Abstract
The natural habitat of Gardnerella vaginalis is a vagina since it could be located among 69% of women who have no signs of vaginal infection and in the vagina of as many as 13,5% girls. G. vaginalis is almost certainly identified among women diagnosed with bacterial vaginosis as well as in the urethra of their sexual partner. The increase in prevalence and concentration of G. vaginalis among patients diagnosed with this syndrome confirms that G. vaginalis plays a significant role in its pathogenesis. In our research, based on Amsel criteria for three or more clinical signs of bacterial vaginosis, it was diagnosed in 20,5% of women with subjective problems of vaginal infection, and in 48,80% of women with subjective symptoms characteristic of this disease. G. vaginalis was isolated from vaginal secretion of women without clinical signs characteristic of bacterial vaginosis. In 2,58% of cases it was solitary, while in 1,28% it was found in combination with other aerobic and anaerobic bacteria and, in 1,28% women combined with Candida albicans. The isolation of G. vaginalis was significantly increased (p<0,05) in the group of women with clinical signs of bacterial vaginosis in comparison to the group of women without these signs. Frequent recurrence of bacterial vaginosis, which is found in 20-30% of women within a three months treatment, is explained as reinfection with other biotype of G. vaginalis, different from a source biotype or as a consequence of wrong treatment. Following Piot biotype scheme, biotypes 2., 3. and 7. G. vaginalis are significantly more often isolated from women who suffer from bacterial vaginosis. Biotype 7. G. vaginalis, isolated from the group of women without clinical signs of bacterial vaginosis, accounted for 2,58% cases. Following Benit biotype scheme, biotypes IVa, IVc and Ilc were identified in 12,90% cases, while biotypes Illa, IIa, Ia, IVb, IIb were found in 6,45% cases. Lipase-positive isolates of G. vaginalis were significantly more frequently accompanied by the syndrome of bacterial vaginosis.
Keywords: Gardnerella vaginalis, bacterial vaginosis, biotypization
INTRODUCTION
Bacterial vaginosis is chronic or recurrent syndrome related to unidentified factors, and its pathogenesis is polymicrobic and multicausal (1). It was internationally defined in 1984 as “replacement of vaginal Lactoba- cillus with specific group of bacteria due to which the characteristics of vaginal discharge are changed” (2). Bacterial vaginosis is a result of replacement of normal vaginal flora (Lactobacillus) with mixed bacterial flora including Gardnerella vaginalis, anaerobic bacteria (Prevotella biva, P. disiens, P. species, Peptostreptococcus spp., Mobiluncus species) and Mycoplasme hominis. It has been proven that G. vaginalis is a dominant microorganism in 95% of women with clinical signs of vaginosis even though it is isolated from vaginal discharge of 40 to 50% of healthy women. In the last (9th) edition of “Bergey’s Manual of Systematic Bacteriology”, G. vaginalis is listed in volume 1, section 5 of Facultative anaerobic Gram-negative rods. This is oxidasis and catalase negative, noncapsulated immobile pleomorphic rod. Indole, nitrate and urea are negative. In order to grow, it requires thiamine, riboflavin, niacin, folic acid, biotin and two or more purine and pyrimidine bases. It is incubated at 35 °C in an environment enriched with carbon dioxide. It produces diffuse β hemolysis in blood agar with human but not with sheep blood. It ferments raffinose, glucose, maltose and sucrose but not mannitol and melibios, and hydrolyses starch and sodium hippurate (3). Based on biochemical characteristics such as the activity of lipase and β galactosidase as well as hippurate hydrolysis, G. vaginalis is classified into specific biotypes (4, 5). By using their own biotype scheme, Piot and associates (4) established eight separate biotypes, while Benito and associates (5) identified seventeen biotypes. Distribution of specific biotypes of G. vaginalis from female vaginal discharge differs in various geographic regions. Among the isolates of G. vaginalis from vaginal discharge of women with symptoms of bacterial vaginosis, Scot and associates (6) and Ison and associates (7) found that biotypes 1., 2. and 5. were the most prevalent while the data on presence of certain types of G. vaginalis in asymptomatic women have not been released since they have not been tested yet. Benito and associates, based on their own identification scheme confirmed that biotypes IIa, IIa1, IIb, IIIa, IIIc and IVa1 were more frequent among women diagnosed with vaginosis, while biotype IVa was more frequent among women without symptoms of this disease. Briselden and Hillier (8), using slightly changed typization scheme, found that lipase-positive biotypes were more frequently associated with clinical signs of bacterial vaginosis. The natural habitat of G. vaginalis is a vagina since it could be identified among 69% of women who have no signs of vaginal infection and in the vagina of as many as 13,5% girls. G. vaginalis is almost certainly located among women diagnosed with bacterial vaginosis as well as in the urethra of their sexual partners. Urethra of male sexual partners is colonized with the same type of G. vaginalis that infects the female partner. Certain biotypes of G. vaginalis are accompanied by clinical signs of bacterial vaginosis (5). The increase in prevalence and concentration of G. vaginalis among patients with this syndrome emphasizes the fact that G. vaginalis plays a significant role in pathogenesis of bacterial vaginosis since it presents a pre-condition for its development. Pathogenesis of bacterial vaginosis is very complex and has not been fully clarified. However, it is evident that numerous factors are included in this process, such are adhesines, cytotoxines and enzymes of G. vaginalis, which enable its colonization and invasion of vaginal and urethral epithelia. G. vaginalis adheres to epithelial cells with the assistance of submicroscopic extensions, cilia. Adherence to urogenital epithelial cells enables the colonization of those organs by G. vaginalis from within thus reducing the possibility of its being washed out by urinal or vaginal discharge (3). G. vaginalis produces cytotoxin, which enables its incorporation into lipid membranes. It is known nowadays that bacteria morphotypes G. vaginalis, Bacterodes spp., and Prevotella spp. excrete enzymes: mucinase, sialidase and IgA protease. These enzymes are factors of virulence since they destroy mucines, which play a significant role in functioning of female reproductive tract, and they also facilitate adherence of bacteria to epithelial cells of urogenital tract. Sialidase also influences the reduction of unspecified defensive mechanism of the host. (9) Previous studies (10, 11, 12, 13) did not establish any significant differences in distribution of certain biotypes of G. vaginalis among women with clinical signs of vaginosis (increased vaginal discharge of fishy odor, test result “clue cells”, positive amino odor test, pH above 4,5) and those without signs of vaginosis. By using a modified biotype scheme it was proven that certain biotypes such as 2., 4., 5. and 7. of G. vaginalis were more frequently accompanied by clinical signs of bacterial vaginosis (5). Frequent recurrence of bacterial vaginosis, which appear in 20-30% of women during a three-month treatment, is explained with reinfection caused by other biotype of G. vaginalis, different from the source biotype, or as a consequence of wrong treatment.
Among microbiological laboratory methods for the diagnosis of bacterial vaginosis is the method of cultivation of G. vaginalis on human two-layer Tween blood agar (10). Minimal diagnostic criteria for identifying G. vaginalis are: appearance of β hemolysis on two-layer Tween blood agar, typical morphology of the colonies, and typical morphology of microorganism using Gram-color staining set, negative catalase test and positive test for hippurate hydrolysis. Additional differential-diagnostic characteristics of G. vaginalis are negative mannitol fermentation as well as the appearance of inhibition zones on nutritive agar with 50 micrograms of metronidazole and 5 micrograms of trimethoprim. Enzymatic tests (ELISA), molecularbiological (DNA-DNA-hybridization), direct or indirect immunofluorescence (DIF and HF) with polyclonal antibodies are applied for specific detection of G. vaginalis.
Our study goals were to:
-
Determine frequency of Gardnerella vaginalis isolates from vaginal swab among:
- -women with clinical signs of bacterial vaginosis,
- -women without clinical signs of bacterial vaginosis;
Perform biotypization of isolated types of G. vaginalis;
Study relation between infection caused by certain biotypes of G. vaginalis and clinical symptoms and signs of bacterial vaginosis.
MATERIAL AND METHODS
The research was conducted at the Department for Microbiology and at the Gynecological and Obstetric Department at the Tuzla University Clinics Center, as well as at the Clinic for Women Health Care at the Tuzla Health Center. A total of 200 women 20-51 years of age were included in the prospective study. Based on clinical examination and presence of one or more signs of internationally accepted Amsel criteria, the examinees were divided into the test and the control group (11). Test group consisted of 84 subjects with one or several Amsel signs of bacterial vaginosis. Control group consisted of 116 subjects without Amsel signs of bacterial vaginosis. Each subject, besides registering personal and anamnestic data relevant for diagnosing bacterial vaginosis, was subjected to clinical and microbiological examination. Three swabs of vaginal discharge were taken for microbiological examination, and they were subjected to series of tests. A direct microscopic preparation was made from the material taken by one swab, using Gram staining method. Using the system for counting characteristic microorganisms of Gardnerella, Prevotella, Mobiluncus and Lactobacilus morphotypes, the condition of vaginal flora was assessed following Nugent method (12). The test results values from 0 to 3 are marked as normal vaginal flora, from 4 to 6 as changed vaginal flora while the values from 7 to 10 signify bacterial vaginosis. The second swab sample was cultivated in commercial selective medium for G. vaginalis produced by “Sanofi” Pasteur. After the cultivation for 48 hours at 37°C, in atmosphere enriched with CO2, the identification of grown colonies was conducted by application of standard microbiological methods. Isolated types of G. vaginalis were identified based on reaction to the following tests: catalase, oxidase, hippurate hydrolysis, activity of lipase and β galactosidase, arabinose and xylose. Test results are presented both graphically and in tabular form. χ2 test and student t-test were used for statistical data processing, student t-test (Computer application SPSS for Windows release). Rate variations χ2 > 3,804 were considered statistically significant.
RESULTS
Test results of clinical signs of bacterial vaginosis according to Amsel
After clinical examination, 42% (84/200) of the subjects were found with one or more clinical signs of bacterial vaginosis according to Amsel (Table 1.)
TABLE 1.
Distribution of subjects according to the number of Amsel signs present
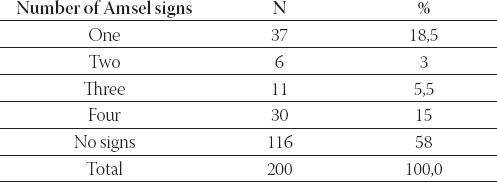
Based on finding of three or more clinical signs of bacterial vaginosis according to Amsel, bacterial vaginosis was diagnosed in 20,5% of women who were examined at the Department for Women Health Care in Tuzla, after complaining of subjective discomfort, characteristic of this disease.
Comparative analysis of the results of microbiological and parasitological examination of vaginal swab between the groups
Anaerobic and aerobic bacteria and yeast were isolated from the samples of vaginal discharge of examinees from both control and test group, but protozoa were isolated only from vaginal discharge examinees test group (Table 2).
TABLE 2.
Various bacteria, yeast and protozoa found in women in the control and test group
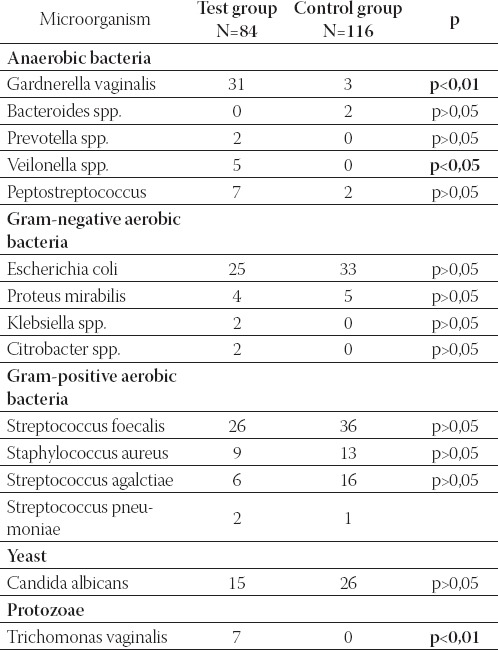
In comparison to the control group, G. vaginalis, Trichomonas vaginalis and Veilonella species were significantly more abundant in the test group. The following biotypes were found among the isolated types of G. vaginalis: biotype 1 (1 isolate), biotype 2 (7 isolates), biotype 3 (10 isolates), biotype 4 (3 isolates), biotype 5 (3 isolates), biotype 7 (10 isolates). The most common biotypes were: 3, 7 and 2. Among the total of 200 tested samples of vaginal swabs, Candida albicans was isolated in 41 samples (20,5%), and Trichomonas vaginalis was found in 7 samples (3,5%).
Analysis of G. vaginalis isolates
After the analysis of biochemical characteristics of all G. vaginalis isolates, biotypization was completed for all types 34/34 (100%) following Piot method, while Benit method produced the results in 24 of 34 (70,58%). Significant difference was registered in abundance of biotypes 2., 3. and 7. between the test and control group (p<0,05) (Table 3).
TABLE 3.
Bíotypes of G, vaginalis, following Plot method, isolated from samples of vaginal discharge of subjects in both test and control group
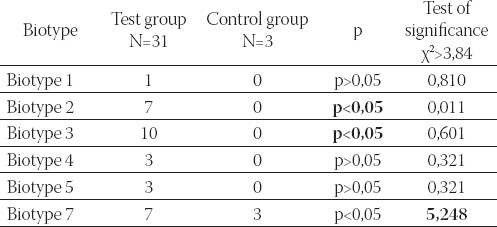
The biotypization analysis results showed that biotype 3. G. vaginalis was significantly more present in comparison to the other causative agents that are associated with the clinical signs of bacterial vaginosis. Following Piot method based on χ2 test, a significant difference related to the presence of biotype 7 between the test and control group is registered (Table 3).
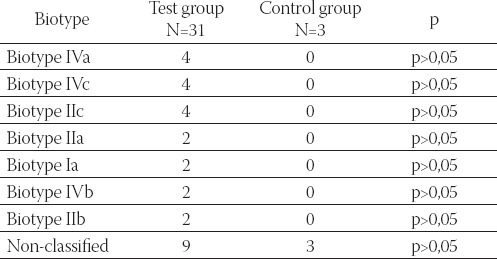
A total of nine types of G. vaginalis from test group and three types of G. vaginalis from control group could not be typed following Benit scheme. The remaining G. vaginalis belonged to following biotypes, according to Benit: Ia, IIa, IIb, IIc, IIIa, IVa, and IVc (Table 4).
TABLE 4.
Bíotypes of G, vaginalis, according to Benit, isolated from samples of vaginal discharge of subjects in test and control group
Relation between signs of bacterial vaginosis and isolation of G. vaginalis
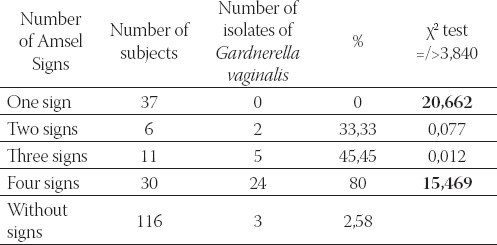
The isolation of G. vaginalis directly depends on the number of noticeable clinical signs of bacterial vaginosis. The highest percentage of G. vaginalis isolates was found among persons who exhibited all four clinical signs. G. vaginalis was not isolated from samples of vaginal discharge of subjects who exhibited only one clinical sign. χ2 test also confirmed that significantly larger number (χ2=15,469) of isolates were found in persons with all four clinical signs. G. vaginalis was not isolated from the samples of vaginal discharge of subjects with only one clinical sign, which is significantly less (χ2=20,662) in comparison to subjects with several signs (Table 5).
TABLE 5.
Correlation between isolation of G, vaginalis and number of present clinical signs of bacterial vaginosis
DISCUSSION
The increased prevalence and concentration of G. vaginalis in patients with bacterial vaginosis confirms the fact that G. vaginalis plays a significant role in pathogenesis of bacterial vaginosis since it actually is a precondition for its development. Pathogenesis of bacterial vaginosis is very complex and has not been clarified yet. However, it is evident that numerous factors participate in this process, such as adhesines, cytotoxins, and enzymes of G. vaginalis and anaerobic bacteria morphotypes Bacterodes spp, Prevotella spp., Peptostreptococcus spp., Mobiluncus species and Mycoplasma hominis. G. vaginalis is proven to be a dominant microorganism in 95% of women with signs of this disease. Almost all authors agree in assessment that culture testing of vaginal discharge with intent to establish routine diagnosis of bacterial vaginosis is not a method of choice since the process itself is very complicated and expensive (13, 14, 15). Positive test result for G. vaginalis does not necessarily imply the disease since G. vaginalis can be isolated in 5-60% of healthy women (16, 15). On the other hand, Gardner and Dukes isolated G. vaginalis in 93% of women diagnosed with bacterial vaginosis (17). Totten found higher number of isolates in women with bacterial vaginosis amounting to as high as 100% (10). In this study, G. vaginalis was isolated in 80% of subjects with all signs of bacterial vaginosis, and in 36,90% of subjects with one or more clinical signs of bacterial vaginosis, as well as in 2,58% of examinees without clinical signs.
Even though the percentage of isolated G. vaginalis is not high in tested subjects, and the interpretation of positive and negative test results is difficult, it is still recommended to cultivate vaginal discharge, for the purpose of isolating and biotyping the microorganisms. Biotypization of isolated types of G. vaginalis, based on hippurate hydrolysis, activities of lipase and β galactosidasis, as well as on fermentation of arabinose, xylose and galactose, serve as an attempt to understand its role in pathogenesis of bacterial vaginosis. Commonly accepted schemes for biotypization of G. vaginalis, Benit and Piot’s schemes, differ in the specter of tested enzymes (5,4%).
Based on numerous studies of biotypes of G. vaginalis in the world, different distribution was found in different geographical regions. Pandit and associates (18) and Piot and associates (4) in their research conducted in the USA, Kenya, India and Belgium found that the biotypes 1. (26-60%), 5. (16-31%) and 2. (8-24%) were the most frequent. In their research, they found no significant difference in the presence of certain biotypes of G. vaginalis in women with and without clinical signs of bacterial vaginosis. Among the isolates of G. vaginalis from vaginal discharge of women with symptoms of bacterial vaginosis, Scot and associates (6) and Ison and associates (7) found that biotypes 1., 2. and 5. were the most prevalent while the data on the presence of certain types of G. vaginalis in asymptomatic women were not released since they had not been tested. The analysis of Piot biotypization results in this study confirmed that biotype 3. was significantly more frequent in comparison to other biotypes in women diagnosed with bacterial vaginosis. This study showed that biotypes 2., 3. and 7. of G. vaginalis were significantly more frequently isolated in women diagnosed with bacterial vaginosis. Benit and associates (5), using their own identification scheme, confirmed that biotypes IIa, IIa1, IIb, IIIa, IIIc and IVa1 were more frequently present in women diagnosed with vaginosis, while biotype IVa was more frequent in women without symptoms of this disease, but this difference was not statistically significant. After the biotypization of isolated types of G. vaginalis, following Benit scheme, this study showed that biotypes IVa, IVc and IIc were more frequently isolated in comparison to other biotypes. Among the types that we isolated, there were 12 types (9 isolates from vaginal discharge of women with vaginosis and 3 from vaginal discharge of women without symptoms) that could not be biotyped following the above-mentioned scheme. These types draw attention due to differences in biochemical activity and they will be the subject of our further studies. The analysis of biochemically-defined types of G. vaginalis in this study confirmed that lipase positive types were more significantly present. At the same time, these types were accompanied by the clinical syndrome of bacterial vaginosis. Very similar results were achieved by Brieselden and Hiller (8), who used slightly changed biotypization scheme. Since bacterial vaginosis is a clinical syndrome that results from disturbance in mutual relations among various bacterial species that are normally present in vagina, it is to be expected that, besides G. vaginalis, other gram positive and gram negative aerobic and anaerobic bacteria may be isolated in abundance. In the study, G. vaginalis was isolated in pure culture in only 11.76% samples, while in the remaining 88.24% it was accompanied by other bacteria such as: Enterococcus foecalis (28,40%); Escherichia coli (25%), Staphylococ- cus aureus (13,63%); Streptococcus agalactiae (6,81%); Peptostreptococcus (7,95%); Proteus spp. (4,54%); Veil- lonela spp. (7.95%); and Pseudomonas aeruginosa, Citrobacter freundi, Bacteroides spp, Prevotella spp. and Streptococcus pneumoniae (5,65%). With the exception of G. vaginalis and Veillonella spp., which were isolated in significantly larger number in women with bacterial vaginosis, the results for other bacterial species did not significantly differ between test and control group. Other authors reached similar results referring to correlation between G. vaginalis and other bacterial species. According to Gupta and associates (19) G. vaginalis was isolated in pure culture in 5.8% of samples, while in 48,3% it was accompanied by other bacteria, namely: Escherichia coli (11,7%); Klebsiella spp. (9,2%); Enterococcus foecalis (7,3%); Proteus spp. (5,8%) and Staphylococcus albus (5%).
CONCLUSION
Bacterial vaginosis is diagnosed in 20,5% (41/200) women with subjective discomfort characteristic of vaginal infection, and in 48,80% (41/84) women with subjective discomfort characteristic of bacterial vaginosis.
G. vaginalis was isolated from vaginal discharge of women without clinical signs characteristic of bacterial vaginosis. It was solitary in 2.58% of women, associated with other aerobic and anaerobic bacteria in 1,28%, and along Candida albicans in 1,28% of women.
Isolation of G. vaginalis was significantly more frequent (p<0,05) in the group of women with clinical signs of bacterial vaginosis in comparison to the group of women without these signs.
Piot biotypization scheme showed that biotypes 2., 3. and 7. of G. vaginalis were significantly more frequently isolated in women diagnosed with bacterial vaginosis.
In the group of women without clinical signs of bacterial vaginosis, biotype 7. of G. vaginalis was isolated in 2,58% of samples.
Benit biotypization showed that biotypes IVa, IVc and IIc were identified in 12,90% of cases, and biotypes IIIa, IIa, Ia, IVb, IIb in 6,45% of cases. Lipase positive types of G. vaginalis were significantly more frequently associated with the syndrome of bacterial vaginosis.
REFERENCES
- 1.Royen P, Avonts D, Piot P. Third International Symposium on Vaginitis/Vaginosis. Madeira, Portugal: 1994. Epidemiology of bacterial vaginosis; pp. 15–24. [Google Scholar]
- 2.Westrom L, Evaldson G, Holmes KK, van der Meijden W, Rylander E, Fredriksson B. Taxonomy of vaginosis;bacterial vaginosisa definition. Scand. J. Urol. Nephrol. 1984;86:259–260. [Google Scholar]
- 3.Catlin BW. Gardnerella vaginalis Characteristics, clinical considerations, and controversies. Clin. Microbiol. Rev. 1992;5:213–237. doi: 10.1128/cmr.5.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piot E, van Dyck E, Peeters M, Hale J, Totten PA, Holmes KK. Biotypes of Gardnerella vaginalis J. Clin. Microbiol. 1984;20:677–679. doi: 10.1128/jcm.20.4.677-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benito R, Vasques JA, Berron S, Fenoll A Saez-Nieto. A modified scheme for biotyping Gardnerella vaginalis. J. Med. Microbiol. 1986;21:357–359. doi: 10.1099/00222615-21-4-357. [DOI] [PubMed] [Google Scholar]
- 6.Scott TG, Smyth CJ, Keane CT. In vitro adhesiveness and biotype of Gardnerella vaginalis strains in relation to the occurrence of clue cells in vaginal discharge. Genitourin. Med. 1987;63:47–53. doi: 10.1136/sti.63.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ison CA, Harvey DG, Tanna A, Easman CSF. Development and evaluation of scheme for serotyping Gardnerella vaginalis. Genitourin. Med. 1987;63:196–201. doi: 10.1136/sti.63.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briselden AM, Hillier SH. Longitudinal study of the biotypes of Gardnerella vaginalis J. Clin. Microbiol. 1990;28:2761–2764. doi: 10.1128/jcm.28.12.2761-2764.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd FR. Basic Medical Microbiology. 5th ed. Little, Brown and Company; 1995. Miscellaneous Pathogens; pp. 374–376. [Google Scholar]
- 10.Totten PA, Amsel R, Hale J, Piot P, Holmas KK. Selective differential human blood bilayer media for isolation of Gardnerella vaginalis (Haemophilus) vaginalis J. Clin. Microbiol. 1982;15:141–147. doi: 10.1128/jcm.15.1.141-147.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amsel R, Totten P, Spiegel CA, Chen KCS, Eschenbach D, Holmes KK. Nonspecific vaginitis, diagnostic criteria and microbial epidemiologic association. Am. J. Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 12.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J. Clin. Microbiol. 1991;29:1266–1271. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzales-Pedraza-Aviles A, Inzunza-Montiel A, Ortiz-Zaragoza C, Ponce-Rosas R, Irigoyen-Coria A. A comparison of 2 clinical laboratory methods in the diagnosis of bacterial vaginosis. Atencion Primaria. 1997;19:357–360. 26. [PubMed] [Google Scholar]
- 14.Krohn MA, Hillier SL, Eschenbach DA. Comparison of methods for diagnostic bacterial vaginosis among pregnant women. J. Clin. Microbiol. 1989;27:1266–1271. doi: 10.1128/jcm.27.6.1266-1271.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lien EA, Hillier SL. Evaluation of the enhanced rapid identification method for Gardnerella vaginalis J. Clin. Microbiol. 1989;3:566–567. doi: 10.1128/jcm.27.3.566-567.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCormack WM, Hayes CH, Rosner B, Evrard JR, Crockett VA, Alpert S, Zinner SH. Vaginal colonization with Corynebacterium vaginale (Haemophilus vaginalis) J. Infect. Dis. 1977;136:740–745. doi: 10.1093/infdis/136.6.740. [DOI] [PubMed] [Google Scholar]
- 17.Gardner HL, Dukes CD. Haemophilus vaginalis vaginitis. A newly defined specific infection previously classified “nonspecific” vaginitis. Am. J. Obstet. Gynecol. 1955;69:962–976. [PubMed] [Google Scholar]
- 18.Pandit DV, Varoe SM, Deodhar LP. Biotypes of Gardnerella vaginalis isolated from non-specific vaginitis patients in Bombay. Indian. J. Med. Res. 1989;89:435–438. [PubMed] [Google Scholar]
- 19.Gupta BK, Kumar R, Sofat R, Khurana S Deepinder. The role of Gardnerella vaginalis in nonspecific vaginitis in intrauterine contraceptive device users. Ind. J. Pathology & Microbiology. 1998;41:67–70. [PubMed] [Google Scholar]


