Abstract
Rationale: Individuals with cystic fibrosis (CF) experience frequent acute pulmonary exacerbations, which lead to decreased lung function and reduced quality of life.
Objectives: The goal of this study was to determine if an intervention directed toward early detection of pulmonary exacerbations using home spirometry and symptom monitoring would result in slower decline in lung function than in control subjects.
Methods: We conducted a multicenter, randomized trial at 14 CF centers with subjects at least 14 years old. The early intervention arm subjects measured home spirometry and symptoms electronically twice per week. Sites were notified if a participant met criteria for an exacerbation and contacted participants to determine if treatment for acute exacerbation was required. Participants in the usual care arm were seen every 3 months and were asked to contact the site if they were concerned about worsening pulmonary symptoms.
Measurements and Main Results: The primary outcome was the 52-week change in FEV1. Secondary outcomes included time to first exacerbation and subsequent exacerbation, quality of life, and change in weight. A total of 267 patients were randomized, and the study arms were well matched at baseline. There was no significant difference between study arms in 52-week mean change in FEV1 slope (mean slope difference, 0.00 L, 95% confidence interval, −0.07 to 0.07; P = 0.99). The early intervention arm subjects detected exacerbations more frequently than usual care arm subjects (time to first exacerbation hazard ratio, 1.45; 95% confidence interval, 1.09 to 1.93; P = 0.01). Adverse events were not significantly different between treatment arms.
Conclusions: An intervention of home monitoring among patients with CF was able to detect more exacerbations than usual care, but this did not result in slower decline in lung function.
Clinical trial registered with www.clinicaltrials.gov (NCT01104402).
Keywords: cystic fibrosis, pulmonary exacerbation, clinical trial, home monitoring
At a Glance Commentary
Scientific Knowledge on the Subject
Pulmonary exacerbations are key clinical events in the lives of patient with cystic fibrosis (CF). Identifying pulmonary exacerbations earlier in their time course could improve clinical outcomes in CF.
What This Study Adds to the Field
An intervention of home monitoring in CF was associated with shorter time to first exacerbation than usual care. However, identification of these events did not result in slower decline in lung function. A better understanding of the underlying pathophysiology leading to CF pulmonary exacerbations is essential to developing better approaches to prevention and treatment of exacerbations.
Cystic fibrosis (CF) is the most common life-shortening inherited disease in white individuals and affects approximately 30,000 people in the United States (1). Advances in care for individuals with CF have resulted in dramatic improvements in survival, but people with CF still have debilitating symptoms and die far too early (2, 3). Acute pulmonary exacerbations (PEs) are frequent and central events in the lives of individuals with CF. They result in permanent loss of lung function, worse quality of life, and shortened survival (4–8).
Several studies have shown that in approximately 25% of exacerbations, patients do not return to within 90% of their baseline lung function after treatment for the exacerbation (9, 10). One factor associated with poor response to exacerbation treatment may be longer time from symptom onset to exacerbation treatment, suggesting that delayed treatment results in worse treatment outcomes. There is also evidence suggesting that CF centers that see patients more frequently and treat patients more aggressively (e.g., with more antibiotic use) provide better clinical outcomes (11, 12).
Most individuals with CF do not measure lung function or objectively track symptoms at home and contact their health care providers only during routinely scheduled appointments or if their symptoms worsen to a point that they feel they need treatment. This approach could lead to delays in exacerbation treatment, thus preventing important health outcomes, as seen in other studies (13, 14).
We hypothesized that use of a protocol consisting of electronic home monitoring of FEV1 and respiratory symptoms, as well as notifying care teams when patients had objective evidence of deterioration, would result in less decline in lung function over 12 months than in a group that received usual care. To test this, we performed a multicenter, nonblinded, randomized clinical trial of a protocol for early intervention in CF exacerbation.
Methods
Study Design and Population
We conducted the eICE (Early Intervention in Cystic Fibrosis Exacerbation) study, a 1-year, randomized, nonblinded, multicenter, two-arm trial that ran from October 6, 2011, to July 7, 2015, and involved adolescents and adults with CF, to assess whether early treatment of CF PEs was beneficial. Participants with CF were randomized 1:1 to either an early intervention (EI) arm or a usual care (UC) arm. The EI arm participants used home-based spirometers and patient-reported respiratory symptoms (using the Cystic Fibrosis Respiratory Symptom Diary [CFRSD]) (15), completed twice weekly via the Viasys AM2 device (CareFusion, Yorba Linda, CA) to identify and trigger the treatment of PEs. The AM2 system alerted sites to contact patients for an acute PE evaluation whenever (1) FEV1 values (in liters) fell by greater than 10% from baseline or (2) CFRSD worsened from baseline in two or more of eight respiratory symptoms. The UC arm participants, in contrast, had quarterly CF visits in addition to acute visits based on calls from the participant to the clinic triage telephone line (see earlier publication [16]). The study is registered with www.clinicaltrials.gov (NCT01104402). The institutional review boards at each participating center approved the study.
The 52-week duration of the study consisted of five in-person clinic visits (see online supplement and Figure E1 in the online supplement). Eligibility for the study required that participants be at least 14 years of age, be clinically stable at baseline, and have FEV1 percent predicted greater than 25%. After confirmation of eligibility, participants were randomized using an adaptive randomization algorithm (see online supplement) (17).
Primary and Secondary Outcomes
The primary outcome variable was the 52-week change in FEV1 volume (in liters). Pulmonary function testing was performed in accordance with American Thoracic Society standards (18–20). See Table 1 and the online supplement for details of secondary outcome measures.
Table 1.
Secondary Outcome Measures with Defined Units
| Outcome Measure | Unit |
|---|---|
| CFQ-R (21) | 52-wk change |
| CFRSD (15) | 52-wk change |
| FEV1, % predicted | 52-wk change |
| FVC, L | 52-wk change |
| FEF25–75%, L/s | 52-wk change |
| Time to first acute protocol-defined PE | Time in days |
| Time from first acute PE to subsequent PE | Time in days |
| Number of hospitalization days | Time in days |
| Number of hospitalizations | n |
| Pseudomonas aeruginosa | % Change in prevalence |
| Staphylococcus aureus | % Change in prevalence |
| Global assessment of protocol burden | 0–10 scale (10 = great burden) |
Definition of abbreviations: CFQ-R = Cystic Fibrosis Questionnaire–Revised; CFRSD = Cystic Fibrosis Respiratory Symptom Diary; FEF25–75% = forced expiratory flow, midexpiratory phase; PE = pulmonary exacerbation.
Statistical Analysis
Our primary hypothesis was that the EI arm participants would have a lower rate of FEV1 decline over 52 weeks than the UC arm. In the primary analysis, we used a linear mixed effects model to estimate the difference in the 52-week mean change in FEV1 between arms. The random slope and intercept model with unstructured covariance was fit with FEV1 (in liters) measured at quarterly study visits as the response and predictors for baseline FEV1 percent predicted group (<50%, 50 to 75%, >75%), age group (14–18 yr, ≥19 yr), treatment arm, time in weeks, and the interaction between treatment and time. The slope of the treatment by time interaction was used to estimate change from baseline to 52 weeks. All primary and secondary analyses were based on the intention-to-treat (ITT) population, defined as all participants who were randomized. A per-protocol population was defined as the ITT population subset not having a major protocol violation. Additional sensitivity analyses of the primary endpoint included estimating the 52-week change among those with at least 80% compliance in the EI arm. See the online supplement for additional analytic methods, including multivariate models and sample size estimates. The study was originally designed for enrollment of 140 participants with 80% power to detect a 110-ml (SD, 22 ml) 52-week change in lung function, assuming a 7% dropout rate. In August 2012, enrollment was increased to 320 participants (160 per arm) after the addition of 12 new sites, resulting in an improved ability to detect a smaller difference (72 ml) with 80% power. Safety outcomes were monitored throughout the study by an independent data monitoring committee (DMC), which was approved by NHLBI officials. After a post hoc futility analysis of the primary endpoint, the DMC recommended stopping enrollment early on June 24, 2014. Nevertheless, we enrolled 83% (267 of 320) of the study’s intended sample size.
All testing, including P values and confidence intervals (CIs), were performed using a two-sided 0.05 significance level. All analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) or R 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria) software.
Results
Recruitment and Follow-up
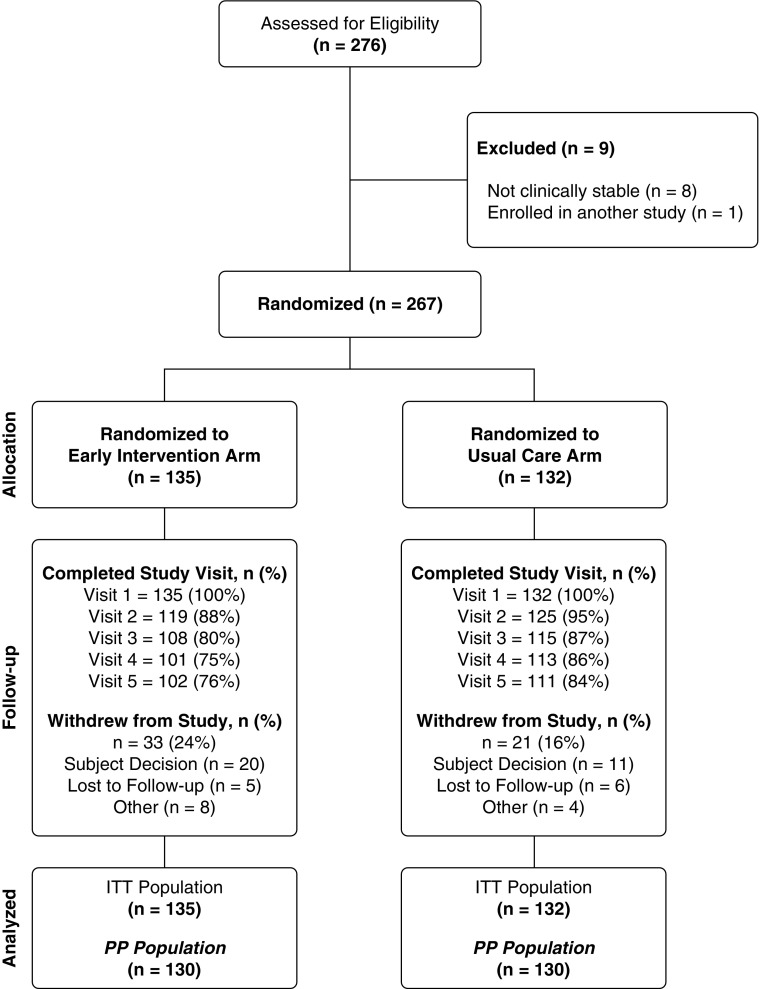
Of the 276 screened participants, 267 passed the eligibility criteria and were randomized (the ITT population): 135 to the EI arm and 132 to the UC arm (Figure 1; see Figure E2 and Table E1). After enrolling 267 adolescents and adults with CF, the DMC recommended stopping recruitment because of a projected inability for the study to detect a difference between the EI and UC arms. Twenty percent (54 of 267) of participants withdrew from the study, with more withdrawals occurring in the EI arm (33 of 135 [24%]) than in the UC arm (21 of 132 [16%]). Across groups, the reasons for withdrawals were comparable and distributed as follows: 57% (n = 31) were due to subject decision, 20% (n = 11) were lost to follow-up, and 22% (n = 12) withdrew for other reasons.
Figure 1.
Consolidated Standards of Reporting Trials diagram showing participant flow in the eICE (Early Intervention in Cystic Fibrosis Exacerbation) study. ITT = intention-to-treat; PP = per protocol.
The mean follow-up times were 46.8 weeks per participant in the EI arm compared with 50.9 weeks per participant in the UC arm. The two study arms were well matched with respect to demographic and clinical characteristics at baseline (Table 2). Overall, 49% of the participants were female; 94% were white; 45% were F508del homozygous; and the mean age was 27 years, with 29% younger than 18 years of age.
Table 2.
Demographics and Baseline Characteristics by Study Arm
| Characteristic | EI Arm (n = 135) | UC Arm (n = 132) | Total (n = 267) |
|---|---|---|---|
| Female sex, n (%) | 68 (50%) | 68 (52%) | 136 (51%) |
| Age, yr, mean (SD) | 26.5 (11.5) | 27.8 (12.5) | 27.1 (12.0) |
| Age distribution, n (%) | |||
| 14 to <18 yr | 38 (28%) | 39 (30%) | 77 (29%) |
| 18 to <30 yr | 55 (41%) | 41 (31%) | 96 (36%) |
| ≥30 yr | 42 (31%) | 52 (39%) | 94 (35%) |
| Race, n (%) | |||
| White | 126 (93%) | 125 (95%) | 251 (94%) |
| Hispanic | 6 (4%) | 4 (3%) | 10 (4%) |
| African American | 2 (1%) | 2 (2%) | 4 (1%) |
| Unknown/other* | 1 (1%) | 1 (1%) | 2 (1%) |
| CFTR genotype, n (%) | |||
| F508del homozygous | 55 (41%) | 65 (49%) | 120 (45%) |
| F508del heterozygous | 64 (47%) | 55 (42%) | 119 (45%) |
| Other | 14 (10%) | 10 (8%) | 24 (9%) |
| Not available | 2 (1%) | 2 (2%) | 4 (1%) |
| FEV1, % predicted†, mean (SD) | 80.0% (22.9%) | 79.0% (24.7%) | 79.5% (23.8%) |
| FEV1, % predicted distribution, n (%) | |||
| 25 to <50% | 18 (13%) | 19 (14%) | 37 (14%) |
| 50 to <75% | 33 (24%) | 31 (23%) | 64 (24%) |
| 75 to <100% | 60 (44%) | 52 (39%) | 112 (42%) |
| ≥100% | 24 (18%) | 30 (23%) | 54 (20%) |
| Pseudomonas aeruginosa–positive sputum, n (%) | 70 (52%) | 69 (52%) | 139 (52%) |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator gene; EI = early intervention; UC = usual care.
“Other” refers to participants with two known non-ΔF508 cystic fibrosis mutations.
FEV1 % predicted was calculated using Wang/Hankinson age-dependent equations.
Home Spirometry Adherence
Adherence with once-weekly data transmission was 50%, with 67 of 135 participants using the device at least once per week in more than 80% of their follow-up weeks. Adherence with twice-weekly transmission, however, was lower, with only 19% (26 of 135) of participants being greater than 80% adherent. A total of 524 alarms among 97 participants (72%) in the EI arm required physician follow-up (see online supplement for reasons for alarms). Symptoms triggered the alarms less than home spirometry did.
Primary Endpoint
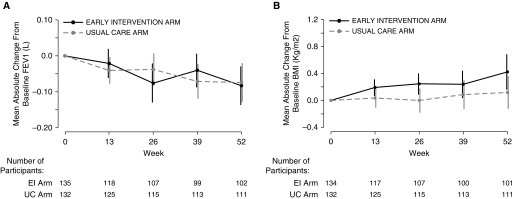
Absolute change in mean FEV1 volume (in liters) from baseline to Week 52 in the EI arm was −0.08 L (95% CI, −0.13 to −0.03; P = 0.002), as compared with a −0.07-L change in the UC arm (95% CI, −0.13 to −0.02; P = 0.006) (Figure 2A). Covariate adjusted analysis of the primary endpoint showed no difference between study arms in terms of the 52-week mean change in FEV1 slope (mean difference, 0.00 L; 95% CI, −0.07 to 0.07; P = 0.991). Sensitivity analyses of the primary endpoint, including spirometry from withdrawal visits, yielded estimates of 52-week mean difference similar to the primary endpoint. Similarly, the per-protocol population analysis removing seven participants with major protocol violations did not change the results (Table E2). The two study arms were also not significantly different in terms of mean change in FEV1 from baseline at the withdrawal visit (mean change EI arm, −0.04 L [n = 22] vs. 0.01 L [n = 10] in the UC arm [difference between arms, −0.05 L; 95% CI, −0.18 to 0.10; P = 0.526]). In additional analyses, we noted similar results when evaluating only the results from the two highest-enrolling sites (Table E2). Also, there was no difference in effect between those who had high adherence and those subjects without high adherence to the intervention (mean difference in FEV1, 0.02 L; 95% CI, −0.09 to 0.12; P = 0.776) (see Table E3).
Figure 2.
(A) Mean absolute change from baseline in FEV1. (B) Mean absolute change from baseline in body mass index. Data are presented as means and 95% confidence intervals. BMI = body mass index; EI = early intervention; UC = usual care.
Secondary Efficacy Endpoints
Absolute change in mean FEV1 (percent predicted) from baseline to Week 52 in the EI arm was −3.58% (95% CI, −4.93 to −2.24%) as compared with a −3.45% change in the UC arm (95% CI, −4.72 to −2.16%) (Figure E3). Mixed model results for the 52-week mean change in FEV1 (percent predicted) showed no difference between treatment arms (mean difference, 0.01%; 95% CI, −1.65 to 1.67%; P = 0.986). The mean change in FEV1 volume (in liters) from an acute visit at diagnosis of a PE to the 2-week follow-up visit after initiation of antibiotics was not statistically different between the two study arms (mean, 0.19 L [n = 61 events] in EI arm vs. 0.12 L [n = 26 events] in UC arm), although the confidence boundaries were wide (difference, 0.07 L; 95% CI, −0.07 to 0.20; P = 0.309). The 52-week mean absolute change in body mass index (BMI) was 0.42 kg/m2 in the EI arm compared with 0.12 kg/m2 in the UC arm (mean difference, 0.30 kg/m2; 95% CI, −0.04 to 0.65; P = 0.081) (Figures 2B and E4).
The prevalence of Pseudomonas aeruginosa at baseline (Table 2) did not differ between treatment arms, and the prevalence of P. aeruginosa postbaseline as captured at routine clinic visits was comparable between study arms (46% P. aeruginosa–positive in EI arm vs. 51% P. aeruginosa–positive in UC arm; difference, −5%; 95% CI, −19 to 8%; P = 0.411). Similarly, the prevalence of Staphylococcus aureus was not significantly different postbaseline between study arms (34% S. aureus–positive in EI arm vs. 30% S. aureus–positive in UC arm; difference, 4%; 95% CI, −9 to 16%; P = 0.658). There was also no significant difference between study arms in terms of newly emergent P. aeruginosa or S. aureus over the follow-up period.
Of 135 participants in the EI arm, 101 (75%) experienced a protocol-defined PE, as compared with 92 (70%) of 132 participants in the UC arm (difference, 5%; 95% CI, −6 to 17%; P = 0.412). The EI arm was associated with a significantly higher hazard ratio (HR) for time to first PE and increased risk for subsequent PE (time to first PE HR, 1.45; 95% CI, 1.09 to 1.93; P = 0.011; time to subsequent PE HR, 1.34; 95% CI, 0.95 to 1.89; P = 0.091) (see Figure E5). Hospitalization event rates were not significantly different between study arms (0.64 hospitalizations/participant-year in EI arm vs. 0.59 in UC arm; relative risk, 0.90; 95% CI, 0.65 to 1.23; P = 0.509).
Acute visits were much more likely to occur in the EI arm than in the UC arm (participants with at least one acute visit, 77 [57%] in EI arm vs. 38 [29%] in UC arm; difference, 28%; 95% CI, 16 to 40%) (Table 3). More than twice as many acute visits occurred in the EI arm as in the UC arm (153 in EI arm vs. 64 in UC arm). Similar proportions of acute visits in each study arm fulfilled our protocol-defined criteria for an acute PE. The main difference between the treatment arms was that the EI arm received a higher proportion of oral antibiotics as the treatment for protocol-defined PEs and a lower proportion of intravenous antibiotics than the UC arm (Table 3). We found that, regarding PEs at both acute visits at the start of treatment and 2-week follow-up spirometry, fewer patients in the EI arm recovered to 5% of baseline FEV1 percent predicted compared with the UC arm (28 of 60 acute visits in EI arm vs. 5 of 24 visits in UC arm) (see Table E4).
Table 3.
Summary of Acute Visits
| EI Arm (n = 135) | UC Arm (n = 132) | P Value* | Total (n = 267) | |
|---|---|---|---|---|
| Participants with at least one acute visit, n (%) | 77 (57%) | 38 (29%) | <0.001 | 115 (43%) |
| Distribution of acute visits per subject, n (%) | ||||
| 0 | 58 (43%) | 94 (71%) | <0.001 | 152 (57%) |
| 1 | 37 (27%) | 20 (15%) | 57 (21%) | |
| 2–3 | 30 (22%) | 17 (13%) | 47 (18%) | |
| ≥4 | 10 (7%) | 1 (1%) | 11 (4%) | |
| Total number of acute visits | 153 | 64 | 0.818 | 217 |
| Acute visits missing PE evaluation†, n (%) | 16 (10%) | 8 (12%) | 0.642 | 24 (11%) |
| Acute visits not meeting protocol-defined PE†, n (%) | 29 (19%) | 12 (19%) | 1.000 | 33 (19%) |
| Requiring oral antibiotics‡ | 12 (41%) | 4 (33%) | 0.734 | 16 (48%) |
| Requiring intravenous antibiotics | 4 (14%) | 4 (33%) | 0.202 | 8 (24%) |
| Requiring inhaled antibiotics | 4 (14%) | 2 (17%) | 1.000 | 6 (18%) |
| Requiring any antibiotics | 14 (48%) | 8 (67%) | 0.325 | 22 (67%) |
| Requiring hospitalization | 5 (17) | 4 (33%) | 0.408 | 9 (27%) |
| Acute visits meeting protocol-defined PE†, n (%) | 108 (71%) | 44 (69%) | 0.871 | 152 (70%) |
| Requiring oral antibiotics‡ | 72 (67%) | 19 (43%) | 0.010 | 97 (64%) |
| Requiring intravenous antibiotics | 35 (32%) | 23 (52%) | 0.027 | 58 (38%) |
| Requiring inhaled antibiotics | 16 (15%) | 10 (23%) | 0.244 | 26 (17%) |
| Requiring any antibiotics | 91 (84%) | 39 (89%) | 0.615 | 130 (86%) |
| Requiring hospitalization | 31 (29%) | 22 (50%) | 0.015 | 53 (35%) |
Definition of abbreviations: EI = early intervention; PE = pulmonary exacerbation; UC = usual care.
All P values are based on Fisher’s exact test.
The denominator for percentages is based on the total number of acute visits in each study arm.
The denominator for percentages is based on the number of acute visits meeting/not meeting protocol-defined PE.
Adverse Events
The number of participants reporting at least one serious adverse event was similar across the two study arms (27% [n = 37] in EI arm vs. 28% [n = 37] in the UC arm) (see Table E3). All serious adverse events were deemed unrelated to study treatment. The EI arm had 1,580 adverse events (AEs) among 124 (92%) participants, whereas the UC arm had 1,307 AEs among 120 (91%) participants (rate ratio, 1.29; 95% CI, 1.19 to 1.38; P < 0.001) (Table E5). One participant in the UC arm had a lung transplant, and two participants in the UC arm had non–respiratory-related life-threatening AEs.
Independent of alarms or treatment allocation, most participants initiated oral antibiotics at least once during the course of the study, with no significant difference between study arms (92% in EI arm vs. 91% in UC arm; difference, 1%; 95% CI, −6 to 8%; P = 0.830). Despite differences in how exacerbations were treated (Table 3), no significant differences were seen between study arms in the proportion receiving inhaled or intravenous antibiotics at least once for the duration of the study, with or without a protocol-defined exacerbation.
Quality-of-Life Surveys and Protocol Burden
Participants in the EI arm had significantly fewer respiratory symptoms than the UC arm participants as measured by the CFRSD (52-wk mean change in EI arm vs. UC arm, 0.45 vs. 4.56; difference, −4.1; 95% CI, −7.8 to −0.5; P = 0.028). The model-based estimate of the 52-week difference between study arms for slope of change in CFRSD scores was not statistically significant, however (mean, −2.5; 95% CI, −5.7 to 0.6; P = 0.577). Although the EI arm subjects had greater improvement in the Cystic Fibrosis Questionnaire–Revised respiratory domain scores from baseline to Week 52, the difference was not statistically significant (52-wk mean change in EI arm vs. UC arm, −0.44 vs. −3.07; difference, 2.6; 95% CI, −1.8 to 7.1; P = 0.244) (21). Among participants who completed the study, the EI arm scored the protocol as significantly more burdensome (0–10 scale) than the UC arm (mean score in EI arm vs. UC arm, 2.9 vs. 0.6; P < 0.001). Subjects in the EI arm had significantly worse burden scores than patients completing the study (mean burden score, 4.45 among withdrawals vs. 2.92 among completers; P = 0.026).
Discussion
The primary objective of this randomized trial was to determine whether an intervention of home spirometry and home symptom monitoring would lead to earlier detection of CF PEs, which would in turn lead to earlier exacerbation treatment and result in slower decline in FEV1 over 12 months. Importantly, the DMC recommended stopping recruitment because of a projected inability for the study to detect a difference between the treatment arms. Although acute PEs were more frequent in the EI arm, the decline in FEV1 was small in both study arms and was not significantly different. There were trends toward greater improvement in BMI, respiratory symptoms, and quality of life in the EI arm, but these differences did not reach significance. The intervention was associated with an increased burden that may have led to decreased adherence to the intervention.
This is the first large-scale, multicenter, randomized trial of a home monitoring intervention in CF. Earlier studies of home monitoring in CF showed encouraging results, and home monitoring has demonstrated efficacy in patients with other disease states, such as lung transplants (22). A retrospective study was performed in the late 1980s by investigators at the University of Minnesota with 50 individuals with CF (23). Twenty-five participants were selected randomly from a group that had used home monitoring, and they were matched to 25 participants who had not done home monitoring. Over 4 years, FEV1 declined from 73.1% predicted to 70.1% predicted in the home monitoring group (not significant) and from 72.3% predicted to 60.8% (P < 0.001) in the control group. More recently, in an Italian study of telehealth in 16 individuals with CF, investigators reported a significant increase in FEV1 compared with a matched control group (24). Although these were small observational studies, the results suggest that home monitoring was acceptable to patients, did not adversely affect patients, and may have resulted in beneficial health effects. However, these studies were not randomized, and they may have been subject to selection bias. It is likely that individuals who were receptive to home spirometry and followed through with it for prolonged periods of time had characteristics that were different from a selected control group. Factors such as treatment adherence and social support are extremely important in CF and could not be controlled for in the aforementioned studies. Our study differed in that it was a randomized, multicenter clinical trial, which removed selection bias from the use of home monitoring. Integrating devices in the home has been evaluated for other aspects of CF care centered in Australia (25–27). These studies were pilot studies that may fail to be validated in a broader population much like ours. In CF, achieving adherence to medications, much less extra home monitoring, can be very challenging when extended to a broader population (28).
Despite encouraging results of earlier studies in CF, our study did not demonstrate clinical efficacy and was unlikely to do so with continued enrollment (29). There are other factors that may explain the negative results of the present study. Adherence with the EI protocol was lower than desired, with only 19% of participants in the intervention arm transmitting their home data twice weekly 80% of the time or greater. However, adherence with once-weekly data transmission was 50% using the device at least once per week on more than 80% of the follow-up weeks, and a total of 524 alarms among 97 participants (72%) in the EI arm required physician follow-up. Thus, even with less-than-ideal adherence to the intervention, the intervention did lead to clinical encounters and a shorter time to diagnosis of a protocol-defined PE. In addition, a subgroup analysis of only the participants with high adherence failed to show significant results for the primary endpoint (Table E3). Although this was a rigorously conducted clinical trial, the design was meant to approximate actual clinical care. The inclusion criteria were broad, and the treatment of PEs was not protocolized. Even though more exacerbations were detected in the EI arm, treatment of the PEs was more likely to be with oral antibiotics. This could point to potentially diminished efficacy of oral antibiotics to treat PEs. In fact, as noted above, approximately 47% of the subjects in the EI arm failed to recover to within 5% of baseline in terms of FEV1 percent predicted as compared with only 21% in the UC arm. Despite this fact, 34% of patients in the EI arm did not require acute courses of antibiotics during the study, compared with 36% in the UC arm. Our findings suggest that simply detecting exacerbations more frequently is not sufficient to improve outcomes; improving the approach to management may be key to improving outcomes after PEs. Also, on the basis of subgroup analyses, low adherence with the protocol does not appear to explain our negative results.
One explanation for our negative results could be that, once detected, treatment of the acute exacerbation was suboptimal in the EI arm. Of the patients who had an acute visit, 67% were treated with oral antibiotics in the EI arm as compared with 43% in the observation arm at the time of diagnosis of an acute exacerbation. Given that more PEs were treated with intravenous antibiotics and hospitalizations in the UC arm, the oral antibiotics potentially prevented events that would otherwise have led to use of intravenous antibiotics. Currently, optimal care of an acute PE is not known. This includes number of antibiotics, duration of antibiotics, and location of delivery of antibiotics (at home or inpatient) (30). Thus, identifying PEs alone may not be enough; one needs to optimize management of these events.
Our study did have limitations that could influence the interpretation of the results. First, we encountered a number of challenges with the home monitoring equipment that posed a barrier to some participants. Participants were required to connect the AM2 device to a computer via a serial port cable, and many computer hardware and software incompatibilities were encountered during the trial. There were considerable improvements in computer and communications technology during the study period, and future studies of this nature should use devices with more streamlined data transmission capabilities. Novel technologies and electronic monitoring could markedly enhance the intervention by lowering the burden of monitoring. Next, the study threshold for triggering a notification of an exacerbation was selected on the basis of pilot data, but it was meant to be a compromise that would not burden patients with false-positive alarms. The symptom threshold was triggered less frequently than the home spirometry threshold. It is possible that choosing other cutoffs to detect an exacerbation may have led to different results. However, the alarms that were chosen did identify events more often and earlier during the study in the EI arm; the choice of alarm also would lead most clinicians to consider treating a PE (a 10% drop in spirometry and/or a change in baseline symptoms of two or more of the eight respiratory symptoms in the CFRSD). An additional limitation was that we could not easily blind the intervention; such a design feature could have influenced a positive study but is unlikely to have influenced a negative study. Another possible limitation is that the enrolled patients had relatively preserved lung function, with only 38% of the population having an FEV1 less than 75% of predicted; we cannot rule out that the intervention would be efficacious in those with moderate to severe lung disease.
One additional limitation is worth noting. The study was powered to detect a 72-ml difference with 80% power. Our study was stopped early by the DMC on the basis of an unplanned futility analysis. In our final results, we found a mean difference in the slope of FEV1 between the EI arm and the UC arm of 0.00 L (95% CI, −0.07 to 0.07). Thus, we could not exclude a 70-ml improvement with our intervention or a 70-ml decrement in our intervention compared with the UC arm. A number of current therapeutics that are used in the care of patients with CF have impacts on lung function that are only slightly larger than our a priori treatment effect. Oral azithromycin improved lung function by 94 ml (95% CI, 0.023 to 0.165) (31), and inhaled hypertonic saline improved FEV1 by 68 ml (95% CI, 3 to 132 ml), with no change in the primary endpoint: slope of FEV1 decline (32). However, we felt that to integrate such an approach in CF care of home monitoring, which is associated with clear patient and caregiver burden, we would need to demonstrate clear superiority. In addition, the decline in lung function (approximately 3.5% over 52 wk) observed across both arms appeared large but was more likely an artifact of the study design. At baseline, all subjects were required to be clinically stable (an inclusion criterion), whereas at postbaseline, no such requirement was made. Slightly more than half of all subjects (144 of 267) had a postbaseline visit that met the protocol-defined PE criteria. The 52-week change in FEV1 percent predicted in the remaining 123 subjects was −0.8% in the EI arm versus −1.5% in the UC arm (n = 84; difference, 0.7%; 95% CI, −1.9 to 3.3%; P = 0.601).
What can we take away from this large-scale home monitoring trial in CF? Importantly, our study demonstrated that use of a home symptom and spirometry intervention can be effective for detecting more CF exacerbations with a shorter time to first exacerbation. This may have led to minor improvements in symptoms and BMI, though it did not translate to improved pulmonary outcomes. A better understanding of the underlying pathophysiology leading to CF PEs is essential to developing better approaches to prevention and treatment of exacerbations. Though this study was negative, it demonstrated that home monitoring with spirometry and a symptom diary is feasible and effective for detecting more acute exacerbations at an earlier time period than usual care. Further studies are needed to develop a better approach to treating exacerbations once they are detected.
Acknowledgments
Acknowledgment
The authors extend special thanks to the participants with CF and the families of children with CF who participated in the study, whose dedication to this research made the trial possible. In addition to the NHLBI, the authors also thank Bob Beall, Ph.D.; Preston Campbell, M.D.; and the Cystic Fibrosis Foundation for supporting this clinical trial.
Data and Safety Monitoring Board Members: Lynne Quittell, M.D. (Chair), Cystic Fibrosis Center, Columbia University Medical Center; Patrick A. Flume, M.D. (Executive Secretary); Cystic Fibrosis Center at the Medical University of South Carolina; Shannon S. Carson, M.D., Division of Pulmonary and Critical Care Medicine, University of North Carolina; John Conlon, Ph.D., independent statistician, Blue Bell, PA; and Susan Banks-Schlegel, Ph.D., NHLBI, National Institutes of Health.
Therapeutic Development Network Coordinating Center Staff: Kelli Joubran, programmer; Elena Mullin, data coordinator; Kathy Seidel, clinical research associate; and Dionne Howe, study manager.
eICE Study participating sites and site principal investigators: Johns Hopkins University School of Medicine (Baltimore, MD), Noah Lechtzin; University of Alabama at Birmingham (Birmingham, AL), Veena Antony; University of North Carolina at Chapel Hill (Chapel Hill, NC), Scott Donaldson; Northwestern University (Chicago, IL), Susanna McColley; Cincinnati Children’s Hospital (Cincinnati, OH), John Clancy; Case Western Reserve University (Cleveland, OH), Elliott Dasenbrook; Nationwide Children’s Hospital (Columbus, OH), Karen McCoy; University of Colorado (Denver, CO), Jerry Nick; University of Colorado (Denver, CO), Frank Accurso; University of Iowa (Iowa City, IA), Richard Ahrens; University of Minnesota (Minneapolis, MN), Joanne Billings; Stanford University (Palo Alto, CA), Carlos Milla; University of Pittsburgh (Pittsburgh, PA), David Orenstein; Seattle Children’s Hospital (Seattle, WA), Ronald L. Gibson; and University of Washington School of Medicine (Seattle, WA), Christopher H. Goss.
Footnotes
Supported by NHLBI grant R01 HL103965 and Cystic Fibrosis Foundation Therapeutics. C.H.G. was supported by the Cystic Fibrosis Foundation; National Institutes of Health (NIH) grants R01 HL113382, R01 AI101307, UM1HL119073, and P30DK089507; and U.S. Food and Drug Administration grant R01 FD003704. N.L. was supported by the Cystic Fibrosis Foundation and NIH grant R15 HL126122.
A complete list of eICE Study Team members may be found before the beginning of the References.
Author Contributions: N.L. and C.H.G.: inception of the study, study design, supervision of conduct of the study, enrollment, discussion of data, writing and revising of the manuscript, and co–principal investigators on this study; N.M.-H. and U.K.: contributed to study conception and design, data management, and statistical analyses; B.W.R.: contributed to study inception and ongoing advice during the trial; and M.L.A., R.L.G., E.W., N.E.W., M.P.B., P.J.M., S.A., D.O., C.M., J.P.C., and V.A.: contributed to patient recruitment and enrollment at the study site, provided input regarding study modification and ongoing feedback on the protocol, and participated in review and modifications of the final manuscript. All authors participated in data analysis/interpretation, drafting and/or revising of the manuscript for intellectual content, and editing of the manuscript for final approval.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201610-2172OC on June 13, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: Lynne Quittell, Patrick A. Flume, Shannon S. Carson, John Conlon, Susan Banks-Schlegel, Kelli Joubran, Elena Mullin, Kathy Seidel, Dionne Howe, Noah Lechtzin, Veena Antony, Scott Donaldson, Susanna McColley, John Clancy, Elliott Dasenbrook, Karen McCoy, Jerry Nick, Frank Accurso, Richard Ahrens, Joanne Billings, Carlos Milla, David Orenstein, Ronald L. Gibson, and Christopher H. Goss
References
- 1.Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, Elbert A, Petren KM, Marshall BC. The Cystic Fibrosis Foundation patient registry: design and methods of a national observational disease registry. Ann Am Thorac Soc. 2016;13:1173–1179. doi: 10.1513/AnnalsATS.201511-781OC. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation. Bethesda, MD: Cystic Fibrosis Foundation; 2014. Cystic Fibrosis Foundation patient registry: 2013 annual report. [Google Scholar]
- 3.MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, Marshall BC. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med. 2014;161:233–241. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liou TG, Adler FR, Huang D. Use of lung transplantation survival models to refine patient selection in cystic fibrosis. Am J Respir Crit Care Med. 2005;171:1053–1059. doi: 10.1164/rccm.200407-900OC. [DOI] [PubMed] [Google Scholar]
- 6.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 7.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 8.Dobbin CJ, Bartlett D, Melehan K, Grunstein RR, Bye PT. The effect of infective exacerbations on sleep and neurobehavioral function in cystic fibrosis. Am J Respir Crit Care Med. 2005;172:99–104. doi: 10.1164/rccm.200409-1244OC. [DOI] [PubMed] [Google Scholar]
- 9.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkins MD, Rendall JC, Elborn JS. Incidence and risk factors for pulmonary exacerbation treatment failures in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. Chest. 2012;141:485–493. doi: 10.1378/chest.11-0917. [DOI] [PubMed] [Google Scholar]
- 11.Schechter MS, Leonard A, Nash J, Quinton HB, Richards K, Sabadosa K, VandenBranden S. Benchmarking: signature themes. Pediatr Pulmonol. 2006;41:122–123. [Google Scholar]
- 12.Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest. 2003;123:20–27. doi: 10.1378/chest.123.1.20. [DOI] [PubMed] [Google Scholar]
- 13.International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104:1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354:1077–1083. doi: 10.1016/s0140-6736(99)03428-5. [DOI] [PubMed] [Google Scholar]
- 15.Goss CH, Edwards TC, Ramsey BW, Aitken ML, Patrick DL. Patient-reported respiratory symptoms in cystic fibrosis. J Cyst Fibros. 2009;8:245–252. doi: 10.1016/j.jcf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Lechtzin N, West N, Allgood S, Wilhelm E, Khan U, Mayer-Hamblett N, Aitken ML, Ramsey BW, Boyle MP, Mogayzel PJ, Jr, et al. Rationale and design of a randomized trial of home electronic symptom and lung function monitoring to detect cystic fibrosis pulmonary exacerbations: the early intervention in cystic fibrosis exacerbation (eICE) trial. Contemp Clin Trials. 2013;36:460–469. doi: 10.1016/j.cct.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taves DR. Minimization: a new method of assigning patients to treatment and control groups. Clin Pharmacol Ther. 1974;15:443–453. doi: 10.1002/cpt1974155443. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 21.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of the Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128:2347–2354. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 22.Robson KS, West AJ. Improving survival outcomes in lung transplant recipients through early detection of bronchiolitis obliterans: daily home spirometry versus standard pulmonary function testing. Can J Respir Ther. 2014;50:17–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Finkelstein SM, Wielinski CL, Kujawa SJ, Loewenson R, Warwick WJ. The impact of home monitoring and daily diary recording on patient status in cystic fibrosis. Pediatr Pulmonol. 1992;12:3–10. doi: 10.1002/ppul.1950120104. [DOI] [PubMed] [Google Scholar]
- 24.Murgia F, Bianciardi F, Solvoll T, Tagliente I, Bella F, Carestia A, Bella S. Telemedicine home program in patients with cystic fibrosis: results after 10 years. Clin Ter. 2015;166:e384–e388. doi: 10.7417/T.2015.1905. [DOI] [PubMed] [Google Scholar]
- 25.Roehrer E, Cummings E, Beggs S, Turner P, Hauser J, Micallef N, Ellis L, Reid D. Pilot evaluation of web enabled symptom monitoring in cystic fibrosis. Inform Health Soc Care. 2013;38:354–365. doi: 10.3109/17538157.2013.812646. [DOI] [PubMed] [Google Scholar]
- 26.Roehrer E, Cummings E, Turner P, Hauser J, Cameron-Tucker H, Beggs SA, Micallef NA, Wainwright C, Cheney J, Jessup M, et al. Supporting cystic fibrosis with ICT. Stud Health Technol Inform. 2013;183:137–141. [PubMed] [Google Scholar]
- 27.Cummings E, Hauser J, Cameron-Tucker H, Fitzpatrick P, Jessup M, Walters EH, Reid D, Turner P. Enhancing self-efficacy for self-management in people with cystic fibrosis. Stud Health Technol Inform. 2011;169:33–37. [PubMed] [Google Scholar]
- 28.Quittner AL, Zhang J, Marynchenko M, Chopra PA, Signorovitch J, Yushkina Y, Riekert KA. Pulmonary medication adherence and health-care use in cystic fibrosis. Chest. 2014;146:142–151. doi: 10.1378/chest.13-1926. [DOI] [PubMed] [Google Scholar]
- 29.Goss CH. Early intervention in pulmonary exacerbation [abstract] Pediatr Pulmonol. 2011;46:A331. [Google Scholar]
- 30.Flume PA, Mogayzel PJ, Robinson KA, Goss CH, Rosenblatt RL, Kuhn RJ, Marshall BC. Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. Am J Respir Crit Care Med. 2009;180:802–808. doi: 10.1164/rccm.200812-1845PP. [DOI] [PubMed] [Google Scholar]
- 31.Saiman L, Marshall BC, Mayer-Hamblett N, Burns JL, Quittner AL, Cibene DA, Coquillette S, Fieberg AY, Accurso FJ, Campbell PW., III Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–1756. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 32.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]