USP apparatus 2, Van Kel VK 7010 dissolution tester (Van Kel, Cary). The dissolution apparatus was maintained at 37°C throughout the experiment. Test samples were filtered using a 0,45 μm nylon syringe filters for HPLC (Cronus). All tests were performed in triplicate. Prior to use, the dissolution media and juice samples were also equilibrated at 37°C. Samples were drawn at the following points: at the beginning (immediately thereafter stirring was started) and at the end of test procedure (after one hour for testing in gastric conditions with/without pepsin added and after two hours for testing in intestinal conditions with/without pancreatin added).
HPLC analysis
Chromatographic analysis (gradient HPLC analysis) of anthocyanins was done according to the method of Lee at all. (5) using a Zorbax StableBond-C18 column (250mmx4,6 mmx5 μσι). The analysis was performed on an HPLC system composed of a pump, injection valve, autosampler and variable (DAD) wavelength detector (Shimadzu). Mobile phase A was 100% acetonitrile, while mobile phase B consisted of 1% phosphoric acid and 10% acetic acid (glacial) (v/v) in deionized water. The material used as a reference sample was native juice prepared from fresh black currant fruits using pressure procedure. The program was as follows: 0-25 min linear gradient from 98% to 80% B; 25 - 30 min linear gradient from 80% to 60% B; 30 - 32 min linear gradient from 60% to 98% B and 32-37 min linear gradient 98% B. The flow rate was 1,0 ml/min, the injection volume 25 μ¡, the column temperature 40°C and the detection wavelength 520 nm. Wavelength selection was in accordance with maximum absorption for anthocyanins. Media was selected with the intention to determine possible influence of gastric and intestinal fluids on stability of anthocyanins in acidic/basic conditions in presence/ absence of digestive enzymes (pepsin and pancreatin).
RESULTS AND DISCUSSION
Stability of anthocyanins in commercial black currant juice was analyzed on the basis of four characteristic peaks which were observed in native black currant juice chromatogram (Figure 1.). The native juice was prepared from fresh black currants using pressure procedure. This chromatogram was used as fingerprint chromatogram of black currant juice. The same peaks were observed in chromatograms of the analyzed commercial juice, which conveniently served as identity confirmation as well. Total anthocyanins content and percentage changes during in vitro digestion of black currant juice are presented in Table 1.
FIGURE 1.
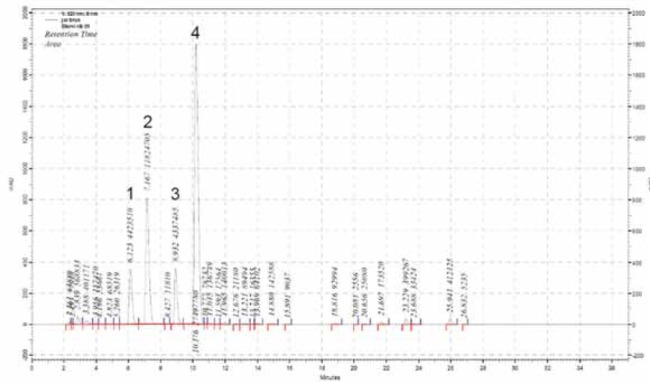
HPLC chromatogram of native black currant juice
TABLE 1.
Total anthocyaníns content and percentage changes during in vitro digestion of black currant juice
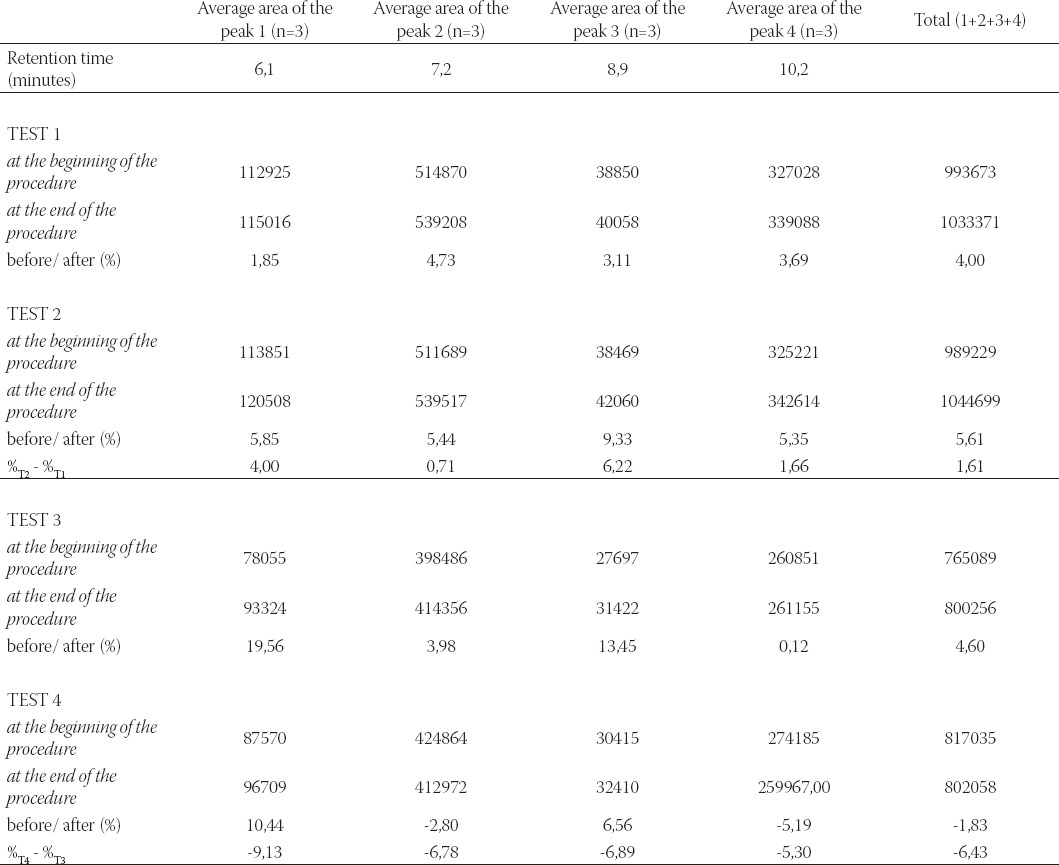
The analysis of content of four basic anthocyanin components in commercial black currant juice in simulated gastric fluid without pepsin indicated slight increase which is in accordance with previously published data (21). Decreased content was noted for anthocyanin components 2 and 4 (-2,80% and 5,19%-) after in vitro digestion in intestinal fluid containing pancreatin. When the intestinal fluid without pancreatin was used, increased content of anthocyanin components 2 and 4 (3,98% and 0,12%) could be determined. The effective content reduction of anthocyanin components 2 and 4 is -6,78% and -5,30%, respectively, and does not represent significant reduction as it was perceived in some previously published studies (21). Negative level of anthocyanin components content (%T4 - %T3) was perceived for components 1 and 3 (-9,13% and -6,78%) in intestinal fluid containing pancreatin, regardless of the fact that if we consider them individually, in media at pH 7,5 with/ without enzyme addition, the content of these components increased.
CONCLUSION
Anthocyanins present in commercial black currant juice remain stable during in vitro digestion in gastric fluid regardless whether pepsin was added into medium or not;
Anthocyanins present in commercial black currant juice remain stable during in vitro digestion in simulated intestinal fluid without pancreatin;
The stability studies of anthocyanins in the intestinal fluid containing pancreatin indicated reduced stability, primarily of anthocyanin components 2 and 4; which also mainly contribute to slight reduction in total anthocyanins content (-1,83%) in commercial black currant juice.
REFERENCES
- 1.Strack D, Wray V. Anthocyanins. In: Harborne JB, editor. Methods in Plant Biochemistry, Plant Phenolics. Vol. 1. London: Academic Press; 1993. pp. 325–356. [Google Scholar]
- 2.Stinzing FC, Carle R. Functional properties of anthocyanins and betalins in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004;15:19–38. [Google Scholar]
- 3.Giusti MM, Rodriguez-Saona LE, Griffin D, Wrolstad RE. Electrospray and tandem mass spectroscopy as tools for anthocyanin characterization. J. Agric. Food Chem. 1999;47:4657–4664. doi: 10.1021/jf981242+. [DOI] [PubMed] [Google Scholar]
- 4.Takeoka G, Dao L. Anthocyanins. In: Hurst WJ, editor. Methods of analysis for functional foods and nutraceuticals. Boca Raton, FL: CRC Press LLC; 2002. pp. 219–241. [Google Scholar]
- 5.Lee J, Rennaker C, Wrolstad RE. Correlation of two anthocyanin quantification methods: HPLC and spectrophotometric methods. Food Chemistry. 2008;110:782–786. [Google Scholar]
- 6.Zhang Y, Vareed SK, Nair MG. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005;76:1465–1472. doi: 10.1016/j.lfs.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Jayaprakasam B, Vareed SK, Olson LK, Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- 8.Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K. a-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J. Agric. Food Chem. 2001;49:1948–1951. doi: 10.1021/jf001251u. [DOI] [PubMed] [Google Scholar]
- 9.Tall JM, Seeram NP, Zhao CS, Nair MG, Meyer RA, Raja SN. Tart cherry anthocyanins suppress inflammation-induced pain behavior in rat. Behav. Brain Res. 2004;153:181–192. doi: 10.1016/j.bbr.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Joseph JA, Shukitt-Hale B, Denisnova NA, Bielinski D, Martin A, McEwan JJ, Bickford PC. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioural deficits with blueberry, spinach, or strawberry dietary supplementation. J. Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez-Tortosa C, Andersen OM, Gardner PT, Morrice PC, Wood SG, Duthie SJ, Collins AR, Duthie GG. Anthocyaninrich extract decreases indices of lipid peroxidation and DNA damage in vitamin E-depleted rats. Free Radic. Biol. Med. 2001 doi: 10.1016/s0891-5849(01)00618-9. [DOI] [PubMed] [Google Scholar]
- 12.Mazza G, Kay CD, Cottrell T, Holub BJ. Absorption of antho-cyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002;50:7731–7737. doi: 10.1021/jf020690l. [DOI] [PubMed] [Google Scholar]
- 13.Serafini M, Maiani G, Ferro-Luzzi A. Alcohol-free red wine enhances plasma antioxidant capacity in humans. J. Nutr. 1998;128:1003–1007. doi: 10.1093/jn/128.6.1003. [DOI] [PubMed] [Google Scholar]
- 14.Bitsch I, Janssen M, Netzel M, Strass G, Frank T. Bioavailability of anthocyanidin-3-glycosides following consumption of elderberry extract and blackcurrant juice. Int. J. Clin. Pharmacol. Therapeut. 2004;42:293–300. doi: 10.5414/cpp42293. [DOI] [PubMed] [Google Scholar]
- 15.Lapidot T, Harel S, Granit R, Kanner J. Bioavailability of red wine anthocyanins as detected in human urine. J. Agric. Food Chem. 1998;46:4297–4302. [Google Scholar]
- 16.Bub A, Watzl B, Heeb D, Rechkemmer G, Briviba K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, de-alcoholised red wine and red grape juice. Eur. J. Nutr. 2001;40:113–120. doi: 10.1007/s003940170011. [DOI] [PubMed] [Google Scholar]
- 17.Frank T, Netzel M, Strass G, Bitsch I. Bioavailability of anthocy-anidin-3-glucosides following consumption of red wine and red grape juice. Can J. Physiol. Pharmacol. 2003;81:423–435. doi: 10.1139/y03-038. [DOI] [PubMed] [Google Scholar]
- 18.van de Waterbeemd H, Lennernas H, Artursson P. Drug Bio-availability. In: Manhold R, Kubinyi H, Folkers G, editors. Methods and Principles in Medicinal Chemistry. Vol. 18. Weinheim: Wiley-VCH; 2004. [Google Scholar]
- 19.Passamonti S, Vrhovsel U, Vanzo A, Mattivi F. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003;544:210–213. doi: 10.1016/s0014-5793(03)00504-0. [DOI] [PubMed] [Google Scholar]
- 20.Gee JM, DuPont MS, Rhodes MJC, Johnson IT. Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic. Biol. Med. 1998;25:19–25. doi: 10.1016/s0891-5849(98)00020-3. [DOI] [PubMed] [Google Scholar]
- 21.Bermudez-Soto MJ, Tomas-Barberan F.-A, Garcia-Conesa M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007;102:865–874. [Google Scholar]


