Abstract
Background: Recommendations regarding key aspects related to the diagnosis and pharmacological treatment of lymphangioleiomyomatosis (LAM) were recently published. We now provide additional recommendations regarding four specific questions related to the diagnosis of LAM and management of pneumothoraces in patients with LAM.
Methods: Systematic reviews were performed and then discussed by a multidisciplinary panel. For each intervention, the panel considered its confidence in the estimated effects, the balance of desirable (i.e., benefits) and undesirable (i.e., harms and burdens) consequences, patient values and preferences, cost, and feasibility. Evidence-based recommendations were then formulated, written, and graded using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach.
Results: For women who have cystic changes on high-resolution computed tomography of the chest characteristic of LAM, but who have no additional confirmatory features of LAM (i.e., clinical, radiologic, or serologic), the guideline panel made conditional recommendations against making a clinical diagnosis of LAM on the basis of the high-resolution computed tomography findings alone and for considering transbronchial lung biopsy as a diagnostic tool. The guideline panel also made conditional recommendations for offering pleurodesis after an initial pneumothorax rather than postponing the procedure until the first recurrence and against pleurodesis being used as a reason to exclude patients from lung transplantation.
Conclusions: Evidence-based recommendations for the diagnosis and treatment of patients with LAM are provided. Frequent reassessment and updating will be needed.
Contents
Overview
Introduction
Methods
Committee Composition
Conflict-of-Interest Management
Guideline Panel Meetings
Formulating Questions and Outcomes
Literature Search and Study Selection
Evidence Synthesis
Development of Recommendations
Manuscript Preparation
Questions and Recommendations
Question 1
Question 2
Question 3
Question 4
Conclusions
Overview
This guideline is the continuation of a prior lymphangioleiomyomatosis (LAM) guideline document developed by the American Thoracic Society (ATS) and the Japanese Respiratory Society (JRS) (1). The current guideline collates the evidence for emerging advancements in LAM and then uses this evidence to formulate recommendations pertaining to the diagnosis and treatment of patients with LAM. The intent of the guideline is to empower clinicians to apply the recommendations in the context of the values and preferences of individual patients and to tailor their decisions to the clinical situation at hand. The guideline panel’s recommendations (Table 1) are as follows:
-
•
For patients who have cystic changes on high-resolution computed tomography (HRCT) of the chest that are characteristic of LAM, but have no additional confirmatory features of LAM (i.e., clinical, radiologic, or serologic), we suggest NOT using the HRCT features in isolation to make a clinical diagnosis of LAM (conditional recommendation, low confidence in the estimated effects).
∘ Remarks: In the guideline panelists’ clinical practices, a clinical diagnosis of LAM is based on a combination of characteristic HRCT features plus one or more of the following: presence of tuberous sclerosis complex (TSC), angiomyolipomas, chylous effusions, lymphangioleiomyomas (lymphangiomyomas), or elevated serum vascular endothelial growth factor-D (VEGF-D) greater than or equal to 800 pg/ml.
-
•
When a definitive diagnosis is required in patients who have parenchymal cysts on HRCT that are characteristic of LAM, but no additional confirmatory features of LAM (i.e., clinical, radiologic, or serologic), we suggest a diagnostic approach that includes transbronchial lung biopsy before a surgical lung biopsy (conditional recommendation, very low confidence in the estimated effects).
∘ Remarks: The advantage of transbronchial lung biopsy is that it offers a less-invasive method to obtain histopathological confirmation of LAM, as compared with surgical lung biopsy. Although not proven, the panelists believed that the yield of transbronchial lung biopsy likely correlates with markers of parenchymal LAM burden (such as cyst profusion, abnormal diffusing capacity of the lung for carbon monoxide, abnormal FEV1) and that appropriate patient selection is required to optimize the safety and efficacy of this diagnostic approach. Consultation with an expert center before undertaking transbronchial lung biopsy, combined with a critical review of the tissue specimens by a pathologist with expertise in LAM, can help avoid false-negative test results and the need for a surgical lung biopsy.
-
•
We suggest that patients with LAM be offered ipsilateral pleurodesis after their initial pneumothorax rather than waiting for a recurrent pneumothorax before intervening with a pleural symphysis procedure (conditional recommendation, very low confidence in the estimated effects).
∘ Remarks: This approach is based on the high rate of recurrence of spontaneous pneumothoraces in patients with LAM. Nonetheless, the final decision to perform pleurodesis and the type of pleurodesis (chemical vs. surgical) should be based on shared decision-making between the clinician(s) and patient, after education about various management options. Every effort must be made to ensure that the pleurodesis is handled by clinicians familiar with management of pleural disease in LAM.
-
•
We suggest that previous unilateral or bilateral pleural procedures (i.e., pleurodesis or pleurectomy) NOT be considered a contraindication to lung transplantation in patients with LAM (conditional recommendation, very low confidence in the estimated effects).
∘ Remarks: Lung transplantation surgery in patients with a history of prior pleurodesis can be challenging. Patients who have undergone prior pleural procedures (i.e., pleurodesis or pleurectomy) should be referred to a lung transplant team with expertise in handling complex pleural dissections.
Table 1.
Summary of the Recommendations Provided in This Guideline
| Context | Recommendation | Strength of Recommendation | Confidence in Estimates of Effect |
|---|---|---|---|
| HRCT as sole confirmatory feature for LAM diagnosis | For patients who have cystic changes on HRCT of the chest that are characteristic of LAM, but have no additional confirmatory features of LAM (i.e., clinical, radiologic, or serologic), we suggest NOT using the HRCT features in isolation to make a clinical diagnosis of LAM. | Conditional | Low |
| Transbronchial lung biopsy for histopathological diagnosis of LAM | When a definitive diagnosis is required in patients who have parenchymal cysts on HRCT that are characteristic of LAM, but no additional confirmatory features of LAM (i.e., clinical, radiologic, or serologic), we suggest a diagnostic approach that includes transbronchial lung biopsy before a surgical lung biopsy. | Conditional | Very low |
| Pleurodesis after a sentinel pneumothorax to prevent recurrence | We suggest that patients with LAM be offered ipsilateral pleurodesis after their initial pneumothorax rather than waiting for a recurrent pneumothorax before intervening with a pleural symphysis procedure. | Conditional | Very low |
| Pleurodesis as a contraindication to future lung transplant | We suggest that previous unilateral or bilateral pleural procedures (i.e., pleurodesis or pleurectomy) NOT be considered a contraindication to lung transplantation in patients with LAM. | Conditional | Very low |
Definition of abbreviations: HRCT = high-resolution computed tomography; LAM = lymphangioleiomyomatosis.
Introduction
This guideline is the continuation of a prior lymphangioleiomyomatosis (LAM) guideline document developed by the ATS and JRS (1). The current guideline collates pertinent evidence and then uses this evidence to formulate recommendations pertaining to the diagnosis and management of LAM. These guidelines are not intended to impose a standard of care. They provide the basis for rational decisions in the diagnosis and treatment of LAM. Clinicians, patients, third-party payers, institutional review committees, other stakeholders, or the courts should never view these recommendations as dictates. No guidelines or recommendations can take into account the entire, often compelling, individual clinical circumstances that guide clinical decision-making. Therefore, no one evaluating clinicians’ actions should attempt to apply the recommendations from these guidelines by rote or in a blanket fashion. Statements about the underlying values and preferences, as well as qualifying remarks accompanying each recommendation, are integral parts and serve to facilitate more accurate interpretation; they should never be omitted when quoting or translating recommendations from these guidelines.
Methods
Committee Composition
The guideline development panel was co-chaired by F.X.M and J.M. and consisted of clinicians and researchers with recognized expertise in LAM (1). A methodologist (K.C.W.) with expertise in the guideline development process and application of the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach (2) was also a member of the panel. Patient perspectives were provided by the LAM Foundation (Table 2).
Table 2.
Summary of Methodology
| Method | Yes | No |
|---|---|---|
| Panel assembly | ||
| Included experts for relevant clinical disciplines | X | |
| Included individuals who represent the views of patients and society at large | X | |
| Included a methodologist with appropriate expertise (documented expertise in conducting systematic reviews to identify the evidence base and the development of evidence-based recommendations) | X | |
| Literature review | ||
| Performed in collaboration with a librarian | X | |
| Searched multiple electronic databases | X | |
| Reviewed reference lists of retrieved articles | X | |
| Evidence synthesis | ||
| Applied prespecified inclusion and exclusion criteria | X | |
| Evaluated studies for sources of bias | X | |
| Explicitly summarized benefits and harms | X | |
| Used PRISMA1 to report systematic review | X | |
| Used GRADE to describe quality of evidence | X | |
| Generation of recommendations | ||
| Used GRADE to rate the strength of recommendations | X |
Definition of abbreviations: GRADE = Grading of Recommendations Assessment, Development, and Evaluation; PRISMA1 = Preferred Reporting Items for Systematic Reviews and Meta-analysis 1.
Conflict-of-Interest Management
Guideline panelists disclosed all potential conflicts of interest according to ATS policies. All conflicts of interest were managed by the ATS conflict of interest and documents departments using the procedures described in the previous LAM guidelines (1). All seven members of the writing group were free of conflicts for all questions related to this version of the guidelines.
Guideline Panel Meetings
Several face-to-face meetings, conference calls, and e-mail discussions were held between 2008 and 2017, during which the guideline development panel discussed the scope of the document, the questions to be addressed, the evidence, and the recommendations. The cosponsoring societies (the ATS and JRS) provided financial support for the meetings and conference calls, as well as travel expenses. Additional support for travel of panelists to meetings was provided by the not-for-profit LAM Foundation and LAM Treatment Alliance. The ATS, JRS, and Foundations had no influence on question selection, evidence synthesis, or recommendations.
Formulating Questions and Outcomes
Clinical questions were developed, circulated among the panelists, and rated according to clinical relevance. Patient-important outcomes were selected a priori for each question and categorized as critical, important, or not important (3). Patient perspectives on the questions to be addressed were obtained via questionnaires distributed by the LAM Foundation.
Literature Search and Study Selection
A detailed description of the search strategy was provided in the recently published LAM guidelines (1). All literature searches were performed by a librarian from the National Institute of Health (K.S.), using four electronic databases: MEDLINE, EMBASE, Web of Science, and Scopus (Table 2). The literature search was originally conducted in 2009, subsequently updated in July 2014, July 2015, and May 2016, and included studies published before March 2016. A smaller working group of seven panelists (C.S., F.X.M., G.A.F., K.C.W., N.G., J.M., and R.M.K.) reviewed the search results and updated them as necessary.
Evidence Synthesis
The body of evidence for each question was summarized in collaboration with one of the methodologists (K.C.W.). When possible, data were pooled to derive single estimates. When this was not possible, the range of results was reported. The quality of the body of evidence was rated using the GRADE approach (Table 2) (4), as described previously (1).
Development of Recommendations
The guideline development panel formulated recommendations on the basis of the evidence synthesis, as described previously (1). Recommendations were formulated by discussion and consensus; none of the recommendations required voting. The final recommendations were reviewed and approved by all members of the entire panel.
The recommendations were rated as strong or conditional in accordance with the GRADE approach. The words “we recommend” indicate that the recommendation is strong, whereas the words “we suggest” indicate that the recommendation is conditional. Table 3 describes the interpretation of strong and conditional recommendations by patients, clinicians, and health care policy makers.
Table 3.
Interpretation of Strong and Conditional Recommendations for Stakeholders
| Implications for | Strong Recommendation | Conditional Recommendation |
|---|---|---|
| Patients | Most individuals in this situation would want the recommended course of action, and only a small proportion would not. | The majority of individuals in this situation would want the suggested course of action, but many would not. |
| Clinicians | Most individuals should receive the intervention. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for individual patients and that you must help each patient arrive at a management decision consistent with his or her values and preferences. Decision aids may be useful in helping individuals to make decisions consistent with their values and preferences. |
| Policy makers | The recommendation can be adopted as policy in most situations. | Policy making will require substantial debate and involvement of various stakeholders. |
Manuscript Preparation
The writing group (C.S., F.X.M., G.A.F., J.M., K.C.W., N.G., and R.M.K.) drafted the final guideline document. The manuscript was then reviewed by the entire guideline development panel, and their feedback was incorporated into the final draft. All members of the panel have reviewed the final version of the document and approve of the document in its entirety.
Questions and Recommendations
Question 1
Should patients be clinically diagnosed with LAM on the basis of their HRCT findings alone if they have cystic changes in the lung parenchyma that are characteristic of LAM but have no additional confirmatory characteristics of LAM (i.e., clinical, radiologic, or serologic)?
Background
The advent of HRCT of the chest has transformed the field of diffuse cystic lung diseases. A critical review of HRCT features can often reveal patterns that are diagnostic in a significant proportion of patients with diffuse cystic lung diseases. The characteristic HRCT pattern of LAM is defined as the presence of multiple, bilateral, uniform, round, thin-walled cysts present in a diffuse distribution. It has been suggested that the diagnosis of LAM can be established with a fair degree of certainty on the basis of the presence of characteristic HRCT features alone (5). However, the rationale for pursuing a definite diagnosis has been strengthened of late. A recent randomized controlled trial demonstrated that sirolimus stabilizes lung function decline and improves quality of life and functional performance in patients with LAM (6). On the basis of these results, sirolimus is now U.S. Food and Drug Administration approved for treatment of LAM and was recommended as the first-line treatment option for qualified patients in the previous LAM guideline document (1). However, effective therapy with sirolimus requires continuous drug exposure and is associated with potential adverse effects. Given the specter of long-term therapy, it is essential to have a firm diagnosis before initiating pharmacotherapy.
Summary of the evidence
Our systematic review identified three studies that evaluated the performance characteristics of HRCT of the chest in establishing the diagnosis of LAM in patients with diffuse cystic lung diseases (7–9). In all three studies, HRCT of the chest from patients with various cystic lung diseases were evaluated, and a diagnosis was rendered by multiple physicians who were blinded to clinical and histopathological information. Clinicians included thoracic radiologists (7–9), pulmonologists (9), and pulmonary fellows (9). Diseases included LAM (7–9), pulmonary Langerhans cell histiocytosis (7–9), emphysema (7–9), usual interstitial pneumonia (8), lymphoid interstitial pneumonia (8, 9), desquamative interstitial pneumonia (8), Birt-Hogg-Dubé syndrome (9), amyloidosis (9), hypersensitivity pneumonitis (9), nonspecific interstitial pneumonia (9), lymphangiomatosis (9), and pleuropulmonary blastoma (9). Two studies included patients without cystic lung disease as control subjects, including normal volunteers and patients with noncystic interstitial lung diseases (7, 9).
We pooled the data from all three studies, which included 72 patients with LAM and 141 patients without LAM. Our analysis revealed that expert thoracic radiologists diagnosed LAM on the basis of HRCT review alone with a sensitivity of 87.5% and a specificity of 97.5%, indicating a false-negative rate of 12.5% and a false-positive rate of 2.5%. Assuming that 30% of patients who present with cystic lung disease of unknown etiology have LAM (1) and that HRCT has a sensitivity and specificity for LAM of approximately 87% and 97%, respectively, then for every 1,000 patients with cystic lung disease who undergo HRCT of the chest, 261 patients will be correctly diagnosed with LAM (true-positive results) and 679 patients will be correctly determined to not have LAM (true-negative results); however, 21 patients will be incorrectly diagnosed as having LAM (false-positive results) and 39 patients will be incorrectly determined to not have LAM (false-negative results).
The guideline panel’s confidence in the estimated sensitivity and specificity was low. The studies appropriately compared the HRCT to a gold standard (histopathology); however, confidence was diminished for two reasons. First, the studies enrolled neither consecutive patients nor patients with true diagnostic uncertainty (potential selection bias). Second, the results may not be generalizable to facilities that do not have access to an expert thoracic radiologist to interpret HRCT (indirectness). Supporting the importance of the latter limitation, the performance characteristics of pulmonary physicians have been shown to be inferior to thoracic radiologists in being able to diagnose LAM on the basis of HRCT review (9). Third, the studies did not include all possible causes of cystic lung disease that could mimic LAM, such as metastatic tumors, light chain deposition disease, etc. Last, isolated cysts have been reported in otherwise asymptomatic, normal individuals and have been postulated to represent an aging manifestation rather than a true pathological disease process (10, 11).
Benefits
The benefit of correctly diagnosing LAM on the basis of HRCT review alone is that HRCT of the chest is noninvasive. If HRCT only could be used to make the diagnosis of LAM, neither transbronchial lung biopsy nor surgical lung biopsy would be required, reducing the risk of complications and the burdens and costs of such procedures.
Harms
False-positive results may lead to missed opportunities to treat the correct disease as well as the adverse effects and costs of inappropriate treatment or management of LAM.
Conclusions and research opportunities
To recommend clinical diagnosis of LAM on the basis of characteristic HRCT findings alone, the guideline panel reasoned that the specificity must be greater than 95%. The rationale was to minimize false-positive results, because such results lead to missed opportunities to treat the correct disease as well as the adverse effects and costs of inappropriate treatment and management of LAM. The specificity of HRCT achieved the prespecified threshold; however, the panel was concerned that the specificity was misleadingly high because it was derived from referral institutions with thoracic radiologists who have expertise in interstitial lung diseases and could have been substantially lower if the studies had been conducted in different medical centers. For this reason, the guideline panel elected to suggest not making a clinical diagnosis of LAM on the basis of HRCT findings alone.
The only noninvasive diagnostic tests for LAM that have been systematically studied are HRCT alone and serum VEGF-D. Therefore, research opportunities exist to study the sensitivity and specificity of combined findings, such as a HRCT plus clinical features of TSC, angiomyolipoma, chylous effusion, or lymphangioleiomyoma.
Recommendation
For patients who have cystic changes on HRCT of the chest that are characteristic of LAM, but have no additional confirmatory features of LAM (i.e., clinical, radiologic, or serologic), we suggest NOT using the HRCT features in isolation to make a clinical diagnosis of LAM (conditional recommendation, low confidence in the estimated effects).
Remarks
In the guideline panelists’ clinical practices, a clinical diagnosis of LAM is based on the combination of characteristic HRCT features plus one or more of the following: presence of TSC, angiomyolipomas, chylous effusions, lymphangioleiomyomas, or elevated serum VEGF-D greater than or equal to 800 pg/ml (Table 4). In certain cases, such as asymptomatic patients with typical clinical presentations for LAM (i.e., young-middle aged, nonsmoking female patients without evidence of underlying connective tissue diseases or other features commonly seen in cystic lung diseases that can mimic LAM, such as emphysema or Sjögren syndrome) and mild cystic change on HRCT, it may be appropriate to base a probable diagnosis of LAM on critical review of HRCT alone (5), especially when a definitive diagnosis is not likely to change management. A second opinion regarding the diagnosis by an expert thoracic radiologist can further strengthen confidence in the diagnosis in these cases. In patients with a probable diagnosis of LAM, the guideline panelists typically monitor disease progression with serial monitoring of their pulmonary function tests. However, there was general agreement that a definite diagnosis should be established with one of the additional criteria (Table 4) before initiation of pharmacotherapy with mechanistic target of rapamycin (mTOR) inhibitors.
Table 4.
Diagnostic Criteria for Lymphangioleiomyomatosis
| Definite LAM |
|---|
| Definite diagnosis of LAM can be established if a patient with compatible clinical history* and characteristic HRCT of the chest† has one or more of the following features: |
| 1. Presence of TSC‡ |
| 2. Renal angiomyolipoma(s)§ |
| 3. Elevated serum VEGF-D ≥ 800 pg/ml |
| 4. Chylous effusion (pleural or ascites) confirmed by tap and biochemical analysis of the fluid |
| 5. Lymphangioleiomyomas (lymphangiomyomas)§ |
| 6. Demonstration of LAM cells or LAM cell clusters on cytological examination of effusions or lymph nodes|| |
| 7. Histopathological confirmation of LAM by lung biopsy or biopsy of retroperitoneal or pelvic masses |
Definition of abbreviations: D2-40 = podoplanin; HMB-45 = human melanoma black-45; HRCT = high-resolution computed tomography; LAM = lymphangioleiomyomatosis; mTOR = mechanistic target of rapamycin; TSC = tuberous sclerosis complex; VEGF-D = vascular endothelial growth factor-D; VEGFR3 = vascular endothelial growth factor receptor 3.
The diagnosis of LAM should be established using the least invasive approach (details in Figure 1). In some cases, such as asymptomatic patients with mild cystic change on HRCT, a probable diagnosis of LAM with serial monitoring may be sufficient, if a definite diagnosis will not change management and some level of diagnostic uncertainty is acceptable to the patient and clinician. Every effort must be made to establish a definite diagnosis of LAM before initiation of pharmacological therapy with mTOR inhibitors.
Compatible clinical history with LAM includes young to middle-aged female patients presenting with worsening dyspnea and/or pneumothorax/chylothorax and the absence of features suggestive of other cystic lung diseases. Typical clues to an alternative etiology of cystic lung disease on history include the presence of sicca symptoms or an underlying diagnosis of connective tissue disease, significant smoking history, personal/family history of non–TSC-related facial skin lesions, and/or kidney tumors. Most patients with LAM will have an obstructive defect on pulmonary function tests. Some patients, especially early in their disease course, may be asymptomatic and have normal pulmonary function tests.
Characteristic HRCT chest features of LAM include the presence of multiple, bilateral, uniform, round, thin-walled cysts present in a diffuse distribution, often with normal-appearing intervening lung parenchyma.
Detailed history and physical examination to investigate for the presence of TSC is needed. The diagnosis of TSC is established based on the proposed criteria in the TSC Guidelines (65). Referral to a TSC specialist may be needed if unsure of the diagnosis.
Angiomyolipoma may be diagnosed on the basis of radiographic appearance of characteristic fat-containing lesions either on computed tomography scan or magnetic resonance imaging. Contrast is not typically required unless the vascular characteristics of the tumor need to be analyzed, such as for evaluation of the potential for hemorrhage or the planning for embolization. Similarly, lymphangioleiomyomas can typically be diagnosed on the basis of characteristic radiographic appearance.
LAM cell cluster refers to a spherical aggregate of LAM cells enveloped by a layer of lymphatic endothelial cells that is found in chylous effusions of patients with LAM. The diagnosis of LAM can be based on typical morphological appearance of LAM cells and positive staining for smooth muscle cell markers and HMB-45 by immunohistochemistry. Lymphatic endothelial cells surrounding the LAM cells can be highlighted by positive immunohistochemical staining for lymphatic endothelial cell markers, including D2-40 and VEGFR-3.
Values and preferences
This recommendation places a high value on avoiding missed opportunities to treat the correct disease, as well as avoiding the adverse effects and costs of inappropriate treatment of LAM. It places a lower value on the potential complications, burdens, and costs of traditional diagnostic testing.
Question 2
Should patients undergo transbronchial lung biopsy for the diagnosis of LAM if they have cystic changes that are characteristic of LAM on HRCT of the chest but have no additional confirmatory characteristics of LAM (i.e., clinical, radiologic, or serologic)?
Background
Typical features on HRCT of the chest can be highly suggestive of LAM (9). Many experts make a diagnosis of LAM if characteristic cystic lung changes on HRCT are accompanied by the presence of tuberous sclerosis complex, angiomyolipomas, chylous effusions, lymphangioleiomyomas, or a serum VEGF-D level greater than or equal to 800 pg/ml (5, 12). Although for some patients, such as those without symptoms and a mild cyst burden, a strategy of close monitoring only may be appropriate, obtaining diagnostic certainty is the optimal approach in those with symptoms or progressive disease before initiating treatment. Video-assisted thoracoscopic surgery (VATS)-guided surgical lung biopsy has been considered the gold standard for obtaining histopathological confirmation of LAM; however, small retrospective series suggest that transbronchial lung biopsy can be safe and effective in a proportion of patients with suspected LAM.
Summary of the evidence
Our systematic review identified 5 case reports (13–17) and 12 case series (18–29) that described transbronchial lung biopsy for the diagnosis of LAM. We did not consider the case reports due to the high risk of publication bias (i.e., patients with successful outcomes are more likely to be submitted by clinicians as case reports). Instead, we considered only the case series to inform the guideline panel’s judgments.
The largest relevant case series reported on 108 Chinese patients with LAM. The diagnosis was confirmed by lung biopsy in 97 patients, including 49 patients who had been diagnosed by transbronchial lung biopsy. The number of patients who had undergone transbronchial lung biopsy was not reported; however, the diagnostic yield would have been 50% if all patients had undergone transbronchial lung biopsy and higher if fewer had undergone transbronchial lung biopsy. The complication rate was similarly unreported (29). Four case series provided sufficient crude data to estimate the diagnostic yield of transbronchial lung biopsy (23, 24, 28, 30). In the largest series, two online surveys were conducted of 1,000 patients with LAM who were registered with the LAM Foundation. Among the 63 patients who underwent transbronchial lung biopsy when they were initially suspected of having LAM, 35 patients (56%) were confirmed to have LAM by the procedure. The self-reported complication rate from transbronchial biopsy was approximately 14% (6% with pneumothorax, 4% with bleeding, 2% with chest pain, and 2% with pneumonia) (30). When we pooled the results from the four case series, it was determined that 48 out of 81 patients (60%) who underwent transbronchial lung biopsy for suspected LAM were confirmed to have LAM (23, 24, 28, 30). Of note, several series reported that the initial diagnosis rendered by the local pathologist was often nondiagnostic or incorrect and was subsequently revised to LAM when reviewed by a pathologist with expertise in LAM.
The guideline panel’s judgments regarding the utility of transbronchial lung biopsy in LAM were informed primarily by small case series, which provided very low confidence in the estimated diagnostic yield and complication rate. The panel’s confidence was further lowered by the fact that only a small proportion of patients in each case series underwent transbronchial lung biopsy, and most of the series did not report the criteria for patient selection for transbronchial biopsy; these limitations collectively increase the possibility of an overestimated diagnostic yield due to selection bias. The panel speculated that the diagnostic yield varies according to the burden of LAM in the lung. Finally, one of the larger series relied on patient-reported results.
Benefits
Transbronchial lung biopsy appears to yield a diagnosis of LAM in greater than 50% of properly selected patients with suspected LAM. Definitive diagnosis by transbronchial lung biopsy renders invasive diagnostic testing, such as surgical lung biopsy, unnecessary.
Harms
Transbronchial lung biopsy is a minimally invasive procedure that may be associated with bleeding, pneumothorax, or adverse medication effects. The overall risk of any complication was 14% in the largest case series that we considered (30) but is generally estimated to be approximately 2% (31, 32), which is substantially less than the risks associated with a VATS-guided surgical lung biopsy, which include a 1.5 to 4.5% mortality rate and 10 to 19% procedure-related complication rate (33–36). In theory, patients with cystic diseases such as LAM may be at greater risk of pneumothorax from transbronchial lung biopsy. However, our evidence synthesis did not support this conclusion, as the pneumothorax rate of 0 to 6% is comparable to the general population (23, 30).
Conclusion and research opportunities
The guideline panel weighed the estimated diagnostic yield (50%) versus the complication rate (2–14%) and cost of bronchoscopy and decided that the benefits of transbronchial lung biopsy outweigh the harms in appropriately selected patients. Generally speaking, we on the guideline panel believe that the diagnosis of LAM should be established in an algorithmic approach that progresses from the least to most invasive method required to confirm the diagnosis of LAM (Table 4 and Figure 1).
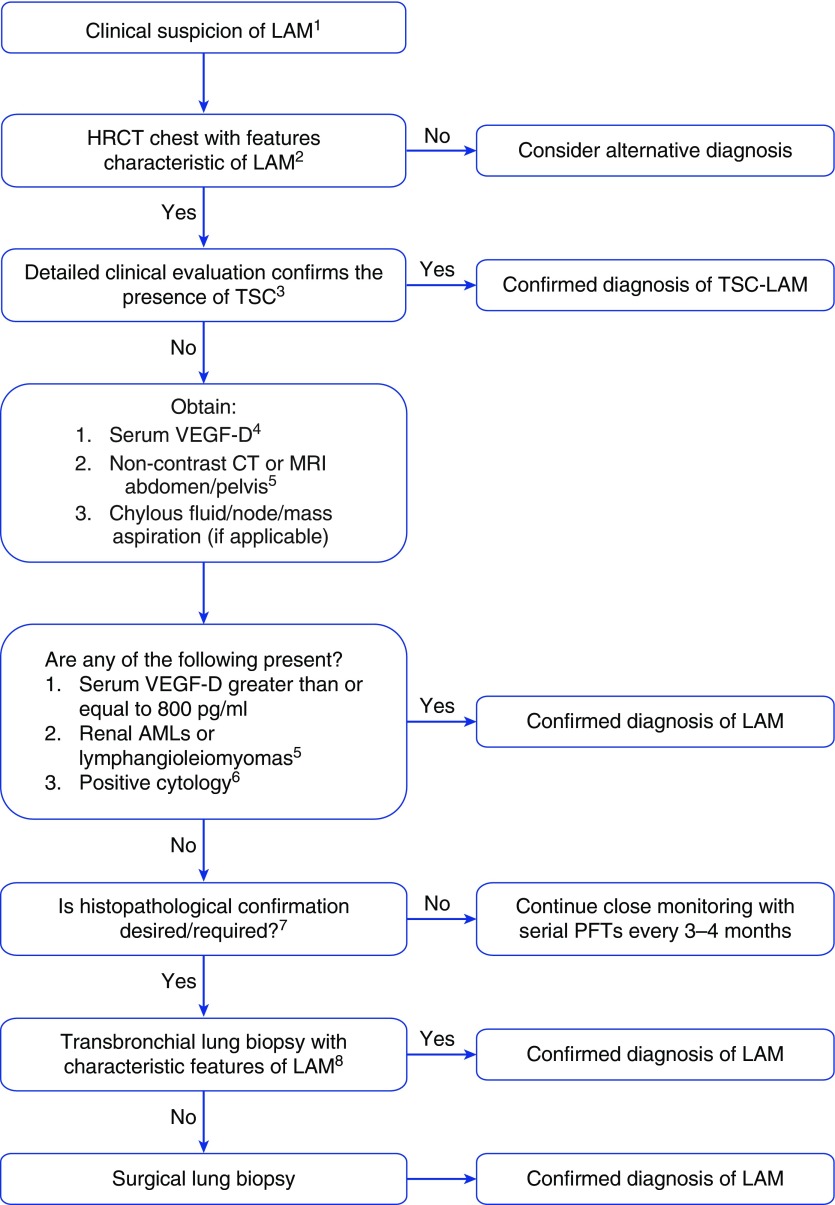
Figure 1.
Proposed algorithm for the diagnosis of lymphangioleiomyomatosis (LAM) in a patient with compatible clinical history. The algorithm is designed as a step-wise, least-invasive approach to confirm the diagnosis of LAM. Modifications on the basis of clinical judgment are frequently required, and diagnostic decisions must be individualized. AML = angiomyolipoma; CT = computed tomography; DlCO = diffusion capacity of the lung for carbon monoxide; HRCT = high-resolution computed tomography; MRI = magnetic resonance imaging; mTOR = mechanistic target of rapamycin; PFTs = pulmonary function tests; TSC = tuberous sclerosis complex; VEGF-D = vascular endothelial growth factor-D.
1Suspect LAM clinically in young to middle-aged female patients presenting with worsening dyspnea and/or pneumothorax/chylothorax. Most patients with LAM will have an obstructive defect on PFTs. Some patients, especially early in their disease course, may be asymptomatic and have normal PFTs.
2Characteristic HRCT features of LAM include the presence of multiple, bilateral, round, well-defined, relatively uniform, thin-walled cysts in a diffuse distribution. The intervening lung parenchyma often appears normal on HRCT. Other associated features that can be seen on HRCT in some patients with LAM include the presence of: chylous pleural effusion, pneumothorax, ground-glass opacity suggestive of chylous congestion, or multiple tiny nodules characteristic of multifocal micronodular pneumocyte hyperplasia (in patients with TSC-LAM).
3Referral to a TSC center should be considered if there is uncertainty regarding the diagnosis of TSC. Features suggestive of TSC include the presence of any of the following: subungual fibromas, facial angiofibromas, hypomelanotic macules, confetti lesions, Shagreen patches, positive family history of TSC, history of seizures or cognitive impairment, or presence of cortical dysplasias, subependymal nodules, and/or subependymal giant cell astrocytomas on brain imaging. Routine brain imaging is not indicated if clinical suspicion for TSC is low. Detailed diagnostic criteria for TSC to establish a definitive diagnosis have been published (65).
4Serum VEGF-D is currently available in the United States as a College of American Pathologists/Clinical Laboratory Improvement Act–certified test only through the Translational Trials Laboratory at Cincinnati Children’s Hospital Medical Center. Detailed instructions for proper collection, handling, and shipping of VEGF-D specimens are available at the laboratory website: www.cincinnatichildrens.org/ttdsl.
5The diagnosis of AML can usually be made radiographically on the basis of the presence of fat in the tumors. Routine use of contrast is not required or recommended for the diagnosis of AMLs. Contrast is useful to define the aneurysmal burden and other vascular characteristics of the tumor, such as for evaluation of the potential for hemorrhage or planning for embolization. Similarly, lymphangioleiomyomas can typically be diagnosed on the basis of characteristic radiographic appearance.
6The sensitivity of cytological analysis of pleural fluid for the diagnosis of LAM requires further investigation and may only be available at select centers. In a majority of patients with chylous effusions, the diagnosis of LAM can be established on the basis of elevated serum VEGF-D.
7The decision to obtain tissue confirmation via invasive means should be individualized. For some patients with mild disease and a paucity of symptoms, a probable clinical diagnosis of LAM with serial monitoring may be sufficient if a definitive diagnosis of LAM would not change management and some level of diagnostic uncertainty is acceptable to the patient and the clinician. Every attempt should be made to establish the diagnosis of LAM with certainty before initiation of pharmacologic therapy with mTOR inhibitors.
8Transbronchial lung biopsy has an estimated yield of greater than 50% for the diagnosis of LAM, and markers of parenchymal LAM burden such as abnormal DlCO are associated with an increased diagnostic yield. Transbronchial lung biopsy appears to be safe in LAM on the basis of case reports and small series, but additional studies are required. Consultation with an expert center is recommended in cases where transbronchial biopsy is being considered, and for interpretation of the biopsy.
Many questions remain unanswered. The diagnostic yield of transbronchial lung biopsy in an unselected patient population is unknown and needs to be determined, as does the relationship between disease burden and yield. The safety profile of transbronchial lung biopsy in LAM, especially pertaining to the risk of pneumothorax, needs to be better understood. The number of biopsies that provides the optimal balance between diagnostic yield and risk of complications in patients with varying severity of LAM needs to be determined. The use of endobronchial ultrasound–guided transbronchial needle aspiration for the diagnosis of LAM has not been reported but may be an attractive option in patients with LAM who have mediastinal or hilar adenopathy, if studies demonstrate a reasonable yield and safety profile. Finally, the safety and efficacy of transbronchial lung cryobiopsy needs to be better understood for patients with suspected LAM (37).
The decision to obtain lung biopsy (transbronchial or surgical) for tissue diagnosis should be individualized for every patient. For some patients with mild disease and a paucity of symptoms, serial monitoring may be sufficient, especially if some level of diagnostic uncertainty is acceptable to the patient and clinician and a definite diagnosis is unlikely to change management. In contrast, VATS-guided surgical lung biopsy may be more appropriate for patients with a low cyst burden, given the potential for sampling error associated with transbronchial lung biopsy. Computed tomography can be used to guide lesion-targeted transbronchial lung biopsy on the basis of distribution of parenchymal abnormalities. In addition, the clinician should take into consideration that the pathological diagnosis of LAM can sometimes be made by less-invasive means, such as by demonstration of LAM cell clusters in chylous effusions (38, 39) or aspirates or core biopsies of pulmonary or extrapulmonary lymph nodes or masses (28, 40–42). The performance characteristics and diagnostic yield of these methodologies, however, is not well established and needs to be studied. Consultation with expert LAM centers is advised to individualize the approach to diagnosis in complex patients. On occasion, re-review of archival tissues from prior procedures by expert pathologists can reveal the diagnosis of LAM and obviate the need for biopsy. Examples include lung tissue obtained from prior bleb resections for pneumothorax or uterine and adnexal tissues from prior hysterectomies. Every attempt should be made to establish the diagnosis of LAM with certainty before initiation of pharmacologic therapy with mTOR inhibitors.
Recommendation
When a definitive diagnosis is required in patients who have parenchymal cysts on HRCT that are characteristic of LAM, but no additional confirmatory features of LAM (i.e., clinical, radiologic, or serologic), we suggest a diagnostic approach that includes transbronchial lung biopsy before a surgical lung biopsy (conditional recommendation, very low confidence in the estimated effects).
Remarks
The advantage of transbronchial lung biopsy is that it offers a less-invasive method to obtain histopathological confirmation of LAM, as compared with surgical lung biopsy. Although not proven, the panelists believed that the yield of transbronchial lung biopsy likely correlates with markers of parenchymal LAM burden (such as cyst profusion, abnormal diffusing capacity of the lung for carbon monoxide [DlCO], abnormal FEV1) and that appropriate patient selection is required to optimize the safety and efficacy of this diagnostic approach. A recent study evaluating the role of transbronchial lung biopsy in 24 consecutive patients presenting to a LAM Clinic revealed a diagnostic yield of 71%, which was inversely correlated with DlCO (43). Consultation with an expert center before undertaking transbronchial lung biopsy, combined with a critical review of the tissue specimens by a pathologist with expertise in LAM, can help avoid false-negative test results and the need for a surgical lung biopsy.
Values and preferences
This recommendation places a high value on the risk reduction and cost savings of the less-invasive and less-expensive approach of transbronchial lung biopsy as opposed to a VATS-guided surgical lung biopsy. It places a lower value on the desire to confirm the diagnosis with a single diagnostic test.
Question 3
Should patients with LAM undergo ipsilateral pleurodesis after an initial pneumothorax or wait for a recurrence before intervening with a pleural symphysis procedure?
Background
LAM is characterized by an increased risk of recurrent spontaneous pneumothoraces. On the basis of the high risk of recurrence in patients with LAM, an expert panel supported pleurodesis after the first episode of pneumothorax in patients with LAM (44).
Summary of the evidence
Our systematic review did not identify any studies that compared outcomes among patients with LAM who underwent pleurodesis after an initial pneumothorax versus those who underwent pleurodesis after a recurrent pneumothorax. The panel, therefore, used seven case series that reported the incidence of pneumothoraces among patients with LAM (19, 21, 28, 29, 44–48) and two observational studies that also compared the incidence of recurrent pneumothorax among those who had undergone pleurodesis to those who had not (44, 46) to inform the guideline panel’s judgments.
Pooling data demonstrated that pneumothorax occurred in 902 out of 1,591 (57%) patients with LAM (19, 21, 28, 29, 44–48). Recurrences were common, with estimates ranging from 29 to 81%, although most estimates were around 70% (28, 29, 44, 46, 48). Patients frequently had multiple recurrences, with estimates ranging from 3.2 to 5.0 pneumothoraces per patient in the pneumothorax-affected groups (21, 44, 45). The observational studies found that approximately 65% of patients who were managed conservatively after their initial pneumothorax had recurrent pneumothoraces, compared with only 18 to 32% of patients who had pleurodesis (44, 46). The rate of complications due to pleurodesis was not reported. The guideline panel had very low confidence in the estimated incidence of pneumothoraces in patients with LAM because they derived from case series and small observational studies.
With multiple recurrent pneumothoraces per patient, the cost of treatment of pneumothoraces can be substantial. In one series, the average time spent in the hospital due to pneumothoraces was approximately 1 month per patient, which was associated with substantial costs due to hospital expenses and lost productivity (44).
Benefits
Early pleurodesis after an initial pneumothorax decreases the risk of recurrent pneumothoraces, thereby decreasing morbidity, burden, and cost.
Harms
Pleurodesis is an invasive procedure associated with pain and potential complications. Prior pleurodesis may also be weighed when considering future candidacy for lung transplant.
Conclusions and research opportunities
The guideline panel weighed the desirable consequences of performing pleurodesis after an initial pneumothorax (i.e., a roughly 30–45% lower risk of recurrent pneumothorax and overall cost savings) against the undesirable consequences (i.e., pain, potential complications, impact on candidacy of transplantation) and decided that the balance favors pleurodesis. Among the panel’s considerations was the fact that the undesirable consequences of pleurodesis are merely delayed rather than avoided, because most patients will eventually suffer a recurrent pneumothorax and require pleurodesis.
There are multiple ways to perform pleurodesis, each with its own set of advantages and disadvantages. Generally speaking, pleurodesis can either be achieved by chemical instillation of a sclerosing agent via a chest tube or by surgical means using mechanical abrasion, talc poudrage, or pleurectomy. Although talc is the most common sclerosant used for pleurodesis, other agents, such as tetracycline derivatives, silver nitrate, iodopovidone, and bleomycin, have been used with varying degrees of success (49–51). In practice, the panelists generally use mechanical abrasion with an earnest attempt to address the entire parietal pleural surface for the initial pneumothorax and reserve more aggressive approaches, such as talc poudrage and pleurectomy, for recurrent and refractory pneumothoraces. The importance of knowledge and prior experience in management of pleural disease in LAM cannot be overstated, and pleural complications in LAM are best handled by thoracic surgeons with expertise in managing patients with LAM.
The ideal method of achieving pleural symphysis in patients with LAM, one that provides the optimal balance between efficacy of preventing future recurrences and the least risk of intra- and postoperative complications during lung transplantation, is not clear. The efficacy of alternative means of achieving pleurodesis, such as total pleural covering, which may prevent adhesions and the associated surgical complications during transplant (52), and autologous blood patch pleurodesis, which is associated with significantly less pain as compared with the traditional means of achieving pleural symphysis (53), needs to be studied in patients with LAM. In addition, a better understanding of pneumothoraces and their impact on long-term disease outcomes needs to be established. With the recognition of mTOR inhibitors as effective therapeutic agents for patients with LAM, the impact and role of mTOR inhibition on pneumothorax occurrence and recurrence needs to be assessed.
Recommendation
We suggest that patients with LAM be offered ipsilateral pleurodesis after their initial pneumothorax rather than waiting for a recurrent pneumothorax before intervening with a pleural symphysis procedure (conditional recommendation, very low confidence in the estimated effects).
Remarks
This approach is based on the high rate of recurrence of spontaneous pneumothoraces in patients with LAM. Nonetheless, the final decision to perform pleurodesis and the type of pleurodesis (chemical vs. surgical) should be based on shared decision-making between the clinician(s) and patient, after education about various management options. Lung biopsy at the time of pleurodesis for pneumothorax may be useful in selected patients who do not already have a confirmed diagnosis of LAM but can be associated with added risk (e.g., prolonged air leak and chronic bronchopleural fistula formation) (54) and should only be used when ATS/European Respiratory Society diagnostic criteria for LAM are not otherwise met and a histologic diagnosis is absolutely necessary. Every effort must be made to ensure that the pleurodesis is handled by clinicians familiar with management of pleural disease in LAM.
Values and preferences
This recommendation places a high value on reduction in the morbidity and cost associated with a recurrent pneumothorax. It places lower value on the adverse effects of pleurodesis.
Question 4
Should patients with LAM who have had a prior pleural intervention (either pleurodesis or pleurectomy) be excluded from consideration for lung transplantation?
Background
LAM typically progresses and may ultimately lead to respiratory insufficiency if it is left untreated. Lung transplantation remains the only treatment modality available for patients with end-stage lung disease due to LAM. A significant proportion of patients with LAM who present for transplant evaluation have undergone prior unilateral or bilateral pleurodesis procedures. These pleural interventions can increase the risk of bleeding complications at the time of lung transplantation, and some centers consider bilateral pleurodesis to be a relative contraindication to lung transplantation (48).
Summary of the evidence
Our systematic review identified five observational studies that enrolled patients with LAM undergoing lung transplantation and compared outcomes among those who had previous pleurodesis or pleurectomy versus those who did not (44, 55–58). In addition, five case series were found that described outcomes of lung transplantation for LAM but did not compare outcomes among patients with and without prior pleural procedure (59–63). Two of the case series were published in foreign languages and, therefore, were not considered (62, 63).
The observational studies collectively included 182 patients with LAM who were undergoing lung transplantation. This included 31 patients (17%) who had undergone unilateral pleurodesis, 55 patients (30%) who had undergone bilateral pleurodesis, 10 patients (5%) who had undergone unilateral pleurectomy, 7 patients (4%) who had undergone bilateral pleurectomy, and 79 patients (43%) who had not undergone a previous pleural procedure. Patients who had undergone a previous pleural procedure were more likely to have intra- or postoperative hemorrhage (48% vs. 7%; relative risk, 6.46; 95% confidence interval, 2.44–17.11) (44, 56, 57), and there was a trend toward such patients being more likely to have pleural adhesions (65% vs. 46%; relative risk, 1.42; 95% confidence interval, 0.96–2.12) (55, 56, 58). However, there was no significant difference in the length of hospital stay (44), lung function (58), mortality (58), or risk of chylous effusions (58). The guideline panel had very low confidence in these estimated outcomes, because the data were derived from small observational studies.
For general pulmonary populations, the International Society for Heart and Lung Transplantation Guideline committee recently recommended that pleurodesis not be considered a contraindication to lung transplantation and that a pneumothorax in a potential future transplant recipient should be given the best immediate management without undue concern that the choice of intervention will influence future acceptance for transplantation (64). The risks associated with transplantation of patients with prior bilateral pleurodesis were not directly addressed in this consensus document, however.
Benefits
Allowing patients with LAM who have had prior pleural procedures to undergo lung transplantation confers all of the potential advantages of lung transplantation in a patient with end-stage lung disease, including improved lung function and increased survival (55).
Harms
Prior pleural procedures increase the risk of intra- and postoperative bleeding and prolong operative time, although other outcomes are the same as those seen in patients who have not had a prior pleural procedure.
Conclusion and research opportunities
The guideline panel weighed the desirable consequences of allowing a patient to be considered for lung transplantation (i.e., hope and, for those who undergo transplant, improved lung function and increased survival) against the undesirable consequences (i.e., more likely to have intra- and postoperative bleeding) and determined that the balance favors lung transplantation. Among the panel’s considerations was the fact that the undesirable consequences are generally short term and rarely fatal, whereas the potential benefits are longer lasting and life-saving.
An unanswered question regarding lung transplantation in LAM is the difference in outcomes after a bilateral lung transplant compared with a single lung transplant. This is especially important, because if there is no difference in outcomes after a single lung transplant, then some patients with prior unilateral pleurodesis may electively undergo single lung transplantation on the contralateral side and potentially avoid the bleeding complications associated with the transplant.
Recommendations
We suggest that previous unilateral or bilateral pleural procedures (i.e., pleurodesis or pleurectomy) NOT be considered a contraindication to lung transplantation in patients with LAM (conditional recommendation, very low confidence in the estimated effects).
Remarks
Lung transplantation surgery in patients with a history of prior pleurodesis can be challenging. Patients who have undergone prior pleural procedures should be referred to a lung transplant team with expertise in handling complex pleural dissections.
Values and preferences
This recommendation places a high value on the later benefits of lung transplantation and a lower value on surgical complications.
Conclusions
The guideline panel used comprehensive evidence syntheses to inform its judgments regarding the balance of benefits versus burdens, adverse effects, and costs; the quality of evidence; the feasibility; and the acceptability of various interventions. For women who have cystic changes on HRCT of the chest characteristic of LAM, but who have no additional confirmatory features of LAM (i.e., clinical, radiologic, or serologic), the guideline panel made conditional recommendations against making a clinical diagnosis of LAM on the basis of the HRCT findings alone and for considering transbronchial lung biopsy as a diagnostic tool. The guideline panel also made conditional recommendations for offering pleurodesis after an initial pneumothorax rather than waiting for a recurrent pneumothorax and against pleurodesis being used as a reason to exclude patients from lung transplantation. Clinicians faced with making management decisions for patients with LAM should individualize their decisions, because the evidence base provided insufficient confidence in the estimated effects to warrant strong recommendations for or against any intervention.
Acknowledgments
This official clinical practice guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Clinical Problems.
Members of the Writing Group are as follows:
Francis X. McCormack, M.D. (Co-Chair)
Joel Moss, M.D., Ph.D. (Co-Chair)
Nishant Gupta, M.D., M.S.
Geraldine A. Finlay, M.D.
Robert M. Kotloff, M.D.
Charlie Strange, M.D.
Kevin C. Wilson, M.D.
Members of the subcommittee are as follows:
Francis X. McCormack, M.D. (Co-Chair)
Joel Moss, M.D., Ph.D. (Co-Chair)
Thomas V. Colby, M.D.
Vincent Cottin, M.D.
Gregory P. Downey, M.D.
Geraldine A. Finlay, M.D.
Nishant Gupta, M.D., M.S.
MeiLan K. Han, M.D.
Yoshikazu Inoue, M.D., Ph.D.
Simon R. Johnson, M.D.
Robert M. Kotloff, M.D.
Cristopher A. Meyer, M.D.
Jay H. Ryu, M.D.
Steven A. Sahn, M.D.
Kuniaki Seyama, M.D., Ph.D.
Karen Smith, M.L.S.
Charlie Strange, M.D.
Angelo M. Taveira-DaSilva, M.D., Ph.D.
Kathryn A. Wikenheiser-Brokamp, M.D., Ph.D.
Kevin C. Wilson, M.D.
Lisa R. Young, M.D.
Footnotes
This Official Clinical Practice Guideline was approved by the American Thoracic Society October 2017 and by the Japanese Respiratory Society August 2017
Author Disclosures: F.X.M. holds a patent for the use of VEGF-D in the diagnosis of lymphangioleiomyomatosis, served as a consultant for LAM Therapeutics, and served on a data and safety monitoring board for Takeda. V.C. served as a speaker for Sanofi-Aventis U.S.; served on a data and safety monitoring board for Promedior; served as a speaker and consultant for and received travel support from Boehringer Ingelheim International, F. Hoffmann-La Roche, and Novartis; and received honoraria for an adjudication committee from Gilead Sciences; and his spouse owns stocks, stock options, or other ownerships interests in Sanofi-Aventis U.S. M.K.H. served as a speaker and consultant for and received research support from Novartis Pharma; served as a consultant for AstraZeneca, Boehringer Ingelheim International, GlaxoSmithKline, and Sunovion; and served as a speaker for Boehringer Ingelheim International. S.R.J. served on an advisory committee for Pfizer, received research support from LAM Therapeutics, and served as a speaker for Novartis. S.A.S. served on a steering committee for InterMune and served as a clinical investigator for Actelion, Arresto, Celgene, and Gilead. C.S. served as a consultant for AstraZeneca Pharmaceuticals; served on a data safety and monitoring board for Arrowhead Pharmaceuticals; received research support from Adverum, CSL Behring, Novartis, Pulmonx Corporation, and Shire; served as a consultant, on an advisory committee, and received research support from BTG International; served as a consultant and received research support from Grifols Therapeutics; and owns stocks, stock options, or other ownerships interests in Abeona. J.M., T.V.C., G.P.D., G.A.F., N.G., Y.I., R.M.K., C.A.M., J.H.R., K. Seyama, K. Smith, A.M.T.-D., K.A.W.-B., K.C.W., and L.R.Y. reported no relationships with relevant commercial interests.
References
- 1.McCormack FX, Gupta N, Finlay GR, Young LR, Taveira-DaSilva AM, Glasgow CG, Steagall WK, Johnson SR, Sahn SA, Ryu JH, et al. ATS/JRS Committee on Lymphangioleiomyomatosis. Official American Thoracic Society/Japanese Respiratory society clinical practice guidelines: lymphangioleiomyomatosis diagnosis and management. Am J Respir Crit Care Med. 2016;194:748–761. doi: 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schünemann HJ. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, Reynaud-Gaubert M, Boehler A, Brauner M, Popper H, et al. Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 6.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonelli FS, Hartman TE, Swensen SJ, Sherrick A. Accuracy of high-resolution CT in diagnosing lung diseases. AJR Am J Roentgenol. 1998;170:1507–1512. doi: 10.2214/ajr.170.6.9609163. [DOI] [PubMed] [Google Scholar]
- 8.Koyama M, Johkoh T, Honda O, Tsubamoto M, Kozuka T, Tomiyama N, Hamada S, Nakamura H, Akira M, Ichikado K, et al. Chronic cystic lung disease: diagnostic accuracy of high-resolution CT in 92 patients. AJR Am J Roentgenol. 2003;180:827–835. doi: 10.2214/ajr.180.3.1800827. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Meraj R, Tanase D, James LE, Seyama K, Lynch DA, Akira M, Meyer CA, Ruoss SJ, Burger CD, et al. Accuracy of chest high-resolution computed tomography in diagnosing diffuse cystic lung diseases. Eur Respir J. 2015;46:1196–1199. doi: 10.1183/13993003.00570-2015. [DOI] [PubMed] [Google Scholar]
- 10.Copley SJ, Wells AU, Hawtin KE, Gibson DJ, Hodson JM, Jacques AE, Hansell DM. Lung morphology in the elderly: comparative CT study of subjects over 75 years old versus those under 55 years old. Radiology. 2009;251:566–573. doi: 10.1148/radiol.2512081242. [DOI] [PubMed] [Google Scholar]
- 11.Araki T, Nishino M, Gao W, Dupuis J, Putman RK, Washko GR, Hunninghake GM, O’Connor GT, Hatabu H. Pulmonary cysts identified on chest CT: are they part of aging change or of clinical significance? Thorax. 2015;70:1156–1162. doi: 10.1136/thoraxjnl-2015-207653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young LR, Vandyke R, Gulleman PM, Inoue Y, Brown KK, Schmidt LS, Linehan WM, Hajjar F, Kinder BW, Trapnell BC, et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138:674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews SM, Avik EL, Evans T. Interstitial infiltrates on computed tomography of the chest in a hispanic patient due to lymphangioleiomyomatosis diagnosed during the second trimester of pregnancy [abstract] Am J Respir Crit Care Med. 2014;189:A6731. [Google Scholar]
- 14.Chen F, Bando T, Fukuse T, Omasa M, Aoyama A, Hamakawa H, Fujinaga T, Shoji T, Sakai H, Hanaoka N, et al. Recurrent lymphangioleiomyomatosis after living-donor lobar lung transplantation. Transplant Proc. 2006;38:3151–3153. doi: 10.1016/j.transproceed.2006.08.145. [DOI] [PubMed] [Google Scholar]
- 15.Guinee DG, Jr, Feuerstein I, Koss MN, Travis WD. Pulmonary lymphangioleiomyomatosis: diagnosis based on results of transbronchial biopsy and immunohistochemical studies and correlation with high-resolution computed tomography findings. Arch Pathol Lab Med. 1994;118:846–849. [PubMed] [Google Scholar]
- 16.Seon CP, Byung HP, Sang YS, Han HJ, Kyung SC, Jun CP, Jeong J, Ji EK, Moo SP, Se KK, et al. A case of lymphangioleiomyomatosis presenting as a lung mass. Tuberc Respir Dis (Seoul) 2007;63:289–293. [Google Scholar]
- 17.Sindhwani G, Shirazi N, Sodhi R, Raghuvanshi S, Rawat J. Transbronchial lung biopsy in patients with diffuse parenchymal lung disease without ‘idiopathic pulmonary fibrosis pattern’ on HRCT scan: experience from a tertiary care center of North India. Lung India. 2015;32:453–456. doi: 10.4103/0970-2113.164148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrington CB, Cugell DW, Gaensler EA, Marks A, Redding RA, Schaaf JT, Tomasian A. Lymphangioleiomyomatosis: physiologic-pathologic-radiologic correlations. Am Rev Respir Dis. 1977;116:977–995. doi: 10.1164/arrd.1977.116.6.977. [DOI] [PubMed] [Google Scholar]
- 19.Taylor JR, Ryu J, Colby TV, Raffin TA. Lymphangioleiomyomatosis: clinical course in 32 patients. N Engl J Med. 1990;323:1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 20.Bonetti F, Chiodera PL, Pea M, Martignoni G, Bosi F, Zamboni G, Mariuzzi GM. Transbronchial biopsy in lymphangiomyomatosis of the lung. HMB45 for diagnosis. Am J Surg Pathol. 1993;17:1092–1102. doi: 10.1097/00000478-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Chu SC, Horiba K, Usuki J, Avila NA, Chen CC, Travis WD, Ferrans VJ, Moss J. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–1052. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 22.Harari S, Barberis M, De Juli E, Colombo F, Cimino G, Sabolla L, Soresi E.The diagnosis of lymphangioleiomyomatosis (LAM) and histiocytosis X (Hx) need to be histologically proven? Chest 19961104168S–172S [Google Scholar]
- 23.Harari S, Torre O, Cassandro R, Taveira-DaSilva AM, Moss J. Bronchoscopic diagnosis of Langerhans cell histiocytosis and lymphangioleiomyomatosis. Respir Med. 2012;106:1286–1292. doi: 10.1016/j.rmed.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naalsund A, Johansen B, Foerster A, Kolbenstvedt A. When to suspect and how to diagnose pulmonary lymphangioleiomyomatosis. Respirology. 1996;1:207–212. doi: 10.1111/j.1440-1843.1996.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 25.Park HY, Nam HS, Chung MP, Jeong SH, Kim YJ, Cha SI, Kim YW, Park JS, Uh ST, Park CS, et al. A nationwide survey of lymphangioleiomyomatosis in Korea: recent increase in newly diagnosed patients. J Korean Med Sci. 2010;25:1182–1186. doi: 10.3346/jkms.2010.25.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seyama K, Kira S, Takahashi H, Ohnishi M, Kodama Y, Dambara T, Kobayashi J, Kitamura S, Fukuchi Y. Longitudinal follow-up study of 11 patients with pulmonary lymphangioleiomyomatosis: diverse clinical courses of LAM allow some patients to be treated without anti-hormone therapy. Respirology. 2001;6:331–340. doi: 10.1046/j.1440-1843.2001.00343.x. [DOI] [PubMed] [Google Scholar]
- 27.Ueng SH, Liu HP, Wu YC, Tsai YH, Lin HC, Lin MC, Lim KE, Huang SF. Pulmonary lymphangioleiomyomatosis: a clinicopathological analysis of ten cases. Chang Gung Med J. 2004;27:201–209. [PubMed] [Google Scholar]
- 28.Urban T, Lazor R, Lacronique J, Murris M, Labrune S, Valeyre D, Cordier JF. Pulmonary lymphangioleiomyomatosis: a study of 69 patients. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) Medicine (Baltimore) 1999;78:321–33. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Ye L, Jin M, Bai C. Clinical analysis of patients with pulmonary lymphangioleiomyomatosis (PLAM) in mainland China. Respir Med. 2010;104:1521–1526. doi: 10.1016/j.rmed.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Meraj R, Wikenheiser-Brokamp KA, Young LR, Byrnes S, McCormack FX. Utility of transbronchial biopsy in the diagnosis of lymphangioleiomyomatosis. Front Med. 2012;6:395–405. doi: 10.1007/s11684-012-0231-5. [DOI] [PubMed] [Google Scholar]
- 31.Rittirak W, Sompradeekul S. Diagnostic yield of fluoroscopy-guided transbronchial lung biopsy in non-endobronchial lung lesion. J Med Assoc Thai. 2007;90:68–73. [PubMed] [Google Scholar]
- 32.Ost DE, Ernst A, Lei X, Kovitz KL, Benzaquen S, Diaz-Mendoza J, Greenhill S, Toth J, Feller-Kopman D, Puchalski J, et al. AQuIRE Bronchoscopy Registry. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions: results of the AQuIRE registry. Am J Respir Crit Care Med. 2016;193:68–77. doi: 10.1164/rccm.201507-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreider ME, Hansen-Flaschen J, Ahmad NN, Rossman MD, Kaiser LR, Kucharczuk JC, Shrager JB. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83:1140–1144. doi: 10.1016/j.athoracsur.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman S, Gleason JB, Ilyas MIM, Martinez F, Mehta JP, Savage EB. assessing the safety and clinical impact of thoracoscopic lung biopsy in patients with interstitial lung disease. J Clin Diagn Res. 2017;11:OC57–OC59. doi: 10.7860/JCDR/2017/20281.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen W, Meyer KC. Surgical lung biopsy for the diagnosis of interstitial lung disease: a review of the literature and recommendations for optimizing safety and efficacy. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:3–16. [PubMed] [Google Scholar]
- 36.Durheim MT, Kim S, Gulack BC, Burfeind WR, Gaissert HA, Kosinski AS, Hartwig MG. Mortality and respiratory failure after thoracoscopic lung biopsy for interstitial lung disease. Ann Thorac Surg. 2017;104:465–470. doi: 10.1016/j.athoracsur.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Fruchter O, Fridel L, El Raouf BA, Abdel-Rahman N, Rosengarten D, Kramer MR. Histological diagnosis of interstitial lung diseases by cryo-transbronchial biopsy. Respirology. 2014;19:683–688. doi: 10.1111/resp.12296. [DOI] [PubMed] [Google Scholar]
- 38.Mitani K, Kumasaka T, Takemura H, Hayashi T, Gunji Y, Kunogi M, Akiyoshi T, Takahashi K, Suda K, Seyama K. Cytologic, immunocytochemical and ultrastructural characterization of lymphangioleiomyomatosis cell clusters in chylous effusions of patients with lymphangioleiomyomatosis. Acta Cytol. 2009;53:402–409. doi: 10.1159/000325340. [DOI] [PubMed] [Google Scholar]
- 39.Hirama M, Atsuta R, Mitani K, Kumasaka T, Gunji Y, Sasaki S, Iwase A, Takahashi K, Seyama K. Lymphangioleiomyomatosis diagnosed by immunocytochemical and genetic analysis of lymphangioleiomyomatosis cell clusters found in chylous pleural effusion. Intern Med. 2007;46:1593–1596. doi: 10.2169/internalmedicine.46.0225. [DOI] [PubMed] [Google Scholar]
- 40.Hecimovic A, Jakopovic M, Pavlisa G, Jankovic M, Vukic-Dugac A, Redzepi G, Brcic L, Samarzija M, Gupta N. Successful treatment of pulmonary and lymphatic manifestations of lymphangioleiomyomatosis with sirolimus. Lymphology. 2015;48:97–102. [PubMed] [Google Scholar]
- 41.Kebria M, Black D, Borelli C, Modica I, Hensley M, Chi DS. Primary retroperitoneal lymphangioleiomyomatosis in a postmenopausal woman: a case report and review of the literature. Int J Gynecol Cancer. 2007;17:528–532. doi: 10.1111/j.1525-1438.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 42.Słodkowska J, Patera J, Breborowicz J, Jarzemska A, Korzeniewska-Kosela M, Siemiatkowska K, Radzikowska E, Przybylski G, Kozłowski W. Extrapulmonary lymphangioleiomyomatosis presented as the asymptomatic retroperitoneal tumours--two cases report. Pol J Pathol. 2006;57:205–207. [PubMed] [Google Scholar]
- 43.Koba T, Arai T, Kitaichi M, Kasai T, Hirose M, Tachibana K, Sugimoto C, Akira M, Hayashi S, Inoue Y.Efficacy and safety of transbronchial lung biopsy for the diagnosis of lymphangioleiomyomatosis: a report of 24 consecutive patients Respirology [online ahead of print] 28 Sept 2017; DOI: 10.1111/resp.13190 [DOI] [PubMed] [Google Scholar]
- 44.Almoosa KF, Ryu JH, Mendez J, Huggins JT, Young LR, Sullivan EJ, Maurer J, McCormack FX, Sahn SA. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129:1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 45.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, Finlay GA, Olson EJ, Ruoss SJ, Maurer JR, et al. NHLBI LAM Registry Group. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson SR, Tattersfield AE. Clinical experience of lymphangioleiomyomatosis in the UK. Thorax. 2000;55:1052–1057. doi: 10.1136/thorax.55.12.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitaichi M, Nishimura K, Itoh H, Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995;151:527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 48.Young LR, Almoosa KF, Pollock-Barziv S, Coutinho M, McCormack FX, Sahn SA. Patient perspectives on management of pneumothorax in lymphangioleiomyomatosis. Chest. 2006;129:1267–1273. doi: 10.1378/chest.129.5.1267. [DOI] [PubMed] [Google Scholar]
- 49.Almind M, Lange P, Viskum K. Spontaneous pneumothorax: comparison of simple drainage, talc pleurodesis, and tetracycline pleurodesis. Thorax. 1989;44:627–630. doi: 10.1136/thx.44.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paschoalini MdaS, Vargas FS, Marchi E, Pereira JR, Jatene FB, Antonangelo L, Light RW. Prospective randomized trial of silver nitrate vs talc slurry in pleurodesis for symptomatic malignant pleural effusions. Chest. 2005;128:684–689. doi: 10.1378/chest.128.2.684. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Efficacy and safety of iodopovidone in chemical pleurodesis: a meta-analysis of observational studies. Respir Med. 2006;100:2043–2047. doi: 10.1016/j.rmed.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Kurihara M, Mizobuchi T, Kataoka H, Sato T, Kumasaka T, Ebana H, Yamanaka S, Endo R, Miyahashira S, Shinya N, et al. A total pleural covering for lymphangioleiomyomatosis prevents pneumothorax recurrence. Plos One. 2016;11:e0163637. doi: 10.1371/journal.pone.0163637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manley K, Coonar A, Wells F, Scarci M. Blood patch for persistent air leak: a review of the current literature. Curr Opin Pulm Med. 2012;18:333–338. doi: 10.1097/MCP.0b013e32835358ca. [DOI] [PubMed] [Google Scholar]
- 54.Cundiff WB, McCormack FX, Wikenheiser-Brokamp K, Starnes S, Kotloff R, Benzaquen S. Successful management of a chronic, refractory bronchopleural fistula with endobronchial valves followed by talc pleurodesis. Am J Respir Crit Care Med. 2014;189:490–491. doi: 10.1164/rccm.201311-1965LE. [DOI] [PubMed] [Google Scholar]
- 55.Boehler A, Speich R, Russi EW, Weder W. Lung transplantation for lymphangioleiomyomatosis. N Engl J Med. 1996;335:1275–1280. doi: 10.1056/NEJM199610243351704. [DOI] [PubMed] [Google Scholar]
- 56.Reynaud-Gaubert M, Mornex JF, Mal H, Treilhaud M, Dromer C, Quétant S, Leroy-Ladurie F, Guillemain R, Philit F, Dauriat G, et al. Lung transplantation for lymphangioleiomyomatosis: the French experience. Transplantation. 2008;86:515–520. doi: 10.1097/TP.0b013e31817c15df. [DOI] [PubMed] [Google Scholar]
- 57.Machuca TN, Losso MJ, Camargo SM, Schio SM, Melo IA, Hochhegger B, Felicetti JC, Camargo JJ. Lung transplantation for lymphangioleiomyomatosis: single-center Brazilian experience with no chylothorax. Transplant Proc. 2011;43:236–238. doi: 10.1016/j.transproceed.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 58.Sakamoto J, Chen F, Chaparro C, Karolak W, Yasufuku K, De Perrot M, Pierre A, Singer LG, Hutcheon M, Waddell T, et al. Impact of pre-transplant pleurodesis in the outcome after lung transplantation for lymphagioleiomyomatosis. J Heart Lung Transplant. 2014;33:S289–S290. [Google Scholar]
- 59.Pechet TT, Meyers BF, Guthrie TJ, Battafarano RJ, Trulock EP, Cooper JD, Patterson GA. Lung transplantation for lymphangioleiomyomatosis. J Heart Lung Transplant. 2004;23:301–308. doi: 10.1016/S1053-2498(03)00195-5. [DOI] [PubMed] [Google Scholar]
- 60.Collins J, Müller NL, Kazerooni EA, McAdams HP, Leung AN, Love RB. Lung transplantation for lymphangioleiomyomatosis: role of imaging in the assessment of complications related to the underlying disease. Radiology. 1999;210:325–332. doi: 10.1148/radiology.210.2.r99fe11325. [DOI] [PubMed] [Google Scholar]
- 61.Benden C, Rea F, Behr J, Corris PA, Reynaud-Gaubert M, Stern M, Speich R, Boehler A. Lung transplantation for lymphangioleiomyomatosis: the European experience. J Heart Lung Transplant. 2009;28:1–7. doi: 10.1016/j.healun.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 62.Adachi K, Kurosawa S, Wagatsuma T, Kameyama E. Seventeen cases of lung transplantations for lymphangioleiomyomatosis [in Japanese] Masui. 2012;61:1239–1244. [PubMed] [Google Scholar]
- 63.Ansótegui Barrera E, Mancheño Franch N, Peñalver Cuesta JC, Vera-Sempere F, Padilla Alarcón J. Lung transplantation in sporadic lymphangioleiomyomatosis: study of 7 cases [in Spanish] Med Clin (Barc) 2013;141:349–352. doi: 10.1016/j.medcli.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 64.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, Lederer DJ, Mulligan MJ, Patterson GA, Singer LG, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 65.Northrup H, Krueger DA International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243–254. doi: 10.1016/j.pediatrneurol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]