Abstract
Background
Fenofibrate is a commonly used hypolipidemic associated with rare instances of hepatotoxicity and routine liver biochemistry monitoring is recommended.
Aims
The aim of this study is to describe the presenting clinical features, liver histopathology and outcomes of 7 cases of acute liver injury associated with fenofibrate.
Methods
All cases of definite, very likely and probable drug induced liver injury (DILI) attributed to fenofibrate enrolled in the DILI Network study between 2004–15 were reviewed.
Results
Among 1229 patients with confirmed DILI, 7 cases (0.6%) were attributed to fenofibrate. The median age was 43 (range 37–61) years, and latency to onset was short (5–8 weeks) in 4 patients but more prolonged (18–56 weeks) in the rest. Laboratory results at presentation showed hepatocellular, mixed and cholestatic injury but 6 cases presented with jaundice. No patient had undergone routine monitoring. Four patients required hospitalization and 2 in whom drug discontinuation was delayed, had a severe outcome, 1 undergoing liver transplantation and 1 developing chronic injury and death. Liver biopsy was available in 4 patients and showed diverse injury patterns. Genetic studies showed the presence of the rare HLA-A*33:01 in 3 patients (43% vs 1% in control populations). The causality scores were highly likely in 5 and probable in 2.
Conclusions
Liver injury after fenofibrate exposure occurs with variable latency, enzyme elevation and histology. Although most cases are self-limited, severe injury and mortality can occur, particularly if drug withdrawal is delayed. Jaundice or abnormal laboratory tests during fenofibrate therapy should trigger prompt discontinuation.
Keywords: Fenofibrate, Hepatotoxicity, Liver injury tests
Introduction
Although hypertriglyceridemia is an independent risk factor for coronary artery disease [1–3], U.S. guidelines focus largely on cholesterol lowering and do not specifically recommend agents to decrease serum triglycerides [4]. These recommendations are based, in part, on large placebo controlled trials of fibrates which demonstrated no reduction in overall coronary events with treatment in patients with hypertriglyceridemia, although there was a reduction in the rate of non-fatal myocardial infarction [5]. Despite this, agents such as nicotinic acid and fibrates are increasingly prescribed for their ability to lower triglyceride levels with an estimated 440 fenofibrate prescriptions per 100,000 population in the U.S. in 2009, an increase of 160% from 2002 [6].
Fenofibrate is a peroxisome proliferator receptor alpha (PPAR-α) activator which was originally approved by the FDA in 1993 as an adjunct to diet for primary hypercholesterolemia or mixed dyslipidemia and for the treatment of severe hypertriglyceridemia. It is given orally at a dose of 48 to 200 mg daily. Since mild liver biochemistry abnormalities develop during fenofibrate therapy in 5–10% of patients, the product label (package insert) recommends baseline and regular periodic monitoring of liver tests for the duration of therapy and discontinuing therapy if serum aminotransferase levels persist above three times the upper limit of normal (ULN). However, the effectiveness of these recommendations in preventing untoward liver toxicity in clinical practice is unknown. Fenofibrate can also lead to CPK elevation in 3% of subjects and long-term use can lead to cholelithiasis and carries a risk of pancreaticobiliary disease.
Fenofibrate has also been linked to cases of clinically apparent idiosyncratic hepatotoxicity. The clinical pattern of fenofibrate associated liver injury has ranged from hepatocellular to cholestatic or mixed hepatitis and from an acute, self-limited hepatitis to a more persistent and even chronic liver injury [7–11]. Clinically significant liver injury from fenofibrate is very rare; indeed less than 0.3% of patients developed serum aminotransferase levels greater than five times the ULN in the FIELD trial that was conducted in almost 5000 patients treated with fenofibrate for an average of 5 years, a rate that was similar to placebo [5]. In addition, fenofibrate is now being investigated as a potential treatment for several liver diseases including primary biliary cholangitis (PBC), fatty liver disease and cirrhosis due to its potential beneficial effects on hepatic steatosis, endothelial dysfunction and fibrosis [12–15].
This study describes the presenting features, liver histopathology and outcomes of 7 patients with liver injury attributed to fenofibrate in a large prospective cohort of patients with drug induced liver injury (DILI) in the United States.
Methods
The Drug-Induced Liver Injury Network (DILIN) prospective study is a National Institutes of Health (NIH) funded multicenter observational cohort study that enrolls patients with suspected DILI. Eligible patients must meet at least one of four predefined laboratory criteria for study entry on two consecutive blood draws: (1) a serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level that exceeded 5 times the ULN (or 5 times a pretreatment baseline value if abnormal), (2) a serum alkaline phosphatase (Alk P) that exceeded 2 times the ULN (or 2 times the pretreatment value if abnormal), (3) any elevation of ALT, AST or Alk P with a total bilirubin of ≥2.5 mg/dL, or (4) any such enzyme elevations with an international normalized ratio (INR) greater than 1.5. Subjects had to be enrolled within 6 months of DILI onset. Patients with acetaminophen hepatotoxicity and those with liver or bone marrow transplant are excluded.
All patients underwent a detailed medical history and laboratory and radiological testing to exclude other causes of liver injury including testing for hepatitis A, B, C, E, HIV, markers of autoimmune disease, cytomegalovirus, Epstein-Barr virus, and biliary tract disease. Patients were asked to return for a follow-up visit at 6 months after enrollment, and those with persistent evidence of liver injury at 6 months to return again at 12 and 24 months.
To assess causality between the drug and liver injury, two methods were employed: a consensus expert opinion by committee using a standardized scoring system [16]: 1 (definite: ≥ 95% likelihood), 2 (highly likely: 75%–94% likelihood), 3 (probable: 50%–74% likelihood), 4 (possible: 25%–49% likelihood) or 5 (unlikely: <25% likelihood); and the standardized Roussel Uclaf Causality Assessment Method (RUCAM) [17]. By convention, RUCAM scores were grouped into likelihood levels as “excluded” (≥ 0), “unlikely” (1–2), “possible” (3–5), “probable” (6–8) and “highly probable” (≥ 9). In subjects where there was more than 1 implicated drug, an overall causality score was assigned and then a causality score was also determined for each individual suspect drug.
To categorize the pattern of liver injury we used the R-ratio: [ALT/ULN] ÷ [Alk P/ULN]. Cases were categorized as hepatocellular when R is greater than 5, cholestatic when less than 2 and mixed when between 2 and 5. A 5-point scale was used to define severity, ranging from 1 (mild), 2 (moderate), 3 (moderate and hospitalized), 4 (severe), and 5 (death or liver transplantation due to DILI within 6 months of onset) [18].
When available, liver biopsy material was reviewed by the DILIN liver histopathologist (DEK) and scored in a formulaic manner for multiple histological features as well as an overall pattern of liver injury [19].
The patients presented here have been included in prior publications of the overall features and outcomes of DILI in the prospective DILIN study [20, 21]. Of the 7 fenofibrate cases presented here, 3 were included in the genome-wide association study (GWAS) phase of the earlier publication, and 3 in the replication phase, where the proxy single nucleotide polymorphism (SNP) rs114577328 was genotyped. The seventh patient was of Hispanic ancestry and not included in the previous European ancestry-focused publication, although this sample had been GWAS genotyped with the Illumina 1MDuo chip. The HLA types for these patients were imputed from the GWAS data using HIBAG.
The study was approved by the Institutional Review Boards at each clinical site and data coordinating center and by a central Data Safety and Monitoring Board appointed by the NIDDK specifically for this study. All enrolled subjects provided written informed consent. The locations and principal investigators of the DILIN participating sites are given in the supplementary material.
Results
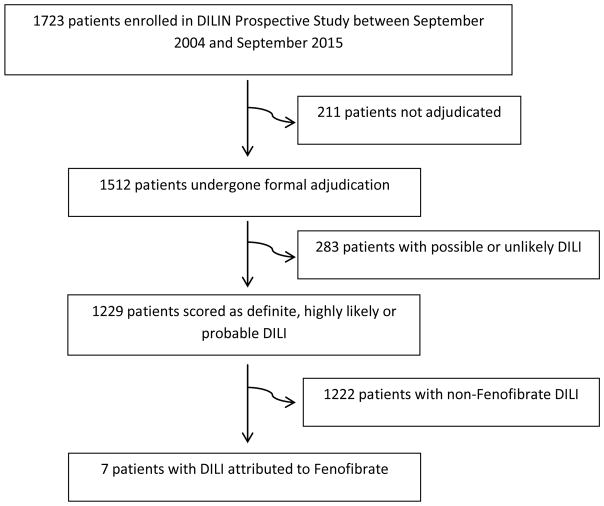
Between September, 2004 and September, 2015, 1723 patients were enrolled in the DILIN Prospective Study and 1512 underwent adjudication (Figure 1). 1229 cases were scored as definite, highly likely or probable DILI. Of these, 7 (0.6%) cases of fenofibrate hepatotoxicity were identified. The subjects included 5 men and 2 women, with a median age of 43 (range: 37–61 years).
Figure 1.
Flow diagram of selection of patients with Fenofibrate DILI in the DILIN Prospective Study
Demographic and clinical features of the 7 patients are shown in Table 1. All patients were prescribed fenofibrate for dyslipidemia, and specifically for elevated triglyceride levels in 6. The doses were all in the prescribed range, 48–160 mg daily. The duration of treatment ranged from 5–56 weeks. Four patients had a short time to onset of 5–8 weeks, the remaining 3 had a more prolonged latency of 18, 43 and 56 weeks, none of whom had had a recent dose increase. All patients were either overweight (body mass index [BMI] 25–30 kg/m2) or obese (BMI > 30 kg/m2) and 2 patients had diabetes mellitus. None had a history of excessive alcohol use or alcoholism. Six patients had been taking medications other than the implicated agent within 2 months of onset, ranging from 1 to 10 medications, but in only two cases (2 and 4) were these agents considered even “possibly” related to the liver injury. All 7 patients were symptomatic at presentation, the most common symptoms being jaundice (n=6), fatigue (n=3), nausea (n=3), itching (n=3) and abdominal pain (n=2). One patient gave a history of rash and one of fever, but these symptoms were not prominent and no patient had documented eosinophilia.
Table 1.
Baseline demographic features of 7 patients with Fenofibrate DILI
| Feature | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Age (years) | 43 | 61 | 37 | 43 | 41 | 54 | 44 |
| Sex | Male | Male | Female | Male | Male | Male | Female |
| Race | Caucasian | Caucasian | Caucasian (Hispanic) | Caucasian | Caucasian | Caucasian (Hispanic) | Unknown |
| BMI (kg/m2) | 26.9 | 50.9 | 26.0 | 30.8 | 28.0 | 25.5 | 33.1 |
| Reason for use | HyperTG | HyperTG | HyperTG | HyperTG | HyperTG | HyperTG | Hyperlipidemia |
| Daily dose | 145mg | 48mg | 160mg | 145mg | 160mg | 134mg | 48mg |
| Duration of therapy | 6 weeks | 16 weeks | 5 weeks | 7 weeks | 43 weeks | 56 weeks | 18 weeks |
| Latency to onset of Laboratory Abnormalities (weeks) | 6 weeks | 8 weeks | 5 weeks | 7 weeks | 40 weeks | 56 weeks | 18 weeks |
| Other implicated drugs (Causality score) | None | Doxazosin (4) Exenatide (4) |
None | Metformin (4) | None | None | None |
| HMG CoA reductase inhibitor use | No | Stopped 18 months prior | No | No | No | Long-term atorvastatin | Long-term atorvastatin |
| Cholelithiasis | No | Multiple small stones in GB | No imaging | No | Post cholecystectomy | No | No |
| Alcohol | None | None | None | Occasional | None | Occasional | Occasional |
| Diabetes | No | Yes | No | Yes | No | No | No |
| Initial symptoms | Jaundice Dark urine Pale stool Pruritis |
Jaundice Rash Pruritis |
Jaundice Nausea Fatigue Myalgia Abdo pain |
Jaundice Nausea Dark urine Pale stool Fever Pruritis |
Jaundice Abdo pain Nausea |
Fatigue | Jaundice Fatigue Dark urine |
The pattern of liver enzyme elevations (based upon R ratios) was hepatocellular in 2, cholestatic in 3 and mixed in 2 (Table 2). The median initial serum ALT was 533 U/L (range 78–2297), AST 245 U/L (63–917) and Alk P 218 U/L (79–518). Six patients presented with jaundice (peak bilirubin levels of 4.7–37.3 mg/dL). No patient had evidence of acute hepatitis A, B, C or E. Initial abdominal imaging was available in 6 patients and showed no evidence of biliary obstruction. However, 3 patients had evidence of hepatic steatosis and 1 patient had questionable coarsened hepatic echotexture on ultrasound. Long-term fenofibrate use has been associated with cholelithiasis but of the 6 patients that had abdominal imaging only 1 patient (case 2) had evidence of gallstones in the gallbladder. Autoantibody testing was negative for ANA in all 7 patients, while two had SMA reactivity.
Table 2.
Laboratory features of seven patients with Fenofibrate DILI
| Feature | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Initial values | |||||||
| ALT (U/L) | 533 | 83 | 332 | 100 | 78 | 584 | 1197 |
| Alk P (U/L) | 440 | 518 | 344 | 218 | 195 | 106 | 79 |
| Bilirubin(mg/dL) | 20.6 | 4.7 | 8.4 | 5.9 | 8.0 | 0.5 | 5.1 |
| INR | 1.04 | 1.40 | 0.80 | 1.00 | 4.3 | Not done | 1.00 |
|
| |||||||
| Peak values | |||||||
| ALT (U/L) | 533 | 123 | 332 | 100 | 161 | 584 | 1197 |
| Alk P (U/L) | 440 | 994 | 344 | 235 | 526 | 106 | 93 |
| Bilirubin(mg/dL) | 20.6 | 37.3 | 8.4 | 12.6 | 22.3 | 0.5 | 5.1 |
| INR | 2.00 | 0.8 | 1.0 | 4.3 | Not done | 0.9 | |
|
| |||||||
| Initial R ratio* | 3.5 | 0.5 | 3.4 | 1.4 | 0.6 | 18.7 | 59.8 |
|
| |||||||
| ANA | Neg | Neg | Neg | Neg | Neg | Neg | Neg |
| SMA | Neg | Neg | Neg | Weak pos | Neg | Neg | Pos |
|
| |||||||
| CPK (U/L) | 42 | Not done | Not done | 98 | Not done | Not done | Not done |
|
| |||||||
| Hospitalized | Yes | Yes | No | No | Yes | No | Yes |
|
| |||||||
| Outcome | Recovery | Chronic | Recovery | Unknown | Chronic | Recovery | Recovery |
|
| |||||||
| Death or liver transplantation | No | Chronic cholestasis and death (renal failure) at 26 months | No | Unknown | Chronic cholestasis and Liver transplant at 8 months | No | No |
|
| |||||||
| DILIN Severity Score | 3 | 4 | 2 | 2 | 4 | 1 | 3 |
|
| |||||||
| RUCAM Score | 7 Probable | 6 Probable | 6 Probable | 3 Possible | 4 Possible | 7 Probable | 7 Probable |
|
| |||||||
| DILIN Causality score | 2 Highly likely | 3 Probable | 2 Highly likely | 2 Highly likely | 2 Highly likely | 2 Highly likely | 3 Probable |
|
| |||||||
| Initial Liver imaging | Fatty infiltration on ultrasound and CT scan | Normal | None | Possible coarsened hepatic echotexture on ultrasound | Fatty liver on MRI | Fatty liver on ultrasound | Normal |
Outcomes
The clinical course was considered mild in one patient, moderate in 4, and severe in 2 (Table 3). The two patients with severe liver injury both had a delay after onset of symptoms in stopping fenofibrate (8 weeks in case 2 and at least 1 week in case 5). Both had persistent liver test abnormalities beyond 6 months, one subsequently undergoing liver transplantation at 8 months (case 5) and one dying of renal failure at 26 months at which time the ALT was 19 U/L, Alk P of 325 U/L and total bilirubin 0.2 mg/dL (case 2).
Table 3.
Timeline of laboratory and clinical parameters in Cases 2 and 5. Case 2 expired 26 months after presentation and case 5 underwent successful liver transplantation 8 months after presentation
| Date | Bilirubin mg/dL | INR | Ascites | Dialysis | |||
|---|---|---|---|---|---|---|---|
| Case 2 | Case 5 | Case 2 | Case 5 | Case 2 | Case 5 | Case 2 | |
| Presentation | 4.7 | 11.0 | - | 4.3 | No | No | No |
| 4–6 weeks | 12.7 | 9.6 | 1.4 | 1.3 | No | No | No |
| 8–10 weeks | 30.0 | 20.5 | 1.6 | 1.7 | Yes | No | Yes |
| 12–16 weeks | 17.0 | 18.5 | 1.4 | 1.2 | Yes | No | Yes |
| 24–30 weeks | 2.4 | 7.8 | 1.2 | 1.4 | No | No | Yes |
Liver Histopathology
Liver biopsy from 4 patients was available for central review, including 6 biopsies and an explant (Table 4). The initial biopsies were taken a mean of 16 days after DILI onset, (range 3 to 35 days). The patterns of injury were diverse, with one case showing chronic hepatitis, two showing cholestasis (one with mild hepatitis and one with steatohepatitis), and one showing chronic cholestasis and prominent zone 3 bile accumulation. Although there was not a single pattern of injury observed, there were common themes. Three patients showed zone 3 cholestasis associated with duct injury that was characterized by reactive epithelial changes rather than direct inflammatory injury. In the 3 cases that did not go to transplant there were eosinophils present in the portal areas but these were rare, similar to the numbers seen in case 5 who underwent liver transplant. The inflammation overall was mild in 3 cases. The one case that showed features of chronic hepatitis had autoimmune hepatitis-like changes with severe inflammatory activity, bridging necrosis, plasma cells and eosinophils. The chronic cholestatic case had two subsequent biopsies and an explant. The follow-up biopsies and the explant showed progressively worsening features of bile accumulation and chronic cholestasis. The explant did not show duct loss or duct injury suggestive of PBC, sclerosing cholangitis or vanishing bile duct syndrome.
Table 4.
Histopathologic features of patients with Fenofibrate DILI
| Description | |
|---|---|
| Case 1 | Steatohepatitis with cholestasis and duct injury. Moderate macrovesicular steatosis in zone 3 associated with mild perisinusoidal fibrosis and small balloon cells. Also mild canalicular cholestasis. The portal areas are expanded with fibrosis and a mild infiltrate with occasional eosinophils. The ducts show injury without loss. |
| Case 4 | Zone 3 cholestasis with mild portal and lobular inflammation, classified as an acute (bland) cholestasis. Eosinophils noted in portal areas. Duct injury present, but no duct loss and no chronic cholestatic features. No fibrosis. |
| Case 5 | Initial biopsy with chronic cholestasis with prominent zone 3 bile accumulation and hepatocyte swelling. Features of chronic cholestasis included both pseudoxanthomatous change and copper accumulation. Duct injury was noted although there were no florid duct lesions or sclerotic lesions. Ducts appeared to be present in most of the portal areas. Inflammation is mild overall. Follow-up biopsies showed similar changes with some worsening of cholestatic features. The explant shows severe cholestasis in the parenchyma along with features of chronic cholestasis. There is no convincing duct paucity and no sclerosing lesions. Inflammation remained mild through the explant. |
| Case 6 | Marked interface hepatitis with bridging necrosis and perivenular hepatitis with hepatocyte drop-out. Numerous foci of lobular inflammation. Plasma cells and eosinophils are prominent in the infiltrate. Duct injury is present, but more like Poulsen lesions than destructive injury. There were no features of chronic cholestasis and the copper stain was negative. There is both periportal and perivenular fibrosis without perisinusoidal fibrosis. Looks very much like autoimmune hepatitis. |
The overall causality scores by the DILIN expert consensus were definite in 1, highly likely in 5 and probable in 1 case; but fenofibrate was assigned causality scores of highly likely in 5 and probable in 2, the difference being in cases where another agent was implicated as “possibly” related (cases 2 and 4).
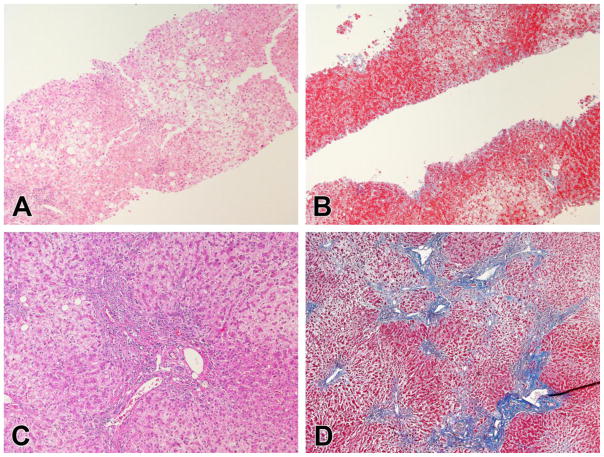
The injury in case 5 involved a 41 year-old male started on fenofibrate 160 mg daily for hypertriglyceridemia. He was on no other medications at the time. Six months later he underwent uneventful cholecystectomy for symptomatic gallbladder disease. He did well but 3 months later he developed abdominal pain, nausea, vomiting and jaundice and the fenofibrate was held. Laboratory studies showed ALT 78 U/L, AST 72 U/L, Alk P 195 U/L and total bilirubin 8.0 mg/dL. Liver imaging showed fatty liver without ductal dilation and he underwent endoscopic retrograde cholangiography which was normal. Viral and autoimmune studies (including AMA) were negative. His symptoms persisted and laboratory studies worsened despite a trial of prednisone. Serial liver histology (Figure 2) showed chronic cholestasis with mild inflammation and no duct loss or sclerosis. He underwent successful liver transplantation 8 months after his initial symptoms and diagnosis of DILI with the explant showing chronic cholestasis and bridging fibrosis without bile duct loss. After transplantation, jaundice resolved rapidly and all liver tests were normal 3 weeks later.
Figure 2.
Representative histological changes in case 5.
A. The initial biopsy showed swelling in zone 3 consistent with cholate stasis. Mild inflammation was present, mainly in portal areas (H&E, 100x). B. Masson trichrome staining showed scant fibrosis (Masson, 100x). C. At the time of transplant, the hepatocytes showed diffuse swelling with cytoplasmic clearing. Mild portal inflammation persisted, but the bile ducts remained intact being found in 20 of 20 consecutive portal tracts. D. Portal-portal and portal-central bridging fibrosis with nodularity were present but without progression to cirrhosis (Masson, 40x).
Genetics
Three of the 7 fenofibrate patients were found to be carriers of the low-frequency HLA type HLA-A*33:01, which has been associated with DILI and has a frequency of 1.1% in European ancestry controls (Table 5). Additionally, the one Hispanic ancestry fenofibrate patient was a carrier for the related HLA types HLA-B*14:02 and HLA-C*08:02, which were found in 8% of Hispanic controls but were found in all of the DILI cases who were carriers of HLA-A*33:01 [22].
Table 5.
HLA genotypes in seven patients with Fenofibrate DILI
| Case | HLA-A*33:01 | HLA-B*14:02 | HLA-C*08:02 | Ancestry |
|---|---|---|---|---|
| 1 | Yes | Yes | Yes | European |
| 2 | No | No | No | European |
| 3 | Yes | Yes | Yes | European |
| 4 | Yes | Yes | Yes | European |
| 5 | No | No | No | European |
| 6 | No | Yes | Yes | Hispanic |
| 7 | No | No | No | European |
Discussion
Fenofibrate accounted for 7 of 1229 patients (0.6%) with DILI enrolled during the first 12 years of the DILIN Prospective study. The 7 cases were enrolled at 6 different medical centers and during 5 different calendar years. The injury did not appear to be dose related, 2 patients receiving the minimal recommended dose and none receiving the maximum dose of 200 mg daily or an overdose. Four cases occurred with a short latency of 5 to 8 weeks and 3 with a longer latency, but none with a recent history of dose escalation. The clinical pattern of injury (based on the R ratio) was variable, but was cholestatic or mixed in 5 and hepatocellular in 2. The two hepatocellular cases had long latency and both had features of autoimmune hepatitis, one with SMA positivitiy and a second with a biopsy interpreted as compatible with autoimmune hepatitis. In both cases, however, the liver injury resolved without corticosteroid therapy once fenofibrate was stopped and did not relapse.
The 5 cholestatic and mixed cases were self-limited in 3 instances, but were prolonged and progressive in 2 others. Both severe cases had an initial delay after onset of jaundice in stopping fenofibrate. Both progressive cases presented in a manner that could be interpreted as “acute-on-chronic” liver failure with early appearance of ascites and features of hepatic dysfunction but without a previous bout of acute liver failure or marked serum aminotransferase elevations. One patient had pre-existing abnormalities in serum enzymes and likely had asymptomatic nonalcoholic steatohepatitis. While vanishing bile duct syndrome was suspected in both cases, liver biopsies did not demonstrate bile duct paucity. One patient (case 5) underwent liver transplantation 8 months after onset and the explant showed severe cholestasis but no ductopenia. The second patient (case 2) recovered from the severe and progressive injury with resolution of jaundice 6 months after onset but had persistence of symptoms of fatigue and weakness, marked elevation in AlkP and ascites. This patient died of renal failure complicated by the accompanying liver dysfunction.
Thus, fenofibrate usually causes an acute cholestatic or mixed hepatitis which rapidly resolves with stopping the drug, but occasionally can lead to progressive cholestasis and hepatic failure that may require liver transplantation. Fenofibrate associated liver injury can also present with a hepatocellular pattern of injury, usually with a longer latency and sometimes with autoimmune features.
The variability in presentation of fenofibrate associated DILI in this cohort mirrors that seen in the published literature. Acute cholestatic injury [9–11] has been described shortly after initiation of therapy with rapid improvement on dechallenge although one case developed a chronic injury. An autoimmune type reaction was seen in a French series of 5 patients [7] with elevated aminotransferase levels, hypergammaglobulinemia and high titers of ANA with liver biopsy demonstrating a lymphoplasmacytic infiltrate. Several patients already had cirrhosis and those who did not went on to develop cirrhosis. Interestingly all the patients had resolution of injury when fibrate therapy was discontinued although immunosuppressive therapy was required in 2 patients.
Given the variability in presentation the mechanism underlying fenofibrate hepatotoxicity is unclear. Hepatic PPAR-α activity has complex effects on lipid metabolism including controlling expression of genes encoding peroxisomal and mitochondrial fatty acid β-oxidation enzymes. There is experimental evidence this may be a useful effect in fatty liver disease [13] and also protection against acetaminophen-induced hepatotoxicity [23].
Recent evidence suggests that altered lipid metabolism involving PPAR-α may also be involved in the pathogenesis and chronicity of cholestatic disease [24]. This finding has led to studies investigating the role of fenofibrate in PBC where activation of PPAR-α has beneficial effects by down-regulating multiple enzymes involved in the synthesis of toxic bile acids [14, 15].
A recent study has examined the association of DILI with polymorphisms in HLA genes and included the 7 cases reported here [22]. Cases 1, 3 and 4 were associated with HLA-A*33:01, and these cases shared a similar short latency of 5–7 weeks, were not taking concomitant HMG-CoA reductase inhibitors and recovered uneventfully (although case 4 was lost to follow up). Interestingly, this HLA type was not seen in the 2 severe cases nor in the 2 with long latency to onset and autoimmune features.
Strengths of the current study include the prospective collection of data and a standardized causality assessment procedure [18] with exhaustive exclusion of other causes of liver disease. Limitations of this study include the lack of an objective diagnostic biomarker for DILI although the available liver histology is typical of what has been reported in other patients with suspected DILI. In addition, pretreatment baseline liver biochemistries were not available in 5 of the 7 treated patients.
Despite the concern for hepatotoxicity and the recommendation to monitor liver tests while on fenofibrate, the FIELD study found no difference in liver test abnormalities between fenofibrate and placebo [5] suggesting that DILI from fenofibrate is a rare event probably arising in fewer than 1:10,000 exposed persons, arguing against routine monitoring of liver tests, particularly as the appropriate interval and frequency is unclear. In addition, while cases of fenofibrate associated liver injury have been prolonged and severe, there have been no instances of acute liver failure attributed to fibrates in the LiverTox database [26]. However, the severe injury in cases 2 and 5 was only noted several weeks after the onset of jaundice. Earlier recognition of the symptoms of liver injury may have led to an opportunity to discontinue the medication and perhaps prevented further deterioration.
In conclusion, fenofibrate is associated with idiosyncratic acute liver injury with a typical latency of 5–8 weeks although this can be more prolonged. The pattern and severity of injury is variable but a chronic progressive cholestatic disease can occur. Although rare, the injury can be severe and patients treated with fenofibrate should be alerted to the possibility of liver injury and importance of discontinuing therapy for unexplained symptoms or signs of possible liver injury.
Supplementary Material
Acknowledgments
Financial Support: DILIN
Funding source: The Drug Induced Liver Injury Network (DILIN) is structured as an U01 cooperative agreement supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) with funds provided by the following grants: U01DK065211 (Indiana University [Purdue]), U01DK065184 (University of Michigan [Ann Arbor]), U01DK065201 (University of North Carolina [Chapel Hill], Asheville, Wake Forest Baptist Medical Center), U01DK083020 (University of Southern California, University of California-Los Angeles [Pfleger Liver Institute]), U01DK083027 (Albert Einstein Medical Center), U01DK100928 (Icahn School of Medicine at Mount Sinai), U01DK065176 (Duke Clinical Research Institute). Additional support was provided by the Intramural Division of the National Cancer Institute (NCI), NIH.
Abbreviations
- ALT
Alanine aminotransferase
- ANA
Antinuclear antibody
- Alk P
Alkaline phosphatase
- AST
Aspartate aminotransferase
- DILI
Drug induced liver injury
- DILIN
Drug Induced Liver Injury Network
- INR
International normalized ratio
- RUCAM
Roussel Uclaf Causality Assessment Method
- ULN
Upper limit of normal
Footnotes
No conflict of interest to disclose
Author contributions: All authors contributed to the collection of clinical data, data analysis, and initial and final drafting of the manuscript. DEK provided expert review of the available liver histopathology.
Conflict of interest disclosure: Dr. Chalasani has ongoing consulting activities (or had in the preceding 12 months) with NuSirt, Abbvie, Eli Lilly, Afimmune (DS Biopharma), Tobira (Allergan), Madrigal, Shire, Cempra, Ardelyx, Gen Fit and Amarin. These consulting activities are generally in the areas of nonalcoholic fatty liver disease and drug hepatotoxicity. Dr. Chalasani receives research grant support from Intercept, Lilly, Gilead, Galectin Therapeutics and Cumberland where his institution receives the funding. Over the last decade, Dr. Chalasani has served as a paid consultant to more than 30 pharmaceutical companies and these outside activities have regularly been disclosed to his institutional authorities. Drs. Kleiner and Hoofnagle have no conflicts of interest to disclose.
References
- 1.Assmann G, Schulte H, von Eckardstein A. Hypertriglyceridemia and elevated lipoprotein(a) are risk factors for major coronary events in middle-aged men. Am J Cardiol. 1996;77(14):1179. doi: 10.1016/s0002-9149(96)00159-2. [DOI] [PubMed] [Google Scholar]
- 2.Dewey FE, Gusarova V, O’Dushlaine C, et al. Inactivating Variants in ANGPTL4 and Risk of Coronary Artery Disease. N Engl J Med. 2016;374(12):1123. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myocardial Infarction Genetics and CARDIoGRAM Exome Consortia Investigators. Coding Variation in ANGPTL4, LPL, and SVEP1 and the Risk of Coronary Disease. N Engl J Med. 2016;374(12):1134. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 5.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 6.Jackevicius CA, Tu JV, Ross JS, et al. Use of fibrates in the United States and Canada. JAMA. 2011;305:1217–24. doi: 10.1001/jama.2011.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganne-Carrié N, de Leusse A, Guettier C, et al. Autoimmune hepatitis induced by fibrates. Gastroenterol Clin Biol. 1998;22:525–9. [PubMed] [Google Scholar]
- 8.Dohmen K, Wen CY, Nagaoka S, et al. Fenofibrate-induced liver injury. World J Gastroenterol. 2005;11:7702–3. doi: 10.3748/wjg.v11.i48.7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucena MI, Andrade RJ, Vicioso L, et al. Prolonged cholestasis after raloxifene and fenofibrate interaction: A case report. World J Gastroenterol. 2006;12:5244–6. doi: 10.3748/wjg.v12.i32.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho CY, Kuo TH, Chen TS, et al. Fenofibrate-induced acute cholestatic hepatitis. J Chin Med Assoc. 2004;67:245–7. [PubMed] [Google Scholar]
- 11.Hajdu D, Aiglová K, Vinklerová I, et al. Acute cholestatic hepatitis induced by fenofibrate. J Clin Pharm Ther. 2009;34:599–602. doi: 10.1111/j.1365-2710.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Vilarrupla A, Laviña B, García-Calderó H, et al. PPARα activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2012;56:1033–9. doi: 10.1016/j.jhep.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Montagner A, Polizzi A, Fouché E, et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65:1202–14. doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegade VS, Khanna A, Walker LJ, et al. Long-Term Fenofibrate Treatment in Primary Biliary Cholangitis Improves Biochemistry but Not the UK-PBC Risk Score. Dig Dis Sci. 2016 Jul 19; doi: 10.1007/s10620-016-4250-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Cheung AC, Lapointe-Shaw L, Kowgier M, et al. Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment Pharmacol Ther. 2016;43:283–93. doi: 10.1111/apt.13465. [DOI] [PubMed] [Google Scholar]
- 16.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–26. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causalityassessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46:1331–6. doi: 10.1016/0895-4356(93)90102-7. [DOI] [PubMed] [Google Scholar]
- 18.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59:661–70. doi: 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110:1450–9. doi: 10.1038/ajg.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalasani N, Bonkovsky HL, Fontana R, et al. United States Drug Induced Liver Injury Network. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicoletti P, Aithal GP, Bjornsson ES, et al. Association of Liver Injury From Specific Drugs, or Groups of Drugs, With Polymorphisms in HLA and Other Genes in a Genome-Wide Association Study. Gastroenterology. 2017;152:1078–1089. doi: 10.1053/j.gastro.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson AD, Shah YM, Matsubara T, et al. Peroxisome proliferator-activated receptor alpha induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology. 2012;56:281–90. doi: 10.1002/hep.25645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moustafa T, Fickert P, Magnes C, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology. 2012;142:140–151. doi: 10.1053/j.gastro.2011.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Russo MW, Hoofnagle JH, Gu J, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60:679–86. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed May 2, 2017]; https://livertox.nih.gov.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.