Abstract
Purpose of Review
To summarize knowledge of the prevalence, relevant physiology and consequences of obesity and visceral adiposity in HIV-infected adults, including highlighting gaps in current knowledge and future research directions.
Recent Findings
Similar to the general population, obesity prevalence is increasing among HIV-infected persons, and obesity and visceral adiposity are associated with numerous metabolic and inflammatory sequelae. However, HIV- and antiretroviral therapy (ART)-specific factors may contribute to fat gain and fat quality in treated HIV infection, particularly to the development of visceral adiposity, and sex differences may exist.
Summary
Obesity and visceral adiposity commonly occur in HIV-infected persons and have significant implications for morbidity and mortality. Future research should aim to better elucidate the HIV- and ART-specific contributors to obesity and visceral adiposity in treated HIV infection, with the goal of developing targeted therapies for the prevention and treatment of obesity and visceral adiposity in the modern ART era.
Keywords: HIV, obesity, visceral fat, lipohypertrophy, antiretroviral therapy
Introduction
In the era of effective antiretroviral therapy (ART), HIV-infected adults can live near normal lifespans but face high rates of metabolic disease stemming from both traditional (Western diet, sedentary lifestyle) and HIV-/ART-related contributors (chronic inflammation and immune activation, gut microbiome disturbances, drug toxicities). Obesity and visceral adiposity are common in treated HIV infection, and have both traditional and HIV-/ART-associated contributors. As in the general population, excess adiposity is associated with numerous metabolic and inflammatory consequences. Although the mechanisms by which HIV and ART contribute to changes in fat quality and quantity are incompletely elucidated, an understanding of the importance of fat health is emerging. Here we review recent advances in our understanding of the prevalence, incidence, pathophysiology and consequences of excess adiposity in treated HIV infection.
Burden of Obesity and Visceral Adiposity
Obesity is traditionally defined as a body mass index (BMI) >30kg/m2. However, in persons with low muscle mass, excess adiposity may exist within the “normal” BMI range of 18.5–24.9 kg/m2. For this reason, some authorities have advocated using body fat >25% for men or >33% for women to define obesity[1], although these cutoffs have not been validated in the setting of HIV infection. Additionally, HIV-infected persons may have increased BMI-to-visceral adipose tissue (VAT) ratios[2–4], creating another scenario where traditional BMI guidelines may underestimate adiposity in this population. Despite this, up to two-thirds of HIV-infected adults are classified as overweight or obese by standard BMI criteria in recent cohorts[5–9], reflecting global HIV obesity rates.
Modern ART initiation is often associated with weight gain. In an AIDS Clinical Trial Group (ACTG) study of ART initiation in resource-diverse settings (A5175), more than 25% of participants were classified as overweight or obese at entry, and approximately 40% of participants were overweight or obese by week 144[8]. Some weight gain following ART initiation may be attributable to a “return to health” phenomenon; however, excessive weight gain can occur, with persons with the highest pre-ART HIV-1 RNA or lowest CD4+ T lymphocyte counts at risk for greater weight gain[10, 11]. Further exemplifying the fact that weight gain can represent differential effects depending on the host, weight gain among underweight persons has been associated with a decline in circulating high-sensitivity C-reactive protein levels[12] and improved survival[13], whereas weight gain among overweight or obese individuals has been associated with significant increases in circulating levels of the monocyte activation marker soluble CD14[12], no mortality benefit[13] and a ≥67% prevalence of multi-morbidity[14].
Importantly, many studies have described weight gain following ART initiation without additional details regarding the type of tissue involved, and lean mass and fat mass may both increase with ART initiation[15, 11]. However, other studies have specifically demonstrated increases in fat following ART initiation. For example, ACTG study A5260, which randomized participants to raltegravir, ritonavir-boosted darunavir or ritonavir-boosted atazanavir, each with a backbone of tenofovir disoproxil fumarate/emtricitabine, demonstrated a mean computed tomography-quantified VAT gain of 26% after 96 weeks of ART that did not vary significantly between randomization arms[11]. However, in subset analyses using waist circumference as a surrogate for VAT (Pearson correlation between waist circumference and VAT=0.52, p<0.0001[16]), women appeared to have greater VAT gain on raltegravir vs protease inhibitors than men[17], suggesting sex differences in drug effects may exist.
Additionally, Grant et al examined longer-term changes in body composition in HIV-infected adults on ART, and demonstrated that after an initial 96-weeks of ART, dual x-ray absorptiometry-quantified trunk fat gains continued at a slower rate than in the first 96 weeks of therapy, but at faster rates than HIV-uninfected controls[15]. As VAT gains of as little as 5% have been associated with increased risk for the metabolic syndrome[18] and central fat accumulation has been associated with short-term mortality risk in HIV infection[19], these size of these VAT changes are highly clinically significant.
Quality and Quantity
Given associations between obesity and immunometabolic disturbances in both the general population and HIV-infected persons, significant emphasis has been placed on the impact of fat quantity to cardiometabolic disease and the inflammatory milieu in treated HIV infection. Fat quality is less well understood, but is likely at least as important as quantity. An example of this is metabolically healthy obesity, in which persons have BMI ≥30 kg/m2 without overt cardiometabolic disease. Indeed, in the general population metabolically healthy obesity has been associated with less VAT and systemic inflammation, more favorable immune cell profiles and higher fat utilization than the metabolically unhealthy obese[20–23]. However, other (but not all) data suggests that this state is not completely benign, and can be associated with increased risk of progression to the metabolic syndrome and/or progression of cardiovascular disease (CVD)[24, 25]. Conflicting data is further complicated by the use of varying populations and definitions to define the metabolically healthy but overweight or obese phenotype. Of note, metabolically healthy obesity has recently been documented in HIV-infected men at a similar prevalence to that observed in HIV-uninfected men[26], and studies of whether metabolically healthy obesity differs in HIV-infected vs HIV-uninfected persons are underway.
Why some persons remain metabolically healthy despite obesity remains unknown, but fat function likely plays a major role: Normal, healthy adipocytes are small, well differentiated and contain a modest lipid droplet. During fat gain, adipocytes either increase in number (hyperplasia) or volume (hypertrophy). While hyperplastic adipocytes maintain normal functioning, large, hypertrophied adipocytes become hypoxic, expand their lipid droplet, and recruit pro-inflammatory immune cells, primarily activated macrophages[27–29].
Activated macrophages stimulate a local, pro-inflammatory type 1 immunologic response and lose the ability to store iron, which leads to iron deposition in the adipose tissue and subsequent reactive oxygen species production and mitochondrial dysfunction[30]. Adipocyte hypertrophy also suppresses adiponectin production, exacerbating the pro-inflammatory environment. Ultimately, increased transforming growth factor-β production[31] triggers pro-fibrotic processes in an attempt to limit further adipocyte hypertrophy[32]. However, when caloric excess persists and fibrosis limits adipocyte expansion, ectopic fat deposition occurs in sites such as the liver and skeletal muscle. Ectopic fat deposition is associated with additional inflammation and metabolic dysregulation[33, 34], including the development of insulin resistance and CVD[35, 36]. Thus, maintaining adipose tissue function, or quality, is critical to preventing immunometabolic consequences of fat dysfunction independent of fat quantity.
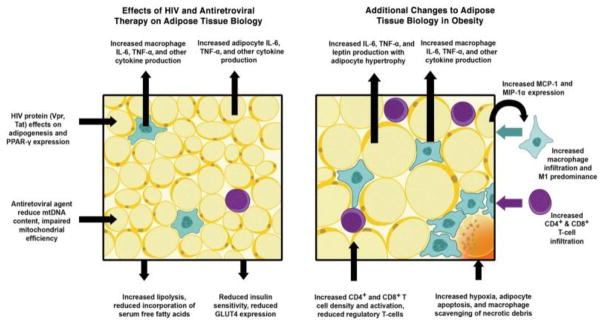
Much of the data on adipose tissue function in the general population is derived from the setting of obesity. Given that both HIV and ART are associated with adipose tissue disturbances (Figure 1[37]), extrapolation of these data to HIV-infected persons must proceed with caution. Much of the available data on adipose tissue dysfunction in HIV-infected persons is in the setting of lipodystrophy. While lipodystrophy in treated HIV infection still occurs, obesity prevalence is increasing, and some persons may experience an overlap syndrome with components of both obesity and lipodystrophy. While central lipohypertrophy is associated with increased adipose tissue inflammation and apoptosis[38], further research is needed to understand the intersections of HIV-, ART- and obesity-induced adipose tissue disturbances, and to define whether these intersections may lead to differential clinical consequences in treated HIV infection.
Figure 1.
Effects of HIV infection, ART and obesity on adipose tissue biology.
Reproduced with permission from J Infect Dis. 2013;208(8):1194–1201. Abbreviations: IL-6, interleukin-6; MCP-1, macrophage chemotactic protein-1; MIP-1α, macrophage inflammatory protein-1α; mtDNA, mitochondrial DNA; PPAR-γ, peroxisome proliferator-activated receptor-γ; TNF-α, tumor necrosis factor-α.
Notably, fibrosis of the subcutaneous adipose tissue in non-obese, HIV-infected adults has recently been documented, accompanied by the finding that adipose tissue fibrosis improves with continued suppressive ART[39]. While additional study is needed, this finding suggests that a pro-fibrotic, pro-inflammatory stimulus in the abdominal adipose tissue of HIV-infected persons exists that is not attributable to ART alone. Whether this stimulus is HIV itself[40–42], microbial products resulting from gut barrier disruption in HIV infection[43, 44] or other currently unidentified triggers remains to be seen. However, these provocative data suggest that adipose tissue dysfunction and its associated metabolic disturbances may be related to a chronic inflammatory stimulus within the adipose tissue, and that continued ART allows for suppression of the inflammatory stimulus such that pro-fibrotic pathways can be suppressed and wound healing can occur. Additional research is needed to determine whether reductions in adipose tissue fibrosis in HIV-infected persons on suppressive ART will translate into improved adipocyte function and clinical benefit.
Consequences of Obesity and Visceral Adiposity
Obesity and visceral adiposity are integral players in the development of multiple non-AIDS comorbid disease states, including CVD and liver disease, which are now leading causes of death among HIV-infected persons[45, 46]. The development of comorbid disease in HIV-infected persons is believed to have both traditional and HIV-/ART-specific contributors: Obesity, and particularly VAT accumulation, are associated with systemic[12, 47] and adipose tissue inflammation[48], insulin resistance, dyslipidemia[49] and increased oxidative stress[50]. At the same time, adipose tissue is a potential HIV reservoir, leading to recruitment of T lymphocytes and macrophages into adipose tissue.[40] HIV also alters adipocyte differentiation[51, 41, 52]. One proposed mechanisms for this is inhibition of peroxisome proliferator-activated receptor-γ (a regulator of adipogenesis, lipogenesis, insulin sensitivity and normal cytokine/adipokine expression) target gene expression and activation of glucocorticoid target gene expression by the HIV protein Vpr, leading to accelerated lipolysis, increased macrophage infiltration into adipose tissue, diminished white adipose tissue quantity and hepatic steatosis[41]. The HIV Tat protein may also impair adipogenesis and promote adipose tissue inflammation[52].
HIV-associated chronic inflammation and immune activation may play a role in the development of visceral adiposity, with circulating CD8+ T lymphocyte activation linked to VAT accumulation[53]. Activation of the renin-angiotensin system has been independently associated with treated HIV infection and VAT accumulation[54, 55], and appears to independently predict insulin resistance[56]. Similar to obesity, HIV-associated hormonal imbalances (e.g., hypogonadism, growth hormone deficiency) and alteration of the gut microbiome may play adjunctive roles[57, 58]. Finally, aging is associated with physiologic central fat redistribution, adipocyte senescence and chronic inflammation[59], which may be enhanced in HIV-infected individuals. Whether the combination of aging, HIV infection and VAT accumulation/obesity has synergistic or additive effects in this population is incompletely understood, however.
Cardiovascular Disease
Obesity and visceral adiposity are documented risk factors for CVD and diabetes mellitus (a CVD risk equivalent) in HIV-infected adults[60, 61], and VAT, intrahepatic fat and epicardial fat are all associated with CVD independent of traditional CVD risk factors in this population[36, 61]. Additionally, HIV infection has been associated with increased type 2 diabetes mellitus[62] and CVD[63, 64] risk. Whether obesity and HIV infection are additive or synergistic to CVD risk, however, is not fully understood. In a recent study of HIV-infected participants, obesity was associated with reduced insulin resistance and greater systemic inflammation but not greater carotid intima media thickness or greater impairment of arterial flow-mediated dilatation compared to normal weight, HIV-infected persons on identical ART regimens[65]. In another study of older HIV-infected adults with traditional cardiovascular risk factors on suppressive ART, high rates of undetectable circulating endothelial progenitor cell levels were observed (suggesting markedly reduced vascular reparative capacity) that did not vary by BMI[66].
Fatty Liver Disease
Approximately 30–40% of HIV-infected adults are estimated to have non-alcoholic fatty liver disease (NAFLD, defined as ≥5% hepatic steatosis without other demonstrable causes)[67–69], and this prevalence may increase substantially among patients with elevated transaminase levels[70]. While NAFLD may be associated with progressive liver disease in the forms of non-alcoholic steatohepatitis (NASH), hepatic fibrosis, and, ultimately, cirrhosis, liver failure and hepatocellular carcinoma[71], CVD accounts for most of the excess morbidity and mortality associated with NAFLD[72, 73]. This association with morbidity and mortality is independent of traditional CVD risk factors[74], but is tightly linked to excess adiposity and its consequences. In fact, 80–90% of adults with NAFLD have generalized obesity, visceral adiposity, metabolic syndrome or type 2 diabetes[67]. Interestingly, intra-hepatic triglyceride accumulation, the root cause of NAFLD, is more closely linked to metabolic complications than VAT quantity[75, 76].
NAFLD may have unique origins in HIV infection. Although the exact mechanisms are not well understood, there may be several contributing factors not common among HIV-uninfected persons. First, HIV infection is characterized by persistent inflammation and immune activation[77], which 1) could help explain higher rates of NASH and liver disease severity in HIV infection (63% vs 37% in HIV-uninfected)[78] and 2) contributes to greater insulin resistance, furthering metabolic dysregulation in both adipose tissue and the liver[79, 80]. In addition to traditional risk factors (older age, sedentary lifestyle), HIV-/ART-specific factors (dyslipidemia, microbial translocation, mitochondrial dysfunction) likely contribute. As such, both traditional and HIV-/ART-specific NAFLD contributors should be considered when developing therapeutics.
Given the high prevalence of NAFLD in HIV infection, NAFLD’s associations with CVD, the independent association of HIV with CVD[81] and increasingly high rates of traditional CVD risk factors in HIV-infected persons on ART, HIV-infected adults with NAFLD are primed for adverse cardiovascular outcomes and aggressive measures should likely be taken to prevent and treat NAFLD in this population. However, no standard of care for NAFLD exists in HIV infection or in the general population beyond diet and exercise recommendations. The thiazolidinedione pioglitazone[82] and growth hormone-releasing factor analog tesamorelin[83] have shown promise for the treatment of NAFLD in HIV/hepatitis C virus co-infected and HIV mono-infected adults, respectively, but have not yet been recommended for use. While a large number of agents are being developed for NAFLD and NASH treatment[84], future research efforts to define how the pathophysiology of NAFLD may differ in HIV infection are needed to allow for the development of targeted NAFLD therapies for this population.
Cognitive Decline
In HIV-infected persons, poorer neurocognitive function has been associated with both increased waist circumference (a marker of visceral adiposity[85])[86] and obesity[87]. Among a subset of middle-aged, HIV-infected and at risk HIV-uninfected women in the Womens Interagency HIV Study, higher leptin levels (indicative of higher adiposity) correlated strongly with poorer neurocognitive testing performance[88]. Similarly, in the Multicenter AIDS Cohort Study, VAT was strongly associated with regional brain atrophy (which precedes neurocognitive decline), irrespective of HIV serostatus[89]. As such, mounting evidence suggests that, similar to middle-aged persons in the general population, obesity and visceral adiposity may have detrimental effects on cognition among HIV-infected persons. Whether these relationships persist into older age and/or weight loss can improve cognitive function in HIV-infected persons requires further study.
Functional Decline
Obesity and the metabolic syndrome are established risk factors for the development of physical function impairment or frailty among middle-aged or older HIV-infected adults[90–93]. Obesity has also been associated with fall risk in HIV-infected women[94]. The exact mechanisms underlying these relationships are incompletely understood, but may be related to adipocytokine imbalances[95] and/or chronic inflammation and immune activation[96, 97]. Complicating this fact is the observation of faster rates of functional decline and high rates of sarcopenia among HIV-infected vs HIV-uninfected persons[98–101, 15], although sex differences may exist. As the obesity and aging epidemics in HIV-infected persons collide, understanding the mechanisms by which obesity and visceral adiposity contribute to functional decline will play an important role in maximizing physical function and preventing further decline in this population.
Interventions
Given the potential consequences of obesity and visceral adiposity in HIV infection, prevention and treatment of these states, including HIV-and ART-specific contributors, are important but incompletely understood components of HIV care. A complete review of therapeutic options for obesity and visceral adiposity in treated HIV infection is beyond the scope of this article, although a consensus guideline has recent been published[102]. To date, data on the efficacy of weight loss and dietary interventions in HIV are mixed, although structured exercise with or without dietary intervention reduces abdominal obesity in most studies[103–107], and disproportionate SAT loss has not been observed, eliminating fear of worsening of lipoatrophy in persons with mixed lipodystrophy. Based on available data, ≥30 minutes of moderate-intensity physical activity most days of the week plus reduction of caloric intake at least 500 kcal/day below usual intake is generally recommended to attain and sustain significant (≥5%) weight loss[108, 102]. While weight loss may be effective, recent data suggests that HIV-related stigma may prevent participation in traditional weight management programs[109], suggesting the possible need for programs specifically targeting HIV-infected persons.
The role of ART in weight management is evolving, as initiation of most preferred ART regimens (whether non-nucleoside reverse transcriptase inhibitor-, protease inhibitor- or integrase strand transfer inhibitor-based) is associated with gain of both subcutaneous adipose tissue and VAT, and data supporting ART switches to improve regional or generalized adiposity are lacking[102].
Tesamorelin is the only FDA-approved intervention to reduce visceral fat in HIV infection, and no pharmacologic weight loss interventions for generalized obesity[110] are specifically approved in HIV infection. As such, additional studies in HIV-infected populations are needed before specific pharmacologic therapies for fat loss can be recommended. However, as in the general population, persons with BMI ≥27 kg/m2 with comorbidity or BMI >30 kg/m2 without comorbidity are likely to receive benefits from weight loss[102], whether it be pharmacologic, diet and exercise-based and/or through behavioral modification.
Conclusions
Obesity and visceral adiposity in HIV-infected persons are frequent and have important physiologic consequences that contribute to morbidity and mortality. The pathophysiology of these states, while overlapping with that of the general population, likely includes HIV- and ART-specific risk factors. Further research is needed to define the pathophysiology of and develop interventions for obesity and lipohypertrophy in HIV-infected persons, with the ultimate goal of preventing morbidity and mortality in this vulnerable population.
Acknowledgments
This work was supported by National Institutes of Health grants K23 AI110532 to JEL.
Footnotes
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflicts of Interest
Jordan E. Lake has received research funding through her institution from Gilead Sciences and GSK, and has served as a consultant to Merck, Sharp and Dohme, Gilead Sciences and GSK.
References
- 1.Hamdy OUG, Oral EA. Obesity Practice Essentials. Medscape. 2016 [Google Scholar]
- 2.Study of Fat Redistribution and Metabolic Change in HIV Infection. Fat distribution in women with HIV infection. J Acquir Immune Defic Syndr. 2006;42(5):562–71. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown TT, Xu X, John M, Singh J, Kingsley LA, Palella FJ, et al. Fat distribution and longitudinal anthropometric changes in HIV-infected men with and without clinical evidence of lipodystrophy and HIV-uninfected controls: a substudy of the Multicenter AIDS Cohort Study. AIDS Res Ther. 2009;6:8. doi: 10.1186/1742-6405-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joy T, Keogh HM, Hadigan C, Dolan SE, Fitch K, Liebau J, et al. Relation of body composition to body mass index in HIV-infected patients with metabolic abnormalities. J Acquir Immune Defic Syndr. 2008;47(2):174–84. doi: 10.1097/QAI.0b013e31815b0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchacz K, Baker RK, Palella FJ, Jr, Shaw L, Patel P, Lichtenstein KA, et al. Disparities in prevalence of key chronic diseases by gender and race/ethnicity among antiretroviral-treated HIV-infected adults in the US. Antivir Ther. 2013;18(1):65–75. doi: 10.3851/IMP2450. [DOI] [PubMed] [Google Scholar]
- 6.Levy ME, Greenberg AE, Hart R, Powers Happ L, Hadigan C, Castel A, et al. High burden of metabolic comorbidities in a citywide cohort of HIV outpatients: evolving health care needs of people aging with HIV in Washington, DC. HIV Med. 2017 doi: 10.1111/hiv.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez D, Kalichman S, Cherry C, Kalichman M, Washington C, Grebler T. Dietary intake and overweight and obesity among persons living with HIV in Atlanta Georgia. AIDS Care. 2017;29(6):767–71. doi: 10.1080/09540121.2016.1238441. [DOI] [PubMed] [Google Scholar]
- 8.Erlandson KM, Taejaroenkul S, Smeaton L, Gupta A, Singini IL, Lama JR, et al. A Randomized Comparison of Anthropomorphic Changes With Preferred and Alternative Efavirenz-Based Antiretroviral Regimens in Diverse Multinational Settings. Open Forum Infect Dis. 2015;2(3):ofv095. doi: 10.1093/ofid/ofv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achhra AC, Mocroft A, Reiss P, Sabin C, Ryom L, de Wit S, et al. Short-term weight gain after antiretroviral therapy initiation and subsequent risk of cardiovascular disease and diabetes: the D:A:D study. HIV Med. 2016;17(4):255–68. doi: 10.1111/hiv.12294. [DOI] [PubMed] [Google Scholar]
- 10.Lakey W, Yang LY, Yancy W, Chow SC, Hicks C. Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses. 2013;29(3):435–40. doi: 10.1089/AID.2012.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.McComsey GA, Moser C, Currier J, Ribaudo HJ, Paczuski P, Dube MP, et al. Body Composition Changes After Initiation of Raltegravir or Protease Inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62(7):853–62. doi: 10.1093/cid/ciw017. A well-designed, head-to-head comparison of the metabolic effects of modern protease inhibitor- vs integrase strand transfer inhibitor-based ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mave V, Erlandson KM, Gupte N, Balagopal A, Asmuth DM, Campbell TB, et al. Inflammation and Change in Body Weight With Antiretroviral Therapy Initiation in a Multinational Cohort of HIV-Infected Adults. J Infect Dis. 2016;214(1):65–72. doi: 10.1093/infdis/jiw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuh B, Tate J, Butt AA, Crothers K, Freiberg M, Leaf D, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 2015;60(12):1852–9. doi: 10.1093/cid/civ192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, et al. Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr. 2012;61(5):600–5. doi: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Grant PM, Kitch D, McComsey GA, Collier AC, Bartali B, Koletar SL, et al. Long-term body composition changes in antiretroviral-treated HIV-infected individuals. Aids. 2016;30(18):2805–13. doi: 10.1097/QAD.0000000000001248. The longest published follow-up of body composiiton changes following ART initiation in HIV-infected persons, with comparison to an HIV-uninfected control group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhagwat P, Ofotokun I, McComsey GA, Brown TT, Moser C, Sugar CA, et al. Changes in abdominal fat following antiretroviral therapy initiation in HIV-infected individuals correlate with waist circumference and self-reported changes. Antivir Ther. 2017 doi: 10.3851/IMP3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhagwat POI, McComsey GA, Brown TT, Moser C, Sugar CA, Currier JS. Raltegravir is Associated with Greater Abdominal Fat Increases after Antiretroviral Therapy Initiation Compared to Protease Inhibitors. Abstracts from the 18th International Workshop of Co-morbidities and Adverse Drug Reactions in HIV; 2016. [Google Scholar]
- 18.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7(12):1221–35. doi: 10.1016/j.jcmg.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherzer R, Heymsfield SB, Lee D, Powderly WG, Tien PC, Bacchetti P, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. Aids. 2011;25(11):1405–14. doi: 10.1097/QAD.0b013e32834884e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch LA, O’Connell JM, Kwasnik AK, Cawood TJ, O’Farrelly C, O’Shea DB. Are natural killer cells protecting the metabolically healthy obese patient? Obesity (Silver Spring) 2009;17(3):601–5. doi: 10.1038/oby.2008.565. [DOI] [PubMed] [Google Scholar]
- 21.Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, et al. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86(3):1020–5. doi: 10.1210/jcem.86.3.7365. [DOI] [PubMed] [Google Scholar]
- 22.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98(10):E1610–9. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 23.Pujia A, Gazzaruso C, Ferro Y, Mazza E, Maurotti S, Russo C, et al. Individuals with Metabolically Healthy Overweight/Obesity Have Higher Fat Utilization than Metabolically Unhealthy Individuals. Nutrients. 2016;8(1) doi: 10.3390/nu8010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon JW, Jung CH, Kim MK, Park HE, Park KS, Jang HC, et al. Influence of the definition of “metabolically healthy obesity” on the progression of coronary artery calcification. PLoS One. 2017;12(6):e0178741. doi: 10.1371/journal.pone.0178741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng R, Liu C, Wang C, Zhou B, Liu Y, Pan F, et al. Natural Course of Metabolically Healthy Overweight/Obese Subjects and the Impact of Weight Change. Nutrients. 2016;8(7) doi: 10.3390/nu8070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lake JELX, Palella FJ, Erlandson K, Wiley D, Kingsley L, Jacobson LP, Brown TT. Metabolic Health Across the Body Mass Index Spectrum in HIV-Infected and HIV-Uninfected Men. Abstracts from the 17th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV; 2015. [Google Scholar]
- 27.Bourlier V, Sengenes C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, et al. TGFbeta family members are key mediators in the induction of myofibroblast phenotype of human adipose tissue progenitor cells by macrophages. PLoS One. 2012;7(2):e31274. doi: 10.1371/journal.pone.0031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haase J, Weyer U, Immig K, Kloting N, Bluher M, Eilers J, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57(3):562–71. doi: 10.1007/s00125-013-3139-y. [DOI] [PubMed] [Google Scholar]
- 29.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161(1):146–60. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19(14):2029–31. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- 32.Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–25. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaggini M, Saponaro C, Gastaldelli A. Not all fats are created equal: adipose vs. ectopic fat, implication in cardiometabolic diseases. Horm Mol Biol Clin Investig. 2015;22(1):7–18. doi: 10.1515/hmbci-2015-0006. [DOI] [PubMed] [Google Scholar]
- 34.Giralt M, Domingo P, Villarroya F. Adipose tissue biology and HIV-infection. Best Pract Res Clin Endocrinol Metab. 2011;25(3):487–99. doi: 10.1016/j.beem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(9):1820–6. doi: 10.1161/ATVBAHA.114.303035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlando G, Guaraldi G, Zona S, Carli F, Bagni P, Menozzi M, et al. Ectopic fat is linked to prior cardiovascular events in men with HIV. J Acquir Immune Defic Syndr. 2012;59(5):494–7. doi: 10.1097/QAI.0b013e31824c8397. [DOI] [PubMed] [Google Scholar]
- 37.Koethe JR, Hulgan T, Niswender K. Adipose tissue and immune function: a review of evidence relevant to HIV infection. J Infect Dis. 2013;208(8):1194–201. doi: 10.1093/infdis/jit324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Souza Dantas Oliveira SH, de Souza Aarao TL, da Silva Barbosa L, Souza Lisboa PG, Tavares Dutra CD, Margalho Sousa L, et al. Immunohistochemical analysis of the expression of TNF-alpha, TGF-beta, and caspase-3 in subcutaneous tissue of patients with HIV lipodystrophy syndrome. Microb Pathog. 2014;67–68:41–7. doi: 10.1016/j.micpath.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Utay NSKD, Fichtenbaum C, Lederman MM, Estes JD, Magyar C, Klingman KL, Currier JS, Lake JE. Telmisartan Does Not Improve Lymph Node or Fat Fibrosis in Treated HIV Infection. Abstracts from the 2017 Conference on Retroviruses and Opportunistic Infections; 2017. [Google Scholar]
- 40••.Damouche A, Lazure T, Avettand-Fenoel V, Huot N, Dejucq-Rainsford N, Satie AP, et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015;11(9):e1005153. doi: 10.1371/journal.ppat.1005153. A landmark paper identifying adipose tisue as a potential reservoir for HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Agarwal N, Balasubramanyam A. Viral mechanisms of adipose dysfunction: lessons from HIV-1 Vpr. Adipocyte. 2015;4(1):55–9. doi: 10.4161/adip.29852. Outlines an animal model important to our understanding of HIV-specific effects on adipose tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X, et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. Aids. 2015;29(6):667–74. doi: 10.1097/QAD.0000000000000599. The second landmark paper identifying adipose tisue as a potential reservoir for HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balagopal A, Philp FH, Astemborski J, Block TM, Mehta A, Long R, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135(1):226–33. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arslan N. Obesity, fatty liver disease and intestinal microbiota. World J Gastroenterol. 2014;20(44):16452–63. doi: 10.3748/wjg.v20.i44.16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masia M, Padilla S, Alvarez D, Lopez JC, Santos I, Soriano V, et al. Risk, predictors, and mortality associated with non-AIDS events in newly diagnosed HIV-infected patients: role of antiretroviral therapy. Aids. 2013;27(2):181–9. doi: 10.1097/QAD.0b013e32835a1156. [DOI] [PubMed] [Google Scholar]
- 46.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14(4):195–207. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 47.Conley LJ, Bush TJ, Rupert AW, Sereti I, Patel P, Brooks JT, et al. Obesity is associated with greater inflammation and monocyte activation among HIV-infected adults receiving antiretroviral therapy. Aids. 2015;29(16):2201–7. doi: 10.1097/QAD.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 48.Bonamichi B, Lee J. Unusual Suspects in the Development of Obesity-Induced Inflammation and Insulin Resistance: NK cells, iNKT cells, and ILCs. Diabetes Metab J. 2017 doi: 10.4093/dmj.2017.41.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stambullian M, Feliu MS, Cassetti LI, Slobodianik NH. Nutritional Status and Lipid Profile in HIV-Infected Adults. Endocr Metab Immune Disord Drug Targets. 2015;15(4):302–7. doi: 10.2174/1871530315666150907111120. [DOI] [PubMed] [Google Scholar]
- 50.Hulgan T, Boger MS, Liao DH, McComsey GA, Wanke CA, Mangili A, et al. Urinary eicosanoid metabolites in HIV-infected women with central obesity switching to raltegravir: an analysis from the women, integrase, and fat accumulation trial. Mediators Inflamm. 2014;2014:803095. doi: 10.1155/2014/803095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal F, Domingo P, Villarroya F, Giralt M, Lopez-Dupla M, Gutierrez M, et al. Adipogenic/lipid, inflammatory, and mitochondrial parameters in subcutaneous adipose tissue of untreated HIV-1-infected long-term nonprogressors: significant alterations despite low viral burden. J Acquir Immune Defic Syndr. 2012;61(2):131–7. doi: 10.1097/QAI.0b013e31825c3a68. [DOI] [PubMed] [Google Scholar]
- 52.Diaz-Delfin J, Domingo P, Wabitsch M, Giralt M, Villarroya F. HIV-1 Tat protein impairs adipogenesis and induces the expression and secretion of proinflammatory cytokines in human SGBS adipocytes. Antivir Ther. 2012;17(3):529–40. doi: 10.3851/IMP2021. [DOI] [PubMed] [Google Scholar]
- 53.Guaraldi G, Luzi K, Bellistri GM, Zona S, Domingues da Silva AR, Bai F, et al. CD8 T-cell activation is associated with lipodystrophy and visceral fat accumulation in antiretroviral therapy-treated virologically suppressed HIV-infected patients. Journal of acquired immune deficiency syndromes. 2013;64(4):360–6. doi: 10.1097/QAI.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 54.Srinivasa S, Fitch KV, Wong K, Torriani M, Mayhew C, Stanley T, et al. RAAS Activation Is Associated With Visceral Adiposity and Insulin Resistance Among HIV-infected Patients. The Journal of clinical endocrinology and metabolism. 2015;100(8):2873–82. doi: 10.1210/jc.2015-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boccara F, Auclair M, Cohen A, Lefevre C, Prot M, Bastard JP, et al. HIV protease inhibitors activate the adipocyte renin angiotensin system. Antivir Ther. 2010;15(3):363–75. doi: 10.3851/IMP1533. [DOI] [PubMed] [Google Scholar]
- 56•.Srinivasa S, Fitch KV, Wong K, Torriani M, Mayhew C, Stanley T, et al. RAAS Activation Is Associated With Visceral Adiposity and Insulin Resistance Among HIV-infected Patients. J Clin Endocrinol Metab. 2015:jc20151461. doi: 10.1210/jc.2015-1461. This study represents an important step in our understanding of the relationship between renin angiotensin system activation, visceral adiposity and its sequelae in HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gerard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73(1):147–62. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rietschel P, Hadigan C, Corcoran C, Stanley T, Neubauer G, Gertner J, et al. Assessment of growth hormone dynamics in human immunodeficiency virus-related lipodystrophy. J Clin Endocrinol Metab. 2001;86(2):504–10. doi: 10.1210/jcem.86.2.7175. [DOI] [PubMed] [Google Scholar]
- 59.Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging cell. 2010;9(5):667–84. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freitas P, Carvalho D, Santos AC, Madureira AJ, Martinez E, Pereira J, et al. Adipokines, hormones related to body composition, and insulin resistance in HIV fat redistribution syndrome. BMC Infect Dis. 2014;14:347. doi: 10.1186/1471-2334-14-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palella FJ, Jr, McKibben R, Post WS, Li X, Budoff M, Kingsley L, et al. Anatomic Fat Depots and Coronary Plaque Among Human Immunodeficiency Virus-Infected and Uninfected Men in the Multicenter AIDS Cohort Study. Open Forum Infect Dis. 2016;3(2):ofw098. doi: 10.1093/ofid/ofw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samaras K. The burden of diabetes and hyperlipidemia in treated HIV infection and approaches for cardiometabolic care. Curr HIV/AIDS Rep. 2012;9(3):206–17. doi: 10.1007/s11904-012-0124-x. [DOI] [PubMed] [Google Scholar]
- 63.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011;57(3):245–53. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 64.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13(8):453–68. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 65.Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. Aids. 2016;30(1):83–91. doi: 10.1097/QAD.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seang SKT, Currier JS, Lake JE. Endothelial Progenitor Cell Production is Suppressed and Associated with Systemic Inflammation and Monocyte Activation in Older HIV-Infected Men. Abstracts from IDWeek 2016. 2016 [Google Scholar]
- 67.Guaraldi G, Squillace N, Stentarelli C, Orlando G, D’Amico R, Ligabue G, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47(2):250–7. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 68.Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50(5):464–73. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46(3):312–7. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 70.Morse CG, McLaughlin M, Matthews L, Proschan M, Thomas F, Gharib AM, et al. Nonalcoholic Steatohepatitis and Hepatic Fibrosis in HIV-1-Monoinfected Adults With Elevated Aminotransferase Levels on Antiretroviral Therapy. Clin Infect Dis. 2015;60(10):1569–78. doi: 10.1093/cid/civ101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. Journal of gastroenterology and hepatology. 2013;28(Suppl 1):68–76. doi: 10.1111/jgh.12212. [DOI] [PubMed] [Google Scholar]
- 72.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 73.Lonardo A, Ballestri S, Guaraldi G, Nascimbeni F, Romagnoli D, Zona S, et al. Fatty liver is associated with an increased risk of diabetes and cardiovascular disease - Evidence from three different disease models: NAFLD, HCV and HIV. World J Gastroenterol. 2016;22(44):9674–93. doi: 10.3748/wjg.v22.i44.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230(2):258–67. doi: 10.1016/j.atherosclerosis.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 75.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci U S A. 2009;106(36):15430–5. doi: 10.1073/pnas.0904944106. 0904944106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–75. doi: 10.1053/j.gastro.2008.01.075. S0016-5085(08)00181-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vallet-Pichard A, Mallet V, Pol S. Nonalcoholic fatty liver disease and HIV infection. Seminars in liver disease. 2012;32(2):158–66. doi: 10.1055/s-0032-1316471. [DOI] [PubMed] [Google Scholar]
- 78.Vodkin I, Valasek MA, Bettencourt R, Cachay E, Loomba R. Clinical, biochemical and histological differences between HIV-associated NAFLD and primary NAFLD: a case-control study. Alimentary pharmacology & therapeutics. 2015;41(4):368–78. doi: 10.1111/apt.13052. [DOI] [PubMed] [Google Scholar]
- 79.Ghazarian M, Revelo XS, Nohr MK, Luck H, Zeng K, Lei H, et al. Type I Interferon Responses Drive Intrahepatic T cells to Promote Metabolic Syndrome. Sci Immunol. 2017;2(10) doi: 10.1126/sciimmunol.aai7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ray I, Mahata SK, De RK. Obesity: An Immunometabolic Perspective. Front Endocrinol (Lausanne) 2016;7:157. doi: 10.3389/fendo.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11(12):728–41. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 82.Matthews L, Kleiner DE, Chairez C, McManus M, Nettles MJ, Zemanick K, et al. Pioglitazone for Hepatic Steatosis in HIV/Hepatitis C Virus Coinfection. AIDS Res Hum Retroviruses. 2015;31(10):961–6. doi: 10.1089/AID.2015.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stanley TL, Feldpausch MN, Oh J, Branch KL, Lee H, Torriani M, et al. Effect of tesamorelin on visceral fat and liver fat in HIV-infected patients with abdominal fat accumulation: a randomized clinical trial. JAMA. 2014;312(4):380–9. doi: 10.1001/jama.2014.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tafesh ZH, Verna EC. Managing nonalcoholic fatty liver disease in patients living with HIV. Curr Opin Infect Dis. 2017;30(1):12–20. doi: 10.1097/QCO.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 85.Lemieux S, Prud’homme D, Bouchard C, Tremblay A, Despres JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64(5):685–93. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 86.Sattler FR, He J, Letendre S, Wilson C, Sanders C, Heaton R, et al. Abdominal obesity contributes to neurocognitive impairment in HIV-infected patients with increased inflammation and immune activation. J Acquir Immune Defic Syndr. 2015;68(3):281–8. doi: 10.1097/QAI.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okafor CN, Kelso NE, Bryant V, Burrell LE, 2nd, Miguez MJ, Gongvatana A, et al. Body mass index, inflammatory biomarkers and neurocognitive impairment in HIV-infected persons. Psychol Health Med. 2017;22(3):289–302. doi: 10.1080/13548506.2016.1199887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gustafson DR, Mielke MM, Keating SA, Holman S, Minkoff H, Crystal HA. Leptin, Adiponectin and Cognition in Middle-aged HIV-infected and Uninfected Women. The Brooklyn Women’s Interagency HIV Study. Journal of gerontology & geriatric research. 2015;4(5) doi: 10.4172/2167-7182.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89•.Lake JE, Popov M, Post WS, Palella FJ, Jr, Sacktor N, Miller EN, Brown TT, Becker JT. Visceral fat is associated with brain structure independent of human immunodeficiency virus infection status. J Neurovirol. 2017 Jun;23(3):385–393. doi: 10.1007/s13365-016-0507-7. This analysis of Multicenter AIDS Cohort participants documents increased VAT quantity as the clinical factor most strongly associated with brain atrophy, irrespective of HIV infection status. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erlandson KM, Wu K, Koletar SL, Kalayjian RC, Ellis RJ, Taiwo B, et al. Association Between Frailty and Components of the Frailty Phenotype With Modifiable Risk Factors and Antiretroviral Therapy. J Infect Dis. 2017;215(6):933–7. doi: 10.1093/infdis/jix063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shah K, Hilton TN, Myers L, Pinto JF, Luque AE, Hall WJ. A new frailty syndrome: central obesity and frailty in older adults with the human immunodeficiency virus. J Am Geriatr Soc. 2012;60(3):545–9. doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Young P, Shah J, Zhang C, Ferris DC, Colon I, Bucovsky M, et al. Frailty in Postmenopausal African American and Hispanic HIV-Infected Women. J Frailty Aging. 2016;5(4):242–6. doi: 10.14283/jfa.2016.104. [DOI] [PubMed] [Google Scholar]
- 93.Bauer LO, Wu Z, Wolfson LI. An obese body mass increases the adverse effects of HIV/AIDS on balance and gait. Phys Ther. 2011;91(7):1063–71. doi: 10.2522/ptj.20100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma A, Hoover DR, Shi Q, Holman S, Plankey MW, Wheeler AL, et al. Falls among middle-aged women in the Women’s Interagency HIV Study. Antivir Ther. 2016;21(8):697–706. doi: 10.3851/IMP3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shah KN, Majeed Z, Yang H, Guido JJ, Hilton TN, Polesskaya O, et al. Functional Limitations and Adipokines in Hiv-Infected Older Adults. J Frailty Aging. 2015;4(1):41–6. [PMC free article] [PubMed] [Google Scholar]
- 96.Margolick JB, Bream JH, Martinez-Maza O, Lopez J, Li X, Phair JP, et al. Frailty and Circulating Markers of Inflammation in HIV+ and HIV− Men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2017;74(4):407–17. doi: 10.1097/QAI.0000000000001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Erlandson KM, Ng DK, Jacobson LP, Margolick JB, Dobs AS, Palella FJ, Jr, et al. Inflammation, Immune Activation, Immunosenescence, and Hormonal Biomarkers in the Frailty-Related Phenotype of Men With or at Risk for HIV Infection. J Infect Dis. 2017;215(2):228–37. doi: 10.1093/infdis/jiw523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schrack JA, Althoff KN, Jacobson LP, Erlandson KM, Jamieson BD, Koletar SL, et al. Accelerated Longitudinal Gait Speed Decline in HIV-Infected Older Men. J Acquir Immune Defic Syndr. 2015;70(4):370–6. doi: 10.1097/QAI.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schrack JA, Jacobson LP, Althoff KN, Erlandson KM, Jamieson BD, Koletar SL, et al. Effect of HIV-infection and cumulative viral load on age-related decline in grip strength. Aids. 2016;30(17):2645–52. doi: 10.1097/QAD.0000000000001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr. 2013;63(2):209–15. doi: 10.1097/QAI.0b013e318289bb7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinto Neto LF, Sales MC, Scaramussa ES, da Paz CJ, Morelato RL. Human immunodeficiency virus infection and its association with sarcopenia. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2016;20(1):99–102. doi: 10.1016/j.bjid.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102••.Lake JE, Stanley TL, Apovian CM, Bhasin S, Brown TT, Capeau J, et al. Practical Review of Recognition and Management of Obesity and Lipohypertrophy in Human Immunodeficiency Virus Infection. Clin Infect Dis. 2017;64(10):1422–9. doi: 10.1093/cid/cix178. This article provides a concise, up-to-date consensus statement for the clinician on the diagnosis, pathophysiology, clinical assessment and treatment of obesity and lipohypertrophy in treated HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Engelson ES, Agin D, Kenya S, Werber-Zion G, Luty B, Albu JB, et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55(10):1327–36. doi: 10.1016/j.metabol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 104.Terry L, Sprinz E, Stein R, Medeiros NB, Oliveira J, Ribeiro JP. Exercise training in HIV-1-infected individuals with dyslipidemia and lipodystrophy. Med Sci Sports Exerc. 2006;38(3):411–7. doi: 10.1249/01.mss.0000191347.73848.80. [DOI] [PubMed] [Google Scholar]
- 105.Mutimura E, Crowther NJ, Cade TW, Yarasheski KE, Stewart A. Exercise training reduces central adiposity and improves metabolic indices in HAART-treated HIV-positive subjects in Rwanda: a randomized controlled trial. AIDS Res Hum Retroviruses. 2008;24(1):15–23. doi: 10.1089/aid.2007.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Becofsky K, Wing EJ, McCaffery J, Bodreau M, Wing RR. A Randomized, Controlled Trial of a Behavioral Weight Loss Program for HIV-Infected Patients. Clin Infect Dis. 2017 doi: 10.1093/cid/cix238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reeds DN, Pietka TA, Yarasheski KE, Cade WT, Patterson BW, Okunade A, et al. HIV infection does not prevent the metabolic benefits of diet-induced weight loss in women with obesity. Obesity (Silver Spring) 2017;25(4):682–8. doi: 10.1002/oby.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munro S, Dinatale E, Hartley S, St Jacques M, Oursler KA. Barriers and Health Beliefs Related to Weight Management Among Veterans With Human Immunodeficiency Virus. Mil Med. 2017;182(1):e1596–e602. doi: 10.7205/MILMED-D-16-00086. [DOI] [PubMed] [Google Scholar]
- 110.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA. 2016;315(22):2424–34. doi: 10.1001/jama.2016.76022528211. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]