Abstract
Rationale: Acute respiratory distress syndrome (ARDS) remains a major cause of respiratory failure in critically ill patients. Mesenchymal stromal cells (MSCs) are a promising candidate for a cell-based therapy. However, the mechanisms of MSCs’ effects in ARDS are not well understood. In this study, we focused on the paracrine effect of MSCs on macrophage polarization and the role of extracellular vesicle (EV)-mediated mitochondrial transfer.
Objectives: To determine the effects of human MSCs on macrophage function in the ARDS environment and to elucidate the mechanisms of these effects.
Methods: Human monocyte–derived macrophages (MDMs) were studied in noncontact coculture with human MSCs when stimulated with LPS or bronchoalveolar lavage fluid (BALF) from patients with ARDS. Murine alveolar macrophages (AMs) were cultured ex vivo with/without human MSC-derived EVs before adoptive transfer to LPS-injured mice.
Measurements and Main Results: MSCs suppressed cytokine production, increased M2 macrophage marker expression, and augmented phagocytic capacity of human MDMs stimulated with LPS or ARDS BALF. These effects were partially mediated by CD44-expressing EVs. Adoptive transfer of AMs pretreated with MSC-derived EVs reduced inflammation and lung injury in LPS-injured mice. Inhibition of oxidative phosphorylation in MDMs prevented the modulatory effects of MSCs. Generating dysfunctional mitochondria in MSCs using rhodamine 6G pretreatment also abrogated these effects.
Conclusions: In the ARDS environment, MSCs promote an antiinflammatory and highly phagocytic macrophage phenotype through EV-mediated mitochondrial transfer. MSC-induced changes in macrophage phenotype critically depend on enhancement of macrophage oxidative phosphorylation. AMs treated with MSC-derived EVs ameliorate lung injury in vivo.
Keywords: acute respiratory distress syndrome, extracellular vesicles, mesenchymal stromal cells, macrophages, mitochondria
At a Glance Commentary
Scientific Knowledge on the Subject
Mesenchymal stromal cells (MSCs) are promising candidates for cell-based therapy for patients with acute respiratory distress syndrome (ARDS). MSC-derived extracellular vesicles (EVs) have been shown to recapitulate many of the beneficial effects of MSCs in lung injury in vivo. These MSC-derived EVs have also been shown to transfer mitochondria to other cell types, including macrophages, improving their bioenergetics. The effect of this EV-mediated mitochondrial transfer on macrophage function in the context of ARDS is currently unknown.
What This Study Adds to the Field
Human MSCs suppress proinflammatory cytokine secretion, enhance phagocytic capacity, and promote M2 macrophage marker expression in human macrophages in the presence of bronchoalveolar lavage fluid from patients with ARDS through paracrine mechanisms. These effects were mediated by mitochondrial transfer from MSCs to macrophages via EVs and were critically dependent on the enhancement of oxidative phosphorylation in macrophages. Moreover, adoptive transfer of murine alveolar macrophages that had been pretreated with MSC-derived EVs ex vivo mitigated endotoxin-induced lung injury in vivo. This study demonstrates an important role of MSC-derived EVs for MSC effects in ARDS, reveals a new mechanism of macrophage polarization, and highlights the essential role of alveolar macrophages as mediators of MSCs’ therapeutic effects.
Acute respiratory distress syndrome (ARDS) is the leading cause of mortality and morbidity in the critically ill. Although mortality rates have fallen with modifications in mechanical ventilation, they remain as high as 25 to 40%, and no effective pharmacological treatments are available (1–3). ARDS results from multiple causes, pneumonia and sepsis being the most common and most devastating. Uncontrolled alveolar inflammation is the hallmark of this disease. Alveolar macrophages (AMs) provide defense against respiratory pathogens and orchestrate inflammatory responses in the distal respiratory tract. Macrophages are polarized by environmental cues and adopt different phenotypes. M1 proinflammatory and M2 antiinflammatory phenotypes are associated with the acute and resolving phases, respectively, of inflammation in ARDS (4, 5). Mesenchymal stromal cells (MSCs) are increasingly recognized as a promising candidate therapy for ARDS (6). We and others have reported that MSCs improve survival, reduce inflammation, and enhance bacterial clearance in preclinical models of lung injury (7–14). Secretion of paracrine factors, modulation of host cells via secretion of extracellular vesicles (EVs), and mitochondrial transfer were shown to be important for the therapeutic effects of MSCs in these studies. These data have lent support to phase I/II clinical trials in which researchers are testing MSC administration to patients with ARDS (15, 16). Currently, however, there are no potency assays in place to aid in the selection of MSC donors before administration of MSCs to patients (17), and despite rapid progression of MSCs to clinical trials, a complete understanding of the mechanisms of MSCs’ immunomodulatory effects remains elusive.
MSCs have the capacity to transfer mitochondria to the alveolar epithelium, enhancing bioenergetics and mitigating lung injury (9, 18). Our group previously demonstrated that MSCs transfer mitochondria to human macrophages via tunneling nanotubes, which enhanced their phagocytic capacity and facilitated MSCs’ antimicrobial effects in murine Escherichia coli pneumonia (19). Importantly, depletion of AMs abrogated MSCs’ protective effects in this model, suggesting that AMs are key cellular mediators of MSCs’ effects. Although contact-dependent mechanisms such as these are important, a large body of evidence suggests that MSCs’ therapeutic effects are mediated by paracrine factors (20–22). Recently, EVs have emerged as an important component of the MSC secretory repertoire. MSC-derived EVs may contain a diverse cargo, including proteins, mRNAs, microRNAs (miRNAs), and mitochondria, the functional effects of which remain largely unknown (9, 23–25). MSC-derived EVs alone are capable of recapitulating many of the beneficial effects of MSC whole-cell therapy (25–28). Phinney and colleagues observed the transfer of MSC mitochondria and miRNAs via EVs to human macrophages in a model of silicosis and found that mitochondria enhanced macrophage bioenergetics, whereas miRNAs suppressed Toll-like receptor signaling (24). They observed that normoxia (21% oxygen) induced oxidative stress, thereby promoting mitophagy in MSCs. Ghanta and colleagues then demonstrated the importance of autophagy in maintaining healthy mitochondrial function and promoting survival in MSCs during oxidative stress (29). The influence of mitochondrial transfer on macrophage phenotype, however, has not been studied extensively. Vats and colleagues showed the importance of mitochondrial oxidative phosphorylation in the induction of IL-4–induced antiinflammatory M2 macrophage polarization (30). Indeed, glucose metabolism is intrinsically linked to a macrophage activation state with M1 proinflammatory macrophages using glycolysis (31).
In the present study, we sought to characterize the effects of MSCs on human macrophage polarization in in vitro models of ARDS. We tested the hypothesis that mitochondrial transfer from MSCs to macrophages via EVs modulates macrophage function through promotion of oxidative phosphorylation. Some of the results of these studies were previously reported in the form of abstracts (32–35).
Methods
See the online supplement for detailed methods.
Human Bone Marrow–derived MSCs
MSCs were acquired from the Texas A&M University Health Science Center College of Medicine Institute for Regenerative Medicine at Baylor Scott & White Hospital (Temple, TX), a National Institutes of Health repository. These cells fulfill all criteria set by the International Society of Cellular Therapy for the definition of MSCs (36). Multiple MSC donors were used for experiments throughout this study.
Human Monocyte–derived Macrophage and MSC Noncontact Coculture
Monocytes were isolated from donor buffy coats as previously described (37). Buffy coats were obtained from the Northern Ireland Blood Transfusion Service. Ethical approval was granted by the School Research Ethics Committee of Queen’s University Belfast. Monocytes were differentiated into macrophages for 7 days in the presence of 10 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF) (R&D Systems, Minneapolis, MN). Macrophages were cultured with MSCs in a Transwell system at a ratio of 1:5 or with MSC conditioned medium (CM). Cells were stimulated with LPS (E. coli O111:B4; List Biological Laboratories, Campbell, CA) at 10 ng/ml or with 30% bronchoalveolar lavage fluid (BALF) pooled from nine patients with ARDS and diluted in RPMI 1640 with 1% fetal bovine serum for 24 or 72 hours. Ethical approval for use of patient samples for research was obtained from the Office for Research Ethics Committees Northern Ireland. In additional experiments, MSC CM was pretreated with CD44 neutralizing antibody (BD Biosciences, San Jose, CA) to assess the importance of EV uptake.
Isolation of MSC-derived EVs
MSC-derived EVs were isolated as previously described (27). Briefly, 15 × 106 cells were cultured in media supplemented with EV-depleted serum for 48 hours. CM was then collected and centrifuged at 10,000 × g for 20 minutes to remove cells and debris, followed by centrifugation at 100,000 × g for 2 hours to isolate EVs. EVs were resuspended in 2 ml of media (7.5 × 106 cells/ml). Flow cytometry was used for characterization of EVs using a FACSCanto II flow cytometer and FACSDiva software (BD Biosciences). Analysis was performed using FlowJo version 7 software (FlowJo, Ashland, OR).
In Vivo LPS-induced Lung Injury Model
C57BL/6 male mice (8 to 10 wk old; Harlan Laboratories Ltd., Shardlow, Derby, UK) were used. Animals were maintained in the Biological Services Unit at Queen’s University Belfast. Experiments were sanctioned and approved by the U.K. Home Office and the Queen’s University Belfast Ethical Review Committee. Mice were anesthetized by isoflurane inhalation, and 20 mg/kg LPS was instilled intranasally. Four hours after injury, AMs from ex vivo culture were given intranasally (2.5 × 105 AMs/mouse) after xylazine/ketamine anesthetization. Twenty-four hours after injury, mice were killed, and BALF was taken for analysis.
Mitochondrial Transfer and Functional Studies
For assessment of mitochondrial transfer, MSC and human monocyte–derived macrophage (MDM) mitochondria were prestained with MitoTracker Deep Red and Green, respectively (Thermo Fisher Scientific, Renfrew, UK). CM was taken from prestained MSCs and added to MDMs for 24 hours to allow EV uptake, and mitochondrial transfer was visualized using the EVOS FL Auto Imaging System (Life Technologies, Carlsbad, CA). For functional studies, MDM mitochondrial function was inhibited with oligomycin at 3 μg/ml, and MSC mitochondria were inhibited by pretreatment for 48 hours with 1 μg/ml rhodamine 6G (both from Sigma-Aldrich, St. Louis, MO), which both target ATP synthase (38, 39). Mitochondrial respiration was assessed using the Seahorse XF Cell Mito Stress Test Kit and an XFe96 Extracellular Flux Analyzer, and analysis was performed using Wave version 2.2 software (all from Agilent Technologies, Santa Clara, CA).
Statistical Analysis
Analysis was performed using Prism 5 software (GraphPad Software, La Jolla, CA). Experiments for each MDM donor were performed at least in triplicate; the average of three technical replicates was taken as a single data point for each donor, and the data points were pooled together for statistical analysis. Pooled data were presented as the mean with SD. For parametric data, Student’s t test or one-way analysis of variance with post hoc analysis using Bonferroni’s selected comparisons was performed. For nonparametric data, the Kruskal-Wallis test with post hoc analysis using Dunn’s selected comparisons was used. The statistical significance level was set at P < 0.05.
Results
MSCs Induce an Unconventional M2-like Phenotype in MDMs with Increased Phagocytic Capacity in the Presence of E. coli LPS
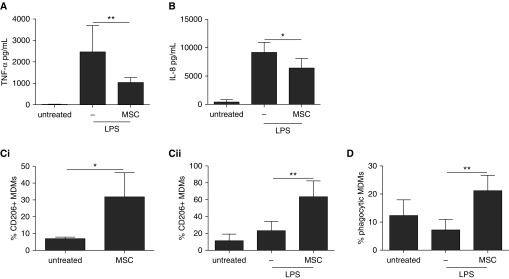
LPS stimulation increased MDM secretion of proinflammatory cytokines tumor necrosis factor (TNF)-α and IL-8. MSCs significantly diminished TNF-α and IL-8 production by 58 ± 8% and 30 ± 15%, respectively (Figures 1A and 1B). Table 1 summarizes the effects of MSCs on MDM production of cytokines and chemokines associated with M1 and M2 macrophage polarization.
Figure 1.
Human mesenchymal stromal cell (MSC) modulation of human monocyte–derived macrophage (MDM) phenotype and function. (A) MSCs in noncontact coculture reduced the production of tumor necrosis factor (TNF)-α by MDMs after 24 hours of LPS treatment. One-way analysis of variance (ANOVA) with Bonferroni’s post hoc test was used (n = 5 per group). (B) IL-8 production by MDMs was reduced by MSC coculture after LPS treatment. One-way ANOVA with Bonferroni’s post hoc test was used (n = 4 per group). (C) MSC coculture increased the percentage of MDMs expressing CD206 on their surface in the (Ci) absence or (Cii) presence of LPS. Unpaired t test (n = 3 per group) and one-way ANOVA with Bonferroni’s post hoc test (n = 4 per group), respectively, were used. (D) MSCs increased the proportion of MDMs that had phagocytosed Escherichia coli pHrodo (Thermo Fisher Scientific, Carlsbad, CA) dye-stained fluorescent bioparticles after LPS. One-way ANOVA with Bonferroni’s post hoc test was used (n = 5 per group). Data are presented as mean ± SD. *P < 0.05; **P < 0.01.
Table 1.
Effect of Human Mesenchymal Stromal Cells on Human Monocyte–derived Macrophage Cytokine and Chemokine Secretion after LPS Stimulation
| Analyte | Concentration (pg/ml) |
P Value | |
|---|---|---|---|
| LPS | LPS + MSC | ||
| M1 associated | |||
| IFN-γ | ND | ND | — |
| IL-1β | ND | ND | — |
| IL-8 | 9,155 ± 1,790 | 6,410 ± 1,676 | <0.05 |
| IL-12p70 | ND | ND | — |
| IL-17 | ND | ND | — |
| IL-23 | 37,856 ± 27,577 | 23,858 ± 17,544 | 0.361 |
| TNF-α | 2,465 ± 1,103 | 1,038 ± 207 | <0.05 |
| CCL5 | 9,571 ± 8,032 | 5,885 ± 4,392 | 0.404 |
| M2 associated | |||
| IL-1ra | 120,143 ± 34,335 | 110,304 ± 27,258 | 0.627 |
| IL-10 | ND | ND | — |
| CCL17 | 678 ± 607 | 341 ± 228 | 0.589 |
| CCL18 | 904 ± 604 | 292 ± 190 | 0.056 |
| CCL22 | 106,211 ± 67,673 | 22,486 ± 13,267 | <0.05 |
Definition of abbreviations: CCL = chemokine (C-C motif) ligand; MSC = human mesenchymal stromal cell; ND = no data; TNF-α = tumor necrosis factor-α.
Data are presented as mean ± SD.
The M1 cytokines IFN-γ, IL-1β, IL-12p70, and IL-17, as well as the M2 antiinflammatory cytokine IL-10, were not detectable in these cocultures. Levels of the M1 cytokine IL-23, the M1 chemokine (C-C motif) ligand 5 (CCL5), the M2 cytokine IL-1ra, and the M2 chemokine CCL17 were unaffected by MSCs. Levels of the M2 chemokines CCL18 (68 ± 21% reduction) and CCL22 (79 ± 12% reduction) were also diminished with MSCs, although the reduction in CCL18 levels was not significant.
In this study, we investigated expression of an array of markers previously shown to be suitable for human macrophages (Table 2). Among them, only expression of CD206 (a marker for M2 polarization [40]) demonstrated differential regulation by LPS and MSCs; MDM expression of CD206 was significantly increased by MSCs in both the presence (40 ± 16% increase) and the absence (25 ± 12% increase) of LPS (Figure 1C). Notably, expression of CD163 (an established M2 marker [41]), although detectable, was not affected by any of the stimulations. MSCs significantly increased the proportion of phagocytic MDMs in the presence of LPS by 2.9-fold compared with LPS alone (Figure 1D). In contrast, fibroblast cell control studies demonstrated that although fibroblasts diminished TNF-α secretion by MDMs, they did not influence IL-8 levels or CD206 expression, indicating that the observed effects were specific to MSCs (see Figure E1 in the online supplement).
Table 2.
Effect of Human Mesenchymal Stromal Cells on Macrophage Marker Expression
| Marker | Expression (%) |
P Value | |
|---|---|---|---|
| Without MSCs | With MSCs | ||
| M1 associated | |||
| No LPS | |||
| CD40 | 70.2 ± 10.8 | 85.3 ± 7.6 | 0.114 |
| CD54 | 97.1 ± 2.5 | 98.6 ± 0.8 | 0.686 |
| LPS | |||
| CD40 | 95.8 ± 1.4 | 97.7 ± 1.4 | 0.147 |
| CD54 | 97.9 ± 0.8 | 98.8 ± 1.1 | 0.200 |
| M2 associated | |||
| No LPS | |||
| CD163 | 11.6 ± 14.5 | 28.0 ± 9.0 | 0.090 |
| CD206 | 7.0 ± 0.8 | 31.8 ± 11.7 | <0.05 |
| LPS | |||
| CD163 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.686 |
| CD206 | 23.3 ± 9.5 | 63.5 ± 16.3 | <0.05 |
Definition of abbreviation: MSCs = human mesenchymal stromal cells.
Data are presented as mean ± SD.
MSCs Modulate MDM Phenotype and Function in BALF from Patients with ARDS
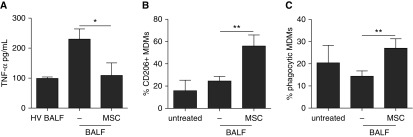
To more closely mimic the ARDS environment, we cocultured MDMs with MSCs in 30% BALF of patients with ARDS or healthy volunteers. ARDS BALF resulted in a sharp up-regulation of TNF-α secretion, which MSCs significantly reduced by 53 ± 16% (Figure 2A). MSCs reduced IL-8 levels in the presence of ARDS BALF; however, this did not reach statistical significance (Figure E2). Consistently, MSCs were able to significantly increase the proportion of MDMs expressing the M2 marker CD206 (Figure 2B). In the presence of ARDS BALF, MSCs were able to double the proportion of phagocytic MDMs (Figure 2C).
Figure 2.
Human mesenchymal stromal cells (MSCs) modulate human monocyte–derived macrophages (MDMs) in the presence of bronchoalveolar lavage fluid (BALF) from patients with acute respiratory distress syndrome (ARDS). (A) MSCs reduced the production of tumor necrosis factor (TNF)-α by MDMs treated with 30% ARDS BALF for 24 hours. Kruskal-Wallis test with Dunn’s post hoc test was used (healthy volunteer [HV] BALF, n = 3; other groups, n = 5). (B) MSCs increased the proportion of MDMs expressing the M2 macrophage surface marker CD206 after 72 hours. One-way analysis of variance with Bonferroni’s post hoc test was used (n = 3 for all groups). (C) MSCs increased the proportion of phagocytic MDMs in the presence of ARDS BALF. One-way analysis of variance with Bonferroni’s post hoc test was used (n = 4 for all groups). Data are presented as mean ± SD. *P < 0.05; **P < 0.01.
MSC-derived EVs Expressing CD44 Are Partially Responsible for the MSC Antiinflammatory Effect and Enhanced Phagocytosis
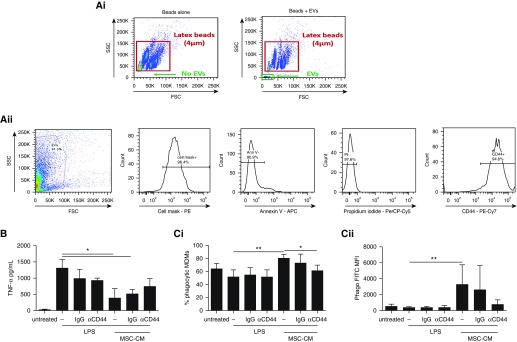
Flow cytometric analysis demonstrated that MSC CM contains a population of particles less than 4 µm in diameter (referred to herein as extracellular vesicles or EVs). This is consistent with the literature showing that MSC-derived EVs range from less than 100 nm to 1,000 nm (24, 27). Further characterization demonstrated that EVs were more than 90% positive for MSC membrane and more than 90% negative for annexin V and propidium iodide staining, indicating minimal contamination with cell debris or apoptotic bodies and, importantly, that EVs demonstrated uniform expression of CD44 (>98% positive) (Figure 3A).
Figure 3.
CD44-expressing extracellular vesicles (EVs) from human mesenchymal stromal cells (MSCs) are partially responsible for their modulatory effects. (A) Flow cytometry demonstrates that MSC-derived EVs are (Ai) smaller in diameter than latex beads of 4-μm diameter and (Aii) positive for CD44 expression on their surface. (B) Preincubation of MSC conditioned medium (CM) with anti-CD44 neutralizing antibody partially reversed MSC suppression of tumor necrosis factor (TNF)-α secretion by human monocyte–derived macrophages (MDMs; n = 4 for all groups) and completely prevented MSC enhancement of (Ci) the proportion of phagocytic MDMs as well as (Cii) their phagocytic index (Phago; n = 5 for all groups). One-way analysis of variance with Bonferroni’s post hoc test was used. Data are presented as mean ± SD. *P < 0.05; **P < 0.01. APC = allophycocyanin; Cy = cyanine; FITC = fluorescein isothiocyanate; FSC = forward scatter; MFI = median fluorescence intensity; PE = phycoerythrin; PerCP = peridinin-chlorophyll-protein complex; SSC = side scatter.
Preincubation of MSC CM with anti-CD44 antibody, but not with IgG, partially abrogated the effect on MDM TNF-α secretion. (Suppression of 70 ± 19% was reduced to 43 ± 15% after CD44 neutralization.) Importantly, anti-CD44 antibody did not alter TNF-α secretion by MDMs without the presence of MSC CM (Figure 3B). As before, MSC CM significantly increased MDM phagocytic activity in the presence of LPS. The percentage of phagocytic MDMs was increased by 28 ± 5%, and the MDM phagocytic index (determined by median fluorescence intensity) was increased ninefold compared with LPS stimulation alone. Anti-CD44, but not IgG, completely reversed these effects. Antibodies administered to MDMs in the absence of MSC CM had no influence on phagocytic activity (Figure 3C).
Adoptive Transfer of Murine AMs Treated by MSC-derived EVs Confer Protection in LPS-induced Lung Injury
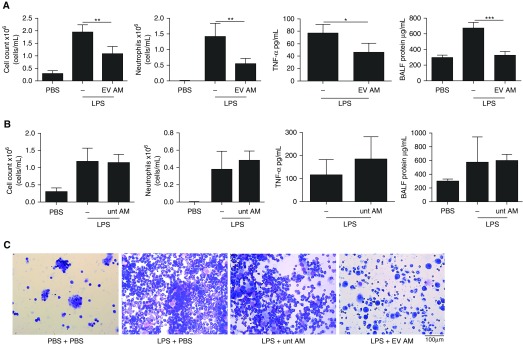
To test if modulation of macrophages by MSC-derived EVs has a therapeutic effect in vivo, AMs were isolated from C57BL/6 mice, treated ex vivo with MSC-derived EVs for 48 hours, and adoptively transferred intranasally into mice, which were challenged with LPS to induce lung injury. Intranasal instillation of LPS into mice resulted in lung injury at 24 hours, as evidenced by increased inflammatory cell infiltration and protein content in the BALF. Treatment of mice with MSC-derived EV–treated AMs 4 hours after LPS challenge reduced total cell counts in the BALF by 45 ± 13%, absolute neutrophil counts by 61 ± 10%, BALF TNF-α levels by 40 ± 15%, and BALF protein by 52 ± 6% compared with LPS-injured mice that received vehicle control treatment (Figure 4A). Importantly, treatment of mice with AMs cultured ex vivo and not treated with MSC-derived EVs had no effect (Figure 4B). Cytospin preparations of BALF demonstrated substantial inflammatory cell recruitment to the alveolar compartment, consisting predominantly of neutrophils in the LPS-injured group, which was reduced by administration of EV-treated AMs (Figure 4C).
Figure 4.
Human mesenchymal stromal cell extracellular vesicle (EV)-treated alveolar macrophages (AMs) reduce LPS-induced lung injury. (A) Treatment of LPS-injured mice with EV-treated AMs reduced total cell counts and neutrophilic cell counts as well as the amount of tumor necrosis factor (TNF)-α and protein in the bronchoalveolar lavage fluid (BALF). One-way analysis of variance with Bonferroni’s post hoc test was used for total cell and neutrophil counts and BALF protein (n = 4 phosphate-buffered saline [PBS]; n = 5 LPS; n = 3 LPS + EV-treated AMs). Student’s unpaired t test was used for TNF-α (n = 3 LPS; n = 3 LPS + EV-treated AMs). (B) Untreated (unt) AMs had no effect on total or neutrophil cell counts and had no effect on BALF TNF-α or protein levels. One-way analysis of variance with Bonferroni’s post hoc test was used for total cell and neutrophil counts and BALF protein (n = 4 PBS; n = 4 LPS; n = 5 LPS + untreated AMs). Student’s unpaired t test was used for TNF-α (n = 5 LPS; n = 5 LPS + untreated AMs). (C) Cytospins of BALF preparations demonstrated inflammatory cell recruitment to the airspaces after LPS injury and reduced cell numbers after treatment with EV-treated AMs but not after treatment with untreated AMs (original magnification, ×20). Data are presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
MSC-derived EVs Transfer Functional Mitochondria to MDMs, Enhancing Macrophage Oxidative Phosphorylation
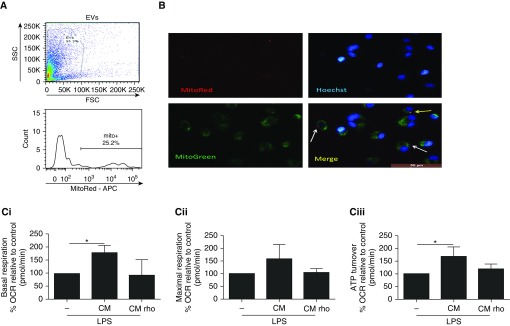
Flow cytometric analysis of MSC CM demonstrated that 25% of MSC-derived EVs detectable by this method were positive for mitochondria (Figure 5A). Mitochondrial transfer from MSCs to MDMs was visualized by fluorescence microscopy. MSCs were prestained with MitoTracker Red FM, and CM was collected and added to MDMs that had been prestained with MitoTracker Green FM and Hoechst nuclear stain (blue in Figure 5B). At 24 hours, there was evidence of MSC mitochondria–containing EVs adhering to the MDMs (red staining, white arrows in Figure 5B) and colocalization of MSC mitochondria into the MDM mitochondrial network (yellow staining, yellow arrow in Figure 5B).
Figure 5.
Human mesenchymal stromal cell (MSC)-derived extracellular vesicles (EVs) transfer functional mitochondria to human monocyte-derived macrophages (MDMs), which enhances macrophage oxidative phosphorylation. (A) Flow cytometry of culture medium taken from MitoTracker Red–prestained MSCs showed that 25% of EVs were positive for mitochondria. (B) EVs contained in MSC culture medium (CM) transfer mitochondria (red) to MDMs (MitoTracker Green) (white arrows). MSC mitochondria are integrated into the MDM mitochondrial network (yellow, yellow arrows) (original magnification, ×20). (C) Treatment of MDMs with MSC CM resulted in increases in (Ci) basal mitochondrial respiration and (Ciii) mitochondrial ATP turnover, as determined by using the Seahorse metabolic analyzer. (Cii) The increase in maximal mitochondrial respiration did not reach statistical significance. Kruskal-Wallis test with Dunn’s post hoc test was used (n = 4 for all groups). Data are presented as mean ± SD. *P < 0.05. APC = allophycyanin; FSC = forward scatter; OCR = oxygen consumption rate; SSC = side scatter.
To specifically inhibit mitochondrial respiration in MSCs, MSCs were pretreated with rhodamine 6G, which irreversibly binds to ATP synthase (38). Rhodamine treatment abrogated MSC mitochondrial respiration while enhancing nonmitochondrial respiration (Figure E3A). Importantly, the capacity of MSCs to secrete paracrine factors (angiopoietin 1, IL-8) was not significantly affected (Figures E3B and E3C), ruling out nonspecific effects of rhodamine on MSCs’ capacity to secrete soluble factors. Rhodamine did not induce cell death in MSCs after 48 hours (Figures E3 and E3D). MSC CM enhanced mitochondrial respiration capacity in MDMs compared with MDMs stimulated with LPS alone. CM from rhodamine-pretreated MSCs had no effect on these parameters (Figure 5C).
Transfer of Mitochondria-Containing, MSC-derived EVs Is Responsible for MSC Modulation of MDMs through Enhanced Oxidative Phosphorylation
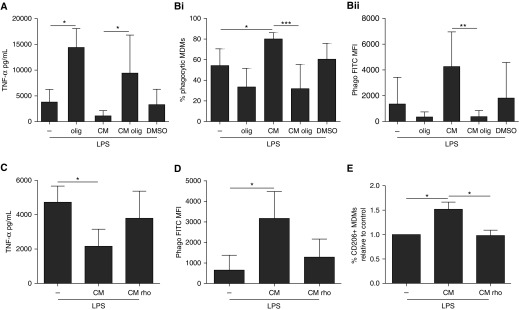
The ATP synthase inhibitor oligomycin drastically increased the MDM TNF-α response to LPS (3.8-fold). Oligomycin treatment had no cytotoxic effects on MDMs after 24 hours (Figure E4). Importantly, the addition of oligomycin completely prevented the antiinflammatory effect of MSC CM (Figure 6A). Similarly, oligomycin completely reversed the effect of MSC CM on MDM phagocytosis (Figure 6B). These results provide evidence for direct involvement of mitochondrial oxidative metabolism in MSC CM–mediated modulation of macrophage function. CM from MSCs pretreated with rhodamine 6G lost the capacity to reduce LPS-induced TNF-α production, enhanced the phagocytic capacity of MDMs, and up-regulated expression of the M2 marker CD206 (Figures 6C–6E). In aggregate, these data demonstrate that MSCs modulate primary human macrophages through EV-mediated transfer of functional mitochondria, which enhances macrophage oxidative phosphorylation.
Figure 6.
Mitochondrial transfer via human mesenchymal stromal cell (MSC)-derived extracellular vesicles modulates human monocyte-derived macrophage (MDM) function through enhancement of macrophage oxidative phosphorylation. (A) Addition of oligomycin (olig; reversible ATP synthase inhibitor) prevented the suppression of tumor necrosis factor (TNF)-α by MSC culture medium (CM) (n = 5 for all groups but dimethyl sulfoxide [DMSO; n = 4]). (B) Oligomycin also prevented MSC CM enhancement of the proportion of (Bi) phagocytic MDMs and (Bii) phagocytic index (Phago; n = 7 for all groups). Pretreatment of MSCs with rhodamine 6G (an irreversible ATP synthase inhibitor) similarly reversed MSC CM capacity to (C) suppress TNF-α production (n = 4 all groups), (D) enhance the phagocytic capacity of MDMs (n = 4 all groups), and (E) promote M2 marker CD206 expression (n = 5 for all groups). All analyses were done by one-way analysis of variance with Bonferroni’s post hoc test. Data are presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. FITC = fluorescein isothiocyanate; MFI = median fluorescence intensity.
Discussion
The following conclusions can be drawn from this study:
-
1.
MSCs reprogram human macrophages in the presence of E. coli LPS and BALF from patients with ARDS via paracrine mechanisms; the MSC-induced macrophage phenotype is characterized by a dampened inflammatory cytokine secretory profile, increases expression of the M2 marker CD206, and enhances phagocytic capacity (Figures 1 and 2).
-
2.
MSC-derived EVs expressing CD44 are partially responsible for the suppression of MDM TNF-α production and promoting phagocytosis (Figure 3).
-
3.
MSC-derived EV–treated murine AMs protect against endotoxin-induced lung injury in vivo (Figure 4).
-
4.
The transfer of functional mitochondria in EVs is responsible for MSC antiinflammatory and phagocytosis-enhancing effects on macrophages in the inflammatory environment through the promotion of oxidative phosphorylation (Figures 5 and 6).
Phinney and colleagues previously showed that MSCs donate their mitochondria to human macrophages under oxidative stress via EV-mediated transport, thereby enhancing their bioenergetics (24). Our group recently showed that mitochondrial transport via tunneling nanotubes from MSCs to MDMs was important for enhancing phagocytic capacity (19). The present study shows, for the first time to our knowledge, that the transfer of mitochondria via MSC-derived EVs promotes phagocytosis and suppresses proinflammatory cytokine secretion by human macrophages. This mitochondrial transfer was associated with increased oxidative phosphorylation in MDMs that was necessary for these modulatory effects. Moreover, this work demonstrates that modulation of AMs by MSC-derived EVs is sufficient to mitigate lung injury in vivo.
We first sought to characterize human macrophage modulation by MSCs in the in vitro models of inflammation by investigating cytokine and chemokine secretion, expression of characteristic surface markers, and phagocytic activity. MSCs were able to reduce MDM production of two major proinflammatory cytokines associated with ARDS severity—TNF-α and IL-8 (42, 43)—in the presence of E. coli LPS (Figures 1A and 1B). This corroborates previous reports showing that MSCs are able to suppress secretion of proinflammatory cytokines by macrophages (40). Other tested M1- and M2-associated cytokines and chemokines were undetectable or unaffected by MSCs, although the M2 chemokine CCL22 was reduced (Table 1). Notably, IL-10 was not detectable in these cultures; this is in contrast with a number of reports describing up-regulation of IL-10 production by macrophages that are cultured with MSCs (40, 44). This discrepancy may be explained by the inherent variability of the immunoregulatory capacity of MSC donors. Moreover, macrophages differentiated in GM-CSF, as in the present study, have been shown to express low levels of IL-10 after LPS treatment (45).
MSCs consistently up-regulated expression of the key M2 macrophage marker CD206 in the presence of LPS, whereas other markers of M1 and M2 macrophages were unaffected (Figure 1C and Table 2). This is in agreement with previous reports describing MSC induction of M2-type macrophages characterized by CD206 expression (40, 46). The lack of effect on other M1/M2 markers is perhaps a reflection of the lack of well-defined markers to unambiguously define the human macrophage activation state (47). It could also be explained by the different methods used for achieving monocyte differentiation and macrophage stimulation; in our studies, we used GM-CSF as a differentiation factor to model AMs (48, 49). GM-CSF also promotes an M1-like phenotype, more closely mimicking activated AMs that would be present in the alveoli of patients with ARDS. MSCs were able to increase the proportion of phagocytic MDMs in the presence of LPS (Figure 1D). This adds to the body of literature supporting the phagocytosis-enhancing effects of MSCs on both macrophages and monocytes (8, 10, 19, 27).
This study demonstrates, for the first time to our knowledge, that MSCs are capable of modulating MDM phenotype and function in the presence of BALF from patients with ARDS. MSCs were able to reduce TNF-α secretion, increase CD206 expression, and promote phagocytosis in MDMs exposed to ARDS BALF (Figure 2), effectively mimicking the distal lung microenvironment of these patients. Induction of a less proinflammatory AM that exhibits increased phagocytic capacity may improve outcomes in ARDS, which is classically associated with a rampant inflammatory response and substantial bacterial burden, such as sepsis- or pneumonia-induced ARDS (1). These data add valuable clinical relevance to these in vitro studies. Importantly, we previously showed that MSCs are able to migrate into the alveolar spaces even when given intravenously (19); therefore, it is plausible that MSCs may produce similar effects on AMs of patients. Additionally, the capacity of MSCs to induce polarization toward M2-type monocytes/macrophages, identified by CD206 expression, may serve as a biomarker for MSC efficacy in clinical samples. EVs have emerged as a major contributor to the therapeutic effects of MSCs in lung injury, capable of recapitulating many of the effects of the whole-cell therapy (25–27). CD44 expression on MSC-derived EVs was shown to be necessary for their uptake and therapeutic effect on target cells (27, 50). In the present study, prevention of EV uptake by MDMs using anti-CD44 neutralizing antibody abrogated the ability of MSC CM to reduce MDM TNF-α secretion and enhance phagocytosis (Figures 3B and 3C). This suggests that MSC-derived EVs are primarily responsible for MSC modulation of MDM function.
To confirm the importance of MSC-derived EV uptake by AMs in lung injury in vivo, endotoxin-injured mice were given murine AMs pretreated with MSC-derived EVs ex vivo. AMs exposed to MSC-derived EVs, but not untreated AMs, were able to significantly reduce the extent of lung injury after 24 hours, as demonstrated by reduced inflammatory cell recruitment and decreased BALF protein levels (Figure 4). These data corroborate our previous findings where clodronate-based depletion of AMs abrogated the beneficial effects of MSCs in lung injury in vivo, demonstrating that AMs are cellular mediators of the MSC effect (19). Importantly, these new data not only highlight AMs as key cellular targets of MSC-derived EVs but also emphasize an essential role of AMs in mitigating lung injury. This is the first study, to our knowledge, of the efficacy of adoptive transfer of AMs in endotoxin-induced lung injury. Adoptive transfer of macrophages has also been investigated in colitis and airway hyperresponsiveness, with promising results (51, 52). Litvack and colleagues also showed that, although not true AMs, stem cell–derived, alveolar-like macrophages improved bacterial and neutrophil clearance in another model of lung injury (49), but further study is required to determine the safety and feasibility of these treatment modalities. Moreover, these data contribute to the growing body of literature that suggests the potential of MSC-derived EVs as a therapy in place of MSCs (25–27). Although there have been no reports of MSC-induced neoplasia to date, EVs cannot themselves transform to form tumors, reducing the potential risk associated with cell-based therapy. It has been shown that different methods of isolation can influence vesicle yield, purity, and contents (53). Obtaining a sufficient quantity of EVs from MSCs for their effective use in the clinic also presents a significant challenge; a consensus must be reached on the optimal culture conditions and EV isolation protocols to standardize this process.
Our group and others’ have previously shown the transfer of mitochondria from MSCs to human macrophages via EVs (19, 24). In this study, MSC-derived EV–mediated mitochondrial transport to MDMs was visualized by fluorescence microscopy (Figure 5B). The treatment of MDMs with MSC CM resulted in enhanced bioenergetics in the macrophages, as evidenced by increased basal mitochondrial respiration as well as ATP turnover. MSC CM taken from rhodamine 6G–pretreated MSCs with dysfunctional mitochondria were unable to affect these parameters (Figure 5C). These findings are in line with previous reports of improved bioenergetics in cells receiving MSC mitochondria, including macrophages, alveolar epithelial cells, and vascular endothelial cells (9, 18, 19, 24, 54). To confirm that production of soluble mediators was sustained in rhodamine 6G–treated MSCs, angiopoietin 1 and IL-8 levels were quantified after LPS treatment with or without rhodamine 6G pretreatment. Although diminished, these paracrine factors were still produced at high levels, suggesting that paracrine secretion by MSCs was not greatly affected by inhibition of their mitochondrial function (Figures E3B and E3C).
M1 proinflammatory macrophages have been shown to use glycolytic metabolism (31). Vats and colleagues highlighted the importance of mitochondrial oxidative phosphorylation in the induction of M2-type macrophages through IL-4 signaling. They observed that mitochondrial inhibition in macrophages blocked the antiinflammatory effects of IL-4 (30). In the present study, addition of the mitochondrial inhibitor oligomycin prevented the suppression of TNF-α and the enhancement of phagocytosis in macrophages by MSC CM, demonstrating that the MSCs’ effect is critically dependent on oxidative phosphorylation (Figures 6A and 6B). We hypothesized that mitochondrial transfer via MSC-derived EVs was responsible for both enhancing MDM phagocytosis and suppressing proinflammatory cytokine production through the promotion of oxidative phosphorylation. Indeed, MSC CM taken from rhodamine 6G–pretreated MSCs with dysfunctional mitochondria had no effect on TNF-α production, phagocytosis, or CD206 expression in LPS-treated MDMs (Figures 6C–6E).
This study has limitations. In the BALF experiments, we used ARDS BALF diluted to 30% by volume; while exposing the MDMs to all of the constituents of the ARDS microenvironment, this effectively reduced the concentrations of the stimuli it contained. The endotoxin-induced lung injury model was relatively mild (14, 25); however, the primary aim of the in vivo experiments was to provide a proof of principle that AMs are important cellular mediators of MSC-derived EV effects using a gain-of-function approach. Additionally, we did not investigate the mechanisms by which MSC-derived EV–treated murine AMs are protective in lung injury.
In conclusion, this study demonstrates that in the inflammatory environment of ARDS, MSCs modulate human macrophages toward decreased production of proinflammatory cytokines, increased expression of the M2 phenotype marker CD206, and enhanced phagocytic capacity. MSC-derived EVs carrying mitochondria are responsible for these effects through the promotion of oxidative phosphorylation in macrophages, uncovering a novel mechanism of modulation of macrophage polarization. Moreover, this work suggests that changes in AMs induced by MSC-derived EVs are sufficient to elicit protection in lung injury in vivo. The ability of MSCs to promote CD206 expression in human macrophages may serve as a biomarker of MSCs’ efficacy in patients with ARDS.
Footnotes
Supported by the Medical Research Council (MR/L017229/1 [A.D.K.]) and the Department for Employment and Learning (A.D.K. and T.J.M.). Some of the materials used in this work were provided by the Texas A&M University Health Science Center College of Medicine Institute for Regenerative Medicine at Baylor Scott & White Hospital through grant P40RR017447 from the National Center for Research Resources of the National Institutes of Health.
Author Contributions: T.J.M.: contributed to overall study design, performance of the experiments, data analysis and interpretation, and writing of the manuscript; M.V.J.: contributed to performance of the experiments, data analysis and interpretation, and writing of the manuscript; E.K.C.: contributed to performance of the experiments as well as data analysis and interpretation; A.K.: contributed to overall study design as well as data analysis and interpretation; D.F.M.: contributed to overall study design as well as data analysis and interpretation and provided samples from patients with acute respiratory distress syndrome; C.M.O’K.: contributed to overall study design as well as data analysis and interpretation; and A.D.K.: contributed to overall study design, performance of experiments, data analysis and interpretation, writing of the manuscript, financial support, and final manuscript approval.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201701-0170OC on June 9, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, et al. American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 3.Pham T, Rubenfeld GD. Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome: a 50th birthday review. Am J Respir Crit Care Med. 2017;195:860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 4.Rosseau S, Hammerl P, Maus U, Walmrath HD, Schütte H, Grimminger F, Seeger W, Lohmeyer J. Phenotypic characterization of alveolar monocyte recruitment in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2000;279:L25–L35. doi: 10.1152/ajplung.2000.279.1.L25. [DOI] [PubMed] [Google Scholar]
- 5.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome: biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196:266–273. doi: 10.1164/rccm.201701-0107CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei SHJ, Haitsma JJ, Dos Santos CC, Deng Y, Lai PFH, Slutsky AS, Liles WC, Stewart DJ. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 8.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003–L1013. doi: 10.1152/ajplung.00180.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curley GF, Ansari B, Hayes M, Devaney J, Masterson C, Ryan A, Barry F, O’Brien T, Toole DO, Laffey JG. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology. 2013;118:924–932. doi: 10.1097/ALN.0b013e318287ba08. [DOI] [PubMed] [Google Scholar]
- 12.Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, Nicholls JM, Fang X, Guan Y, Lee JW, et al. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA. 2016;113:3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curley GF, Jerkic M, Dixon S, Hogan G, Masterson C, O’Toole D, Devaney J, Laffey JG. Cryopreserved, xeno-free human umbilical cord mesenchymal stromal cells reduce lung injury severity and bacterial burden in rodent Escherichia coli–induced acute respiratory distress syndrome. Crit Care Med. 2017;45:e202–e212. doi: 10.1097/CCM.0000000000002073. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, Dominici M, Fibbe WE, Gee AP, Gimble JM, et al. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151–159. doi: 10.1016/j.jcyt.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Zhang Y, Yeung SC, Liang Y, Liang X, Ding Y, Ip MS, Tse HF, Mak JC, Lian Q. Mitochondrial transfer of induced pluripotent stem cell-derived mesenchymal stem cells to airway epithelial cells attenuates cigarette smoke-induced damage. Am J Respir Cell Mol Biol. 2014;51:455–465. doi: 10.1165/rcmb.2013-0529OC. [DOI] [PubMed] [Google Scholar]
- 19.Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O’Kane CM, Krasnodembskaya AD. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang X, Abbott J, Cheng L, Colby JK, Lee JW, Levy BD, Matthay MA. Human mesenchymal stem (stromal) cells promote the resolution of acute lung injury in part through lipoxin A4. J Immunol. 2015;195:875–881. doi: 10.4049/jimmunol.1500244. [DOI] [PubMed] [Google Scholar]
- 22.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839–849. doi: 10.1021/pr200682z. [DOI] [PubMed] [Google Scholar]
- 24.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St. Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW. Microvesicles derived from human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Transplant. 2015;15:2404–2412. doi: 10.1111/ajt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic effects of human mesenchymal stem cell–derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324–336. doi: 10.1164/rccm.201410-1765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6:1018–1028. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghanta S, Tsoyi K, Liu X, Nakahira K, Ith B, Coronata AA, Fredenburgh LE, Englert JA, Piantadosi CA, Choi AMK, et al. Mesenchymal stromal cells deficient in autophagy proteins are susceptible to oxidative injury and mitochondrial dysfunction. Am J Respir Cell Mol Biol. 2017;56:300–309. doi: 10.1165/rcmb.2016-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MAR, Sheedy FJ, Gleeson LE, van den Bosch MWM, Quinn SR, Domingo-Fernandez R, Johnston DG, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the Warburg effect in LPS-activated macrophages Cell Metab 20152165–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison T, Jackson M, Kissenpfennig A, O’Kane C, McAuley D, Krasnodembskaya A. Human mesenchymal stromal cell (hMSC) regulation of human macrophages in in vitro models of the acute respiratory distress syndrome (ARDS) [abstract] Thorax. 2015;70(Suppl 3):A38-A. [Google Scholar]
- 33.Morrison TJ, Jackson MV, O’Kane C, McAuley DF, Krasnodembskaya A. Mesenchymal stromal cells modulate human macrophages in acute respiratory distress syndrome via secretion of extracellular vesicles which enhance oxidative phosphorylation and regulate JAK/STAT signaling [abstract] Thorax. 2016;71(Suppl 3):A46. [Google Scholar]
- 34.Krasnodembskaya AD, Morrison T, Gotts JE, O’Kane C, McAuley DF, Matthay MA. Human mesenchymal stem cells promote M2 macrophage polarization in both in vivo and in vitro models of ARDS [abstract] Am J Respir Crit Care Med. 2014;189:A3964. [Google Scholar]
- 35.Krasnodembskaya A, Morrison T, O’Kane C, McAuley D, Matthay M. Human mesenchymal stem cells (MSC) modulate alveolar macrophage polarization in vivo and in vitro [abstract] Eur Respir J. 2014;44(Suppl 58):3427. [Google Scholar]
- 36.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells: the International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 37.English D, Andersen BR. Single-step separation of red blood cells: granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974;5:249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- 38.Gear AR. Rhodamine 6G: a potent inhibitor of mitochondrial oxidative phosphorylation. J Biol Chem. 1974;249:3628–3637. [PubMed] [Google Scholar]
- 39.Symersky J, Osowski D, Walters DE, Mueller DM. Oligomycin frames a common drug-binding site in the ATP synthase. Proc Natl Acad Sci USA. 2012;109:13961–13965. doi: 10.1073/pnas.1207912109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 42.Aggarwal A, Baker CS, Evans TW, Haslam PL. G-CSF and IL-8 but not GM-CSF correlate with severity of pulmonary neutrophilia in acute respiratory distress syndrome. Eur Respir J. 2000;15:895–901. doi: 10.1034/j.1399-3003.2000.15e14.x. [DOI] [PubMed] [Google Scholar]
- 43.Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer J-M. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992;145:1016–1022. doi: 10.1164/ajrccm/145.5.1016. [DOI] [PubMed] [Google Scholar]
- 44.Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–195. doi: 10.1038/mt.2011.189. [DOI] [PubMed] [Google Scholar]
- 46.Sierra-Filardi E, Vega MA, Sánchez-Mateos P, Corbí AL, Puig-Kröger A. Heme oxygenase-1 expression in M-CSF-polarized M2 macrophages contributes to LPS-induced IL-10 release. Immunobiology. 2010;215:788–795. doi: 10.1016/j.imbio.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Litvack ML, Wigle TJ, Lee J, Wang J, Ackerley C, Grunebaum E, Post M. Alveolar-like stem cell–derived Myb− macrophages promote recovery and survival in airway disease. Am J Respir Crit Care Med. 2016;193:1219–1229. doi: 10.1164/rccm.201509-1838OC. [DOI] [PubMed] [Google Scholar]
- 50.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung G, Wang A, Fernando M, Phan VC, McKay DM. Bone marrow-derived alternatively activated macrophages reduce colitis without promoting fibrosis: participation of IL-10. Am J Physiol Gastrointest Liver Physiol. 2013;304:G781–G792. doi: 10.1152/ajpgi.00055.2013. [DOI] [PubMed] [Google Scholar]
- 52.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2004;31:22–27. doi: 10.1165/rcmb.2003-0229OC. [DOI] [PubMed] [Google Scholar]
- 53.Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles. 2014;3:24858. doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–18. doi: 10.1016/j.mvr.2014.01.008. [DOI] [PubMed] [Google Scholar]