Abstract
Objective
To investigate the safety of umbilical cord milking on both the mother and neonate among very preterm deliveries of less than 33 weeks of gestation.
Methods
Pregnant women who were expected to deliver at between 24 0/7 and 32 6/7 weeks of gestation were randomized to either the umbilical cord milking or immediate cord clamping group. Maternal and neonatal data associated with delivery, in addition to neonatal morbidity and mortality data, were collected and analyzed.
Results
Of the 66 preterm deliveries included in the study, 34 were randomized into the milking and 32 into the clamping group. Differences between maternal pre- and post-partum hemoglobin levels were 1.35 g/dL in the milking and 1.58 g/dL in the clamping group (P=0.451). Neonatal Apgar scores at both 1 and 5 minutes, initial blood gas analysis results, body temperature at admission, need for early intubation, and maximum bilirubin levels were all similar between the 2 groups. However, neonatal hemoglobin levels at birth (15.79 vs. 14.69 g/dL; P<0.05) and at 24 hours of age (14.83 vs. 13.29 g/dL; P<0.05) were significantly higher in the milking group. Neonates in the clamping group required more blood transfusion (1.78 vs. 0.93; P=0.049), and a higher percentage of neonates in the clamping group required inotropic drugs (63% vs. 29%; P=0.007). The mortality rate was significantly lower in the milking group (6% vs. 28%; P=0.015).
Conclusion
Umbilical cord milking can be a safe and beneficial procedure for both the mother and the neonate in deliveries of less than 33 weeks of gestation.
Keywords: Fetomaternal transfusion; Anemia, neonatal; Infant, premature
Introduction
Establishing effective circulation with sufficient blood volume is crucial for the survival of preterm infants. To this end, procedures that enhance autologous transfusion from the placenta to the neonate, such as delayed cord clamping (DCC) or umbilical cord milking have been investigated. These procedures are known to provide up to 30% more blood volume and 60% more red blood cells to neonates than immediate cord clamping [1]. Growing evidence from current studies highlights the neonatal benefits of these volume-enhancing strategies, such as higher levels of hemoglobin [1,2], a lower need for blood transfusions [3], and lower prevalence of necrotizing enterocolitis (NEC) [4] and intraventricular hemorrhage (IVH) [5]. Based on these results, the American College of Obstetricians and Gynecologists have recommended delaying cord clamping for 30 to 60 seconds in preterm deliveries “when feasible” [6].
Despite the expected benefits outlined above, there are still barriers to implementing these blood volume-transferring measures routinely in clinical practice, especially in very preterm deliveries. First, one major concern is the consequences associated with delaying urgent resuscitation, which is usually anticipated in such cases. Wiberg et al. [7] studied the influence of DCC on cord blood gas analysis, and found that it was associated with significantly decreased pH and HCO3- levels, and increased lactic acid levels. Also, delayed resuscitation of the neonate might lead to hypothermia and the need for excessive resuscitation, including early intubation. Up to now, the acceptable duration of delay in resuscitation and the overall outcomes associated with delayed resuscitation in very preterm neonates have not been investigated in detail. Second, there is potentially increased risk of the neonate contracting certain diseases associated with hypervolemia and plethora. An over-distended circulation from placental transfusion may increase the incidence and severity of heart failure, respiratory distress, neurological depression, or jaundice [8,9]. Unlike full-term infants, preterm infants have limited ability to adapt to over-transfusion. In 2006 study, Evans [10] showed that there is a hyperperfusion-reperfusion cycle that occurs over the initial 24 to 48 hours of life in preterm infants born before 30 weeks of gestation, and uncontrolled volume loading by enhanced placental transfusion can be detrimental, with comorbidities such as sepsis or ventricular dysfunction. Moreover, there have been no investigations to determine the ideal blood volume required in preterm infants according to gestational age. Third, very little is known about the influence of iatrogenic manipulation on the fetal to neonatal transition. Milking, a particularly rapid strategy by nature, may bypass the fetoplacental circulation and not allow enough time for a smooth transition of the cardiopulmonary and cerebral circulation, thus rendering the newborn more unstable. In a meta-analysis of 10 studies which included 199 infants born before 30 weeks of gestation, Ghavam et al. [11] concluded that there is a paucity of evidence for the long-term benefits and safety of enhanced placental transfusion strategies. Lastly, delayed placental delivery may increase the amount of third stage bleeding and the associated maternal morbidity. Most reports that studied enhanced placental transfusion focused mainly on the neonatal outcomes, so there is little published data on maternal outcomes, including hemorrhagic incidence.
Providing evidence for the safety of enhanced placental transfusion for both the neonate and mother provides a rationale for its use in very preterm deliveries. Umbilical cord milking may be a better option for placental transfusion than DCC, because it minimizes the delay in resuscitation with a comparable volume expansion [12]. We therefore conducted a prospective study to determine the safety of umbilical cord milking in deliveries less than 33 weeks of gestation for both the neonate and mother by comparing the short-term clinical and laboratory outcomes associated with this procedure with those of immediate cord clamping.
Materials and methods
This phase II randomized controlled study was conducted at a single tertiary care center (Chungnam National University Hospital), and ethical approval was obtained from the Institutional Review Board. Pregnant women expected to deliver between 24 0/7 and 32 6/7 completed weeks of gestation were recruited from March 2012 to June 2015. The inclusion criteria were as follows: a mode of delivery (either vaginal or cesarean delivery), maternal complications such as placental abruption or placenta previa, neonates with nuchal cords, or those in need of immediate resuscitation. Exclusion criteria included multiple gestations, rhesus sensitization, fetal hydrops, or major fetal anomalies. Women without antenatal written consent were also excluded. Enrolled women were randomized into either the milking or clamping group through assignment by computer-generated random numbers just before delivery. All women received 12 mg of betamethasone (Celestone®; Schering-Plough Corporation, Kenilworth, NJ, USA) at admission and, when possible, received an additional dose of 12 mg betamethasone 24 hours after the initial administration.
In the milking group, as a neonate was delivered, the assistant wrapped the neonate with a warm towel and lowered the neonate to 20 cm below the level of the placenta. Then, the obstetrician milked the umbilical cord from the placenta toward the neonate 4 times at a speed of 20 cm/2 seconds before clamping the cord. Between each milking motion, the cord was released and allowed to refill with blood during a 2-second pause. Milking took approximately 15 to 20 seconds for each case. In the immediate clamping group, the cord was immediately clamped after the baby was delivered. After the cord was clamped and cut, the neonate was handed to the resuscitation team for initial support and was transported to the neonatal intensive care unit (NICU) for admission due to prematurity. Following placental delivery, the mother received intravenous oxytocin at a rate of 250 mL/min. Ergometrine or carbetocin was given to the mother if the uterine contractions had not been effective.
Maternal and neonatal data associated with delivery were collected prospectively. Primary outcomes were the short-term safety variables associated with milking for both the neonate and mother. Neonatal data, such as Apgar scores at 1 and 5 minutes, prevalence of hypothermia during the first hour of life, the number of neonates in each group who needed early intubation, initial blood gas analyses, maximum bilirubin levels, duration of phototherapy, use of cross-transfusion, and prevalence of respiratory distress (as assessed by clinical signs, oxygen requirement, and respiratory support), were collected. Maternal data, including changes in maternal hemoglobin levels after delivery, number of transfusions needed, and maternal death, were collected as well. Secondary outcome included neonatal hemodynamic variables, such as neonatal hemoglobin levels at birth and at 24 hours of age, blood pressure at 1 and 4 hours of age, urine output during the first day of life, and number of transfusions needed during the first 30 days of life. Major morbidities, such as sepsis, IVH (staging according to Papile et al. [13]), NEC, retinopathy of prematurity, length of hospital stay, and death of the newborn, were also included in secondary outcomes.
Optimal sample sizes were calculated based on a previously published randomized controlled study of umbilical cord milking in preterm neonates [14]. With an α level of 0.05 and a β level of 0.80, we determined that at least 25 infants in each group would be required to demonstrate a 10% difference in initial hemoglobin levels. Allowing for a 20% dropout rate increased the total number of required neonates to 29 for each group. Statistical analysis was performed using Microsoft Excel and International Business Machines (IBM) Statistical Package for the Social Sciences statistics software version 22.0 (SPSS; IBM Corp., Armonk, NY, USA). The 2-sample t-test was used for analysis of normally distributed continuous variables. A χ2 or Fisher's exact test was used for categorical variables. Relative risk was reported as a risk ratio (RR) with a 95% confidence interval (CI). Kaplan-Meier analysis was used to compare survival between the 2 groups. A P<0.05 and a CI of 95% were considered significant.
Results
A total of 66 preterm deliveries were enrolled. Maternal demographics and delivery data are presented in Table 1. The 2 groups were similar with respect to antenatal baseline characteristics, including the presence of complicated medical conditions, delivery mode, receipt of antenatal steroids, and premature rupture of membranes before 24 hours of delivery. Betamethasone was initially administered to all mothers, but only 24 (70.6%) in the milking group and 19 (59.4%) in the clamping group completed the protocol of 2 doses at an interval of 24 hours due to obstetrical emergencies that required prompt delivery. These obstetrical emergencies, including fetal distress, placental problems (placental abruption or placenta previa with bleeding) and deteriorating maternal conditions due to severe pre-eclampsia, were also responsible for the high rate of cesarean delivery in this study (70.6% in the milking group, 78.1% in the clamping group). Differences between pre-partum and post-partum hemoglobin levels (1.35 g/dL in milking vs. 1.58 g/dL in clamping, P=0.451) and the number of transfusions did not differ between the 2 groups, and none of the mothers died.
Table 1. Maternal demographic and delivery data.
| Characteristics | Milking (n=34) | Clamping (n=32) | P-value | |
|---|---|---|---|---|
| Age (yr) | 32.0±4.2 | 33.7±5.5 | 0.151 | |
| BMI | 23.7±5.5 | 21.9±4.5 | 0.157 | |
| Gestational diabetes | 2 (5.9) | 2 (6.3) | 0.950 | |
| Hypertensiona) | 8 (23.5) | 6 (18.8) | 0.635 | |
| Antenatal steroids | 24 (70.6) | 19 (59.4) | 0.339 | |
| Cesarean delivery | 24 (70.6) | 25 (78.1) | 0.484 | |
| CIx for VDb) | 7 (29.2) | 5 (20.0) | ||
| Severe Pre-E | 6 (25.0) | 4 (16.0) | ||
| Fetal distress | 6 (25.0) | 2 (8.0) | ||
| Placental hemorrhagec) | 1 (4.2) | 6 (24.0) | ||
| Others | 4 (16.7) | 8 (32.0) | ||
| PROM >24 hr | 8 (23.5) | 9 (28.1) | 0.670 | |
| Antenatal antibiotics | 14 (41.2) | 12 (37.5) | 0.760 | |
| Hemoglobin difference | 1.4±1.1 | 1.6±1.4 | 0.451 | |
| Blood transfusion | 1 (2.9) | 0 (0.0) | 0.515 | |
Data are presented as number (%) or mean ± standard deviation.
BMI, body mass index; CIx, contraindication; VD, vaginal delivery; Pre-E, pre-eclampsia; PROM, premature rupture of membrane.
a)Hypertension includes pre-eclampsia, chronic hypertension and gestational hypertension. b)Contraindication for vaginal delivery, including fetal malpresentation and history of prior cesarean delivery. c)Placental hemorrhage includes placental abruption and placenta previa with bleeding.
Table 2 presents neonate characteristics and primary outcomes. All of the neonates were admitted to the NICU for evaluation and management of prematurity. The mean gestational age and birth weight were similar between the 2 groups. All short-term safety variables for the neonates were comparable in both groups.
Table 2. Neonatal characteristics and acute outcomes.
| Characteristics | Milking (n=34) | Clamping (n=32) | P-value | |
|---|---|---|---|---|
| Birth weight (g) | 1,256.0±270.8 | 1,256.0±287.8 | 0.170 | |
| Gestational age (wk) | 30.1±2.5 | 29.0±2.6 | 0.106 | |
| Apgar score (min) | ||||
| 1 | 5.5±2.7 | 5.1±2.4 | 0.488 | |
| 5 | 7.8±1.8 | 7.5±1.7 | 0.448 | |
| Body temperature on admission (℃) | 36.7±0.5 | 36.6±0.6 | 0.880 | |
| Initial intubation | 19 (55.9) | 15 (46.9) | 0.464 | |
| BGA | ||||
| pH | 7.3±0.9 | 7.3±0.2 | 0.435 | |
| Lactic acid | 3.3±2.3 | 4.1±2.9 | 0.213 | |
| HCO3− | 22.6±3.5 | 22.3±4.7 | 0.771 | |
| Oxygen supply | 28 (82.4) | 31 (96.9) | 0.106 | |
| Assisted ventilation | 24 (70.6) | 24 (75.0) | 0.688 | |
| Inotropic drugs use | 10 (29.4) | 20 (62.5) | 0.007a) | |
| Maximum serum bilirubin (mmol/L) | 8.8±2.5 | 9.1±4.9 | 0.738 | |
| Duration of phototherapy (day) | 6.7±3.7 | 6.1±4.3 | 0.546 | |
Data are presented as number (%) or mean ± standard deviation.
BGA, blood gas analysis.
a)P<0.05, χ2 test.
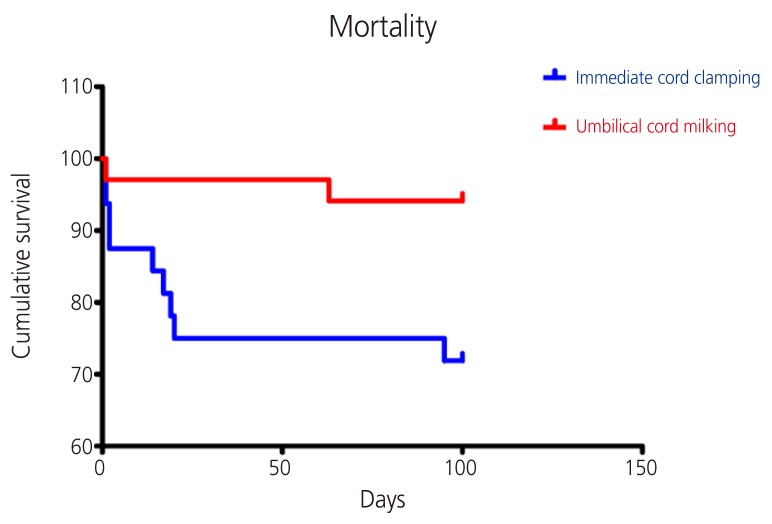
Secondary outcomes are presented in Table 3. Neonatal serum hemoglobin level at birth (15.79 vs. 14.69, respectively; P=0.018) and 24 hours of age (14.83 vs. 13.29, respectively; P=0.046) were significantly higher in the milking group than the clamping group. In accordance with this, the milking group required, on average, fewer packed red blood cell transfusions than the clamping group during the first 24 days of life (0.93 in milking vs. 1.78 in clamping, respectively; P=0.049). Additionally, the number of infants requiring inotropic drugs was significantly lower in the milking group (29.4% vs. 62.5%; RR, 0.47; 95% CI, 1.18–3.82; P=0.007). The incidence of various morbidities, including sepsis, IVH, NEC, and retinopathy of prematurity, was not different between the 2 groups. However, with regard to survival, the milking group showed a lower mortality rate than the clamping group (5.9% vs. 28.1%, respectively; RR, 0.21; 95% CI, 1.12–20.47; P=0.015) (Fig. 1).
Table 3. Neonatal hemodynamic outcomes and major morbidities.
| Characteristics | Milking (n=34) | Clamping (n=32) | P-value | |
|---|---|---|---|---|
| Initial hemoglobin (g/dL) | 15.8±1.6 | 14.7±2.1 | 0.018a) | |
| Hemoglobin at 24 hr (g/dL) | 14.8±2.8 | 13.3±3.1 | 0.046a) | |
| Mean BP at 1 hr of life (mmHg) | 31.7±6.2 | 29.6±6.7 | 0.219 | |
| Mean BP at 4 hr of life (mmHg) | 33.0±5.5 | 32.7±7.5 | 0.893 | |
| Urine output during 24 hr | 109.2±35.8 | 95.0±46.7 | 0.173 | |
| No. of PRBC transfusion during 30 day | 0.9±1.6 | 1.8±2.5 | 0.049a) | |
| Surfactant use | 21 (61.8) | 20 (62.5) | 0.951 | |
| Sepsis | 23 (67.6) | 25 (78.1) | 0.460 | |
| IVH | ||||
| Any grade | 1 (2.9) | 2 (6.3) | 0.512 | |
| Severe | 0 (0) | 2 (6.3) | 0.231 | |
| Necrotizing enterocolitis | 0 (0) | 1 (3.1) | 0.299 | |
| Retinopathy of prematurity | 0 (0) | 2 (6.3) | 0.214 | |
| Length of initial hospital stay (day) | 54.7±19.3 | 51.5±44.8 | 0.718 | |
| Deaths | 2 (5.9) | 9 (28.1) | 0.015a) | |
Data are presented as number (%) or mean ± standard deviation.
BP, blood pressure; PRBC, packed red blood cell; IVH, intraventricular hemorrhage.
a)P< 0.05, 2 sample t-test or χ2 test.
Fig. 1.
Survival of preterm infants in milking and clamping group.
Discussion
This study demonstrated that umbilical cord milking in preterm deliveries of less than 33 weeks of gestation did not affect the acute variables associated with delivery, including Apgar scores at 1 minute and 5 minutes, the occurrence of hypothermia during the first hour of life, the incidence of intubation, and values obtained from blood gas analysis. Short-term clinical outcomes, such as respiratory distress or jaundice, were not influenced by umbilical cord milking as well. Although many studies have reported conflicting data regarding these issues, our results supported the safety of umbilical cord milking in very preterm neonates. These results may be explained by the short duration of umbilical cord milking used in this study, which can minimize the delay of neonatal resuscitation. Other possible explanations include successful prevention of rapidly falling neonatal body temperature, a significant risk associated with prematurity, through the warmth of the additional placental blood and by wrapping the neonate with a warm towel immediately after birth, which prevents the heat loss caused by immature skin and a large surface area-to-body mass ratio. Establishing a protocol to minimize the time for cord milking, and wrapping a newborn with a polyethylene bag instead of a warm towel may be helpful in terms of preventing delayed resuscitation and hypothermia.
For the maternal aspect, delayed delivery of the placenta can theoretically cause excessive maternal bleeding, since the blood flow through the spiral vessel reaches up to 600 mL/min at term [4]. But, there was no difference in maternal hemoglobin levels after delivery and hemorrhagic incidence between the 2 groups in our study. One subject in the milking group experienced postpartum hemorrhage with 2,000 mL of blood loss. She delivered via cesarean section at 32 weeks of gestation with preterm labor. Two packs of red blood cells were transfused, and she was discharged without other complications. Overall, these results indicate the safety of umbilical cord milking for both the neonate and mother with regards to delivery-associated outcomes in very preterm deliveries.
For hemodynamic variables, we found that umbilical cord milking was associated with a significant increase in neonatal serum hemoglobin levels, a decreased use of inotropic drugs, and, on average, fewer packed red blood cell transfusions. Even though the incidence of major morbidities was similar between groups, the mortality rate was significantly lower in the milking group than the clamping group. A recent meta-analysis of 12 studies involving a total of 531 infants delivered at less than 32 weeks of gestation affirmed the effects of enhanced placental transfusion (including both DCC and umbilical cord milking) in reducing the need for blood transfusion, the incidence of IVH of any grade, and mortality [15]. Other studies have also suggested the favorable effect of placental transfusion on neonatal morbidities and mortality [3,5,16,17] (Table 4). These studies offered the decreased incidence of IVH associated with umbilical milking as a possible mechanism for improved survival. Moreover, the milking procedure during delivery might increase systemic blood volume and stabilize cerebral perfusion immediately after birth, thus reducing the incidence of IVH and death. In 2013, March et al. [17] demonstrated the protective effect of umbilical cord milking in extremely preterm infants (born at <29 weeks of gestation) with a 50% reduction in the total incidence of IVH.
Table 4. Summary of recent studies on umbilical cord milking.
| Author | Year | Study design | Enrollment | Result |
|---|---|---|---|---|
| Katheria et al. [20] | 2015 | RCT | 154 babies, GA <32 wk | Higher systematic blood flow with UCM compared to DCC |
| Dang et al. [21] | 2015 | Meta-analysis | 292 babies, GA <32 wk (6 studies) | Improved neonatal outcomes with UCM compared to ICC |
| Backes et al. [15] | 2014 | Meta-analysis | 531 babies, GA <32 wk (12 studies) | Improved neonatal outcomes with enhanced placental transfusion |
| Ghavam et al. [11] | 2014 | Meta-analysis | 199 babies, GA <30 wk (10 studies) | Possible improvement in short-term neonatal outcomes with enhanced placental transfusion |
| Rabe et al. [12] | 2011 | RCT | 58 babies, GA <33 wk | Similar amount of placenta-fetal blood transfusion between UCM and DCC |
RCT, randomized controlled study; GA, gestational age; UCM, umbilical cord milking; DCC, delayed cord clamping; ICC, immediate cord clamping.
The improved survival in the milking group, despite similar incidences of morbidities between the groups, seen in our results may be explained as follows. Although our study failed to show a significant difference in IVH incidence between the groups, there were some trends towards less severe IVH in the milking group. Only one neonate in the milking group was diagnosed with grade I IVH, whereas 2 neonates in the clamping group were diagnosed with grade III IVH. Given that high grade IVH is a major cause of mortality, this favorable trend may explain the improved survival observed in the milking group. In addition, we found that the death of newborns was mostly (7/11 deaths) related to sepsis. Umbilical cord milking facilitates the transfer of hematopoietic stem cells toward the neonate, and these cells can enhance the neonate's resistance to bacterial infection and sepsis. Lastly, the decreased number of transfusions in the milking group might have led to a lower incidence of transfusion-related complications, such as gut injuries, acute lung injuries [18], immunosensitization, and metabolic imbalances, including hyperkalemia. By stabilizing the hemodynamic status with an increase in blood volume, umbilical cord milking can decrease the need for red blood cell transfusion, thus preventing the neonates from potential adverse outcomes following transfusion.
The major limitation of this study was its small sample sizes, which may account for the failure to demonstrate significant differences in major morbidities between the 2 groups. Enhanced placental transfusion among neonates at extremely low gestational age remains controversial because of the susceptibility of such neonates to rapid changes in their hemodynamic status due to their immature cardiovascular systems [19] and a paucity of data regarding the long-term outcomes of such volume-enhancing methods. However, we could not analyze the data according to gestational age, due to the small sample sizes, and the follow-up period was not long enough to evaluate long-term outcomes. Further studies with a larger number of infants with extremely low gestational age are required, and there must also be a focus on long-term outcomes. Despite these limitations, this study has strength in that it was prospective and randomized. Moreover, this study emphasized maternal data and outcomes associated with umbilical cord milking, unlike other studies that were focused mainly on the effects of milking on the neonates.
In conclusion, umbilical cord milking for enhancing placental transfusion in deliveries of less than 33 weeks of gestation is a safe, feasible method for both the mother and neonate to improve neonatal outcomes.
Footnotes
This abstract was presented at 25th Asian & Oceanic Congress of Obstetrics and Gynaecology as poster presentation in Hong Kong, 2017.
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Strauss RG, Mock DM, Johnson KJ, Cress GA, Burmeister LF, Zimmerman MB, et al. A randomized clinical trial comparing immediate versus delayed clamping of the umbilical cord in preterm infants: short-term clinical and laboratory endpoints. Transfusion. 2008;48:658–665. doi: 10.1111/j.1537-2995.2007.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, Raker C, et al. Hemodynamic effects of delayed cord clamping in premature infants. Pediatrics. 2012;129:e667–e672. doi: 10.1542/peds.2011-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinmond S, Aitchison TC, Holland BM, Jones JG, Turner TL, Wardrop CA. Umbilical cord clamping and preterm infants: a randomised trial. BMJ. 1993;306:172–175. doi: 10.1136/bmj.306.6871.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabe H, Diaz-Rossello JL, Duley L, Dowswell T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev. 2012:CD003248. doi: 10.1002/14651858.CD003248.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Mercer JS, Vohr BR, McGrath MM, Padbury JF, Wallach M, Oh W. Delayed cord clamping in very preterm infants reduces the incidence of intraventricular hemorrhage and late-onset sepsis: a randomized, controlled trial. Pediatrics. 2006;117:1235–1242. doi: 10.1542/peds.2005-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Committee on Obstetric Practice, American College of Obstetricians and Gynecologists. Committee opinion no.543: timing of umbilical cord clamping after birth. Obstet Gynecol. 2012;120:1522–1526. doi: 10.1097/01.AOG.0000423817.47165.48. [DOI] [PubMed] [Google Scholar]
- 7.Wiberg N, Källén K, Olofsson P. Delayed umbilical cord clamping at birth has effects on arterial and venous blood gases and lactate concentrations. BJOG. 2008;115:697–703. doi: 10.1111/j.1471-0528.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 8.Saigal S, Usher RH. Symptomatic neonatal plethora. Biol Neonate. 1977;32:62–72. doi: 10.1159/000240996. [DOI] [PubMed] [Google Scholar]
- 9.Saigal S, O'Neill A, Surainder Y, Chua LB, Usher R. Placental transfusion and hyperbilirubinemia in the premature. Pediatrics. 1972;49:406–419. [PubMed] [Google Scholar]
- 10.Evans N. Assessment and support of the preterm circulation. Early Hum Dev. 2006;82:803–810. doi: 10.1016/j.earlhumdev.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Ghavam S, Batra D, Mercer J, Kugelman A, Hosono S, Oh W, et al. Effects of placental transfusion in extremely low birthweight infants: meta-analysis of long- and short-term outcomes. Transfusion. 2014;54:1192–1198. doi: 10.1111/trf.12469. [DOI] [PubMed] [Google Scholar]
- 12.Rabe H, Jewison A, Alvarez RF, Crook D, Stilton D, Bradley R, et al. Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates: a randomized controlled trial. Obstet Gynecol. 2011;117:205–211. doi: 10.1097/AOG.0b013e3181fe46ff. [DOI] [PubMed] [Google Scholar]
- 13.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 14.Kumar B, Upadhyay A, Gothwal S, Jaiswal V, Joshi P, Dubey K. Umbilical cord milking and hematological parameters in moderate to late preterm neonates: a randomized controlled trial. Indian Pediatr. 2015;52:753–757. doi: 10.1007/s13312-015-0711-1. [DOI] [PubMed] [Google Scholar]
- 15.Backes CH, Rivera BK, Haque U, Bridge JA, Smith CV, Hutchon DJ, et al. Placental transfusion strategies in very preterm neonates: a systematic review and meta-analysis. Obstet Gynecol. 2014;124:47–56. doi: 10.1097/AOG.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 16.Hosono S, Mugishima H, Fujita H, Hosono A, Minato M, Okada T, et al. Umbilical cord milking reduces the need for red cell transfusions and improves neonatal adaptation in infants born at less than 29 weeks' gestation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F14–F19. doi: 10.1136/adc.2006.108902. [DOI] [PubMed] [Google Scholar]
- 17.March MI, Hacker MR, Parson AW, Modest AM, de Veciana M. The effects of umbilical cord milking in extremely preterm infants: a randomized controlled trial. J Perinatol. 2013;33:763–767. doi: 10.1038/jp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rashid N, Al-Sufayan F, Seshia MM, Baier RJ. Post transfusion lung injury in the neonatal population. J Perinatol. 2013;33:292–296. doi: 10.1038/jp.2012.114. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds GJ. Beyond sweetness and warmth: transition of the preterm infant. Arch Dis Child Fetal Neonatal Ed. 2008;93:F2–F3. doi: 10.1136/adc.2007.120790. [DOI] [PubMed] [Google Scholar]
- 20.Katheria AC, Truong G, Cousins L, Oshiro B, Finer NN. Umbilical cord milking versus delayed cord clamping in preterm infants. Pediatrics. 2015;136:61–69. doi: 10.1542/peds.2015-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang D, Zhang C, Shi S, Mu X, Lv X, Wu H. Umbilical cord milking reduces need for red cell transfusions and improves neonatal adaptation in preterm infants: meta-analysis. J Obstet Gynaecol Res. 2015;41:890–895. doi: 10.1111/jog.12657. [DOI] [PubMed] [Google Scholar]