Abstract
Rationale: Minority drug-resistant Mycobacterium tuberculosis subpopulations can be associated with phenotypic resistance but are poorly detected by Sanger sequencing or commercial molecular diagnostic assays.
Objectives: To determine the role of targeted next-generation sequencing in resolving these minor variant subpopulations.
Methods: We used single molecule overlapping reads (SMOR), a targeted next-generation sequencing approach that dramatically reduces sequencing error, to analyze primary cultured isolates phenotypically resistant to rifampin, fluoroquinolones, or aminoglycosides, but for which Sanger sequencing found no resistance-associated variants (RAVs) within respective resistance-determining regions (study group). Isolates also underwent single-colony selection on antibiotic-containing agar, blinded to sequencing results. As a positive control, isolates with multiple colocalizing chromatogram peaks were also analyzed (control group).
Measurements and Main Results: Among 61 primary culture isolates (25 study group and 36 control group), SMOR described 66 (49%) and 45 (33%) of 135 total heteroresistant RAVs at frequencies less than 5% and less than 1% of the total mycobacterial population, respectively. In the study group, SMOR detected minor resistant variant subpopulations in 80% (n = 20/25) of isolates with no Sanger-identified RAVs (median subpopulation size, 1.0%; interquartile range, 0.2–3.9%). Single-colony selection on drug-containing media corroborated SMOR results for 90% (n = 18/20) of RAV-containing specimens, and the absence of RAVs in 60% (n = 3/5) of isolates. Overall, Sanger sequencing was concordant with SMOR for 77% (n = 53/69) of macroheteroresistant (5–95% total population), but only 5% of microheteroresistant (<5%) subpopulations (n = 3/66) across both groups.
Conclusions: Cryptic minor variant mycobacterial subpopulations exist below the resolving capability of current drug susceptibility testing methodologies, and may explain an important proportion of false-negative resistance determinations.
Keywords: next-generation sequencing, Sanger sequencing, drug-resistant tuberculosis, diagnostics
At a Glance Commentary
Scientific Knowledge on the Subject
Heteroresistance is a potential diagnostic, prognostic, and therapeutic problem for diseases caused by pathogenic bacteria, viruses, and parasites, as well as human malignancies. Microheteroresistant Mycobacterium tuberculosis subpopulations (<5% of the total sampled population) are poorly described by conventional Sanger sequencing or commercial molecular tuberculosis assays, and hitherto uninvestigated using ultradeep sequencing approaches.
What This Study Adds to the Field
Using a targeted next-generation sequencing approach that dramatically reduces sequencing error relative to conventional next-generation sequencing, we describe the presence of resistant variant subpopulations in a majority of phenotypically resistant isolates without Sanger-identified resistance-associated variants, and corroborate our findings via single-colony selection on drug-containing media. Our study provides further evidence for a rich diversity of minor variant subpopulations below the resolving capability of Sanger sequencing. These microheteroresistant M. tuberculosis subpopulations convey phenotypic resistance and may explain an important proportion of false-negative resistance determinations by conventional sequencing and molecular tuberculosis assays.
Multidrug-resistant tuberculosis (MDR TB), defined as resistance to the essential first-line agents isoniazid and rifampin (RIF), affects nearly half a million people each year and is a major obstacle to TB elimination (1). Programmatic surveys (2) and clinical trials (3) suggest that approximately one-half of MDR TB isolates demonstrate resistance to at least one second-line drug, and that acquired resistance to fluoroquinolones (FQ) or second-line injectable (SLI) medicines, the backbone of current standardized regimens, occurs during treatment in up to 15% of patients (4). Although treatment failure and amplified drug resistance impose major human and programmatic costs (5), including ongoing transmission of drug-resistant strains (6, 7), laboratory capacity for the diagnosis of drug-resistant TB in most high-burden settings remains limited.
Although several existing assays detect resistance to first-line drugs (8), the first commercial molecular diagnostic for second-line Mycobacterium tuberculosis drug susceptibility testing (DST) (the reverse line blot hybridization assay, GenoType MTBDRsl version 2; Hain Lifescience, Nehren, Germany) (9, 10) was only recently endorsed by the World Health Organization in May 2016, including for direct testing on sputum smear-positive and smear-negative specimens. Although MTBDRsl and other current molecular assays are transformative advances, suboptimal sensitivity has been commonly ascribed to incomplete characterization of drug resistance loci. Furthermore, no commercial assay is yet available for many components of the MDR TB short-course regimen (e.g., clofazimine, ethionamide, ethambutol, pyrazinamide) (11).
Suboptimal sensitivity of commercial molecular DST assays might also be a function of their limited resolution for detection of heteroresistance (Xpert MTB/RIF, able to detect resistant subpopulations ≥65% of total sampled population [12, 13]; Xpert Ultra, 5–10% [S531L], 20–40% [other rpoB mutations] [14]; pyrosequencing, ≥10% [15]; line probe assay, ≥5–10% [16]). Heteroresistance indicates the coexistence of drug-resistant and drug-susceptible strains, or MDR strains with discrete haplotypes (i.e., combinations of resistance-associated variants (RAVs) at several loci that are transmitted together), and may occur among 5–38% of resistant isolates, depending on antibiogram, population sampling, and detection method (17–23). Genetic heterogeneity at drug resistance loci may indicate mixed infection/reinfection (with strains typically differing by 100s of single-nucleotide polymorphisms) (24, 25); reflect sampling bias (26); and/or characterize the interplay of various subclones responding to ongoing selection pressure (27–30; see Figure 6 in Reference 31). Microheteroresistance may be present at the time of diagnosis in patients with active disease, persist for years, contribute to relapse, and complicate successful treatment (32).
Although Sanger sequencing (33) has traditionally been the reference standard for pathogen RAV verification, it has been replaced over the last 5 years with next-generation sequencing (NGS) technologies. NGS provides rapid, accurate, cost- and process-efficient (34) translation of sequence data into actionable knowledge from clinical specimens (25, 35, 36). However, conventional library preparation processes and the intrinsic error rate have limited the depth of coverage of conventional NGS to hundreds of reads, which are inadequate to resolve rare M. tuberculosis subpopulations. We recently demonstrated a highly sensitive, targeted NGS technique based on complete overlap of forward and reverse paired-end reads from the same DNA molecule (single molecule overlapping reads [SMOR]), providing 10,000–100,000X coverage and able to demonstrate an M. tuberculosis mutant spectrum to a subpopulation resolution of 0.1% (37).
We examined the utility of these advancements in targeted sequencing in resolving isolates phenotypically resistant to RIF, FQs, or SLIs, but without known genotypic correlate (i.e., wild-type) following analysis with Sanger sequencing, therefore hypothesizing that isolates may harbor “microheteroresistant” M. tuberculosis subpopulations below the resolving capability of Sanger sequencing. As a positive control, we analyzed isolates demonstrating Sanger-presumptive heteroresistance according to the presence of multiple colocalizing chromatogram peaks within respective resistance-determining regions (RDRs) submitted during the same time period.
Methods
Specimen Selection
Between 2006 and 2008, in accordance with the National TB Control Program of South Africa, sputum specimens from patients previously treated for TB, failing first-line therapy, or in contact with a patient with drug-resistant TB were submitted to the South African National Health Laboratory Service for DST. RIF-monoresistant (1 μg/ml) isolates grown from sputum and, as previously described (38), isoniazid and RIF-resistant isolates demonstrating additional resistance to ofloxacin (2 μg/ml) and/or amikacin (AMK; 4 μg/ml) on Middlebrook 7H11 (Becton Dickinson, Sparks, MD) underwent genotyping and Sanger sequencing of RDRs (rpoB, gyrA, and rrs, respectively) at Stellenbosch University. Resistant isolates without Sanger sequencing–determined RAVs, or with multiple convergent chromatographic peaks, were selected for our study. Mixed infections were identified by spoligotyping and pncA gene sequencing (38, 39), and were excluded. We excluded mixed strain infection to focus specifically on microevolutionary processes of in situ–derived individual clonal founder strains.
Definitions
We defined heteroresistance as coexisting subpopulations of drug-resistant and drug-susceptible M. tuberculosis organisms, or two or more separate populations of drug-resistant strains within the same patient specimen, detected by conventional Sanger sequencing or targeted deep sequencing, and not in the setting of mixed infection. Microheteroresistance, macroheteroresistance, and full drug resistance were defined as drug-resistant subpopulations less than 5%, 5–95%, and greater than 95% of the total M. tuberculosis population, respectively.
Culturing and DNA Extraction
Decontaminated and liquefied sputum was cultured in the MGIT 960 (Becton Dickinson) system until positive (by acid-fast bacilli smear microscopy and M. tuberculosis speciation), after which DST was done on Middlebrook 7H11 slants (Becton Dickinson). We estimate that at least 1,000 CFUs were plated on the DST control slant following inoculation with 1 ml diluted (1/100) from the MGIT culture; this was then added to 400 μl Tween80 saline solution (0.001% Tween80 and 0.08M NaCl). We then generated a crude DNA lysate (200 μl) by incubation of the cells at 100°C for 30 minutes.
Sanger Sequencing
We sequenced amplification products using an ABI 3130XL genetic analyzer (Applied Biosystems, Foster City, CA), and the resulting chromatograms were analyzed using Chromas software (Technelysium Pty Ltd, South Brisbane, Australia). To identify mutations conferring resistance to RIF, the rifampicin RDR (RRDR) of rpoB (amplification product nucleotides 1,016–1,452) was subjected to DNA sequencing. The presence of more than one nucleotide at a defined sequence position was assigned if the peak height of the underlying nucleotide was greater than or equal to two times the height of the highest background peak. Mutations conferring ofloxacin resistance were determined by DNA sequencing of the quinolone RDR (QRDR) of the gyrA gene and flanking sequences (amplification product codons 18–132), and mutations conferring AMK resistance were determined by DNA sequencing of the region encompassing nucleotides 1,401 and 1,484 of the rrs gene (amplification product nucleotides 1,339–1,528).
Single-Colony Selection on Antibiotic-Containing Agar
Isolates that demonstrated phenotypic resistance in the absence of RAVs detected by Sanger sequencing were subcultured onto Middlebrook 7H10 medium with and without ofloxacin (2 μg/ml), AMK (4 μg/ml), or RIF (1 μg/ml) according to World Health Organization recommendations (40) for 3–4 weeks at 37°C. Thereafter, 8–10 individual CFUs were picked from the drug-containing plate and 1–2 CFUs from the control plate, suspended in 1 ml of enriched 7H9 medium (supplemented with oleic albumin dextrose catalase growth supplement), and incubated at 37°C for 4 days. Thereafter, a 500-μl aliquot was stored at −80°C, whereas the remaining aliquot was heat inactivated at 100°C to generate a crude DNA lysate for Sanger sequencing, as described previously.
Targeted Deep Sequencing
Isolates for which crude M. tuberculosis DNA extracted from the original DST 7H11 agar was available and viable were selected for targeted deep sequencing. DNA specimens were coded, blinded, amplified, and prepared for targeted SMOR sequencing, as described previously (37), with the following modifications. Following the gene-specific multiplex polymerase chain reaction, primer-dimer artifacts were removed using a single 0.8X, Agencourt AMPure XP bead (Beckman Coulter, Brea, CA) cleanup, instead of two sequential bead cleanups, eluting the amplicons in 15 μl of a 10 mM Tris-HCl 0.05% Tween20 solution. The SMOR assay’s gene-specific multiplex polymerase chain reaction contains gene regions critical for detecting mutations associated with the extensively drug resistant phenotype: rpoB to characterize RIF resistance; gyrA to characterize FQ resistance; rrs to characterize AMK resistance; and the eis promoter and rrs to characterize kanamycin resistance (37). All RAVs were covered with 10 or more SMOR reads (i.e., ≥20 standard reads, a pair of reads for each sequenced amplicon molecule), and 58% were covered with 100 or more SMOR reads (≥200 standard reads). Numerous no-template controls were used throughout the preparation process to ensure lack of well-to-well sample or amplicon contamination. DNA from a confirmed pan-susceptible M. tuberculosis H37Rv strain was used as a sequencing error control throughout the SMOR assay, as described previously (36). All sequencing read files were deposited to the Sequence Read Archive under accession numbers SRR5488531-SRR5488590.
Targeted Deep Sequencing Analysis
The previously published SMOR analysis tool (37) was incorporated into the TB Amplicon Sequencing Analysis Pipeline software (35). Briefly, this software automates the process of quantifying the alleles of interest within gene regions of interest, for every overlapping read pair. Paired reads from the same DNA molecule that disagree invariably indicate sequencing error, and were excluded. Therefore, the use of overlapping reads allows for high confidence of low-level subpopulation (>0.1%) detection, well below standard sequencing error rates (37). Targeted sequencing additionally allows for the detection of multiple RDR-associated RAVs within individual amplicons (i.e., haplotype analysis). The Amplicon Sequencing Analysis Pipeline software detects and quantifies the presences of multiple RAV haplotypes among the amplicons to further analyze the nature of heteroresistance within resistant subpopulations.
Results
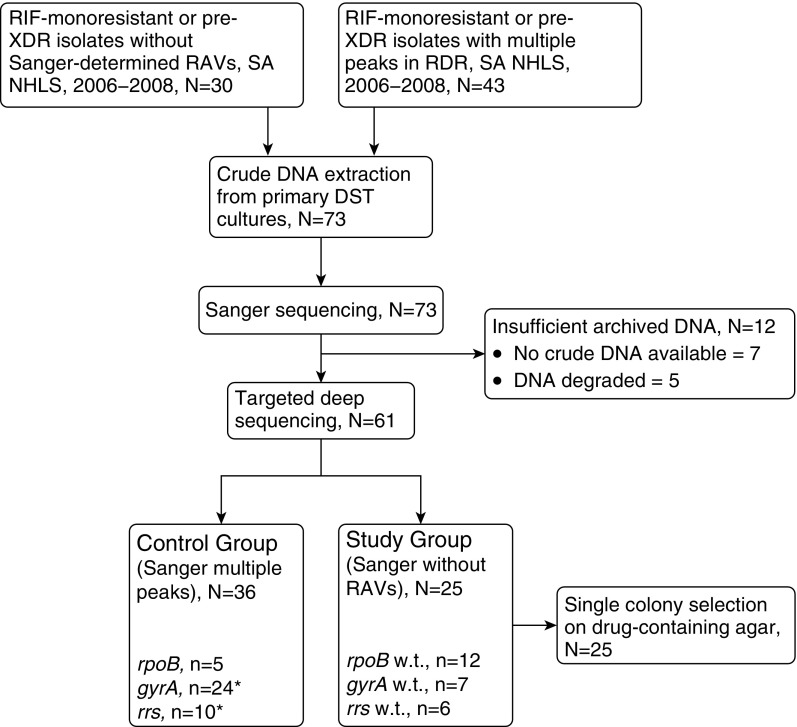
We assessed 73 primary cultured isolates, and analyzed 61 total primary isolates with both Sanger and targeted deep sequencing. These included 25 phenotypically resistant isolates demonstrating absence of RAVs within respective target RDRs (Study Group; RRDR, n = 12; QRDR, n = 7; rrs, n = 6), and 36 phenotypically resistant primary isolate controls in which Sanger sequencing demonstrated colocalizing chromatogram peaks (Control Group; RRDR, n = 5; QRDR, n = 24; rrs, n = 10) (Figure 1). Clinical records from 24 (39%) patients were available and showed that 13 (54%) received or were receiving treatment at the time of sputum collection (Tables 1 and 2).
Figure 1.
Flow diagram of specimen selection and analysis. DST = drug susceptibility testing; RAV = resistance-associated variant; RDR = resistance-determining region; RIF = rifampin; SA NHLS = South African National Health Laboratory Service; w.t. = wild-type; XDR = extensively drug resistant. *Three isolates in the control group were analyzed for both gyrA and rrs.
Table 1.
Targeted Deep Sequencing of Resistance-Determining Regions Wild-Type by Sanger Sequencing in the Setting of Phenotypic Resistance (Study Group)
| Strain Identifier | Sanger | SMOR |
Plating on Drug-Containing Agar Confirms SMOR* | |||
|---|---|---|---|---|---|---|
| Gene/Position | Codon | RAV Population Size per SMOR (%) | Other Heteroresistance Detected within RDR? | |||
| R_1941† | gyrA w.t. | gyrA 94 | GGC | 13 | Micro (multiple) | Yes |
| X_7‡ | gyrA w.t. | gyrA 94 | GGC | 4.2 | Micro (multiple) | Yes |
| X_62† | gyrA w.t. | gyrA 94 | GGC | 14 | Micro (multiple) | Yes |
| X_112‡ | gyrA w.t. | gyrA 90, 94 | GTG, GGC | 1.2; 3.2 | Micro (multiple) | Yes |
| X_139‡ | gyrA w.t. | — | — | None; w.t. | — | Yes |
| X_142‡ | gyrA w.t. | gyrA 94 | GGC | 0.3 | Micro (multiple) | Yes |
| X_173 | gyrA w.t. | — | — | None; w.t. | — | Yes |
| R_3271 | rrs w.t. | rrs 1401, 1484 | G, T | 3.9; 1.2 | No | Yes |
| R_3315 | rrs w.t. | rrs 1401 | G | 5.1 | No | Yes |
| R_3720 | rrs w.t. | rrs 1401 | G | 6.4 | No | Yes |
| X_96 | rrs w.t. | rrs 1401 | G | 99 | No | Yes |
| X_100 | rrs w.t. | rrs 1401 | G | 8.6 | No | Yes |
| X_130 | rrs w.t. | — | — | None; w.t.§ | eis -12T, 100% | No (1401G) |
| R_2362 | rpoB w.t. | rpoB | Unknown (del516_525) | — | Yes | |
| R_3119 | rpoB w.t. | rpoB 533 | CCG | 4.4 | Micro (multiple) | No (del516_531) |
| R_3486 | rpoB w.t. | rpoB 516 | GTC, TAC | 43; 39 | Micro (multiple) | Yes |
| R_4093 | rpoB w.t. | rpoB 531 | TTG | 2.7 | No | Yes |
| R_4271 | rpoB w.t. | rpoB 531 | TTG | 0.1 | No | Yes |
| R_4370 | rpoB w.t. | — | — | None; w.t. | — | No (531TTG) |
| R_4485 | rpoB w.t. | rpoB 526 | TAC | 2.4 | No | Yes |
| R_4927 | rpoB w.t. | rpoB 526 | TAC | 6.5 | No | Yes |
| R_4956 | rpoB w.t. | rpoB 526, 531 | AAC, TTG | 0.1; 0.1 | No | No (w.t.) |
| R_5491 | rpoB w.t. | — | — | None; w.t. | — | Yes |
| R_5603 | rpoB w.t. | rpoB 531 | TTG | 0.2 | No | Yes |
| R_5650 | rpoB w.t. | rpoB 526 | GAC | 0.25 | Micro (multiple) | Yes |
Definition of abbreviations: RAV = resistance-associated variant; RDR = resistance-determining region; SMOR = single molecule overlapping reads; w.t. = wild-type.
Sanger sequencing of single colonies selected following plating on drug-containing agar consistent with largest determined SMOR-resistant subpopulation.
Participant had no history of treatment and was not taking fluoroquinolones at time of sampling.
Participant was on treatment with fluoroquinolones at time of sampling.
Rare minor variants (0.4% 1401G) were detected in X_130 but did not meet criteria for minimum number of reads.
Table 2.
Targeted Deep Sequencing of Resistance-Determining Regions with Colocalizing Chromatogram Peaks by Sanger Sequencing in the Setting of Phenotypic Resistance (Control Group)
| Strain Identifier | Sanger Sequencing |
SMOR |
Prior Treatment | Current Treatment | |||
|---|---|---|---|---|---|---|---|
| Gene/Position | Codon | Gene/Position | Codon | RAV Population Size per SMOR (%) | |||
| R_2335 | gyrA 90 | GTG | gyrA 90 | GTG | 21 | Yes | Yes |
| R_2335 | — | — | gyrA 91 | CCG | 11 | Yes | Yes |
| R_2335 | gyrA 94 | GGC | gyrA 94 | GGC | 17 | Yes | Yes |
| R_2335 | gyrA 94 | TAC | gyrA 94 | TAC | 39 | Yes | Yes |
| R_2335 | gyrA 94 | AAC | gyrA 94 | AAC | 9 | Yes | Yes |
| R_2335 | — | — | gyrA | mult. | <1 | Yes | Yes |
| R_2652 | gyrA 90 | GTG | gyrA 90 | GTG | 74 | No | No |
| R_2652 | — | — | gyrA 94 | mult. | <1 | No | No |
| R_2658 | gyrA 94 | GGC | gyrA 94 | GGC | 24 | Yes | Yes |
| R_2658 | gyrA 94 | GCC | gyrA 94 | GCC | 75 | Yes | Yes |
| R_2934 | gyrA 94 | GGC | gyrA 94 | GGC | 61 | No | No |
| R_2934 | gyrA 94 | GCC | gyrA 94 | GCC | 39 | No | No |
| R_2934 | — | — | gyrA | mult. | <1 | No | No |
| R_3034 | gyrA 94 | AAC | gyrA 94 | AAC | 69 | — | — |
| R_3034 | gyrA 94 | GGC | gyrA 94 | GGC | 31 | — | — |
| R_3034 | — | — | gyrA 94 | TAC | 0.1 | — | — |
| R_3206 | gyrA 90 | GTG | gyrA 90 | GTG | 25 | No | No |
| R_3206 | gyrA 94 | GGC | gyrA 94 | GGC | 63 | No | No |
| R_3206 | gyrA 94 | AAC | gyrA 94 | AAC | 9 | No | No |
| R_3206 | — | — | gyrA | mult. | <1 | No | No |
| R_3428 | gyrA 88 | TGC | gyrA 88 | TGC | 6 | Yes | Yes |
| R_3428 | gyrA 94 | AAC | gyrA 94 | AAC | 91 | Yes | Yes |
| R_3658 | gyrA 90 | GTG | gyrA 90 | GTG | 8 | — | — |
| R_3658 | gyrA 94 | GGC | gyrA 94 | GGC | 91 | — | — |
| R_3658 | — | — | gyrA 94 | AAC | 0.3 | — | — |
| R_3731 | gyrA 94 | AAC | gyrA 94 | AAC | 48 | — | — |
| R_3731 | gyrA 94 | GGC | gyrA 94 | GGC | 21 | — | — |
| R_3731 | gyrA 94 | TAC | gyrA 94 | TAC | 30 | — | — |
| R_3731 | — | — | gyrA 94 | CAC | 0.1 | — | — |
| R_3731 | — | — | gyrA 88 | TGC | 0.4 | — | — |
| R_4007 | gyrA 90 | GTG | gyrA 90 | GTG | 50 | — | — |
| R_4007 | gyrA 94 | GGC | gyrA 94 | GGC | 43 | — | — |
| R_4007 | gyrA 94 | AAC | gyrA 94 | AAC | 5.7 | — | — |
| R_4007 | — | — | gyrA | mult. | <1 | — | — |
| X_1 | gyrA 90 | GTG | gyrA 90 | GTG | 13 | No | No |
| X_1 | — | — | gyrA | mult. | <5 | No | No |
| X_6 | gyrA 90 | GTG | gyrA 90 | GTG | 6 | Yes | Yes |
| X_6 | gyrA 94 | GGC | gyrA 94 | GGC | 32 | Yes | Yes |
| X_6 | gyrA 94 | GCC | gyrA 94 | GCC | 27 | Yes | Yes |
| X_6 | — | — | gyrA 91 | CCG | 20 | Yes | Yes |
| X_6 | — | — | gyrA 94 | TAC | 14 | Yes | Yes |
| X_8 | gyrA 91 | CCG | gyrA 91 | CCG | 44 | No | No |
| X_12 | gyrA 90 | GTG | gyrA 90 | GTG | 55 | — | — |
| X_12 | — | — | gyrA 91 | CCG | 15 | — | — |
| X_14 | gyrA 94 | GGC | gyrA 94 | GGC | 56 | — | — |
| X_14 | gyrA 94 | AAC | gyrA 94 | AAC | 24 | — | — |
| X_14 | — | — | gyrA 94 | TAC | 12 | — | — |
| X_14 | — | — | gyrA | mult. | <5 | — | — |
| X_25 | gyrA 94 | GGC | gyrA 94 | GGC | 77 | Yes | No |
| X_25 | gyrA 94 | AAC | gyrA 94 | AAC | 23 | Yes | No |
| X_25 | — | — | gyrA 94 | TAC | 0.2 | Yes | No |
| X_25 | — | — | gyrA | mult. | <1 | Yes | No |
| X_58 | gyrA 94 | GCC | gyrA 94 | GCC | 2.6 | Yes | Yes |
| X_58 | — | — | gyrA | mult. | <1 | Yes | Yes |
| X_101 | gyrA 94 | GGC | — | — | — | Yes | No |
| X_101 | gyrA 94 | TAC | — | — | — | Yes | No |
| X_101 | — | — | gyrA 91 | CCG | 13 | Yes | No |
| X_116 | gyrA 90 | GTG | gyrA 90 | GTG | 58 | Yes | Yes |
| X_116 | gyrA 94 | GGC | gyrA 94 | GGC | 34 | Yes | Yes |
| X_116 | — | — | gyrA | mult. | <5 | Yes | Yes |
| X_125 | gyrA 94 | GGC | gyrA 94 | GGC | 99.7 | No | No |
| X_126 | gyrA 94 | GGC | gyrA 94 | GGC | 80 | — | — |
| X_126 | — | — | gyrA | mult. | <1 | — | — |
| X_134 | gyrA 94 | GGC | gyrA 94 | GGC | 93 | Yes | No |
| X_134 | gyrA 94 | AAC | gyrA 94 | AAC | 6 | Yes | No |
| X_160 | gyrA 90 | GTG | gyrA 90 | GTG | 90 | — | — |
| X_160 | gyrA 94 | GGC | gyrA 94 | GGC | 10 | — | — |
| X_160 | — | — | gyrA | mult. | <1 | — | — |
| X_184 | gyrA 90 | GTG | gyrA 90 | GTG | 76 | No | No |
| X_184 | gyrA 94 | GGC | gyrA 94 | GGC | 23 | No | No |
| R_2652 | rrs 1401 | G | rrs 1401 | G | 18 | — | — |
| R_2654 | rrs 1401 | G | rrs 1401 | G | 35 | — | — |
| R_2934 | rrs 1401 | G | rrs 1401 | G | 71 | No | No |
| R_3275 | rrs 1401 | G | rrs 1401 | G | 36 | — | — |
| X_4 | rrs 1401 | G | rrs 1401 | G | 100 | — | — |
| X_105 | rrs 1401 | G | rrs 1401 | G | 100 | — | — |
| X_113 | rrs 1401 | G | rrs 1401 | G | 92 | — | — |
| X_124 | rrs 1401 | G | rrs 1401 | G | 98 | No | No |
| X_125 | rrs 1401 | G | rrs 1401 | G | 100 | — | — |
| X_148 | rrs 1401 | G | rrs 1401 | G | 100 | — | — |
| R_2115 | rpoB 531 | TTG | rpoB 531 | TTG | 62 | — | — |
| R_2115 | rpoB 533 | CCG | rpoB 533 | CCG | 32 | — | — |
| R_2115 | — | — | rpoB 516 | GTC | 7 | — | — |
| R_4024 | rpoB 531 | TTG | rpoB 531 | TTG | 4.1 | — | — |
| R_4387 | rpoB 531 | TGG | rpoB 531 | TGG | 63 | — | — |
| R_4387 | rpoB 531 | TTG | rpoB 531 | TTG | 20 | — | — |
| R_4387 | — | — | rpoB 526 | AAC | 0.3 | — | — |
| R_4664 | rpoB 531 | TTG | rpoB 531 | TTG | 3 | — | — |
| R_4664 | — | — | rpoB 533 | CCG | 14 | — | — |
| R_4849 | rpoB 531 | TGG | rpoB 531 | TGG | 16 | — | — |
| R_4849 | — | — | rpoB 531 | TTG | 2.4 | — | — |
Definition of abbreviations: mult. = multiple; RAV = resistance-associated variant; SMOR = single molecule overlapping reads.
Prior treatment refers to ≥30 days of ofloxacin or kanamycin for gyrA- and rrs-associated specimens, respectively.
Mutation Detection within Sanger-Determined Wild-Type Gene Regions (Study Group)
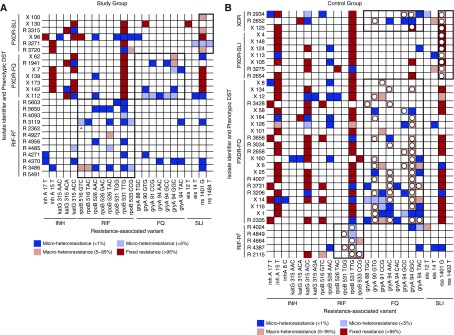
SMOR detected minor resistant variant subpopulations in 80% (n = 20/25) of isolates with no Sanger-identified RAVs, at a median population size of 1.0% (interquartile range, 0.2–3.9%) within respective RDRs. Single-colony selection on drug-containing media corroborated SMOR results for 90% (n = 18/20) of RAV-containing specimens, and absence of RAVs in 60% (n = 3/5) of SMOR-determined wild-type RDRs (Table 1). Relative to phenotypic DST, the sensitivity of SMOR in this group was 80% (95% confidence interval, 59–93%). Additional coexisting microheteroresistant subpopulations were noted within the target regions of half (50%; n = 10/20) of non-wild-type specimens (Figure 2A).
Figure 2.
Distribution of single molecule overlapping reads–determined microheteroresistant subpopulations. The heat maps indicate targeted deep sequencing–determined resistant Mycobacterium tuberculosis populations as follows: dark blue, minor resistant subpopulation, 1% of the total M. tuberculosis population; light blue, minor resistant subpopulation, between 1% and 5% of the total M. tuberculosis population; light red, macroheteroresistant subpopulation, 5–95% of the total M. tuberculosis population; red, fixed resistance mutations, >95% total M. tuberculosis population. (A) Study group. The study group consisted of isolates with phenotypic drug resistance (subheading, y-axis) without Sanger sequencing–determined genotypic resistance within respective resistance-determining regions (black outlined boxes). Note that microheteroresistant subpopulations were often detected within rpoB and gyrA, consistent with phenotypic resistance, despite lack of Sanger-identified resistance-associated variant. (B) Control group. The control group was selected on the basis of multiple chromatographic peaks within resistance-determining regions corresponding to phenotypic drug resistance (subheading, y-axis) for each analyzed drug (black outlined boxes). Open circles indicate resistance-associated variants also detected by Sanger sequencing. *Most reads for sample R_2362 identified the 9-bp deletion del516_525. †According to national tuberculosis control program policy at the time, second-line DST was not performed for the RIF-monoresistant group. DST = drug susceptibility testing; FQ = fluoroquinolone; INH = isoniazid; PXDR = pre–extensively drug resistant (RIF and INH resistance, with additional resistance to either FQ or SLI); RIF = rifampin; RIF-R = rifampin monoresistance; SLI = second-line injectable medication; XDR = extensively drug resistant (RIF and INH resistance, with additional resistance to FQ and SLI).
Mutation Detection within Sanger-Determined Heteroresistant Gene Regions (Control Group)
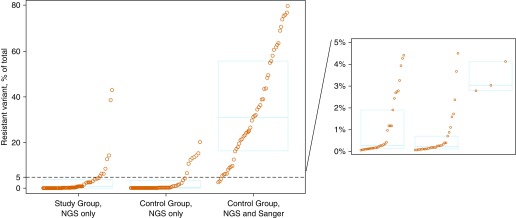
Sanger sequencing identified multiple chromatogram peaks at 64 individual loci (RAVs) among 36 isolates. SMOR determined six isolates (17%) to be unexpectedly fully resistant (most [n = 5/6] within rrs) and two (6%) to be unexpectedly wild-type. The remaining isolates (78%; n = 28/36) were correspondingly heteroresistant by Sanger sequencing and SMOR at 56 individual loci (seven within RRDR, 44 within QRDR, and five within rrs). Subpopulation median size of these 56 heteroresistant RAVs was 33% (interquartile range, 18–62%) of total M. tuberculosis population (Figure 2B), consistent with the known limited resolution of Sanger sequencing. In addition to those RAVs identified by Sanger, SMOR detected 38 additional RAVs across eight loci; subpopulations detected by SMOR only were significantly smaller than those detected by both Sanger and SMOR (P < 0.01) (Figure 3).
Figure 3.
Comparison of Sanger sequencing and single molecule overlapping reads (SMOR) for detection of Mycobacterium tuberculosis heteroresistant subpopulations. Each circle represents 1 of 135 total heteroresistant resistance-associated variants detected by next-generation sequencing (NGS) within the resistance-determining regions of interest, stratified by study group (Sanger-identified wild-type resistance-determining regions) or control group (Sanger-identified multiple colocalizing chromatogram peaks within resistance-determining regions), and whether they were detected by SMOR but not by Sanger (NGS Only), or by both SMOR and Sanger sequencing (NGS and Sanger). The dashed line represents the 5% resistant subpopulation cut point defining microheteroresistance. The blue box plots represent the first quartile, median, and third quartile of the heteroresistant subpopulation distribution in each category. Note that two resistance-associated variants were detected by Sanger sequencing but not by SMOR; these were omitted for clarity.
Spectrum of M. tuberculosis Heteroresistant Variants (Combined Groups)
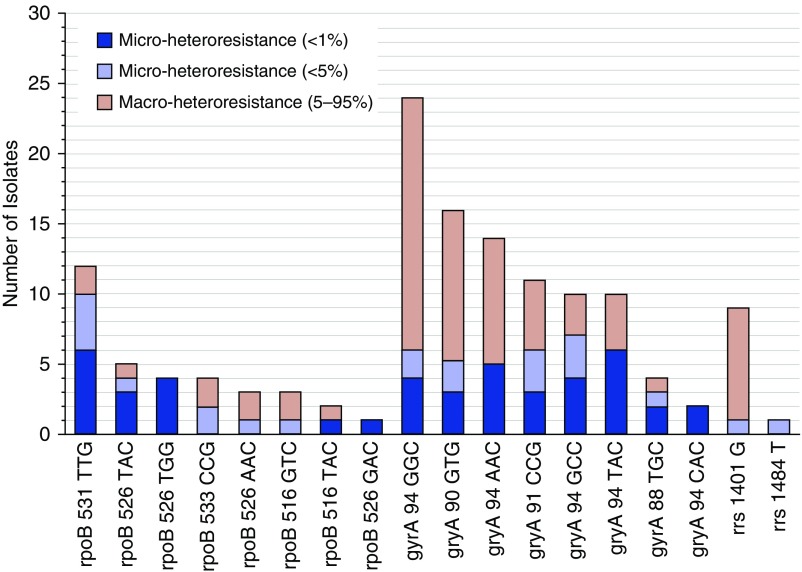
Based on an average sequencing depth ranging from 63,000 to 144,000X, and excluding six fully resistant RAVs, SMOR described 66 (49%) and 45 (33%) of 135 total heteroresistant RAVs within Sanger-analyzed RDRs at frequencies less than 5% and less than 1%, respectively (Figures 2A and 2B). Sanger sequencing was concordant with SMOR for 77% (n = 53/69) of macroheteroresistant (5–95% total population) subpopulations, but only 5% of microheteroresistant (<5%) subpopulations (n = 3/66) across both groups (Figure 3). Individual mutations within each gene exhibited a spectrum of existence as subphenotypic (<1%), microheteroresistant (<5%), or macroheteroresistant variants (Figure 4). Microheteroresistant subpopulations occurred more commonly within rpoB or gyrA than rrs (P = 0.03). The median number of microheteroresistant subpopulations was two per RDR among those with RDR-specific treatment history, and one per RDR among those with no history of treatment (P = 0.6, by Wilcoxon rank sum test). Haplotype mixtures (i.e., multiple RDR-associated RAVS within individual amplicons), however, were most commonly found within gyrA, including up to six separate heteroresistant subpopulations within a single isolate (see Figure E1 in the online supplement).
Figure 4.
Proportional subpopulation size stratified by resistance-associated variant. The frequencies and relative proportions of subphenotypic (dark blue), microheteroresistant (light blue), and macroheteroresistant (light red) subpopulations are presented for each resistance-associated variant analyzed.
Discussion
NGS has an emerging role in rapidly informing treatment of drug-resistant pathogens because of unprecedented resolution, high efficiency, and increasing portability. We analyzed phenotypically RIF-, FQ-, and SLI-resistant isolates without genotypic correlate by Sanger sequencing. Our targeted deep sequencing assay revealed complex microheteroresistant subpopulation structures, often within baseline, pretreatment samples and among patients without prior treatment history, and was validated by growth selection on drug-containing media. These results provide further evidence that discrete bacterial subpopulations may independently contribute to suboptimal sensitivity of commercial molecular TB assays, and that such sensitivity can be improved with greater sequencing depth.
We used a targeted NGS approach combining ultradeep sequencing with validated control of intrinsic sequencing error (35) to uncover a rich diversity of rare variant resistant subpopulations, often coexisting as haplotypes (multiple mutations on a single sequencing read) at presumably subphenotypic (<1%) levels. Our findings were corroborated down to 0.1% of the total sampled population through single-colony analysis following enrichment on drug-containing agar in 90% of cases, and are consistent with M. tuberculosis heteroresistance described in recent reports using deep sequencing (22, 30, 32, 41). As with other pathogenic bacteria (42, 43), malaria (44), HIV (45), and human malignancies (46, 47), M. tuberculosis exhibits dynamic microvariation within genes whose products interface directly with selective pressure, the clinical outcome of which is mediated by host immunity, extent of disease, drug exposure, and fitness cost.
The gradual emergence of drug-resistant M. tuberculosis from very low pretreatment frequencies (∼1 × 10−5) has been known since studies of streptomycin monotherapy in the 1940s (48, 49), a process increasingly appreciated as biologically complex (50). Contemporary genomics studies using minimally invasive autopsies (26), careful characterization of serial within-host diversity (31), and physiologic imaging (32) now collectively describe substantially greater intrahost genetic diversification than previously appreciated, and confirm positive selection as a driving force during periods of ineffective treatment. Our findings complement this literature and underscore a depth of discrete resistant variant subpopulations, an abundant “playing field” for clonal interference. Although appropriately controlled longitudinal studies are required to characterize the fate of individual RAVs, consideration of the genetic heterogeneity we describe vis-a-vis the most common fixed mutations in resistant clinical strains may be instructive. For example, in our study the gyrA 94GAC->TAC (D94Y) mutation occurred commonly, although often as a rare (<1%) variant, and never as a fixed mutation. Although considered a high-confidence mutation in association studies (51), gyrA D94Y is described relatively less often in multinational cohorts of FQ-resistant strains (22, 41, 52), suggesting that fitness cost associated with this mutation (well-demonstrated for gyrA among other human pathogens [53–55], despite long-standing selective pressure) (56) may be difficult to overcome with epistatic mechanisms. In contrast, the gyrA 94GAC->GGC (D94G) mutation, associated with high-level resistance to newer generation FQ (57), was the most prevalent fixed gyrA mutation in our cohort and several others (22, 41, 52); it occurred proportionally far less often as a rare variant. Correspondingly, within rpoB, the most prevalent mutation in our study and globally (531TCG->TTG [S531L]), is known to confer little to no fitness cost (58), and occurred in approximately equal proportions as a subphenotypic and larger variant.
Consistent with prior comparisons with pyrosequencing (35), we found that SMOR was superior to Sanger (or first-generation) sequencing for detection of low-level resistant variants. Sanger sequencing has been used to systematically confirm single-nucleotide polymorphisms calls in whole genome sequencing studies, and as a reference standard for assessments of novel molecular TB assays (59). However, because of off-target wild-type amplification, it has a well-described 10–20% detection threshold for minor components in a mixed sample that relies on subjective interpretation of chromatogram peaks (33, 60). This detection threshold is largely congruent with our findings, where Sanger detected only one in five heteroresistant subpopulations below a 10% threshold. Furthermore, Sanger sequencing is low-throughput and unable to discern the components of M. tuberculosis haplotype mixtures, an aspect of subpopulation complexity that may be clinically relevant given that accumulated mutations are known to increase minimum inhibitory concentration (57).
There are some limitations to our study. First, there is no accepted gold standard for determination of M. tuberculosis biologic variability at subphenotypic levels. Use of completely overlapping reads in SMOR reduces sequencing error by orders of magnitude; has been established in vitro through use of contrived control mixtures (37); and is validated in the current study through demonstration of minor variants following selection on drug-containing media, a process known to increase the sensitivity for detection of low-frequency events by several logs (61). Second, although a strength of our study is that patients were diagnosed and treated under programmatic conditions, increasing relevance, an accompanying consequence is that treatment data are limited, and treatment outcomes along with important comorbidities including HIV coinfection are unavailable. Relatedly, our study revealed some instances of false-negative phenotypic DST reporting (in particular for SLIs), although this is not unusual for ultrahigh-throughput laboratories. Third, because deep whole genome sequencing was not performed in parallel, we cannot comment on other areas of the genome determinative for drug resistance or its adaptation (62, 63). Lastly, microbiology laboratory procedures (e.g., M. tuberculosis expansion in subculture media, drug concentrations, bacterial dilutions) likely influence the spectrum of microheteroresistance detectable by deep sequencing (64), although the relative impact of each factor remains poorly characterized.
The incorporation of novel targeted sequencing technologies into the care of patients with drug-resistant TB will contribute to an evidence base around the clinical impact of microheteroresistance and, most importantly, allow early, comprehensive, and personalized profiling of drug resistance. Accordingly, a user-friendly kit-based version of our assay (35) is currently under development within a tabletop NGS platform for rapid DST in limited resource settings (65). Large, prospective studies with well-characterized drug exposure, comprehensive clinical annotation including surrogates of host immune response, and serial deep sequencing at clinically relevant time points will contribute substantially to characterization of the drivers of M. tuberculosis microheteroresistance and its clinical significance.
Acknowledgments
Acknowledgment
The authors thank M. de Kock and H. Pretorius for their technical assistance.
Footnotes
Supported by the Doris Duke Charitable Foundation (2015094, J.Z.M.), the National Institute of Allergy and Infectious Diseases (ACTG Supplement 111558, J.Z.M.), a National Research Foundation (NRF) Research Career Advancement Award (E.S.), NRF Incentive Funding (R.W. and G.T.), the European & Developing Countries Clinical Trials Partnership 2 program (G.T.), and the South African Medical Research Council (SAMRC) (R.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NRF or the SAMRC.
Author Contributions: Conception or design, J.Z.M., E.S., G.T., R.W., and D.M.E. Acquisition of data, E.S. and R.W. Analysis and interpretation of data, J.Z.M., E.S., C.A., D.L., and D.M.E. Drafting the work, J.Z.M., E.S., and D.M.E. Revising for important intellectual content, G.T., R.E.C., and R.W.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201703-0556OC on June 14, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization. Global Tuberculosis Report. 2016 [accessed 2017 May 1]. Available from: http://www.who.int/tb/publications/global_report/en/
- 2.Dalton T, Cegielski P, Akksilp S, Asencios L, Campos Caoili J, Cho SN, Erokhin VV, Ershova J, Gler MT, Kazennyy BY, et al. Global PETTS Investigators. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380:1406–1417. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sotgiu G, Tiberi S, D’Ambrosio L, Centis R, Alffenaar JW, Caminero JA, Abdo Arbex M, Alarcon Guizado V, Aleksa A, Dore S, et al. International Carbapenem Study Group. Faster for less: the new “shorter” regimen for multidrug-resistant tuberculosis. Eur Respir J. 2016;48:1503–1507. doi: 10.1183/13993003.01249-2016. [DOI] [PubMed] [Google Scholar]
- 4.Cegielski JP, Dalton T, Yagui M, Wattanaamornkiet W, Volchenkov GV, Via LE, Van Der Walt M, Tupasi T, Smith SE, Odendaal R, et al. Global Preserving Effective TB Treatment Study (PETTS) Investigators. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2014;59:1049–1063. doi: 10.1093/cid/ciu572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzon D, Mirzayev F, Wares F, Baena IG, Zignol M, Linh N, Weyer K, Jaramillo E, Floyd K, Raviglione M. Multidrug-resistant tuberculosis around the world: what progress has been made? Eur Respir J. 2015;45:150–160. doi: 10.1183/09031936.00101814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pietersen E, Ignatius E, Streicher EM, Mastrapa B, Padanilam X, Pooran A, Badri M, Lesosky M, van Helden P, Sirgel FA, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383:1230–1239. doi: 10.1016/S0140-6736(13)62675-6. [DOI] [PubMed] [Google Scholar]
- 7.Shah NS, Auld SC, Brust JC, Mathema B, Ismail N, Moodley P, Mlisana K, Allana S, Campbell A, Mthiyane T, et al. Transmission of extensively drug-resistant tuberculosis in South Africa. N Engl J Med. 2017;376:243–253. doi: 10.1056/NEJMoa1604544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalokhe AS, Shafiq M, Lee JC, Ray SM, Wang YF, Metchock B, Anderson AM, Nguyen ML. Multidrug-resistant tuberculosis drug susceptibility and molecular diagnostic testing. Am J Med Sci. 2013;345:143–148. doi: 10.1097/MAJ.0b013e31825d32c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO treatment guidelines for drug-resistant tuberculosis. 2016 Update [accessed 2016 June 8]. Available from: http://www.who.int/tb/MDRTBguidelines2016.pdf?ua=1.
- 10.Theron G, Peter J, Richardson M, Warren R, Dheda K, Steingart KR. GenoType(®) MTBDRsl assay for resistance to second-line anti-tuberculosis drugs. Cochrane Database Syst Rev. 2016;9:CD010705. doi: 10.1002/14651858.CD010705.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowdy DW, Theron G, Tornheim JA, Warren R, Kendall EA. Of testing and treatment: implications of implementing new regimens for multidrug-resistant tuberculosis. Clin Infect Dis. 2017;65:1206–1211. doi: 10.1093/cid/cix486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blakemore R, Story E, Helb D, Kop J, Banada P, Owens MR, Chakravorty S, Jones M, Alland D. Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol. 2010;48:2495–2501. doi: 10.1128/JCM.00128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zetola NM, Shin SS, Tumedi KA, Moeti K, Ncube R, Nicol M, Collman RG, Klausner JD, Modongo C. Mixed Mycobacterium tuberculosis complex infections and false-negative results for rifampin resistance by GeneXpert MTB/RIF are associated with poor clinical outcomes. J Clin Microbiol. 2014;52:2422–2429. doi: 10.1128/JCM.02489-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, Banada PP, Deshpande S, Shenai S, Gall A, et al. The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio. 2017;8:e00812–17. doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SY, Rodwell TC, Victor TC, Rider EC, Pham L, Catanzaro A, Desmond EP. Pyrosequencing for rapid detection of extensively drug-resistant Mycobacterium tuberculosis in clinical isolates and clinical specimens. J Clin Microbiol. 2014;52:475–482. doi: 10.1128/JCM.01821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkvardsen DB, Svensson E, Thomsen VO, Rasmussen EM, Bang D, Werngren J, Hoffner S, Hillemann D, Rigouts L. Can molecular methods detect 1% isoniazid resistance in Mycobacterium tuberculosis? J Clin Microbiol. 2013;51:1596–1599. doi: 10.1128/JCM.00472-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofmann-Thiel S, van Ingen J, Feldmann K, Turaev L, Uzakova GT, Murmusaeva G, van Soolingen D, Hoffmann H. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur Respir J. 2009;33:368–374. doi: 10.1183/09031936.00089808. [DOI] [PubMed] [Google Scholar]
- 18.Nikolayevskyy V, Balabanova Y, Simak T, Malomanova N, Fedorin I, Drobniewski F. Performance of the Genotype MTBDRPlus assay in the diagnosis of tuberculosis and drug resistance in Samara, Russian Federation. BMC Clin Pathol. 2009;9:2. doi: 10.1186/1472-6890-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Zhao B, Liu L, Zhu Y, Zhao Y, Jin Q. Subpopulation analysis of heteroresistance to fluoroquinolone in Mycobacterium tuberculosis isolates from Beijing, China. J Clin Microbiol. 2012;50:1471–1474. doi: 10.1128/JCM.05793-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P, Balooni V, Sharma BK, Kapil V, Sachdeva KS, Singh S. High degree of multi-drug resistance and hetero-resistance in pulmonary TB patients from Punjab state of India. Tuberculosis (Edinb) 2014;94:73–80. doi: 10.1016/j.tube.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Bradley P, Gordon NC, Walker TM, Dunn L, Heys S, Huang B, Earle S, Pankhurst LJ, Anson L, de Cesare M, et al. Rapid antibiotic-resistance predictions from genome sequence data for Staphylococcus aureus and Mycobacterium tuberculosis. Nat Commun. 2015;6:10063. doi: 10.1038/ncomms10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eilertson B, Maruri F, Blackman A, Herrera M, Samuels DC, Sterling TR. High proportion of heteroresistance in gyrA and gyrB in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother. 2014;58:3270–3275. doi: 10.1128/AAC.02066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolani MP, D’souza DT, Mistry NF. Drug resistance mutations and heteroresistance detected using the GenoType MTBDRplus assay and their implication for treatment outcomes in patients from Mumbai, India. BMC Infect Dis. 2012;12:9. doi: 10.1186/1471-2334-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theisen A, Reichel C, Rüsch-Gerdes S, Haas WH, Rockstroh JK, Spengler U, Sauerbruch T. Mixed-strain infection with a drug-sensitive and multidrug-resistant strain of Mycobacterium tuberculosis. Lancet. 1995;345:1512. doi: 10.1016/s0140-6736(95)91073-5. [DOI] [PubMed] [Google Scholar]
- 25.Köser CU, Bryant JM, Becq J, Török ME, Ellington MJ, Marti-Renom MA, Carmichael AJ, Parkhill J, Smith GP, Peacock SJ. Whole-genome sequencing for rapid susceptibility testing of M. tuberculosis. N Engl J Med. 2013;369:290–292. doi: 10.1056/NEJMc1215305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieberman TD, Wilson D, Misra R, Xiong LL, Moodley P, Cohen T, Kishony R. Genomic diversity in autopsy samples reveals within-host dissemination of HIV-associated Mycobacterium tuberculosis. Nat Med. 2016;22:1470–1474. doi: 10.1038/nm.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Post FA, Willcox PA, Mathema B, Steyn LM, Shean K, Ramaswamy SV, Graviss EA, Shashkina E, Kreiswirth BN, Kaplan G. Genetic polymorphism in Mycobacterium tuberculosis isolates from patients with chronic multidrug-resistant tuberculosis. J Infect Dis. 2004;190:99–106. doi: 10.1086/421501. [DOI] [PubMed] [Google Scholar]
- 28.Black PA, de Vos M, Louw GE, van der Merwe RG, Dippenaar A, Streicher EM, Abdallah AM, Sampson SL, Victor TC, Dolby T, et al. Whole genome sequencing reveals genomic heterogeneity and antibiotic purification in Mycobacterium tuberculosis isolates. BMC Genomics. 2015;16:857. doi: 10.1186/s12864-015-2067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun G, Luo T, Yang C, Dong X, Li J, Zhu Y, Zheng H, Tian W, Wang S, Barry CE, III, et al. Dynamic population changes in Mycobacterium tuberculosis during acquisition and fixation of drug resistance in patients. J Infect Dis. 2012;206:1724–1733. doi: 10.1093/infdis/jis601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D, Gomez JE, Chien JY, Haseley N, Desjardins CA, Earl AM, Hsueh PR, Hung DT. Genomic analysis of the evolution of fluoroquinolone resistance in Mycobacterium tuberculosis prior to tuberculosis diagnosis. Antimicrob Agents Chemother. 2016;60:6600–6608. doi: 10.1128/AAC.00664-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trauner A, Liu Q, Via LE, Liu X, Ruan X, Liang L, Shi H, Chen Y, Wang Z, Liang R, et al. The within-host population dynamics of Mycobacterium tuberculosis vary with treatment efficacy. Genome Biol. 2017;18:71. doi: 10.1186/s13059-017-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q, Via LE, Luo T, Liang L, Liu X, Wu S, Shen Q, Wei W, Ruan X, Yuan X, et al. Within patient microevolution of Mycobacterium tuberculosis correlates with heterogeneous responses to treatment. Sci Rep. 2015;5:17507. doi: 10.1038/srep17507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larder BA, Kohli A, Kellam P, Kemp SD, Kronick M, Henfrey RD. Quantitative detection of HIV-1 drug resistance mutations by automated DNA sequencing. Nature. 1993;365:671–673. doi: 10.1038/365671a0. [DOI] [PubMed] [Google Scholar]
- 34.Mardis ER. Next-generation DNA sequencing methods. Annu Rev Genomics Hum Genet. 2008;9:387–402. doi: 10.1146/annurev.genom.9.081307.164359. [DOI] [PubMed] [Google Scholar]
- 35.Colman RE, Anderson J, Lemmer D, Lehmkuhl E, Georghiou SB, Heaton H, Wiggins K, Gillece JD, Schupp JM, Catanzaro DG, et al. Rapid drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates directly from clinical samples by use of amplicon sequencing: a proof-of-concept study. J Clin Microbiol. 2016;54:2058–2067. doi: 10.1128/JCM.00535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witney AA, Gould KA, Arnold A, Coleman D, Delgado R, Dhillon J, Pond MJ, Pope CF, Planche TD, Stoker NG, et al. Clinical application of whole-genome sequencing to inform treatment for multidrug-resistant tuberculosis cases. J Clin Microbiol. 2015;53:1473–1483. doi: 10.1128/JCM.02993-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colman RE, Schupp JM, Hicks ND, Smith DE, Buchhagen JL, Valafar F, Crudu V, Romancenco E, Noroc E, Jackson L, et al. Detection of low-level mixed-population drug resistance in Mycobacterium tuberculosis using high fidelity amplicon sequencing. PLoS One. 2015;10:e0126626. doi: 10.1371/journal.pone.0126626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streicher EM, Bergval I, Dheda K, Böttger EC, Gey van Pittius NC, Bosman M, Coetzee G, Anthony RM, van Helden PD, Victor TC, et al. Mycobacterium tuberculosis population structure determines the outcome of genetics-based second-line drug resistance testing. Antimicrob Agents Chemother. 2012;56:2420–2427. doi: 10.1128/AAC.05905-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Said HM, Kushner N, Omar SV, Dreyer AW, Koornhof H, Erasmus L, Gardee Y, Rukasha I, Shashkina E, Beylis N, et al. A novel molecular strategy for surveillance of multidrug resistant tuberculosis in high burden settings. PLoS One. 2016;11:e0146106. doi: 10.1371/journal.pone.0146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization Global TB Programme. Updated interim critical concentrations for first-line and second-line DST. [accessed 2017 March 1]. Available from: http://www.stoptb.org/wg/gli/assets/documents/Updated%20critical%20concentration%20table_1st%20and%202nd%20line%20drugs.pdf.
- 41.Farhat MR, Sultana R, Iartchouk O, Bozeman S, Galagan J, Sisk P, Stolte C, Nebenzahl-Guimaraes H, Jacobson K, Sloutsky A, et al. Genetic determinants of drug resistance in Mycobacterium tuberculosis and their diagnostic value. Am J Respir Crit Care Med. 2016;194:621–630. doi: 10.1164/rccm.201510-2091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rinder H. Hetero-resistance: an under-recognised confounder in diagnosis and therapy? J Med Microbiol. 2001;50:1018–1020. doi: 10.1099/0022-1317-50-12-1018. [DOI] [PubMed] [Google Scholar]
- 43.Morand B, Mühlemann K. Heteroresistance to penicillin in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 2007;104:14098–14103. doi: 10.1073/pnas.0702377104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Day KP, Artzy-Randrup Y, Tiedje KE, Rougeron V, Chen DS, Rask TS, Rorick MM, Migot-Nabias F, Deloron P, Luty AJF, et al. Evidence of strain structure in Plasmodium falciparum var gene repertoires in children from Gabon, West Africa. Proc Natl Acad Sci USA. 2017;114:E4103–E4111. doi: 10.1073/pnas.1613018114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Casadellà M, Paredes R. Deep sequencing for HIV-1 clinical management. Virus Res. 2017;239:69–81. doi: 10.1016/j.virusres.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Jamal-Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, Shafi S, Johnson DH, Mitter R, Rosenthal R, et al. TRACERx Consortium. Tracking the evolution of non-small-cell lung cancer. N Engl J Med. 2017;376:2109–2121. doi: 10.1056/NEJMoa1616288. [DOI] [PubMed] [Google Scholar]
- 47.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13:e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 48.Pyle MM. Relative numbers of resistant tubercle bacilli in sputa of patients before and during treatment with streptomycin. Proc Staff Meet Mayo Clin. 1947;22:465–473. [PubMed] [Google Scholar]
- 49.Mitchison DA. Development of streptomycin resistant strains of tubercle bacilli in pulmonary tuberculosis; results of simultaneous sensitivity tests in liquid and on solid media. Thorax. 1950;5:144–161. doi: 10.1136/thx.5.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kester JC, Fortune SM. Persisters and beyond: mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Crit Rev Biochem Mol Biol. 2014;49:91–101. doi: 10.3109/10409238.2013.869543. [DOI] [PubMed] [Google Scholar]
- 51.Sandgren A, Strong M, Muthukrishnan P, Weiner BK, Church GM, Murray MB. Tuberculosis drug resistance mutation database. PLoS Med. 2009;6:e2. doi: 10.1371/journal.pmed.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodwell TC, Valafar F, Douglas J, Qian L, Garfein RS, Chawla A, Torres J, Zadorozhny V, Kim MS, Hoshide M, et al. Predicting extensively drug-resistant Mycobacterium tuberculosis phenotypes with genetic mutations. J Clin Microbiol. 2014;52:781–789. doi: 10.1128/JCM.02701-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rozen DE, McGee L, Levin BR, Klugman KP. Fitness costs of fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2007;51:412–416. doi: 10.1128/AAC.01161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han J, Wang Y, Sahin O, Shen Z, Guo B, Shen J, Zhang Q. A fluoroquinolone resistance associated mutation in gyrA Affects DNA supercoiling in Campylobacter jejuni. Front Cell Infect Microbiol. 2012;2:21. doi: 10.3389/fcimb.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunz AN, Begum AA, Wu H, D’Ambrozio JA, Robinson JM, Shafer WM, Bash MC, Jerse AE. Impact of fluoroquinolone resistance mutations on gonococcal fitness and in vivo selection for compensatory mutations. J Infect Dis. 2012;205:1821–1829. doi: 10.1093/infdis/jis277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roumagnac P, Weill FX, Dolecek C, Baker S, Brisse S, Chinh NT, Le TA, Acosta CJ, Farrar J, Dougan G, et al. Evolutionary history of Salmonella typhi. Science. 2006;314:1301–1304. doi: 10.1126/science.1134933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farhat MR, Jacobson KR, Franke MF, Kaur D, Sloutsky A, Mitnick CD, Murray M. Gyrase mutations are associated with variable levels of fluoroquinolone resistance in Mycobacterium tuberculosis. J Clin Microbiol. 2016;54:727–733. doi: 10.1128/JCM.02775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gagneux S, Long CD, Small PM, Van T, Schoolnik GK, Bohannan BJ. The competitive cost of antibiotic resistance in Mycobacterium tuberculosis. Science. 2006;312:1944–1946. doi: 10.1126/science.1124410. [DOI] [PubMed] [Google Scholar]
- 59.Roh SS, Smith LE, Lee JS, Via LE, Barry CE, III, Alland D, Chakravorty S. Comparative evaluation of sloppy molecular beacon and dual-labeled probe melting temperature assays to identify mutations in Mycobacterium tuberculosis resulting in rifampin, fluoroquinolone and aminoglycoside resistance. PLoS One. 2015;10:e0126257. doi: 10.1371/journal.pone.0126257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z, Wang Y, Pang Y, Liu C. Comparison of different drug susceptibility test methods to detect rifampin heteroresistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014;58:5632–5635. doi: 10.1128/AAC.02778-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchison DA. The segregation of streptomycin-resistant variants of Mycobacterium tuberculosis into groups with characteristic levels of resistance. J Gen Microbiol. 1951;5:596–604. doi: 10.1099/00221287-5-3-596. [DOI] [PubMed] [Google Scholar]
- 62.Farhat MR, Shapiro BJ, Kieser KJ, Sultana R, Jacobson KR, Victor TC, Warren RM, Streicher EM, Calver A, Sloutsky A, et al. Genomic analysis identifies targets of convergent positive selection in drug-resistant Mycobacterium tuberculosis. Nat Genet. 2013;45:1183–1189. doi: 10.1038/ng.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang H, Li D, Zhao L, Fleming J, Lin N, Wang T, Liu Z, Li C, Galwey N, Deng J, et al. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat Genet. 2013;45:1255–1260. doi: 10.1038/ng.2735. [DOI] [PubMed] [Google Scholar]
- 64.Metcalfe JZ, Streicher E, Theron G, Colman RE, Penaloza R, Allender C, Lemmer D, Warren R, Engelthaler DM. Mycobacterium tuberculosis subculture results in loss of potentially clinically relevant heteroresistance. Antimicrob Agents Chemother. doi: 10.1128/AAC.00888-17. [online ahead of print] 11 Sept 2017; DOI: 10.1128/AAC.00888-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dolinger DL, Colman RE, Engelthaler DM, Rodwell TC. Next-generation sequencing-based user-friendly platforms for drug-resistant tuberculosis diagnosis: a promise for the near future. Int J Mycobacteriol. 2016;5:S27–S28. doi: 10.1016/j.ijmyco.2016.09.021. [DOI] [PubMed] [Google Scholar]