Abstract
Inositol pyrophosphates are small, diffusible signaling molecules that possess the most concentrated three-dimensional array of phosphate groups in Nature; up to eight phosphates are crammed around a six-carbon inositol ring. This review discusses the physico-chemical properties of these unique molecules, and their mechanisms of action. Also provided is information on the enzymes that regulate the levels and hence the signaling properties of these molecules. This review pursues the idea that many of the biological effects of inositol pyrophosphates can be rationalized by their actions at the interface of cell signaling and metabolism that is essential to cellular and organismal homeostasis.
Keywords: cell signaling, inositol pyrophosphates, kinase, metabolism, phosphatase
1 | BACKGROUND
The dovetailing of metabolic circuitry with signal transduction cascades has constructed an extensive framework for multiple molecular interactions that are essential to cellular and organismal homeostasis (Gomes & Blenis, 2015). Among key players that act at this functionally bidirectional interface is an evolutionarily ancient group of signaling molecules known as the inositol pyrophosphates (PP-InsPs; Figure 1). In particular, several recent studies have shown that metabolic status can modulate the signaling activities of the PP-InsPs. Moreover, there is growing evidence that PP-InsPs themselves can manipulate cellular metabolism.
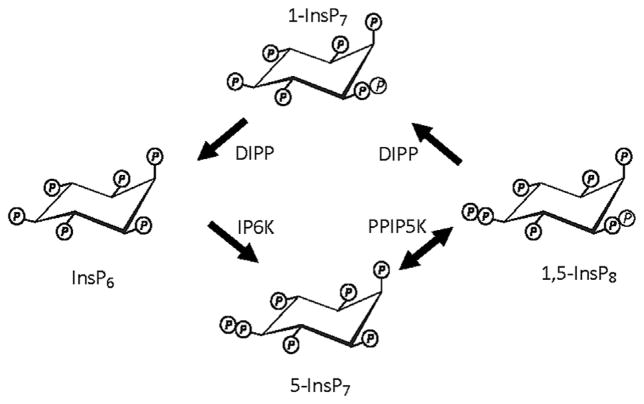
FIGURE 1.
A cyclical pathway for PP-InsP turnover. Current evidence (described in the text) indicates that the pathway for PP-InsP turnover may be described as a cyclical interconversion of InsP6, 5-InsP7, 1,5-InsP8, and 1-InsP7, upon which is superimposed “futile” cycling between 5-InsP7 and 1,5-InsP8 (see text for details). IP6K, inositol hexakisphosphate kinase; PPIP5K, diphosphoinositolpentakisphosphate kinase; DIPP, diphosphoinositol polyphosphate phosphatase
The importance of signaling cascades that regulate metabolism cannot be overstated: all biological programs place bioenergetic demands upon an organism; its ability to adapt to environmental challenges—that is, its long-term survival—is reliant upon dynamic control over uptake, storage, and utilization of various metabolic fuels. As noted previously (Wilson, Livermore, & Saiardi, 2013), many of the diverse activities of PP-InsPs can be rationalized by their being downstream of one fundamental function, namely, information transfer at the interface of metabolism and signaling. The purpose of this review is to assess our current understanding of the participation of PP-InsPs in these intricate regulatory processes, and to highlight future challenges and opportunities. This goal is assisted by an update on recent developments in our understanding of the enzymology of PP-InsP synthesis and metabolism.
2 | INTRODUCTION: 5-INSP7 , INSP8 , AND THEIR METABOLIC ENZYMES
PP-InsPs comprise a six-carbon inositol ring (“Ins”), around which are placed unique patterns of monophosphates (“P”) and pyrophosphates (“PP”). The PP-InsPs are members of the wider inositol phosphate signaling family, which all originate from inositol 1,4,5-trisphosphate (Ins(1,4,5)P3); the latter is released into cellular cytoplasm through the phospholipase C-mediated hydrolysis of PtdIns(4,5)P2, an inositol lipid, or “phosphoinositide.” Despite this metabolic connection between phosphoinositides and inositol phosphates, they each belong to two physicochemically and functionally distinct classes of signaling molecules. Through their diacylglycerol backbone, the phosphoinositides are embedded into membranes, or in some cases a hydrophobic pocket within certain, specialized proteins (Blind, Suzawa, & Ingraham, 2012). Clearly, phosphoinositides are not diffusible. In contrast PP-InsPs, like all soluble inositol phosphates, have the mobility to access the entire cytoplasm (it is presumed they can pass through nuclear pores, but there is no indication they can cross other membrane barriers).
PLC-generated Ins(1,4,5)P3 is phosphorylated by a series of kinase activities to inositol hexakisphosphate (InsP6), which comprises a relatively abundant precursor pool for two PP-InsPs in particular (Figure 1): 5-InsP7 (more technically: 5-diphosphoinositol 1,2,3,4,6- pentakisphosphate, or 5-PP-InsP5, so as to describe the presence of five monophosphates and one diphosphate at C-5; see Figure 1); 1,5-InsP8 (officially: 1,5-bis-diphosphoinositol 2,3,4,6-tetrakisphosphate, or 1,5-[PP]2-InsP4, so as to indicate four monophosphates, and two diphosphates at C-1 and C-5; Figure 1). Both 5-InsP7 and 1,5-InsP8 are near ubiquitous in yeast and animal cells. However, it is worth noting that certain Dictyostelids have been reported to contain a second, more predominant InsP8 isomer, with diphosphates at positions 5 and 6 (Laussmann et al., 1998). The biological significance of this alternative PP-InsP remains to be established; what we know about the metabolism and function of “InsP8” comes from studies of the 1,5-isomer.
InsP6 kinases—or IP6Ks (kcs1 in Saccharomyces cerevisiae)—utilize ATP as a phosphate donor to add a 5-β-phosphate to InsP6 (i.e., they exhibit a 5-kinase activity). Mammals express three IP6Ks, types 1, 2, and 3, that range in mass from 46 to 49 kDa. By solving the structure of an IP6K homologue from Entamoeba histolytica (Wang, DeRose, London, & Shears, 2014), it has been established that this family of kinases binds ATP between two lobes formed by the N- and C-termini. A third —”IP-binding”—lobe grips InsP6 through opposing helical structures that have been likened to the arrangement of an open clamshell, that lies in a shallow depression in the enzyme surface (Wang, DeRose, et al., 2014). IP6K2 is unique within this family in having a regulatory 12 amino-acid insert that inhibits kinase activity upon its association with heat-shock protein-90 (HSP90) (Chakraborty et al., 2008).
A separate family of enzymes adds a second β-phosphate at C-1 (i.e., they exhibit a 1-kinase activity); these are the PPIP5Ks—the standard abbreviation for diphosphoinositol-pentakisphosphate kinases. The primary substrate is 5-InsP7, with lesser activity shown toward InsP6 (Weaver, Wang, & Shears, 2013). The PPIP5K orthologues in S. cerevisiae and Schizosaccharomyces pombe are known as vip1 and asp1, respectively; two corresponding proteins in Arabidopsis have been named VIH1 and VIH2 (Laha et al., 2015).
There are two PPIP5K genes in mammals: types 1 and 2 (Figure 2). These encode relatively large proteins: 160 (for PPIP5K1) and 140 kDa (for PPIP5K2). The kinase domain (Figures 2 and 3a) is self-contained within the N-terminal one-third of their amino-acid sequences (Fridy, Otto, Dollins, & York, 2007; Mulugu et al., 2007). This domain binds ATP between two sets of anti-parallel beta sheets—a so-called “ATP-grasp” kinase (Wang, Falck, Hall, & Shears, 2012). To ensure that only InsP6 or 5-InsP7 may enter the active site and be phosphorylated, there are two near parallel grooves on the surface of the kinase domain that form a sterically constraining, “staggered-H” pocket (Figure 3a). For such highly charged substrates, it could be anticipated that an important role in ligand–protein binding is played by electrostatic steering; electropositive residues that act as a “tractor-beam” that pulls negatively charged substrate toward the entrance of the catalytic site (Wade, Gabdoulline, Ludemann, & Lounnas, 1998). However, PPIP5K utilizes a more advanced and highly unusual mechanism to acquire substrate from the bulk phase: an actual ligand-binding site on the protein s surface (Figure 3b). Once within this “capture-site,” the bound substrate is optimally oriented for being flipped into the catalytic pocket (Wang et al., 2012; Wang, Godage, et al., 2014). This catch-and-pass reaction mechanism is proposed to significantly enhance catalytic activity (Figure 3b–d).
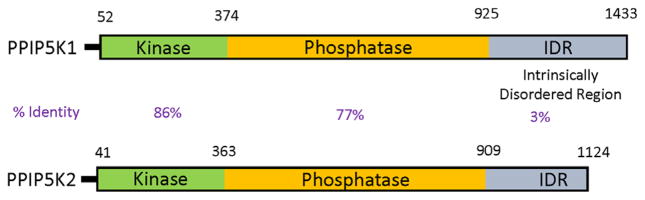
FIGURE 2.
Domain graphics of human PPIP5K1 and PPIP5K2. Domain graphics are shown for the human PPIP5K1 (Accession number BC057395.1) and PPIP5K2 (accession number XM_005271938); IDR, intrinsically disordered domain. The derivation of the amino-acid boundaries of each domain is as previously described (Gu et al., 2017). The % sequence identities across each of the three domains are provided
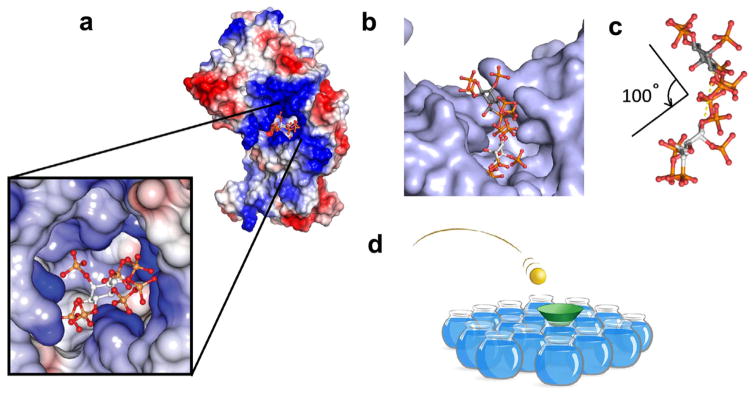
FIGURE 3.
PPIP5Ks utilize a substrate capture site and a catalytic site for an unusual “catch-and-pass” reaction mechanism. (a) Electrostatic surface rendering of the structure of the kinase domain of human PPIP5K2. Blue represents electropositive, and red indicates electronegative. A magnified image of the catalytic site is also shown. Note that two near parallel grooves form a “staggered-H” that ensures only InsP6 or 5-InsP7 may enter the active site and be phosphorylated. (b) Surface representation of PPIP5K highlighting the binding of a 5-phosphonoacetate analogue of 5-InsP7 to both a surface mounted “substrate capture” site and a catalytic site (note that steric constraints prevent both sites from being occupied simultaneously) (Wang, Godage, et al., 2014). (c) Depiction of the molecular gymnastics required for delivery of substrate between the two sites: a 100° ring flip and a lateral movement (broken yellow line) of 7 Å (Wang, Godage, et al., 2014). (d) To avoid the sterically restricted active site constraining catalytic activity, does substrate in the bulk phase (the ping-pong ball) have an increased probability of being appropriately oriented to enter the active site (the neck of the goldfish bowl) because of the proximal substrate capture site (the green cone)?
Another unconventional feature of members of the PPIP5K family—as was initially recognized by York and coworkers (Fridy et al., 2007; Mulugu et al., 2007)—is that they also contain a phosphatase domain. Only 1-InsP7 and 1,5-InsP8 are substrates, and in each case it is the 1-β-phosphate that is hydrolyzed (Gu et al., 2017; Wang et al., 2015). This exquisite specificity remains to be rationalized at a structural level. We will return in section 8 to studies into the significance of this 1-kinase/1-phosphatase substrate cycle being catalyzed by a single protein. Finally, the C-termini of both PPIP5K1 and PPIP5K2 are disordered domains (Figure 2) that participate in protein–protein interactions (Machkalyan, Trieu, Petrin, Hebert, & Miller, 2016).
Eukaryotic animal models have been successfully utilized to gain considerable insight into PP-InsP biology. Some murine phenotypes are listed here: IP6K1−/− mice exhibit impaired hemostasis (Ghosh et al., 2013), a 30–35% reduction in circulating insulin levels (Bhandari, Juluri, Resnick, & Snyder, 2008), and decreased susceptibility to carcinogen-induced aerodigestive tract carcinoma, likely through a reduction in rates of cell migration and invasion (Jadav et al., 2016). Male IP6K1−/− mice are also infertile (Bhandari et al., 2008). Interestingly, some of these phenotypes are not observed in IP6K2−/− mice. These particular animals have higher susceptibility to the same carcinoma-inducing regimen (Morrison et al., 2009), male fertility is not compromised (Morrison et al., 2009), and insulin levels are normal (Morrison et al., 2009). IP6K3−/− mice also breed normally (Moritoh et al., 2016). These differences between the knock-out strains may reflect tissue-specific differences in expression of the alternative IP6K genes. IP6K3−/− mice display several features that are indicative of an activation of glycolysis: reduced serum glucose levels, elevated lactate levels, and a reduction in expression of pyruvate dehydrogenase 4 in skeletal muscle, where IP6K3 is highly expressed (Moritoh et al., 2016). Such metabolic changes have been theorized to contribute to the extended lifespan of the IP6K3−/− mice (Moritoh et al., 2016). At the current time, there are no published investigations into the phenotypes of PPIP5K knock-out mice.
Animal knock-out models are known to have some limitations for characterizing the physiological significance of a particular PP-InsP. For example, an IP6K gene deletion does not solely compromise the synthesis of 5-InsP7; also attenuated is the production of 1,5-InsP8 (see Figure 1) and the pyrophosphate derivatives of Ins(1,3,4,5,6)5 (see section 4). Thus, additional experiments are required, before loss of a particular PP-InsP can be held responsible for a phenotype. A separate confounding issue is that the functional consequence of an IP6K deletion may not even reflect loss of PP-InsP production, but instead the elimination of crucial, non-catalytic protein–protein interactions. Indeed, in one illustrative study, morphological defects in cerebellar Purkinje cells of IP6K3−/− mice were found to result from disruption of the binding to IP6K3 of the cytoskeletal proteins adducin and spectrin (Fu et al., 2015). Those seeking access to the literature that provides more information on non-catalytic effects of IP6Ks and PPIP5Ks could begin with other reviews that cover this topic (Thota & Bhandari, 2015). Such work is not the primary focus of this review, which is more specifically concerned with the PP-InsPs themselves. Nevertheless, the occurrence of these functionally significant protein–protein interactions illustrates how helpful it is to use pharmacology as well as genetics to pursue functions of IP6Ks and PPIP5Ks. A pan-IP6K inhibitor, TNP (N2-(m-Trifluorobenzyl), N6-(p-nitrobenzyl) purine) (Padmanabhan, Dollins, Fridy, York, & Downes, 2009), has been deployed in some recent studies. For example, dosing of mice with TNP recapitulates both the elevated insulin sensitivity and the resistance to diet-induce obesity that are evident in the IP6K1−/−mouse model (Ghosal et al., 2016). However, there are concerns that arise from reports of some off-target effects of TNP (Ghosal et al., 2016), so it would be useful if more specific inhibitors could be developed. The preparation of isoform-selective IP6K inhibitors would be of particular benefit, since the three mammalian IP6K genes are not redundant (see above in Section 2).
Naturally, what goes up must come down. Thus, 1,5-InsP8 is dephosphorylated back to InsP6 (Figure 1). A family of diphosphoinositol polyphosphate phosphatases (DIPPs) cleave both the 1- and 5-diphosphate groups from all PP-InsPs. Nevertheless, enzymatic data that we obtained in vitro indicate that DIPPs preferentially hydrolyze the 5-phosphate from 1,5-InsP8 (Kilari, Weaver, Shears, & Safrany, 2013). The latter work supports our contention that net metabolic flux through the PP-InsP pathway is cyclical in nature (Figure 1, and see section 3).
Mammals contain four genes that encode five slightly different DIPP proteins (Kilari et al., 2013); the selective advantage of such diversity has not been adequately rationalized. These phosphatases are distributed through animals and yeasts; they are all approximately 20 kDa in size. Somewhat disappointingly (at least for those who have studied them!), there is no demonstrated mechanism by which the phosphatase activities of any members of the DIPP family might be regulated. On the other hand, polymorphism of the NUDT3 gene that encodes DIPP1 has been associated with an increased body mass index and obesity (Goumidi, Cottel, Dallongeville, Amouyel, & Meirhaeghe, 2014); such data strongly hint at the relevance of properly regulated PP-InsP catabolism to metabolic homeostasis.
Recently, there has been a description of a novel PP-InsP 5-phosphatase that is expressed in S. cerevisiae: siw14 (Steidle et al., 2016). This protein hosts a C(X5)R signature that is also found in some protein- and phosphoinositide-phosphatases (Pulido, Stoker, & Hendriks, 2013). The discovery that Siw14 is a PP-InsP phosphatase (Steidle et al., 2016) establishes the first member of a new subgroup within the C(X5)R family. Whether or not there may be others, especially in animal cells, is a question that needs to be addressed.
3 | ANOTHER INOSITOL PYROPHOSPHATE: 1-INSP7
In addition to 5-InsP7, there is a second, naturally occurring InsP7 isomer: 1-InsP7 (Lin et al., 2009; Wang et al., 2012). This can be synthesized by direct phosphorylation of InsP6 by the PPIP5Ks (Mulugu et al., 2007). Steady-state levels of 1-InsP7 in yeasts and mammalian cells are as little as 0.05 μM, which is hovering around the limits of detection, and much lower than any other inositol phosphate signal (Lin et al., 2009; Onnebo & Saiardi, 2009; Gu, Wilson, Jessen, Saiardi, & Shears, 2016). This observation may be considered evidence that the 1-kinase phosphorylation of InsP6 is a quantitatively minor reaction within the PP-InsP pathway. In fact, additional genetic (Onnebo & Saiardi, 2009) and pharmacological data (Padmanabhan et al., 2009), as well as kinetic considerations (Weaver et al., 2013), also strongly support the idea that the predominant pathway for 1,5-InsP8 synthesis is through 5-InsP7 (as depicted in Figure 1). Indeed, in S. cerevisiae, significant net phosphorylation of InsP6 to 1-InsP7 requires not one but two gene deletions: first, kcs1 (the IP6K ortholog), to prevent synthesis of 5-InsP7, the preferred substrate for vip1; second, ddp1 (the DIPP ortholog) to eliminate all 1-InsP7 dephosphorylation. Indeed, what little 1-InsP7 does accumulate in cells may actually derive largely from 1,5-InsP8 (see Figure 1 and Kilari et al., 2013).
One study with S. cerevisiae described many-fold elevations in 1-InsP7 levels upon depletion of extracellular inorganic phosphate (Pi) (Lee, Mulugu, York, & O’Shea, 2007) (note that 1-InsP7 is mis-referenced as 4/6-InsP7 in some prior literature). The 1-InsP7 was shown to augment inhibition by Pho81 of the cyclin kinase activity of Pho80 (Lee et al., 2007; Lee, Huang, Quiocho, & O’Shea, 2008), rendering the latter incapable of phosphorylating the transcription factor Pho4. In such a situation, Pho4 becomes competent to enter the nucleus to drive the transcription of genes important for phosphate generation and assimilation, such as a phosphate transporter and a secreted acid phosphatase. Thus, 1-InsP7 was put forward as mediating an adaptive response to Pi limitation: increased expression of gene products that can scavenge phosphate from organic molecules. This discovery generated considerable excitement; in its organic form, Pi is a component of genomic material, it is ubiquitous in cell signaling, and it serves as an energy currency. Thus, 1-InsP7 appeared at the epicenter of a metabolic response—Pi accumulation—that is fundamental to metabolic regulation. Unfortunately, others have been unable to reproduce the key finding that 1-InsP7 levels increase during phosphate starvation (Lonetti et al., 2011; Wild et al., 2016). As a consequence, although the specific binding of 1-InsP7 by the Pho80/Pho85/Pho81 complex is well-characterized, its biological significance is now uncertain. Ironically, quite different links between PP-InsP turnover and Pi homeostasis have recently emerged, although 1-InsP7 is not involved (see section 8).
The 1-kinase activity of PPIP5Ks has also been shown to have a role in certain innate immune responses, such as those that occur in response to viral invasion (Pulloor et al., 2014). In this situation, double stranded viral DNA is detected by the retinoic-acid inducible gene (RIG-1) product, which phosphorylates the IRF3 transcription factor, thereby promoting interferon transcription (Pulloor et al., 2014). Both 1-InsP7 and 1,5-InsP8 have been shown to stimulate this immune response, by enhancing the degree of IRF3 phosphorylation (Pulloor et al., 2014). In reconstituted IRF3 phosphorylation assays, 1-InsP7 is the more potent of the two PP-InsPs, but its levels in mammalian cells are 10-fold or more lower than those of 1,5-InsP8 (Gu et al., 2016); the latter may, therefore, be the more physiologically active product of PPIP5K 1-kinase activity. It has not yet been determined how either of these PP-InsPs might influence IRF3 phosphorylation; perhaps there is regulation of an IRF3 kinase and/or phosphatase.
Immune responses are bioenergetically expensive; it takes a considerable energy investment by innate immune cells to synthesize and secrete a battery of cytokines and inflammatory mediators. However, immune cells lack significant stores of nutrients, so these protective responses require uptake of significant quantities of metabolic fuels (Ganeshan & Chawla, 2014). It might not be a coincidence that changes in PP-InsP turnover that drive immune responses may also have roles in directing homeostatic metabolic responses.
Although orthologs of the RIG-1/IRF3 pathway have not been found in plants (Williams, Gillaspy, & Perera, 2015), it is intriguing that innate immunity in Arabidopsis is also enhanced by a PPIP5K activity (VIH2) (Laha et al., 2015). In seedlings of this particular plant, cellular levels of InsP8 are almost doubled by the actions of methyl-jasmonate, one of a group of plant hormones that helps protect plants from herbivorous insects and necrotrophic fungi (Laha et al., 2015). This protective response is impaired in lines of A. thaliana in which the kinase domain of VIH2 is disrupted by insertional mutagenesis (Laha et al., 2015). It has been suggested that InsP8 may augment transcriptional events that are activated by methyl-jasmonate (Laha et al., 2015).
In no member of the plant kingdom has the positions of the two diphosphate groups of InsP8 been determined; this situation is rendered more uncertain by the failure to identify an InsP6 kinase that might synthesize 5-InsP7 (Laha et al., 2015; Williams et al., 2015). Nevertheless, when a vip1Δ strain of S. cerevisiae was transformed with AtVIH2, levels of 5-InsP7 were reduced and InsP8 synthesis was rescued (Laha et al., 2015). Such data indicate that AtVIH2 is capable of transforming 5-InsP7 into 1,5-InsP8, if given that opportunity. Perhaps in plants there is an alternative InsP6 5-kinase activity, the nature of which remains to be described (Williams et al., 2015). It has also been speculated that, in plants, the InsP8 may be a novel diphosphate isomer, perhaps 1,3-InsP8, or even a triphosphate, 1-PPP-InsP5, perhaps synthesized by further phosphorylation of 1-InsP7 by PPIP5Ks; if so, this would mean these enzymes are more catalytically flexible than their mammalian counterparts (Williams et al., 2015). Hopefully these uncertainties will shortly be resolved.
4 | INOSITOL PYROPHOSPHATES SYNTHESIZED FROM INSP5
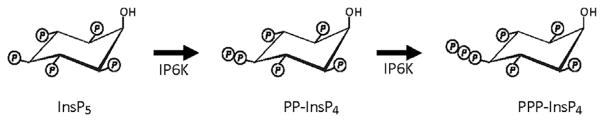
Although the catalytic activity of the IP6Ks is primarily studied for its role in 5-InsP7 synthesis, Ins(1,3,4,5,6)P5 is a secondary substrate (Figure 4). The immediate product that is formed is typically denoted as “PP-InsP4” (Saiardi, Caffrey, Snyder, & Shears, 2000); the latter, in vitro, can be further phosphorylated to a limited extent by IP6Ks to yield a second, triphosphorylated molecule, 5-PPP-InsP4 (Draskovic et al., 2008). However, there is no good evidence this last reaction has physiological relevance, and the phenomenon may instead reflect the patience of the experimenter who has access to large amounts of recombinant enzyme. These pyrophosphates of Ins(1,3,4,5,6)P5 (Figure 4) are not well studied, outside two separate studies with yeasts which describe evidence that these PP-InsPs may inhibit Tel1, a phosphoinositide 3-kinase related kinase that normally maintains telomere length (Saiardi, Resnick, Snowman, Wendland, & Snyder, 2005; York, Armbruster, Greenwell, Petes, & York, 2005). The human orthologue of Tel-1 is ataxia telangiectasia mutated (ATM). It is intriguing to consider that PP-InsPs may also inhibit ATM. One of the (many) consequences of down-regulation of ATM is metabolic reprogramming: increased glucose and glutamine consumption, and up-regulation of the pentose phosphate pathway (Aird et al., 2015). This could provide another link between PP-InsPs and metabolic regulation.
FIGURE 4.
Phosphorylation of Ins(1,3,4,5,6)P5 by IP6Ks. The figure summarizes data obtained in vitro which demonstrate that two PP-InsPs can be generated by IP6K-mediated phosphorylation of Ins(1,3,4,5,6)P5 (Draskovic et al., 2008)
5 | PHYSICOCHEMICAL PROPERTIES OF PP-INSPs DRIVE MECHANISMS OF ACTION: PROTEIN PYROPHOSPHORYLATION
The PP-InsPs have an intense concentration of phosphate groups within a relatively small three-dimensional space—as illustrated by the electron density map of an 1,5-InsP8 molecule (Figure 5) that was captured in the active site of a crystal complex of the kinase domain of PPIP5K (Capolicchio, Wang, Thakor, Shears, & Jessen, 2014). Since phosphates are strongly electronegative at physiological pH, PP-InsPs exhibit a mother-of-all degree of charge density. These phosphate groups are also bulky. Thus, a significant free energy change occurs upon hydrolysis of a PP-InsP’s diester-phosphate, due to significant electrostatic, solvation, and resonance stabilization phenomena. Indeed, PP-InsPs are often said to be highly “energetic” (Wilson et al., 2013). Hence the origin of the idea that this free energy change might be sufficient to promote protein phosphorylation (Stephens et al., 1993; Voglmaier et al., 1996). The first demonstrations of this phenomenon are contained in two influential papers published by Snyder and coworkers (Saiardi et al., 2004; Bhandari et al., 2007). These studies also show that the specific target is a Ser that is surrounded by acidic residues. This residue must first be primed by its phosphorylation by casein kinase 2 (CK2). Then comes the highly “energetic” step: the PP-InsP donates a β-phosphate group to this phosphoserine in a Mg2+-dependent but non-enzymatic reaction. That is, the net result is protein pyrophosphorylation.
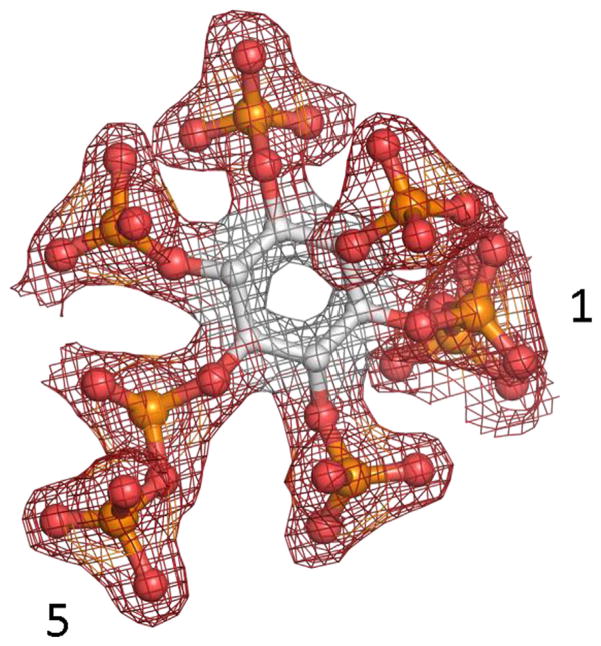
FIGURE 5.
Electron density map of 1,5-InsP8. 1,5-InsP8 is depicted as a refined 2 Fo-Fc map contoured at 2.0 ςthat is shown in blue mesh. This information was captured from crystals of the kinase domain of PPIP5K2 that also contained ADP (Capolicchio et al., 2014). The 1- and 5-diphosphate groups are numbered
In vitro, protein pyrophosphorylation has been observed by monitoring the direct transfer to proteins of [32P] from a [32P]-labeled PP-InsP. The efficiency of protein pyrophosphorylation depends only on the concentration of the PP-InsP donor, rather than the precise arrangement of the diphosphate groups (Bhandari et al., 2007). It might seem a little puzzling from a signaling perspective that each of the individual PP-InsPs have similar abilities to phosphorylate proteins in vitro. Such a scenario might appear to make redundant those molecular mechanisms that are known to independently regulate levels of 5-InsP7 or 1,5-InsP8 (such as that described by Gu et al., 2017; also see section 8). Nevertheless, under most physiological circumstances, protein pyrophosphorylation could in practice be specific to 5-InsP7, by virtue of it being the predominant PP-InsP species (1–5 μM (see Ingram, Safrany, & Barnes, 2003; Illies et al., 2007; Wilson et al., 2013). In fact, the irrelevance of isomer specificity to one important function for 5-InsP7 is clear from experiments in which this PP-InsP was shown to stimulate insulin secretion from pancreatic β-cells (Illies et al., 2007). In that study, each of the other five InsP7 isomers were chemically synthesized and found to as effective as 5-InsP7 in the insulin-secretion assay (Illies et al., 2007). But in vivo, only 5-InsP7 would attain the levels required to accomplish this function.
The role of 5-InsP7 in insulin secretion is very pertinent to metabolic homeostasis in higher animals; it will be important to determine the underlying molecular mechanisms. It seems that 5-InsP7 increases the number of secretory vesicles that are primed to release insulin during the initial phase of glucose-induced exocytosis (Illies et al., 2007). Nevertheless, 5-InsP7 is not a general activator of exocytosis. In fact, 5-InsP7 inhibits neurotransmitter release from synaptic vesicles (Lee et al., 2016). In the latter study, by binding to the C2AB Ca2+-sensing domain of synaptotagmin-1, 5-InsP7 appeared to attenuate vesicle fusion with the plasma membrane. Interestingly, this response showed isomer specificity: 1-InsP7 and InsP6 were consider-ably less effective (Lee et al., 2016). Synaptotagmins are also functionally important for insulin secretion; the contrasting effects of 5-InsP7 upon two exocytic processes (insulin secretion versus neurotransmitter release) reinforce the differences in the mechanisms by which large dense core vesicles or synaptic vesicles are commanded to release their cargo.
The best-characterized targets of protein pyrophosphorylation by 5-InsP7 can be found in five separate studies with the following proteins: AP3B1, the β-subunit of the adaptor protein complex AP-3 (Azevedo, Burton, Ruiz-Mateos, Marsh, & Saiardi, 2009); dynein intermediate chain (Chanduri et al., 2016); three subunits of the RNA polymerase complex (Pol I) in S. cerevisiae, (Thota, Unnikannan, Thampatty, Manorama, & Bhandari, 2015) and the glycolytic genes transcriptional activator 1 (Szijgyarto, Garedew, Azevedo, & Saiardi, 2011). Nevertheless, it has not yet been directly demonstrated that protein pyrophosphorylation by PP-InsPs occurs in vivo; there are several technical limitations. To illustrate, no-one has so far produced any pan-specific, anti-serine-pyrophosphate antibodies. As noted elsewhere (Williams & Fiedler, 2015), it is a challenge to develop such an antibody, since anti-phosphoserine antibodies generally tend to be context specific; the sequence surrounding the serine is expected to contribute to the epitope, thereby restricting the general utility of any antibody that might be produced. There are also serious impediments to the identification of pyrophosphorylated peptides by mass spectrometry (see Williams & Fiedler, 2015): the lability of phospho-serines, the possibility of low stoichiometry of the modification, and an inability to distinguish a diphosphate within a peptide sequence from two proximal monophosphates. Thus, other options are being considered. Rather promising is the recent design of a fluorescent sensor based on a dinuclear zinc complex that was shown to have nanamolar affinity for a serine pyrophosphate within a synthetic polypeptide (Williams & Fiedler, 2015).
Notwithstanding an ultimate goal that methods be developed that directly assay protein pyrophosphorylation in vivo, Saiardi and coworkers (Azevedo et al., 2009) introduced an indirect means to study this phenomenon in cell lysates. These experiments were performed in their work with AP3B1, pyrophosphorylation of which was found to reduce its ability to associate with Kinesin Family Member 3A (Azevedo et al., 2009), a microtubule-based motor that participates in bidirectional transport of organelles, granules and vesicles. Saiardi’s group realized that any protein that is amenable to pyrophosphorylation in intact cells may also be pyrophosphorylated by 5-InsP7 in vitro, but only if it were not to be naturally pyrophosphorylated before the lysate is prepared; this is the basis for a so-called back-phosphorylation assay (Figure 6a,b). Thus, exogenous GST-tagged human AP3B1 was exogenously expressed in three strains of S. cerevisiae: wild-type, ksc1Δ (with negligible levels of 5-InsP7) and vip1Δ (elevated levels of 5-InsP7; Onnebo & Saiardi, 2009). Next, the AP3B1 was immunoaffinity purified and incubated with [32P]-labeled 5-InsP7 in vitro. The degree of pyrophosphorylation in vitro was inversely proportional to the cellular levels of 5-InsP7 (and hence the presumed degree of AP3B1 pyrophosphorylation in intact cells) (Azevedo et al., 2009).
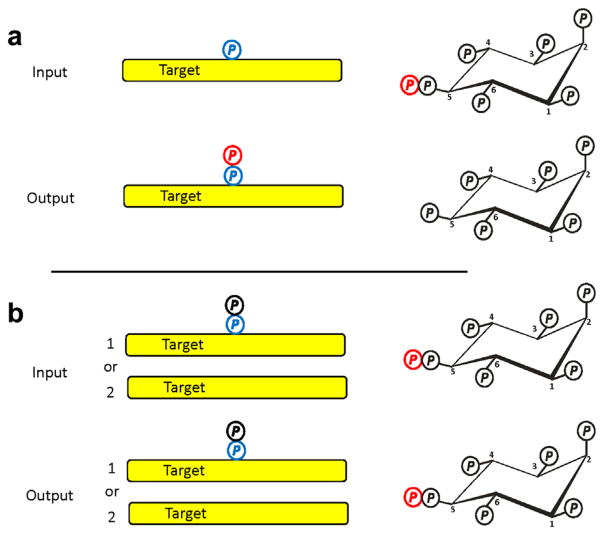
FIGURE 6.
Assessing the status of protein pyrophosphorylation in vivo by a back-pyrophosphorylation assay in vitro. (a) Idealized assay input and output are shown for an in vitro, back-phosphorylation assay. The input is 5-β-32P-InsP7 and a purified target protein that, in vivo, had been phosphorylated by CK2 (blue “P”), but not pyrophosphorylated by a PP-InsP. The assay “output” is pyrophosphorylated target (red “P”) and InsP6. The conclusion to be drawn from this outcome is that the target was not pyrophosphorylated in vivo. (b) Describes two different assay inputs that both lead to an assay output showing no protein pyrophosphorylation: condition 1 is target pyrophosphorylation in vivo; condition 2 is no target phosphorylation by CK2 in vivo. Thus, the assay output data are ambiguous as to the phosphorylation status of the target in vivo. This problem can be resolved with a second, independent back-phosphorylation assay in vitro, using CK2. The latter will only phosphorylate the target if condition 2 is satisfied. The absence of CK2-mediated phosphorylation in vitro would support condition 1: target pyrophosphorylation in vivo
However, Ser pyrophosphorylation in vivo first requires that the residue be primed by CK2-mediated phosphorylation (see Figure 6). Furthermore, CK2 activity is itself activated by 5-InsP7 (Rao et al., 2014). Thus, changes in cellular levels of 5-InsP7 can modify the extent to which a target protein is phosphorylated by CK2. This situation creates ambiguities for the back-phosphorylation assay: a Ser in a target protein that is pyrophosphorylated cannot be distinguished from one that is not mono-phosphorylated (Figure 6b).
Thus, Chanduri et al. (2016) added an additional experimental step in their study with dynein intermediate chain: back-phosphorylation of their target protein by CK2. In that study, the extent to which immunoaffinity purified dynein intermediate chain was back-phosphorylated by CK2 in vitro was similar for material obtained from either wild-type and IP6K1−/− MEFs. This observation confirmed that CK2 activity toward dynein intermediate chain in vivo is not modified by the IP6K knock-out. These data strengthen the conclusion derived from their back-phosphorylation assays with [32P]-labeled 5-InsP7 that were performed in parallel: that is, knock-out of the IP6K1 gene inhibited pyrophosphorylation of dynein intermediate chain in vivo.
As for the biological significance, pyrophosphorylation of dynein intermediate chain stabilizes its interaction with p150Glued in the dynactin motor protein complex, facilitating its membrane binding (Chanduri et al., 2016). Furthermore, trafficking processes that are regulated by dynein intermediate chain were both attenuated in IP6K1−/− MEFs: transferrin transport and phagasome mobility (Chanduri et al., 2016).
A separate study of the biological consequences of protein pyrophosphorylation concluded that this was the mechanism by which 5-InsP7 stimulates Pol I mediated transcriptional elongation (Thota et al., 2015). The latter study also noted that Pol I-mediated rRNA synthesis is sensitive to cellular energy status, and so it was hypothesized that the linking of 5-InsP7 levels to ribosome biogenesis might reflect an adaptive mechanism to adjust energy consumption (Thota et al., 2015).
A more direct link between inositol pyrophosphate signaling and bioenergetic homeostasis emerged from the studies with S. cerevisiae into pyrophosphorylation by 5-InsP7 of glycolytic genes transcriptional activator 1 (GCR1) (Szijgyarto et al., 2011). This pyrophosphorylation destabilizes the association of GCR1 with GCR2 and RAP1, thereby reducing the binding affinity of this transcriptional complex for the corresponding promotor of glycolytic genes (Szijgyarto et al., 2011). Thus, in a ksc1Δ strain of S. cerevisiae, there are higher levels of expression of glycolytic genes, and elevated ATP levels (Szijgyarto et al., 2011). These observations speak directly to a role for PP-InsPs in cellular energy homeostasis. But it is arguable that this work yielded an even more dramatic observation: the ksc1Δ strain of S. cerevisiae also possesses poorly functioning mitochondria (Szijgyarto et al., 2011). The mechanism underlying this phenotype is not known, but it appears to be conserved, since mitochondria are also defective in IP6K1−/− MEFs (Szijgyarto et al., 2011). This particular link between PP-InsP signaling and metabolism deserves further study, not in the least because the division of energy production between glycolysis and oxidative phosphorylation has particular relevance to tumor biology.
As in any signaling cascade, the “off-switch” is as critical as the “on-switch” in defining the parameters of a cell-signaling process. Thus, efforts have been made to identify phosphatases that will dephosphorylate pyrophosphorylated proteins. Initial experiments failed to detect [32P]-Pi release when cell lysates were added to peptides that had been pre-labeled with [32P] by using [32P]-5-InsP7 as a donor (Bhandari et al., 2007). The apparent metabolic stability of the serine-pyrophosphate group was argued to be biologically significant, by ensuring that signaling through this process is long-lived (Burton, Hu, & Saiardi, 2009). Nevertheless, the identification of the requisite phosphatase, even if it is not very active, is key to bolstering the credentials of this hypothesis. The recently described chemical synthesis of pyrophosphorylated peptides has led to phosphatase activities being detected; this is an important advance in the field (Yates & Fiedler, 2015). In the latter study, HPLC was used to separate the pyro-phosphorylated peptide from the dephosphorylated product; levels of each were determined by measuring amide-bond absorbance at 214 nm. The pyrophosphate group was not hydrolyzed by either DIPP1, nor by PP1 and PP2C protein phosphatases (Yates & Fiedler, 2015). In contrast, the pyrophosphorylated peptides were dephosphorylated by lysates prepared from either S. cerevisiae or HeLa cells, in 24 hr assays performed at either 25 or 37°C. These hydrolytic activities are unstable to heat treatment and sensitive to EDTA, indicative of the participation of metal-dependent enzymatic reactions (Yates & Fiedler, 2015). Considering the extended time-frame of the assay conditions, these data support the idea that Ser-pyrophosphates have a relatively long half-life in vivo (Burton et al., 2009).
6 | PHYSICOCHEMICAL PROPERTIES AND MECHANISMS OF ACTION: “ BRUTE-FORCE” ELECTROSTATICS
In addition to protein pyrophosphorylation (section 5), PP-InsPs can also regulate protein function through non-covalent electrostatic interactions with target proteins. This is, of course, true for all of the other inositol phosphates and the phosphoinositide; each of them have a particular arrangement of phosphate groups around the inositol ring that imparts specificity of function. There is no reason that receptors for PP-InsPs might not follow that paradigm. Indeed, the specific binding of 1-InsP7 by the Pho80/Pho85/Pho81 complex in S. cerevisiae (Lee et al., 2007, 2008) is arguably the most well-characterized example, although its biological significance is now uncertain (see section 3).
However, a 2003 study by Snyder and coworkers reported that PP-InsP binding to a group of plecktrin-homology (PH) domains appeared to contradict pre-defined topological requirements for ligand specificity (Luo et al., 2003). The latter group studied PH domains that had been considered specific for the phosphate code of the 3-phosphorylated phosphoinositides PtdIns(3,4)P2 and PtdIns(3,4,5)P3. This ligand specificity has even been well characterized by structural analysis (e.g., Thomas, Deak, Alessi, & van Aalten, 2002). Proteins that host these particular PH domains are normally translocated to the plasma membrane following accelerated PtdIns- (3,4,5)P3 synthesis, in turn driven by stimulus-dependent activation of phosphoinositide 3-kinase (PI3K). This compartmentalization promotes assembly of multiprotein signaling complexes and facilitates the activation of kinase cascades (Cantley, 2002). There are many cellular consequences to these regulatory events, including control over protein synthesis, actin polymerization, cell survival, cell cycle entry and—of particular relevance to this review—metabolic homeostasis (Cantley, 2002).
Snyder’s group (Luo et al., 2003) reported that 5-InsP7 was an effective competing ligand for PtdIns(3,4,5)P3 binding to PH domains. This binding of PP-InsPs may not be primarily reliant upon a particular topology of phosphate groups. Instead, the density of phosphate groups in the PP-InsPs has attained a functionally critical point at which delocalized (i.e., non-specific) electrostatic interactions become possible (Lemmon, Ferguson, & Abrams, 2002). In other words, PH-domain specificity for PtdIns(3,4,5)P3 may be over-ridden by 5-InsP7, largely by virtue of its highly electronegative character—a “brute-force” (Shears, 2015) electrostatic association with positively charged protein domains. This represents another functionally important property of the crowded array of phosphate groups in the PP-InsPs.
Binding of 5-InsP7 to PH domains has not been rationalized at a structural level. However, it is known that these domains possess several electropositive amino acid residues that are not required for binding PtdIns(3,4,5)P3 (Thomas et al., 2002). These “spare” residues have previously received attention for their electrostatic interactions with phosphatidylserine, which is considered to aid PH domain recruitment to membranes (Lai et al., 2013). Perhaps 5-InsP7 interacts with a sufficient number of residues so that it competes with PH domain binding to both phosphatidylserine and the 3-phosphorylated phosphoinositides.
The PH domain of AKT can also bind InsP6, although its potency is lower than that of 5-InsP7 (Gokhale, Zaremba, Janoshazi, Weaver, & Shears, 2013; Luo et al., 2003), consistent with the idea (Lemmon et al., 2002) that delocalized electrostatic interactions of inositol phosphates increase with the number of charged phosphate groups. Total cellular levels of InsP6 are usually at least 20-fold greater than those of 5-InsP7, but there are reasons to believe that much of the cell’s InsP6 is not free in the cytoplasm: InsP6 binds to membranes (Poyner, Cooke, Hanley, Reynolds, & Hawkins, 1993), and it is a structural cofactor for certain proteins (Macbeth et al., 2005). Additionally, data showing that an InsP6-phosphatase is sequestered inside endoplasmic reticulum (Chi et al., 2000) suggest that this organelle may also contain InsP6 itself. Thus, an idea that pervades the PP-InsP literature is that intracellular compartmentalization of InsP6 prevent its cytoplasmic levels from attaining a level that could significantly compete with 5-InsP7 function (Burton et al., 2009; Gokhale et al., 2013; Saiardi et al., 2004). This is not only important for 5-InsP7 binding to PH domains, but also protein pyrophosphorylation by 5-InsP7, which itself faces inhibition by InsP6 (Saiardi et al., 2004). However, InsP6 compartmentalization has yet to be directly verified in any animal cell type; this is a critical gap in the field. Thus, there is considerable need for probes that can specifically assay free cytoplasmic concentrations of InsP6 and the individual PP-InsPs.
It is such concerns that illustrate the importance of the demonstration that inhibition of AKT activation by 5-InsP7 is biologically relevant. This information was obtained by pre-loading a caged version of 5-InsP7 into HeLa cells using a guanidinium-rich molecular transporter; photo-uncaging of the 5-InsP7 inhibited receptor-dependent recruitment of AKT to the plasma membrane (Pavlovic et al., 2016). Nevertheless, there is one study that appears inconsistent with this idea: in HEK293 cells, an increase in [5-InsP7] by over-expression of IP6K activity was shown to slightly stimulate AKT, rather than inhibit it (Nagata et al., 2010). The degree of IP6K over-expression is quite striking, which may have complicated the phenotype. This same study also presented evidence that IP6K over-expression promotes autophagy (Nagata et al., 2010). This is a process by which proteins are selected for degrading and recycling to fuel biosynthetic capacity and metabolic homeostasis during periods of energy stress and nutrient starvation. Such a potentially important effect of PP-InsPs upon metabolic homeostasis deserves further study, provided it can be shown to be promoted by an increase in IP6K expression and/or elevated 5-InsP7 levels that are physiologically relevant.
Many proteins which exhibit PtdIns(3,4,5)P3-binding to PH domains may be regulated by 5-InsP7 (Gokhale et al., 2013; Luo et al., 2003), but the biological consequences of this ligand competition have mainly been elucidated from studies with AKT (Chakraborty et al., 2010; Gokhale et al., 2013; Pavlovic et al., 2016). For example, it has been shown that 5-InsP7 attenuates insulin-mediated translocation of this protein kinase to the plasma membrane. This biological effect of 5-InsP7 has implications for metabolic homeostasis, since AKT is upstream of mTOR, a protein kinase that balances cell growth with nutrient supply (Chakraborty et al., 2010). Indeed, over-expression of IP6K activity inhibits mTOR activity (Nagata et al., 2010). In part at least, this can explain why impaired 5-InsP7 synthesis in IP6K1 knock-out mice promotes insulin hypersensitivity, and reduces serum glucose levels (Chakraborty et al., 2010). Such mice are also resistant to weight gain when placed on a high-fat diet (Chakraborty et al., 2010). Knockout of IP6K1 leads to higher rates of fat oxidation (by an unknown mechanism), and elevates expression in adipose tissue of the mitochondrial uncoupling protein UCP1 (Zhu, Ghosal, Tyagi, & Chakraborty, 2016). These thermogenic consequences of IP6K1 deletion offer it as a candidate target for treatment of obesity. Nevertheless, the IP6K1−/− phenotype is complex, with several other contributing factors. For example, it has been discovered that InsP6 promotes LKB1-mediated phosphorylation and activation of AMPK, thereby promoting metabolic energy expenditure (Zhu, Ghoshal, Rodrigues, et al., 2016). By associating with AMPK, IP6K1 may locally deplete InsP6 levels; this is a restraint upon AMPK activity that is lost in the IP6K1−/− mice (Zhu, Ghosal, Tyagi, et al., 2016).
Returning to the topic of 5-InsP7 attenuating PtdIns(3,4,5)P3- mediated activation of AKT, it has been shown that this may protect against inadvertent inflammatory responses in neutrophils; these are cells that contain unusually high levels of 5-InsP7 (Prasad et al., 2011). This can be viewed as a mechanism of coincidence detection, whereby PtdIns(3,4,5)P3 accumulation and a decrease in 5-InsP7 levels are both required to recruit and activate AKT, in order to prevent any non-sustained, stochastic increases in PtdIns(3,4,5)P3 alone acting inappropriately. One mechanism to relieve inhibition by 5-InsP7 of PH domain recruitment involves PPIP5K. Our group (Gokhale, Zaremba, & Shears, 2011) has found that PPIP5K1 itself contains a cryptic PtdIns(3,4,5)P3-binding module. Thus, sustained stimulus-dependent increases in PtdIns(3,4,5)P3 can cause PPIP5K1 to translocate to the plasma membrane (Gokhale et al., 2011, 2013). Here, we propose, PPIP5K1 can promote a subplasmalemmal depletion of 5-InsP7, by phosphorylating it to 1,5-InsP8. In this way, PPIP5K1 can remove an inhibitor of PtdIns(3,4,5)P3-signaling, because the PH domain of AKT binds 5-InsP7 with at least sevenfold higher affinity than 1,5-InsP8 (Gokhale et al., 2013).
There is further significance to the demonstration that the 1-diphosphate group alters ligand affinity of PP-InsPs for certain target proteins; such data show that delocalized electrostatic interactions are not the sole determinant of their protein-binding affinity. On the other hand, it has yet to be rationalized how the 1-diphosphate might reduce PP-InsP affinity for a PH domain. Thus, it may be worth noting that in order for a ligand to bind to a protein, they must both lose some of their interactions with water; this is a desolvation penalty that opposes electrostatic driving forces for ligand/protein association (Shoichet, 2007). Indeed, there are instances where ligand affinity may be improved more by reducing desolvation penalties than by optimizing interaction energies (Shoichet, 2007). Binding energetics may also be affected by rearrangements of Mg2+-polyphosphate chelates, in which the 1,2,3-monophosphate grouping of an inositol phosphate has particularly significance (Torres et al., 2005). Perhaps the 1-β-phosphate and 5-β-phosphate differentially influence Mg2+-chelation by PP-InsPs, and the nature of their solvation shells, and this contributes to differences in their affinities for a target protein. Studies into these physicochemical properties of PP-InsPs would seem to be warranted.
Another type of PP-InsP binding domain has recently been found to be exposed on the surface of the SPX domain (Wild et al., 2016). In yeasts and plants, SPX domains are widely distributed in proteins that mediate phosphate homeostasis, which is a process that directs the life of all living organisms: in its organic form, Pi is a component of genomic material, it is frequently the basis of cell signaling mechanisms, and it serves as an energy currency.
Proteins that contain an SPX domain can mediate functions for PP-InsPs that are relevant to metabolic homeostasis. For example, 5-InsP7 stimulates ATPase-dependent synthesis of inorganic poly-phosphate (polyP) by the vacuolar transporter chaperone (VTC) in S. cerevisiae (Wild et al., 2016); the synthesis and degradation of polyP is a process by which free cytoplasmic Pi can be buffered (Azevedo & Saiardi, 2017). PolyP itself is ubiquitous in all organisms, but it is not known which proteins synthesize this polymer in higher animals, which lack orthologs of the yeast VTC complex (Azevedo & Saiardi, 2017). Nevertheless, the possibility of a requirement for PP-InsPs in polyP synthesis in mammals is raised by the demonstration that polyP levels are substantially reduced in platelets isolated from IP6K1−/− mice (Ghosh et al., 2013).
Studies of ligand specificity for the activation of VTC revealed some characteristics that are consistent with delocalized electrostatics playing a role: InsP6 is ineffective at stimulating polyP synthesis, 1-InsP7 and 5-InsP7 are almost equipotent (EC50 = 350–500 nM), and 1,5-InsP8 is approximately 20-fold more potent (Gerasimaite et al., 2017). That is, the number of phosphates is important. However, more detailed work has revealed that phosphate placement also makes key contributions: 1,5-InsP8 is several-fold more potent than 3,5-InsP8, and 20-fold more potent than 5-PPP-InsP5 (Gerasimaite et al., 2017), which also hosts eight phosphates (Figure 4). After taking into account the relative levels of different PP-InsPs in intact cells, it was concluded that 5-InsP7 is likely the most physiologically relevant ligand; that conclusion was supported by analyzing the effects in intact cells upon polyP synthesis upon selective genetic interventions in PP-InsP turnover (Gerasimaite et al., 2017). In any case, this work offers further evidence that ligand specificity overlays delocalized interactions in specifying PP-InsP functions.
X-ray crystallography has characterized the PP-InsP binding site in SPX domains as surface mounted, leaving one face of the ligand available for co-ordination with other target proteins (Wild et al., 2016); that is, ligand may be sandwiched between two proteins. Such a phenomenon may be key to directing specificity of action, since InsP6 will bind to the SPX domain of subunit 2 of VTC, but it has no effect upon polyP synthesis by the multimeric VTC complex (Wild et al., 2016). This possible role for PP-InsPs in directing specificity of interactions between two proteins is also evident from experiments with an SPX protein from rice, SPX4. Analysis by isothermal titration calorimetry indicated that 5-InsP7 is more potent than InsP6 at promoting the association of SPX4 with its cognate transcription factor PHR2, which then becomes competent to suppress the expression of genes that respond to Pi-starvation (Wild et al., 2016).
The only human protein known to contain an SPX domain is XPR1, which transports Pi out of cells (Wild et al., 2016). There are as yet no published studies to indicate that PP-InsPs might regulate XPR1. However, it has been speculated that Pi efflux through XPR1 might be inhibited by 5-InsP7 (Azevedo & Saiardi, 2017), since net Pi uptake into Xenopus oocytes is stimulated by heterologous expression of rabbit IP6K2 (Norbis et al., 1997) accompanied, presumably, by elevated 5-InsP7 synthesis.
Other candidate binding domains for PP-InsPs may be formed at the interface of class 1 histone deacetylases (HDACs) and their cognate transcriptional co-repressors such as MTA1 or SMRT (Watson et al., 2016). Such ligand binding activates HDAC activity (Watson et al., 2016; Watson, Fairall, Santos, & Schwabe, 2012), which is significant because reversible acetylation of lysine residues in the tails of histone proteins regulates eukaryotic gene expression. Initial studies (Watson et al., 2012) focused on the active ligand as being Ins(1,4,5,6) P4, but later work (Watson et al., 2016) showed that a similar degree of HDAC activation could be elicited by either 5-PP-InsP4 or by a 1-phosphonoacetate analogue of 1-InsP7. That is, one or more PP-InsPs might be physiological regulators of HDAC. However, neither 5-InsP7 nor 1,5-InsP8 were tested, so a profile of PP-InsP specificity remains to be obtained. Nevertheless, in an independent genetic study with yeast (Worley, Luo, & Capaldi, 2013), the activity of the HDAC Rpd3L was found to be compromised upon deletion of both kcs1 and vip1. As a consequence, there was attenuation of stress-dependent transcription of genes that regulate glycolysis and ribosome assembly (Worley et al., 2013), which further links PP-InsP turnover to energetic processes. Others have confirmed that ribosomal biogenesis—which comprises 80% of the ongoing cellular energy demand—is reduced in kcs1Δ yeast (Thota et al., 2015).
A large number of proteins have electropositive patches on their surface (Paz, Kligun, Bengad, & Mandel-Gutfreund, 2016); how many of these might be significant for PP-InsP function remains to be seen. Nevertheless, at the very least, there is a possibility that the littering of cells with such electropositive hot-spots imposes a significant drag on the diffusion of highly electronegative entities such as the PP-InsPs (somewhat analogously, there has been a recent challenge to the dogma that there is essentially unhindered diffusion of Ins(1,4,5)P3 through cell cytoplasm; the true rate of diffusion may be 25-fold lower than earlier estimates, the slowdown being caused by the large number of cellular binding sites for this inositol phosphate; Dickinson, Ellefsen, Dawson, Pearson, & Parker, 2016). It is also worth considering that the more ponderous the rate of diffusion of a PP-InsP, the greater the opportunity for it to attain a locally elevated concentration at its site of synthesis. This might facilitate spatially restricted signaling activities.
A search for more specific PP-InsP receptors is ongoing. A recent study screened yeast cell lysates for proteins that would bind to a bead-immobilized, non-hydrolyzable, 5-bisphosphonate analogue of 5-InsP7 (5-PCP-InsP5) (Wu, Chong, Perlman, Resnick, & Fiedler, 2016). In parallel, the authors also screened with bead-immobilized InsP6. Many of the isolated target proteins displayed comparable affinities for both ligands (Wu et al., 2016), although it is nevertheless interesting that gene ontology analysis showed a significant over-representation of proteins with bioenergetically important roles, such as nucleotide metabolism, glucose metabolism and ribosome biogenesis (Wu et al., 2016). A separate group of proteins can be identified from this study that exhibit a distinct ligand preference for 5-PCP-InsP5. After filtering out those in this particular list that have CK2-consensus phosphor-ylation sites (and hence are candidates for PP-InsP mediated pyrophosphorylation), a subset remains (Table 1) that may be considered as potentially hosting new examples of specific, non-covalent PP-InsP binding domains.
TABLE 1.
Candidates for “receptors” with selectivity for 5-InsP7
| Systematic name | Gene name | Description |
|---|---|---|
| YDL083C | RPS16B | Component of the small (40S) ribosomal subunit |
| YGL252C | RTG2 | Sensor of mitochondrial dysfunction |
| YCL043C | PDI1 | Protein disulfide isomerase |
| YPR074C | TKL1 | Transketolase (in the pentose phosphate pathay) |
| YLR293C | GSP1 | Ran GTPase |
| YBR221C | PDB1 | E1 beta subunit of the pyruvate dehydrogenase (PDH) complex |
| YGL202W | ARO8 | Aromatic aminotransferase I |
| YER126C | NSA2 | Constituent of 66S pre-ribosomal particles |
| YJR063W | RPA12 | RNA polymerase I subunit A12.2 |
| YIL051C | MMF1 | Mitochondrial protein required for transamination of isoleucine |
| YCR053W | THR4 | Threonine synthase |
| YPL127C | HHO1 | Histone H1; linker histone with roles in meiosis and sporulation |
| YOL109W | ZEO1 | Peripheral membrane protein of the plasma membrane |
| YLR058C | SHM2 | Cytosolic serine hydroxymethyltransferase |
| YIL078W | THS1 | Threonyl-tRNA synthetase |
| YGL245W | GUS1 | Glutamyl-tRNA synthetase |
| YLR043C | TRX1 | Cytoplasmic thioredoxin isoenzyme |
| YLR083C | EMP70 | Protein with roles in cellular adhesion, filamentous growth and endosome-to- vacuole sorting |
| YDL185W | VMA1 | Subunit A of the V1 peripheral membrane domain of V-ATPase |
| YKL056C | TMA19 | Ribosome-associated protein |
| YGR124W | ASN2 | Asparagine synthetase |
| YLR109W | AHP1 | Thiol-specific peroxiredoxin |
The listed genes (as described in the Saccharomyces genome database, http://www.yeastgenome.org/) encode proteins in S. cerevisiae cell-lysates that were found to bind to a bead-immobilized, non-hydrolyzable, 5-bisphosphonate analogue of 5-InsP7 (5-PCP-InsP5) with >2-fold enrichment compared to binding to bead-immobilized InsP6 (Wu et al., 2016). Not listed are proteins that have CK2-consensus phosphorylation sites (and hence are candidates for PP-InsP mediated pyrophosphor-ylation); these were filtered out using Scansite, with medium stringency (http://scansite3.mit.edu/#home). Those proteins that remain may be considered as potentially hosting new examples of PP-InsP-specific, non-covalent binding domains. An important caveat is that there has not been a direct demonstration of direct binding of a PP-InsP to any of these candidates; any protein in this list could be pulled down through its association with another PP-InsP-binding protein. The author is grateful to Dr. Dorothea Fiedler for providing the information presented in this table.
7 | EXAMPLES OF METABOLIC STIMULI THAT PROMOTE CHANGES IN CELLULAR LEVELS OF 5-INSP 7 AND 1,5-INSP8
A prototypical feature of an intracellular messenger is that its intracellular levels change in response to a defined extracellular stimulus. Proof of principle that this signaling paradigm applies to cellular PP-InsPs levels has largely come from the HPLC analysis of cell extracts pre-labeled with [3H]inositol (e.g., Gu et al., 2016). It is unfortunate that this is a tedious and low-throughput assay, and expensive too, both in terms of equipment and reagents; this has disincentivized its widespread use. Some doubts have even been raised that such assays faithfully record intracellular PP-InsP levels, on the basis that these molecules may be unstable, both in the acidic conditions typically used to quench cells, and the acidic (pH 3–4) HPLC buffers (Pisani et al., 2014). An electrophoretic method for assaying PP-InsPs was introduced to bypass this potential problem, and also to increase analytical throughput (Pisani et al., 2014). Nevertheless, in a later study, similar levels of PP-InsPs were recorded when either HPLC or electrophoretic assays were used (Gu et al., 2016); concerns that PP-InsPs are lost during HPLC analysis now appear unwarranted. In any case, HPLC is still generally considered the more sensitive method, particularly for assaying 1,5-InsP8.
One mechanistically well-characterized connection between 5-InsP7 levels and metabolic homeostasis is provided by the low affinity for ATP of the IP6Ks (Km = 1 mM (Saiardi, Erdjument-Bromage, Snowman, Tempst, & Snyder, 1999; Voglmaier et al., 1996). This is a unique characteristic among mammalian inositol phosphate kinases, which generally exhibit Km values for ATP which are less than 100 μM, as is the case for most other small molecule kinases and protein kinases. The atypically low affinity of IP6Ks for ATP explains why intact cells exhibit a rapid loss of 5-InsP7, without impacting InsP6 levels, when ATP levels fall in response to metabolic poisons (Nagel, Barker, Berggren, & Illies, 2010; Oliver, Obie, & Shears, 1992) or depletion of extracellular Pi (Gu et al., 2017). Under more physiologically relevant conditions, the prevailing levels of 5-InsP7 may also mirror the cell’s bioenergetic status. Just 1 min treatment of HL60 cells with the chemoattractant fMLP is sufficient to deplete 5-InsP7 levels, which facilitates neutrophil activation (Prasad et al., 2011). Furthermore, fMLP promotes ATP release from neutrophils to activate cell-surface purinergic receptors as part of an autocrine feedback loop (Bao et al., 2014); perhaps IP6K activity decreases in response to this export of cellular ATP, thereby accounting for the reduction in 5-InsP7 synthesis. It might be worth investigating if changes in ATP levels underlie the decrease in 5-InsP7 levels in HL60 cells that were incubated with nicotine for 15 hr (Xu et al., 2013).
There may be certain experimental situations in which ATP-mediated changes in IP6K activity can have unexpected consequences. For example, in a study with HEP2G cells, 5-InsP7 levels that had been substantially reduced by 24 hr serum starvation were quickly restored by addition of either insulin or IGF (Chakraborty et al., 2010). Rather than this reflecting direct, agonist-mediated control of inherent IP6K activity, it could be a consequence of growth factors rescuing ATP levels after their depletion by serum starvation (Su et al., 2010). Such a suggestion also questions the value of using long-term serum starvation as an experimental protocol in this field, if it may perturb PP-InsP turnover as an indirect consequence of promoting a bioenergetic imbalance.
In addition to sensing metabolic homeostasis, could 5-InsP7 regulate it too? There is evidence for such an activity in a dramatic 2011 study (Szijgyarto et al., 2011). Herein, it was shown that a sustained decrease in 5-InsP7 levels may promote metabolic adjust- ments to restore bioenergetic health: kcs1Δ yeast, and IP6K1−/− mouse embryonic fibroblasts, both exhibit elevated ATP levels compared to corresponding wild-type cells. However, if this phenomenon represents a homeostatic metabolic program in the mutant cells, its mechanistic basis is counter-intuitive; mitochondrial ATP synthesis is much more efficient than that produced by glycolysis, but loss of IP6K activity was associated with up-regulation of glycolytic gene expression and a profound reduction in mitochondrial function (Szijgyarto et al., 2011). Perhaps other phenotypes of kcs1Δ yeast that conserve ATP usage are more significant metabolic adjustments: a decrease in the rate of cell growth and reduction in general synthetic activity (Szijgyarto et al., 2011).
Evidence for metabolic drivers of PP-InsP turnover has also been discovered in Chlamydomonas, a unicellular green algae (Couso et al., 2016). In the latter study, cells were treated with rapamycin to inhibit TORC1. Only 1 hr of drug treatment was sufficient to near-deplete levels of both InsP7 and InsP8 (Couso et al., 2016). Furthermore, disruption of the PPIP5K orthologue in Chlamydomonas had significant metabolic consequences: increased synthesis of the lipid storage molecule, triacylglycerol, and altered profiles of intermediates of the tricarboxylic acid cycle (Couso et al., 2016).
As for 1,5-InsP8, there is evidence that it is an acute sensor of bioenergetic health in mammalian cells (Choi, Mollapour, Choi, & Shears, 2008): levels of this particular PP-InsP decrease following relatively mild bioenergetic challenges, such as those known to elevate AMP rather than decrease ATP levels (Choi et al., 2008). Other experiments with the epithelial cell-line, HCT116, have shown that severe bioenergetic stress, such as that which can be induced by Pi starvation, also promotes substantial loss of 1,5-InsP8 (Gu et al., 2017). Conversely, an elevation in 1,5-InsP8 levels has been observed in response to raising extracellular [Pi] from 1 to 6 mM (Gu et al., 2017); we will return to this topic in section 8.
Fluctuations in cellular levels of PP-InsPs can also occur over a time frame of hours rather than minutes. For example, long-term regulation of level of 5-InsP7 might be effected by changes in IP6K levels. Indeed, IP6K3 expression in murine skeletal muscle is up-regulated during fasting, and in experimental models of diabetes and muscle disuse (Moritoh et al., 2016). These may reflect metabolic sensing adaptations (Moritoh et al., 2016). Another example of long-term fluctuation in PP-InsP levels is provided by a cell-cycle study with S. cerevisiae; it was concluded that PP-InsPs have an important role in cell cycle progression (Banfic, Bedalov, York, & Visnjic, 2013). In that work, PP-InsP levels were monitored in synchronized cultures of S. cerevisiae transiting into S-phase after being released from α-factor mediated G1 arrest; levels of InsP7 and 1,5-InsP8 approximately doubled within the first hour (Banfic et al., 2013). It is conceivable that such changes in PP-InsP levels could be associated with fluctuating metabolic demands. However, in a separate but less detailed study with a mammalian cell type (WRK-1), entry into S-phase showed a decrease in InsP7 levels (Barker, Wright, Hughes, Kirk, & Michell, 2004), the opposite of the aforementioned result obtained with yeast (Banfic et al., 2013).
Finally, two independent studies have indicated a link between aging and 5-InsP7: using either young or aged mice as sources, 5-InsP7 levels were determined in either hepatocytes (Chakraborty et al., 2010) or bone marrow-derived mesenchymal stem cells (Zhang et al., 2014). In both cases, the cells from the older animals contained the higher levels of 5-InsP7. It could be that such changes in PP-InsP turnover are associated with aging-related metabolic imbalance (Chakraborty et al., 2010; Zhang et al., 2014).
8 | THE SIGNIFICANCE OF A BIFUNCTIONAL 5-INSP7 1-KINASE/1,5-INSP8 PHOSPHATASE IN THE REGULATION OF PP-INSP TURNOVER
As discussed in section 2, PPIP5Ks catalyze a 1-kinase/1-phosphatase substrate cycle (Figure 1). It is exceedingly rare for such a cycle to be catalyzed by a single protein. PPIP5Ks from humans, yeasts, and plants contain both kinase and phosphatase domains (Mulugu et al., 2007), indicating that this arrangement has survived at least 1.5 billion years of evolutionary pressure (see timetree.org). Understanding what are the selective advantages of such bifunctionality is a topic of considerable general interest (Dasgupta et al., 2014).
One important characteristic of the PPIP5Ks in vivo is that their kinase domains operate under zero-order conditions, whereas the activities of the phosphatase domains are substrate limited (i.e, first-order) (Gu et al., 2017). Mathematical modeling of just such a situation for a kinase/phosphatase bifunctional protein has established that a biological outcome is a degree of concentration robustness for the kinase product (Straube, 2013), in this case, 1,5-InsP8. In other words, 1,5-InsP8 may be considered to be inherently insensitive to changes in 5-InsP7 levels. Thus, PPIP5Ks can ensure specificity of PP-InsP signaling, by stabilizing 1,5-InsP8 levels during periods of stimulus-dependent regulation of 5-InsP7 levels.
Another, well-known advantage of all substrate cycles lies in their bringing amplification to the regulation of metabolic flux (Newsholme, 1971). Nevertheless, any such cycle that is directed by separate proteins is susceptible to incoherent output within a particular tissue, due to stochastic cell-to-cell variation in the expression of each of the individual proteins. This potential for incoherent output is avoided when co-expression of the competing catalytic activities is strictly enforced—such as when they both occur in a single protein (Dasgupta et al., 2014). That is one key property of the PPIP5Ks.
It might seem inevitable that there should be mechanisms by which both the kinase phosphatase activities should be regulated. But to date, only two examples are known; one has been described for the S. pombe orthologue, Asp1. This protein hosts an Fe-S cluster (in this case, [2Fe-2S]2+) that inhibits the 1-phosphatase (Wang et al., 2015). It has not yet been determined if this potential regulatory mechanism has any link to metabolic homeostasis. Any regulatory impact of altered [2Fe-2S]2+ cluster assembly upon PP-InsP metabolism might be expected to be a relatively long-term adaptive process—Fe sensing for example—since the mechanism by which phosphatase activity is inhibited appears not to be readily reversible, at least in vitro (Wang et al., 2015).
It is not yet known which other members of the PPIP5K family might host Fe-S clusters. There is little predictive value in these cofactors being ligated by Cys residues, since their arrangements are so diverse (White & Dillingham, 2012). The spectroscopic and/or structural studies that are essential for defining Fe-S clusters require milligram quantities of pure protein, which is a tall order for proteins as large as the PPIP5Ks. Thus, the degree of evolutionary conservation of this Fe–S cluster is as yet unclear.
Another regulatory mechanism that has a more obvious role in metabolic homeostasis is exhibited by PPIP5K2: inorganic Pi inhibits its phosphatase activity and stimulates its kinase activity (Gu et al., 2017). Reciprocal regulation of the competing catalytic activities of PPIP5Ks is a particularly sensitive mechanism (Newsholme, 1971), by which quite small fluctuations in [Pi] can exert proportionately larger effects upon 1,5-InsP8 levels (Gu et al., 2017). Since PPIP5Ks are predominantly located in the cytoplasm (Yong et al., 2015), that is where such Pi-sensing would have to occur. Unfortunately, cytoplasmic Pi homeostasis is not a well-studied topic, due to considerable technical hurdles. No fluorescent probes to record cytoplasmic Pi have been developed. Moreover, chemical assays of Pi in cell lysates do not resolve cytoplasmic Pi from the independently regulated Pi pools in mitochondria, lysosomes, and endoplasmic- or sarcoplasmic-reticulum (Bergwitz & Juppner, 2011). There are 31P-NMR studies of mammalian systems in which a particular resonance signal has been attributed to cytosolic Pi (e.g., Bevington et al., 1986; Kan et al., 2010), but generally without direct confirmation of its subcellular origin. This is a solid roadblock to the further study of the apparent metabolic relationship between cytoplasmic Pi and 1,5-InsP8.
One potential means to influence cytoplasmic [Pi] might be fluctuations in its levels in extracellular fluids. However, serum Pi levels are normally tightly controlled at around 1.5 mM (Giachelli, 2003). In any case, work with human erythrocytes (which lack organelles that contain their own Pi pools), indicates that cytoplasmic [Pi] can be somewhat insulated from fluctuations in extracellular [Pi]: cytoplasmic [Pi] was found to rise by only 35%, to approx 0.8 mM, when extracellular [Pi] was more than doubled from 1 to 2.3 mM (Bevington et al., 1986).
9 | CONCLUDING COMMENTS
The PP-InsPs are a unique group of cellular signals. As explained in the sections above, the crowded phosphate array of PP-InsPs allows them to exhibit unique mechanisms of action: protein pyrophosphorylation, and “brute-force” electrostatic interactions with target proteins. Although such molecular processes have the potential for a myriad of biological outcomes, many of the diverse activities of PP-InsPs appear to derive from one fundamental function, namely, information transfer at the interface of metabolism and signaling. In particular, as noted here and in previous work (Azevedo & Saiardi, 2017), PP-InsPs are key to reciprocal interplay between Pi, polyP and ATP. PP-InsPs have obtained front row status as important intracellular signals. We expect even more from them in the near future.
Acknowledgments
Work in the author’s laboratory was supported by the Intramural Research Program of the NIH/National Institute of Environmental Health Sciences. I am grateful to Dr. Dorothea Fiedler for providing the information described in Table 1.
Funding information
Intramural Research Program of the NIH/National Institute of Environmental Health Sciences
References
- Aird KM, Worth AJ, Snyder NW, Lee JV, Sivanand S, Liu Q, … Zhang R. ATM couples replication stress and metabolic reprogramming during cellular senescence. Cell Reports. 2015;11:893–901. doi: 10.1016/j.celrep.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C, Burton A, Ruiz-Mateos E, Marsh M, Saiardi A. Inositol pyrophosphate mediated pyrophosphorylation of AP3B1 regulates HIV-1 Gag release. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21161–21166. doi: 10.1073/pnas.0909176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo C, Saiardi A. Eukaryotic phosphate homeostasis: The inositol pyrophosphate perspective. Trends in Biochemical Sciences. 2017;42:219–231. doi: 10.1016/j.tibs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Banfic H, Bedalov A, York JD, Visnjic D. Inositol pyrophosphates modulate S phase progression after pheromone-induced arrest in Saccharomyces cerevisiae. Journal of Biological Chemistry. 2013;288:1717–1725. doi: 10.1074/jbc.M112.412288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Ledderose C, Seier T, Graf AF, Brix B, Chong E, Junger WG. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. Journal of Biological Chemistry. 2014;289:26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker CJ, Wright J, Hughes PJ, Kirk CJ, Michell RH. Complex changes in cellular inositol phosphate complement accompany transit through the cell cycle. Biochemical Journal. 2004;380:465–473. doi: 10.1042/BJ20031872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwitz C, Juppner H. Phosphate sensing. Advances in Chronic Kidney Disease. 2011;18:132–144. doi: 10.1053/j.ackd.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevington A, Mundy KI, Yates AJ, Kanis JA, Russell RG, Taylor DJ, … Radda GK. A study of intracellular orthophosphate concentration in human muscle and erythrocytes by 31P nuclear magnetic resonance spectroscopy and selective chemical assay. Clinical Science. 1986;71:729–735. doi: 10.1042/cs0710729. [DOI] [PubMed] [Google Scholar]
- Bhandari R, Juluri KR, Resnick AC, Snyder SH. Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermio-genesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2439–2353. doi: 10.1073/pnas.0712227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari R, Saiardi A, Ahmadibeni Y, Snowman AM, Resnick AC, Kristiansen TZ, … Snyder SH. Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15305–15310. doi: 10.1073/pnas.0707338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blind RD, Suzawa M, Ingraham HA. Direct modification and activation of a nuclear receptor-PIP2 complex by the inositol lipid kinase IPMK. Science Signaling. 2012;5:ra44. doi: 10.1126/scisignal.2003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A, Hu X, Saiardi A. Are inositol pyrophosphates signalling molecules? Journal of Cellular Physiology. 2009;220:8–15. doi: 10.1002/jcp.21763. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Capolicchio S, Wang H, Thakor DT, Shears SB, Jessen HJ. Synthesis of densely phosphorylated bis-1,5-Diphospho-myo-Inositol tetrakisphosphate and its enantiomer by bidirectional P-Anhydride formation. Angewandte Chemie International Edition In English. 2014;53:9508–9511. doi: 10.1002/anie.201404398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Koldobskiy MA, Bello NT, Maxwell M, Potter JJ, Juluri KR, … Snyder SH. Inositol pyrophosphates inhibit akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty A, Koldobskiy MA, Sixt KM, Juluri KR, Mustafa AK, Snowman AM, … Snyder SH. HSP90 regulates cell survival via inositol hexakisphosphate kinase-2. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1134–1139. doi: 10.1073/pnas.0711168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanduri M, Rai A, Malla AB, Wu M, Fiedler D, Mallik R, Bhandari R. Inositol hexakisphosphate kinase 1 (IP6K1) activity is required for cytoplasmic dynein-driven transport. The Biochemical Journal. 2016;473:3031–3047. doi: 10.1042/BCJ20160610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H, Yang X, Kingsley PD, O’Keefe RJ, Puzas JE, Rosier RN, … Reynolds PR. Targeted deletion of Minpp1 provides new insight into the activity of multiple inositol polyphosphate phosphatase in vivo. Molecular and Cell Biology. 2000;20:6496–6507. doi: 10.1128/mcb.20.17.6496-6507.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Mollapour E, Choi JH, Shears SB. Cellular energetic status supervises the synthesis of bis-diphosphoinositol tetrakisphosphate independently of AMP-activated protein kinase. Molecular Pharmacology. 2008;74:527–536. doi: 10.1124/mol.107.044628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couso I, Evans B, Li J, Liu Y, Ma F, Diamond S, … Umen JG. Synergism between inositol polyphosphates and TOR kinase signaling in nutrient sensing, growth control and lipid metabolism in Chlamydomonas. Plant Cell. 2016;28:2026–2042. doi: 10.1105/tpc.16.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta T, Croll DH, Owen JA, Vander Heiden MG, Locasale JW, Alon U, … Gunawardena J. A fundamental trade-off in covalent switching and its circumvention by enzyme bifunctionality in glucose homeostasis. Journal of Biological Chemistry. 2014;289:13010–13025. doi: 10.1074/jbc.M113.546515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson GD, Ellefsen KL, Dawson SP, Pearson JE, Parker I. Hindered cytoplasmic diffusion of inositol trisphosphate restricts its cellular range of action. Science Signaling. 2016;9:ra108. doi: 10.1126/scisignal.aag1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draskovic P, Saiardi A, Bhandari R, Burton A, Ilc G, Kovacevic M, … Podobnik M. Inositol hexakisphosphate kinase products contain diphosphate and triphosphate groups. Chemistry & Biology. 2008;15:274–286. doi: 10.1016/j.chembiol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Fridy PC, Otto JC, Dollins DE, York JD. Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. Journal of Biological Chemistry. 2007;282:30754–30762. doi: 10.1074/jbc.M704656200. [DOI] [PubMed] [Google Scholar]
- Fu C, Xu J, Li RJ, Crawford JA, Khan AB, Ma TM, … Snyder SH. Inositol hexakisphosphate kinase-3 regulates the morphology and synapse formation of cerebellar purkinje cells via spectrin/adducin. Journal of Neuroscience. 2015;35:11056–11067. doi: 10.1523/JNEUROSCI.1069-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annual Review of Immunology. 2014;32:609–634. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimaite R, Pavlovic I, Capolicchio S, Hofer A, Schmidt A, Jessen HJ, Mayer A. Inositol pyrophosphate specificity of the SPX-dependent polyphosphate polymerase VTC. ACS Chemical Biology. 2017;12:648–653. doi: 10.1021/acschembio.7b00026. [DOI] [PubMed] [Google Scholar]
- Ghosal S, Zhu Q, Asteian A, Lin H, Xu H, Ernst G, … Chakraborty A. TNP [N2-(m-trifluorobenzyl), N6-(p-nitrobenzyl)purine] ameliorates diet induced obesity and insulin resistance via inhibition of the IP6K1 pathway. Molecular Metabolism. 2016;5:903–917. doi: 10.1016/j.molmet.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Shukla D, Suman K, Lakshmi BJ, Manorama R, Kumar S, Bhandari R. Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood. 2013;122:1478–1486. doi: 10.1182/blood-2013-01-481549. [DOI] [PubMed] [Google Scholar]
- Giachelli CM. Vascular calcification: In vitro evidence for the role of inorganic phosphate. Journal of the American Society of Nephrology. 2003;14:S300–S304. doi: 10.1097/01.asn.0000081663.52165.66. [DOI] [PubMed] [Google Scholar]
- Gokhale NA, Zaremba A, Janoshazi AK, Weaver JD, Shears SB. PPIP5K1 modulates ligand competition between diphosphoi-nositol polyphosphates and PtdIns(3,4,5)P3 for polyphosphoinositide-binding domains. The Biochemical Journal. 2013;453:413–426. doi: 10.1042/BJ20121528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhale NA, Zaremba A, Shears SB. Receptor-dependent compartmentalization of PPIP5K1, a kinase with a cryptic poly-phosphoinositide binding domain. The Biochemical Journal. 2011;434:415–426. doi: 10.1042/BJ20101437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Blenis J. A nexus for cellular homeostasis: The interplay between metabolic and signal transduction pathways. Current Opinion in Biotechnology. 2015;34:110–117. doi: 10.1016/j.copbio.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumidi L, Cottel D, Dallongeville J, Amouyel P, Meirhaeghe A. Effects of established BMI-associated loci on obesity-related traits in a French representative population sample. BMC Genetics. 2014;15:62. doi: 10.1186/1471-2156-15-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Nguyen HN, Hofer A, Jessen HJ, Dai X, Wang H, Shears SB. The significance of the bifunctional kinase/phosphatase activities of PPIP5Ks for coupling inositol pyrophosphate cell-signaling to cellular phosphate homeostasis. Journal of Biological Chemistry. 2017;292:4544–4555. doi: 10.1074/jbc.M116.765743. https://doi.org/10.1074/jbc.M116.765743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Wilson MSC, Jessen HJ, Saiardi A, Shears SB. Inositol pyrophosphate profiling of two HCT116 cell lines uncovers variation in InsP8 levels. PLoS ONE. 2016;11:e0165286. doi: 10.1371/journal.pone.0165286. https://doi.org/10.1371/journal.pone.0165286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illies C, Gromada J, Fiume R, Leibiger B, Yu J, Juhl K, … Berggren P-O. Inositol pyrophosphates determine exocytic capacity. Science. 2007;318:1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- Ingram SW, Safrany ST, Barnes LD. Disruption and overexpression of the Schizosaccharomyces pombe aps1 gene and the effects on growth rate, morphology, and intracellular diadenosine 5’, 5”’-P1, P5-pentaphosphate and diphosphoinositol polyphosphate concentrations. The Biochemical Journal. 2003;369:519–528. doi: 10.1042/BJ20020733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadav RS, Kumar D, Buwa N, Ganguli S, Thampatty SR, Balasubramanian N, Bhandari R. Deletion of inositol hexakisphosphate kinase 1 (IP6K1) reduces cell migration and invasion, conferring protection from aerodigestive tract carcinoma in mice. Cellular Signalling. 2016;28:1124–1136. doi: 10.1016/j.cellsig.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan HE, Klomp DW, Wong CS, Boer VO, Webb AG, Luijten PR, Jeneson JA. In vivo 31P MRS detection of an alkaline inorganic phosphate pool with short T1 in human resting skeletal muscle. NMR in Biomedicine. 2010;23:995–1000. doi: 10.1002/nbm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilari RS, Weaver JD, Shears SB, Safrany ST. Understanding inositol pyrophosphate metabolism and function: Kinetic characterization of the DIPPs. FEBS Letters. 2013;587:3464–3470. doi: 10.1016/j.febslet.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha D, Johnen P, Azevedo C, Dynowski M, Weiss M, Capolicchio S, … Schaaf G. VIH2 regulates the synthesis of inositol pyrophosphate InsP8 and jasmonate-dependent defenses in Arabidopsis. Plant Cell. 2015;27:1082–1097. doi: 10.1105/tpc.114.135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CL, Srivastava A, Pilling C, Chase AR, Falke JJ, Voth GA. Molecular mechanism of membrane binding of the GRP1 PH domain. Journal of Molecular Biology. 2013;425:3073–3090. doi: 10.1016/j.jmb.2013.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laussmann T, Hansen A, Reddy KM, Reddy KK, Falck JR, Vogel G. Diphospho-myo-inositol phosphates in Dictyostelium and Polysphondylium: Identification of a new bisdiphospho-myo-inositol tetrakisphosphate. FEBS Letters. 1998;426:145–150. doi: 10.1016/s0014-5793(98)00329-9. [DOI] [PubMed] [Google Scholar]
- Lee TS, Lee JY, Kyung JW, Yang Y, Park SJ, Lee S, … Kim S. Inositol pyrophosphates inhibit synaptotagmin-dependent exocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:8314–8319. doi: 10.1073/pnas.1521600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Huang K, Quiocho FA, O’Shea EK. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nature Chemical Biology. 2008;4:25–32. doi: 10.1038/nchembio.2007.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Mulugu S, York JD, O’Shea EK. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, Abrams CS. Pleckstrin homology domains and the cytoskeleton. FEBS Letters. 2002;513:71–76. doi: 10.1016/s0014-5793(01)03243-4. [DOI] [PubMed] [Google Scholar]
- Lin H, Fridy PC, Ribeiro AA, Choi JH, Barma DK, Vogel G, … Mayr GW. Structural analysis and detection of biological inositol pyrophosphates reveals that the VIP/PPIP5K family are 1/3-kinases. Journal of Biological Chemistry. 2009;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetti A, Szijgyarto Z, Bosch D, Loss O, Azevedo C, Saiardi A. Identification of an evolutionarily conserved family of inorganic polyphosphate endopolyphosphatases. Journal of Biological Chemistry. 2011;286:31966–31974. doi: 10.1074/jbc.M111.266320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo HR, Huang YE, Chen JC, Saiardi A, Iijima M, Ye K, … Snyder SH. Inositol pyrophosphates mediate chemotaxis in Dictyos-telium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Macbeth MR, Schubert HL, Vandemark AP, Lingam AT, Hill CP, Bass BL. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science. 2005;309:1534–1539. doi: 10.1126/science.1113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machkalyan G, Trieu P, Petrin D, Hebert TE, Miller GJ. PPIP5K1 interacts with the exocyst complex through a C-terminal intrinsically disordered domain and regulates cell motility. Cell Signal. 2016;28:401–411. doi: 10.1016/j.cellsig.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Moritoh Y, Oka M, Yasuhara Y, Hozumi H, Iwachidow K, Fuse H, Tozawa R. Inositol hexakisphosphate kinase 3 regulates metabolism and lifespan in mice. Scientific Reports. 2016;6:32072. doi: 10.1038/srep32072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BH, Haney R, Lamarre E, Drazba J, Prestwich GD, Lindner DJ. Gene deletion of inositol hexakisphosphate kinase 2 predisposes to aerodigestive tract carcinoma. Oncogene. 2009;28:2383–2392. doi: 10.1038/onc.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE, … York JD. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- Nagata E, Saiardi A, Tsukamoto H, Satoh T, Itoh Y, Itoh J, … Takagi S. Inositol hexakisphosphate kinases promote autophagy. International Journal of Biochemistry & Cell Biology. 2010;42:2065–2071. doi: 10.1016/j.biocel.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Nagel A, Barker CJ, Berggren PO, Illies C. Diphosphosinositol polyphosphates and energy metabolism: Assay for ATP/ADP ratio. Methods in Molecular Biology. 2010;645:123–131. doi: 10.1007/978-1-60327-175-2_8. [DOI] [PubMed] [Google Scholar]
- Newsholme EA. The regulation of phosphofructokinase in muscle. Cardiology. 1971;56:22–34. doi: 10.1159/000169338. [DOI] [PubMed] [Google Scholar]
- Norbis F, Boll M, Stange G, Markovich D, Verrey F, Biber J, Murer H. Identification of a cDNA/protein leading to an increased Pi-uptake in Xenopus laevis oocytes. Journal of Membrane Biology. 1997;156:19–24. doi: 10.1007/s002329900183. [DOI] [PubMed] [Google Scholar]
- Oliver KG, Putney JW, Jr, Obie JF, Shears SB. The interconversion of inositol 1,3,4,5,6-pentakisphosphate and inositol tetrakisphosphates in AR4-2J cells. Journal of Biological Chemistry. 1992;267:21528–21534. [PubMed] [Google Scholar]
- Onnebo SM, Saiardi A. Inositol pyrophosphates modulate hydrogen peroxide signalling. The Biochemical Journal. 2009;423:109–118. doi: 10.1042/BJ20090241. [DOI] [PubMed] [Google Scholar]
- Padmanabhan U, Dollins DE, Fridy PC, York JD, Downes CP. Characterization of a selective inhibitor of inositol hexaki-sphosphate kinases: Use in defining biological roles and metabolic relationships of inositol pyrophosphates. Journal of Biological Chemistry. 2009;284:10571–10582. doi: 10.1074/jbc.M900752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic I, Thakor DT, Vargas JR, McKinlay CJ, Hauke S, Anstaett P, … Jessen HJ. Cellular delivery and photochemical release of a caged inositol-pyrophosphate induces PH-domain translocation in cellulo. Nature Communications. 2016;7:10622. doi: 10.1038/ncomms10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz I, Kligun E, Bengad B, Mandel-Gutfreund Y. BindUP: a web server for non-homology-based prediction of DNA and RNA binding proteins. Nucleic Acids Research. 2016;44:W568–W574. doi: 10.1093/nar/gkw454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani F, Livermore T, Rose G, Chubb JR, Gaspari M, Saiardi A. Analysis of Dictyostelium discoideum inositol pyrophosphate metabolism by gel electrophoresis. PLoS ONE. 2014;9:e85533. doi: 10.1371/journal.pone.0085533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner DR, Cooke F, Hanley MR, Reynolds DJM, Hawkins PT. Characterization of metal ion-induced 3hinositol hexakisphosphate binding to rat cerebellar membarnes. Journal of Biological Chemistry. 1993;268:1032–1038. [PubMed] [Google Scholar]
- Prasad A, Jia Y, Chakraborty A, Li Y, Jain SK, Zhong J, Roy SG, … Luo HR. Inositol hexakisphosphate kinase 1 regulates neutrophil function in innate immunity by inhibiting phosphatidylinositol-(3,4,5)-trisphosphate signaling. Nature Immunology. 2011;12:752–760. doi: 10.1038/ni.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R, Stoker AW, Hendriks WJ. PTPs emerge as PIPs: Protein tyrosine phosphatases with lipid-phosphatase activities in human disease. Human Molecular Genetics. 2013;22:R66–R76. doi: 10.1093/hmg/ddt347. [DOI] [PubMed] [Google Scholar]
- Pulloor NK, Nair S, Kostic AD, Bist P, Weaver JD, Tyagi R, … Krishnan MN. Human genome-wide RNAi screen identifies an essential role for inositol pyrophosphates in type-I interferon response. PLoS Pathogens. 2014;10:e1003981. doi: 10.1371/journal.ppat.1003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao F, Cha J, Xu J, Xu R, Vandiver MS, Tyagi R, … Snyder SH. Inositol pyrophosphates mediate the DNA-PK/ATM-p53 cell death pathway by regulating CK2 phosphorylation of Tti1/Tel2. Molecular Cell. 2014;54:119–132. doi: 10.1016/j.molcel.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiardi A, Bhandari A, Resnick R, Cain A, Snowman AM, Snyder SH. Inositol pyrophosphate: Physiologic phosphorylation of proteins. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- Saiardi A, Caffrey JJ, Snyder SH, Shears SB. The inositol hexakisphosphate kinase family: Catalytic flexibility, and function in yeast vacuole biogenesis. Journal of Biological Chemistry. 2000;275:24686–24692. doi: 10.1074/jbc.M002750200. [DOI] [PubMed] [Google Scholar]
- Saiardi A, Erdjument-Bromage H, Snowman A, Tempst P, Snyder SH. Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Current Biology. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- Saiardi A, Resnick AC, Snowman AM, Wendland B, Snyder SH. Inositol pyrophosphates regulate cell death and telomere length via PI3K-related protein kinases. Proceedings of the National Academy of Sciences United States of America. 2005;102:1911–1914. doi: 10.1073/pnas.0409322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears SB. Inositol pyrophosphates: Why so many phosphates? Advances in Biological Regulation. 2015;57:203–216. doi: 10.1016/j.jbior.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoichet BK. No free energy lunch. Nature Biotechnology. 2007;25:1109–1110. doi: 10.1038/nbt1007-1109. [DOI] [PubMed] [Google Scholar]
- Steidle EA, Chong LS, Wu M, Crooke E, Fiedler D, Resnick AC, Rolfes RJ. A novel inositol pyrophosphate phosphatase in Saccharomyces cerevisiae: Siw14 selectively cleaves the beta-phosphate from 5-diphosphoinositol pentakisphosphate (5PP-IP5) Journal of Biological Chemistry. 2016;291:6772–6783. doi: 10.1074/jbc.M116.714907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens LR, Radenberg T, Thiel U, Vogel G, Khoo KH, Dell A, … Mayr GW. The detection, purification, structural characterization and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s) Journal of Biological Chemistry. 1993;268:4009–4015. [PubMed] [Google Scholar]
- Straube R. Sensitivity and robustness in covalent modification cycles with a bifunctional converter enzyme. Biophysical Journal. 2013;105:1925–1933. doi: 10.1016/j.bpj.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su MY, Hsieh SY, Lee YR, Chang MC, Yuan TT, Chang JM. The relationship between energy status and AMP-activated protein kinase in human H460 lung cancer cells. Cell Biochemistry and Function. 2010;28:549–554. doi: 10.1002/cbf.1685. [DOI] [PubMed] [Google Scholar]
- Szijgyarto Z, Garedew A, Azevedo C, Saiardi A. Influence of inositol pyrophosphates on cellular energy dynamics. Science. 2011;334:802–805. doi: 10.1126/science.1211908. [DOI] [PubMed] [Google Scholar]
- Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Current Biology. 2002;12:1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- Thota SG, Bhandari R. The emerging roles of inositol pyrophosphates in eukaryotic cell physiology. Journal of Biosciences. 2015;40:593–605. doi: 10.1007/s12038-015-9549-x. [DOI] [PubMed] [Google Scholar]
- Thota SG, Unnikannan CP, Thampatty SR, Manorama R, Bhandari R. Inositol pyrophosphates regulate RNA polymerase I-mediated rRNA transcription in Saccharomyces cerevisiae. The Biochemical Journal. 2015;466:105–114. doi: 10.1042/BJ20140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J, Domínguez S, Cerdá FM, Obal G, Mederos A, Irvine RF, … Kremer C. Solution behaviour of myoinositol hexakisphos-phate in the presence of multivalent cations. Prediction of a neutral pentamagnesium species under cytosolic/nuclear conditions. Journal of Inorganic Biochemistry. 2005;99:828–840. doi: 10.1016/j.jinorgbio.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Voglmaier SM, Bembenek ME, Kaplin AI, Dormán G, Olszewski JD, Prestwich GD, Snyder SH. Purified inositol hexaki-sphosphate kinase is an ATP synthase: Diphosphoinositol pentaki-sphosphate as a high-energy phosphate donor. Proceedings of the National Academy of Sciences United States of America. 1996;93:4305–4310. doi: 10.1073/pnas.93.9.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade RC, Gabdoulline RR, Ludemann SK, Lounnas V. Electrostatic steering and ionic tethering in enzyme-ligand binding: Insights from simulations. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5942–5949. doi: 10.1073/pnas.95.11.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, DeRose EF, London RE, Shears SB. IP6K structure and the molecular determinants of catalytic specificity in an inositol phosphate kinase family. Nature Communications. 2014;5:4178. doi: 10.1038/ncomms5178. https://doi.org/10.1038/ncomms5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Falck JR, Hall TM, Shears SB. Structural basis for an inositol pyrophosphate kinase surmounting phosphate crowding. Nature Chemical Biology. 2012;8:111–116. doi: 10.1038/nchembio.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Godage HY, Riley AM, Weaver JD, Shears SB, Potter BVL. Synthetic inositol phosphate analogs reveal that PPIP5K2 has a surface-mounted substrate capture site that is a target for drug discovery. Chemistry & Biology. 2014;21:689–699. doi: 10.1016/j.chembiol.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Nair VS, Holland AA, Capolicchio S, Jessen HJ, Johnson MK, Shears SB. Asp1 from Schizosaccharomyces pombe binds a [2Fe-2S](2+) cluster which inhibits inositol pyrophosphate 1-phosphatase activity. Biochemistry. 2015;54:6462–6474. doi: 10.1021/acs.biochem.5b00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PJ, Fairall L, Santos GM, Schwabe JW. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature. 2012;481:335–340. doi: 10.1038/nature10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PJ, Millard CJ, Riley AM, Robertson NS, Wright LC, Godage HY, … Schwabe JW. Insights into the activation mechanism of class I HDAC complexes by inositol phosphates. Nature Communications. 2016;7:11262. doi: 10.1038/ncomms11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Wang H, Shears SB. The kinetic properties of a human PPIP5K reveal that its kinase activities are protected against the consequences of a deteriorating cellular bioenergetic environment. Bioscience Reports. 2013;33:228–241. doi: 10.1042/BSR20120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF, Dillingham MS. Iron-sulphur clusters in nucleic acid processing enzymes. Current Opinion in Structural Biology. 2012;22:94–100. doi: 10.1016/j.sbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Wild R, Gerasimaite R, Jung JY, Truffault V, Pavlovic I, Schmidt A, … Mayer A. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science. 2016;352:986–990. doi: 10.1126/science.aad9858. [DOI] [PubMed] [Google Scholar]
- Williams FJ, Fiedler D. A fluorescent sensor and gel stain for detection of pyrophosphorylated proteins. ACS Chemical Biology. 2015;10:1958–1963. doi: 10.1021/acschembio.5b00256. [DOI] [PubMed] [Google Scholar]
- Williams SP, Gillaspy GE, Perera IY. Biosynthesis and possible functions of inositol pyrophosphates in plants. Frontiers in Plant Science. 2015;6:67. doi: 10.3389/fpls.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MS, Livermore TM, Saiardi A. Inositol pyrophos-phates: Between signalling and metabolism. The Biochemical Journal. 2013;452:369–379. doi: 10.1042/BJ20130118. [DOI] [PubMed] [Google Scholar]
- Worley J, Luo X, Capaldi AP. Inositol pyrophosphates regulate cell growth and the environmental stress response by activating the HDAC Rpd3L. Cell Reports. 2013;3:1476–1482. doi: 10.1016/j.celrep.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Chong LS, Perlman DH, Resnick AC, Fiedler D. Inositol polyphosphates intersect with signaling and metabolic networks via two distinct mechanisms. Proceedings of the National Academy of Sciences United States of America. 2016;113:E6757–E6765. doi: 10.1073/pnas.1606853113. https://doi.org/10.1073/pnas.1606853113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Li H, Bajrami B, Kwak H, Cao S, Liu P, … Luo HR. Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7726–7731. doi: 10.1073/pnas.1302906110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LM, Fiedler D. Establishing the stability and reversibility of protein pyrophosphorylation with synthetic peptides. Chembiochem. 2015;16:415–423. doi: 10.1002/cbic.201402589. [DOI] [PubMed] [Google Scholar]
- Yong ST, Nguyen HN, Choi JH, Bortner CD, Williams J, Pulloor NK, … Shears SB. Identification of a functional nuclear translocation sequence in hPPIP5K2. BMC Cell Biology. 2015;16:17. doi: 10.1186/s12860-015-0063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York SJ, Armbruster BN, Greenwell P, Petes TD, York JD. Inositol diphosphate signaling regulates telomere length. Journal of Biology and Chemistry. 2005;280:4264–4269. doi: 10.1074/jbc.M412070200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao C, Liu B, Liang D, Qin X, Li X, … Cao F. Inositol pyrophosphates mediate the effects of aging on bone marrow mesenchymal stem cells by inhibiting Akt signaling. Stem Cell Research & Therapy. 2014;5:33. doi: 10.1186/scrt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Ghosal S, Tyagi R, Chakraborty A. Global IP6K1 deletion enhances temperature modulated energy expenditure which reduces carbohydrate and fat induced weight gain. Molecular Metabolism. 2016;6:73–85. doi: 10.1016/j.molmet.2016.11.010. https://doi.org/10.1016/j.molmet.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Ghoshal S, Rodrigues A, Gao S, Asterian A, Kamenecka TM, … Chakraborty A. Adipocyte-specific deletion of Ip6k1 reduces diet-induced obesity by enhancing AMPK-mediated thermogenesis. Journal of Clinical Investigation. 2016;126:4273–4288. doi: 10.1172/JCI85510. [DOI] [PMC free article] [PubMed] [Google Scholar]