Abstract
Lassa fever (LF) is increasingly recognized by global health institutions as an important rodent-borne disease with severe impacts on some of West Africa’s poorest communities. However, our knowledge of LF ecology, epidemiology and distribution is limited, which presents barriers to both short-term disease forecasting and prediction of long-term impacts of environmental change on Lassa virus (LASV) zoonotic transmission dynamics. Here, we synthesize current knowledge to show that extrapolations from past research have produced an incomplete picture of the incidence and distribution of LF, with negative consequences for policy planning, medical treatment and management interventions. Although the recent increase in LF case reports is likely due to improved surveillance, recent studies suggest that future socio-ecological changes in West Africa may drive increases in LF burden. Future research should focus on the geographical distribution and disease burden of LF, in order to improve its integration into public policy and disease control strategies.
Keywords: Lassa fever, arenavirus, zoonotic disease, viral haemorrhagic disease, Mastomys natalensis, agriculture, West Africa, One Health
Introduction
Lassa fever is an acute and occasionally severe rodent-borne viral haemorrhagic fever, with cases in humans geographically constrained to sub-Saharan West Africa. Discovered in 1969, Lassa fever (LF) is endemic to much of rural Nigeria and the countries of the Mano River Union (Sierra Leone, Guinea and Liberia; MRU) [1]. International interest in Lassa virus (LASV) has often focused on global health security, since its long incubation period (usually 7–10 days) makes it one of the most commonly exported viral haemorrhagic fevers (VHFs) to countries outside its endemic range [2]. As a result, LASV is now classified as a Select Agent by the US Federal Select Agent Program, requiring Biosafety Level-4 conditions for laboratory study [3]. In contrast, its contribution to the burden of disease in West Africa has historically been under-appreciated, despite suggestions that it causes considerable annual morbidity and mortality in some of Africa’s poorest communities [1,4]. Awareness of LF as a public health issue is increasing, especially following the 2013–2016 Ebola virus disease (EVD) epidemic in the MRU, which has galvanized national and international agencies’ attempts to improve the prediction of and response to disease outbreaks in West Africa. In 2015, the World Health Organisation listed LF among priority diseases requiring urgent research and development attention [5]. In response, LASV was made a priority for vaccine development funding by the multi-agency Coalition for Epidemic Preparedness Innovations (CEPI), alongside several other emerging viruses [6].
Despite growing interest in LF, our knowledge of its ecology, epidemiology and distribution in West Africa is limited. For decades, disease surveillance has piggy-backed on biomedical research projects based in districts where LF is already recognized as a problem. However, previous seroprevalence studies have suggested high numbers of undiagnosed infections in non-endemic areas [7–9] and, in recent years, official incidence reports have seen a substantial increase in the number and geographical extent of cases, which suggests that the true incidence and spatial distribution of the disease may be underestimated (Figure 1). This is a barrier to effective interventions and policy, such as the targeting of future vaccines towards communities at greatest risk, prioritization in health planning and assessments of global health security. In this review, we therefore synthesize current knowledge of the ecology and epidemiology of LF to address several questions with relevance for disease management. Firstly, what are the key knowledge gaps and research priorities with implications for LF control and prevention? Secondly, what is the current understanding of the distribution of LASV in West Africa, and what are the reasons for the recent geographical expansion in LF case reports? Thirdly, how might projected socio-ecological changes impact the incidence and distribution of LF in the coming decades? Much of West Africa is experiencing rapid demographic and environmental shifts that may profoundly affect the transmission dynamics of many important zoonotic diseases [10,11]. Within LF-endemic countries these changes are occurring in societal contexts of high levels of poverty, recent civil conflict, and poor access to healthcare, which increase the complexity of prevention and management of LF and other diseases such as malaria, cholera, yellow fever and EVD [12,13]. There is thus a clear need for better integrated knowledge of the drivers of cross-species LASV transmission, to facilitate disease forecasting and inform interventions that will reduce infection risk in affected communities [10,14].
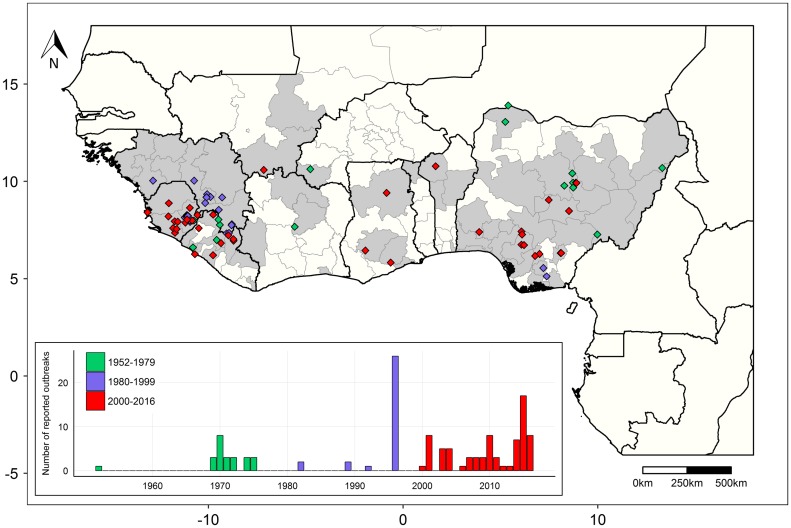
Figure 1.
The known distribution and reported history of Lassa fever and Lassa virus in West Africa.
Points on the map represent human LF outbreak reports inest Africa (both suspected and confirmed) from 1952–2016 with a confirmed geographical locality (n = 102) (colored per time period). Each point represents a separately reported cluster of cases from either a research publication or surveillance report, which may span multiple years of surveillance. Grey shading represents sub-national administrative regions with evidence of LASV or a LASV-like arenavirus in either humans or rodents. Inset shows a bar chart of per-year LF outbreak reports (both with and without a confirmed geographical locality, n = 129), with each denoted by its reported start year. Data and sources are provided in supplementary data.
Disease and diagnosis
In humans LASV causes a wide spectrum of disease manifestations, ranging from asymptomatic infections to acute and severe disease. Onset of acute LF is gradual and nonspecific, often beginning with intermittent fever and malaise followed by myalgia, sore throat, facial oedema and severe headache [15,16]. Recovery begins eight to ten days after onset, while fatal cases progress to shock, organ failure and death, sometimes with haemorrhagic manifestations (see [17] for a recent review of the pathogenesis of LF). However, haemorrhagic signs are often absent, and less common in LF compared to New World arenaviral fevers [15,16,18]. Pregnancy is generally recognized as a risk factor associated with increased LF-related mortality [19,20]. Permanent or temporary sensorineural hearing loss is a common sequela, affecting up to an estimated 25% of all convalescent LF patients [21]. Most LASV infections are mild or subclinical; sampled human populations with high LASV seroprevalence and seroconversion rates often report much lower incidence of LF-like febrile disease, suggesting regular viral exposure but relatively few acute cases [4,7,15,22,23]. The reasons for this variation in pathogenesis are unknown, although proposed mechanisms include variation in LASV strain virulence [7,24,25], and differences in genetic susceptibility to LASV infection between human populations [26]. There is currently no available LF vaccine, and although several candidate vaccines have been developed to various stages, no human trials have occurred to date [27,28]. However, this may change in the near future with the recent prioritisation of LASV by CEPI alongside ongoing research into LF vaccine design [6,29].
One major barrier to LF research is the difficulty of diagnosis based on clinical signs and symptoms alone. Early-stage LF presents similarly to other febrile illnesses (e.g. malaria, typhoid fever, EVD) [15], and LF is often only suspected after development of haemorrhagic symptoms in the late stages of disease. Milder cases in the community therefore often go unrecognized and unreported, with only severely ill people referred for LF testing and treatment [20]. Most health facilities in West Africa have limited access to laboratory testing to confirm diagnosis of LF [30], which requires either viral detection by RT-PCR, antibody or antigen detection by ELISA, or viral isolation (recently reviewed in [31]). The lack of easily-accessible diagnostics for LF has implications for medical treatment since it may delay the commencement of ribavirin therapy, which is most effective when administered during early stage disease [20,32]. Lack of diagnostics has also resulted in under-reporting of LF cases, and a geographical bias in LF detections nearby to established laboratories, such as the dedicated Lassa Fever Ward at Kenema General Hospital, Sierra Leone (KGH) and the Institute of Lassa Fever Research and Control at Irrua Specialist Teaching Hospital, Nigeria [33–35].
The consequence of under-reporting has been to confound understanding of the incidence, geographical distribution and mortality of LF. Annual case estimates are often cited as 100,000–300,000 LASV infections and around 5000 deaths per year across West Africa, but these are extrapolations from one longitudinal study in Sierra Leone in the 1980s [4], and have not been subsequently adjusted for population increases or improvements in diagnostic sensitivity and specificity. Other studies have confirmed that LF is a significant contributor to infectious disease burden in hyper-endemic areas of Nigeria and the MRU [15,23], but its incidence across West Africa remains poorly understood. Under-reporting of milder cases may also be one reason for the marked contrast in reported case fatality rates between community and clinic, which vary from 2% in the community to over 60% in hospital settings [15,16,20]. Despite growing interest in LF from a public health perspective, these knowledge gaps have to date precluded formal quantitative assessments of the total burden of LF and LF-associated sequelae. With diagnostic tests improving, longitudinal studies are needed throughout tropical West Africa to characterise the spectrum of infection severity and assess the disease’s true incidence and burden. This would be an important step towards shifting the global health narrative often associated with LF away from biosecurity [10], and towards a more nuanced understanding of LF as a neglected endemic disease and significant public health concern.
Ecology, epidemiology and transmission of Lassa virus
Reservoir host ecology and epidemiology
Lassa virus is a single-stranded RNA virus (Family: Arenaviridae) which, similarly to most other arenaviruses, is primarily associated with a single rodent reservoir host species. The LASV reservoir host, the Natal multimammate rat Mastomys natalensis [36], is one of the most widespread rodent species in sub-Saharan Africa [37] and its presence is a major spatial predictor of human LF risk [38]. In West Africa, M. natalensis is abundant in rural human homes, surrounding agricultural fields and gardens, occurring at much lower densities in forested and urban areas [39–43]. The high likelihood of human-host contact results in regular spillover and endemic disease in rural areas where LASV is maintained in the rodent population (Figure 2), which is in contrast with many VHFs that occur in sporadic outbreaks such as EVD and yellow fever. LF is primarily a rural disease, with very few cases originating from large urban areas [1]. This may be due to the low abundance of M. natalensis in heavily built-up environments, possibly because of competitive interactions with invasive rodent communities (especially Rattus rattus and Mus musculus) and increased use of concrete for houses and streets in urban areas [42,44].
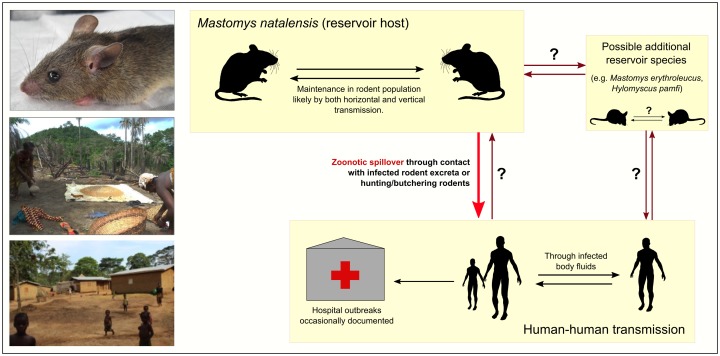
Figure 2.
Transmission dynamics of Lassa virus in rodents and humans.
The primary reservoir host, the Natal multimammate rat (M. natalensis, pictured top left), is a synanthropic species that is widespread throughout rural sub-Saharan West Africa. Other putative rodent reservoir species have been identified, but there is currently little data on LASV prevalence within these populations or routes of transmission to people. It is unknown whether and how often human-to-rodent transmission occurs (photographs © A. Wilkinson).
There has been extensive research into M. natalensis population ecology, motivated by the species’ status as both major agricultural pest and reservoir of several important human diseases, including plague (Yersinia pestis), bartonellosis (Bartonella spp.), leishmaniasis (Leishmania spp.) and leptospirosis (Leptospira spp.) [45–47]. However, the ecology and epidemiology of LASV in M. natalensis is complex and not well understood. Most evidence comes from long-term research projects in Guinea (West Africa), where LASV is endemic, and Tanzania (East Africa), where M. natalensis populations host other arenaviruses including Morogoro virus (MORV) and Gairo virus (GAIV) [40,43,48,49]. The latter two viruses are closely related to LASV but non-pathogenic to humans, and as such are potentially useful model systems for understanding arenavirus ecology in this species. In both regions, M. natalensis shows seasonal population dynamics that are linked to trends in rainfall. Although capable of breeding all year round, the rainy season is associated with higher fecundity and peaks in recruitment, which may be linked to crop maturation and increased food availability, and population crashes often occur in the dry season [39,41]. These dynamics appear to be less strongly pronounced in studies from Guinea, where M. natalensis is found at highest densities inside houses and in proximal cultivated areas [40], than in many trapping studies conducted in Tanzanian agricultural and mosaic landscapes [e.g. 39,50]. Evidence from both laboratory and field studies suggests that LASV, MORV and GAIV are minimally pathogenic in M. natalensis, consistent with long-term coevolution for persistence in strongly fluctuating reservoir host populations [51,52].
The mechanisms of LASV transmission in wild rodent populations are unclear. Several studies have proposed an important role of LASV transmission from mothers to offspring (vertical transmission). For example, Mastomys neonates experimentally infected with LASV or MORV develop chronic infections with persistent viral shedding [51,53]. However, although laboratory studies can shed light on the biological plausibility of transmission mechanisms, their capacity to adequately represent predominant and critical modes of transmission under natural conditions may be more limited [54]. Field research suggests that the main mode of LASV and MORV transmission in wild rodents is between individuals of the same generation (horizontal transmission), leading to short duration infection with transient viral shedding [41]. Evidence for horizontal transmission includes a positive relationship between age and viral antigen prevalence (an indicator of active infection) in juvenile rats [41], and also positive correlations between rodent age and antibody seroprevalence, indicating increasing likelihood of exposure with age [42,52,55]. Horizontal transmission routes are poorly understood, but there is indirect evidence for a role of rodent behavior. For example, seroprevalence data from Tanzania suggest that MORV transmission may be density-independent [43] and that sexual behavior may be involved in GAIV transmission [52].
Overall, the current evidence suggests that most transmission in wild rodents is horizontal, but that a smaller amount of vertical transmission (leading to chronic infections) may contribute to viral persistence in the population [41,43,55]. The highest proportion of live LASV and MORV infections (indicated by presence of viral RNA or antigen) is often found in young animals [41,43], suggesting that the rapid influx of susceptible juveniles during rainy season peaks in recruitment may be a driver of documented seasonal fluctuations in rodent LASV seroprevalence [40,42]. Understanding these dynamics is not merely of ecological interest, since trends in rodent LASV prevalence may drive spatiotemporal variation in the force of zoonotic infection to humans. Uncertainty regarding LASV transmission in rodent populations is a key knowledge gap that could inform, for example, optimal rodent control and LF prevention regimes in endemic areas.
One factor that may limit the usefulness of MORV and GAIV as ecological models for LASV is their seemingly low host plasticity. To date both viruses have only been found in M. natalensis and are non-pathogenic to humans [56]. In contrast, evidence of LASV infections is regularly detected in other rodents, including R. rattus and M. musculus, although these appear to be transient hosts [42]. The picture of LASV ecology has also been complicated by the recent identification of two other putative LASV reservoir hosts, Hylomyscus pamfi and Mastomys erythroleucus [57]. LASV-seropositive M. erythroleucus have been trapped in regions where M. natalensis is absent but seropositivity is documented in humans, suggesting that this species may be involved in zoonotic transmission [57]. This highlights the need for further work to fully understand the spectrum of reservoir and transient hosts, and identify where other species than M. natalensis may be involved in LASV persistence in the reservoir community.
Zoonotic transmission dynamics and human epidemiology
The routes of LASV zoonotic spillover are poorly understood. Similarly to other arenaviruses and hantaviruses, human LASV infections are thought to mostly occur via contact with infected rodent excreta, such as through contaminated food and inhalation of aerosols from dried urine or droppings (Figure 2) [4,58]. Most contact probably occurs in houses, gardens and fields, where rodent densities are highest [59–61]. However, the routes of LASV infection have not been definitively established; for example, to date there has been no testing of environmental samples to identify highest risk rodent-human interfaces (e.g. houses, fields) and to understand the conditions that facilitate persistence of LASV in the external environment. Hunting and butchering of rodents has been shown to be associated with increased risk of LF-like febrile disease [58], although the evidence remains inconclusive, with other studies finding no significant relationship between rodent hunting and butchering and LASV IgG prevalence [22]. The relative risk of infection through butchering may also be affected by preferences for eating particular rodent species. For example, in areas of Sierra Leone, rodents captured from fields, where M. natalensis often occurs at lower densities, are more likely to be butchered and eaten than rodents captured in villages [62]. It is also unknown what modes of contact, such as direct contact with rodent blood or inhalation of urine-contaminated dust particles, are associated with higher risk of virus transmission. Several social factors are also associated with LF risk, including housing quality, evidence of rodent burrows in houses, and high human densities in homes [63].
Like many endemic tropical diseases, LASV spillover dynamics emerge from complex interactions between multiple human and environmental factors [59]. In rodents such factors may include seasonal population and viral transmission dynamics [39,41,42] and behaviour such as consumption of food in people’s homes [40], and in people may include variation in rodent consumption [62], land use practices [59,64] and age and gender-related exposure risk [20,59]. This complexity presents challenges for risk forecasting and planning interventions, since local social-ecological system dynamics may vary across tropical West Africa. There is strong evidence of seasonal patterns in infection risk, with hospitalized cases peaking during the dry season [4,15,20,65], but the drivers of these peaks remain poorly understood. Rainfall is associated with LF risk [66], which may relate to the link between rainfall and M. natalensis recruitment and population density [39]. In Sierra Leone there is evidence that dry season land use practices, such as soil perturbation, burning of fields to prepare for planting, wetland cultivation and rodent pest management, may bring people into closer contact with rodents [59,64,65]. Human-reservoir contact rates may also vary due to seasonal movement of rodents into homes and gardens to seek stored food, but the evidence of seasonal differences in M. natalensis abundance between villages and fields is inconclusive [40,42]. Seasonal and spatial variation in human activities such as rodent butchering and farming may also drive peaks in zoonotic transmission [14,62], but this needs more investigation. There is an urgent need for further combined ecological and social science research into the drivers of LASV spillover dynamics (e.g. [14,59,65]) throughout West Africa. This is required to both improve early-warning LF forecasts, for example by identifying key environmental drivers (e.g. high rainfall and other climatic fluctuations) that are strongly predictive of future outbreaks, and to identify high-risk human-rodent interactions that are suitable for targeted interventions to reduce risk.
Human-to-human LASV transmission can occur via contact with infected bodily fluids, and nosocomial transmission has been documented [67,68]. However, recent research supports the long-held hypothesis that a majority of LF cases are acquired from rodents rather than people. Epidemiological modelling of LF admissions data from KGH showed that around 20% of hospitalized cases were attributable to human-to-human transmission [69], although genomic evidence from LASV isolates from hospital patients in Sierra Leone and Nigeria suggests that human-to-human infections are much rarer [70]. This discrepancy may be related to the apparent role of super-spreaders in driving many of the human-to-human cases in the KGH data [69]. Teasing apart human- from rodent-acquired infections in affected communities is difficult, since in both cases transmission occurs in similar settings (e.g. in the home, on farms). Better knowledge of this gap, such as the factors that increase transmission risk between people and the drivers of super-spreading events, could improve efforts to manage LF in community settings. Interestingly, the long persistence of viruria (3–9 weeks) during the post-recovery phase of human disease also suggests a possible mechanism by which human-to-rodent transmission could contribute to community maintenance of LASV [69], although this has not been investigated.
Distribution and phylogeography of Lassa virus
The geographical distribution of Lassa fever is unusual in that it appears to be non-contiguous, with confirmed LF cases until recently confined almost entirely to areas of Nigeria, Sierra Leone, Guinea and Liberia (Figure 1). The countries in-between have historically been considered non-endemic for LF, despite serological evidence of LASV or a cross-reactive arenavirus in rodents and/or humans in Mali, Cote d’Ivoire, Ghana and Burkina Faso (Figure 1) [1]. However, evidence is growing to support a substantially expanded endemic range of LASV, with human LF outbreaks recently confirmed in southern Mali, Togo, Burkina Faso, Ghana, Benin and Cote d’Ivoire [24,71–73], and serosurveys showing LASV circulation at up to 50% prevalence in M. natalensis populations in southern Mali [74].
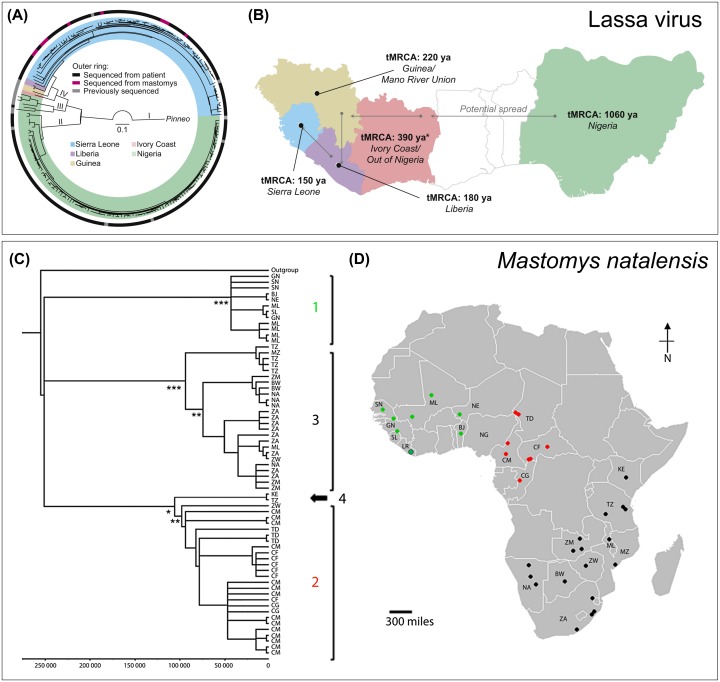
Three distinct LASV lineages currently circulate in Nigeria (lineages I–III) and another (lineage IV) in the MRU area [70] (Figure 3(A)), and a recently proposed lineage V is formed of isolates from Mali and Cote d’Ivoire [73]. Current phylogeographic evidence suggests that this complex of extant LASV strains originated in Nigeria approximately 1060 years ago and spread to neighbouring countries between 300 and 500 years ago, before arriving last in the Mano River Union (MRU) area around 150 years ago [25,70] (Figure 3(B)). Another putative Nigerian strain, related to lineages I, II and III, was recently isolated from proposed reservoir host species H. pamfi, providing further evidence that LASV appears to be more widespread and ecologically complex than previously thought [57].
Figure 3.
Phylogeography of Lassa virus and its reservoir host M. natalensis.
(A) Phylogeny of Lassa virus isolates from humans and rodents, and (B) reconstructed LASV evolutionary history across West Africa. (C) Cytochrome b phylogenetic tree of M. natalensis sampled from across the species’ full range of sub-Saharan Africa, and (D) the geographical distribution of samples. M. natalensis in West Africa form a separate clade from the rest of the continent (clade 1, shown in green). LASV appears to only occur in this western clade. (A–B reproduced from [70], with permission from Elsevier. C–D reproduced from [38], © 2016 The Authors, under CC BY 4.0 license https://creativecommons.org/licenses/by/4.0/).
Knowledge of the true spatial distribution of LASV in rodents across West Africa is confounded by the same problems that impair understanding of LF incidence, i.e. limited access to accurate diagnostics and poor surveillance and monitoring. Surveillance for LASV in rodents has been biased towards known LF-endemic countries, and a lower awareness of LF outside these regions means that past human outbreaks elsewhere may have often gone undiagnosed [75]. Several past risk maps produced for LF (seen in [66] and [76]) probably misrepresent the true LASV distribution, since this geographical reporting bias is often not accounted for during statistical modelling [75]. This problem is compounded by both poor access to diagnostic facilities in more remote areas and the potential for detection errors in serological assays. Multiple arenaviruses have been identified in M. natalensis and other rodents in West and Central Africa, and although LASV assay specificity has improved in recent years, cross-reactivity with other closely-related viruses can lead to false positive detections [55,77,78]. Such uncertainty is likely to apply particularly to older records, for example an arenavirus identified as LASV was reported in several Central African countries in the 1980s [1], but the lack of subsequent detections of LASV or LF outside West Africa suggests these may have been erroneous. False negatives in detection and diagnosis are also possible because of the high genetic diversity among extant LASV strains. LASV is unusually diverse across its range (up to 27% nucleotide and 14.8% amino acid divergence in the nucleoprotein (NP) gene) compared to other VHF agents such as EBOV [25,70,79], prompting recent proposals that LASV might best be defined as a species complex [57].
Other possible reasons for the larger geographical distribution of LASV than of reported human LF may be variations in either the genetic resistance of human populations or the pathogenicity of LASV strains. Such mechanisms might be responsible for the apparent lack of LF in certain regions (such as southern Mali) where substantial rates of human and rodent LASV seroprevalence have been documented [7]. Recent large genomic studies have provided some evidence to support this hypothesis. For example, evidence has been found of positive selection on genes biologically linked to LASV infection in the Yoruba people of Nigeria, suggesting some degree of evolved resistance [26]. An extensive study of LASV isolates from humans and rodents across West Africa reported higher values for viral traits associated with virulence in isolates from Sierra Leone compared to those from Nigeria, which the authors suggested could contribute to the higher case fatality rates recorded in Sierra Leone [70]. The difference in fatality rates could instead be due to socioeconomic (e.g. poverty, nutritional status) or clinical factors [70], but such variation in pathogenicity is plausible given the high genetic diversity of the LASV complex.
Perhaps more difficult to explain, but relevant to disease prevention, are heterogeneities in LASV distribution in rodent populations at both large and local geographical scales. In LF-endemic areas there is large variation in LASV infection prevalence in M. natalensis between neighbouring villages, and sometimes even between houses within a village [42,55]. Population genetic studies suggest that this may be due to the species’ limited dispersal range [56,80,81], but there is also evidence of regular human-mediated movement of LASV and/or infected rodents over longer distances [49,79,82], suggesting that road transport networks have the potential to facilitate virus dispersal to LASV-free areas. However, the current prevalence and genetic diversity of LASV in M. natalensis populations remains unknown for most of West Africa. Future research is needed to address these data gaps and evaluate epidemiological connectivity between rodent subpopulations at both local (within-country) and regional scales, similarly to recent work on fruit bat (Eidolon helvum) hosts of henipaviruses and Lagos bat virus [83].
Another key question concerns the geographical limits of the LASV distribution. Despite M. natalensis occurring throughout sub-Saharan Africa, laboratory-confirmed LF cases have only ever been recorded in West Africa (west of the Nigeria-Cameroon border). Phylogeographic evidence suggests that West African M. natalensis forms a distinct clade from the rest of the continent, with LASV having apparently evolved in this western clade [38,84] (Figure 3(C-D)). It is unknown whether the limited LASV distribution is due to biological factors (e.g. host resistance to infection, coinfection dynamics with other arenaviruses) or geographical barriers to host dispersal (the Sahara Desert to the North and the Cameroonian highlands to the East). In particular, it is unclear whether variation in LASV reservoir host competence exists in different African M. natalensis populations, and how this may affect the distribution of LF. A recent study in Tanzania found strong spatial segregation of MORV and GAIV infection across an apparently geographically contiguous Natal multimammate rat population [56]. This segregation appears to be a result of host-virus coevolution, with the study results showing that MORV and GAIV are each restricted to a separate matrilineal subclade of M. natalensis. These results suggest that genetic variation in host competence between different M. natalensis subclades might restrict LASV to western clade animals [56]; if this were the case, then future range expansions of LASV beyond West Africa would be unlikely. However, as discussed above, LASV shows much greater host plasticity than MORV and GAIV, meaning that this remains an open question. Extensive testing of both human and rodent populations is needed along the eastern boundaries of the known LF distribution (eastern Nigeria and the border with Cameroon), in order to elucidate the role of resistance and coinfection on the spatial distribution of the disease. Unpicking these factors will be key to determining whether projected climate and land use change could drive an eastward range expansion and emergence of LF elsewhere in Africa, and thereby designing appropriate preventative measures.
Forecasting Lassa fever risk and responses to environmental change
Trends in LF during this decade have followed a pattern typical of an emerging pathogen, with a notable increase in suspected and confirmed LF outbreaks across a growing area of West Africa (Figure 1) [20,71,72,85]. Sources of case reports have also shifted from mainly research publications to disease surveillance reports (see supplementary data). Rather than a phase shift in LASV ecology, this apparent trend of emergence is most likely a function of increasing surveillance for LF and other VHFs in West Africa under both public health and biosecurity agendas. For example, the accuracy of LASV diagnostic tools and accessibility of suitable laboratory facilities across West Africa have improved over the last decade [10,20]. There is also increasing political will and institutional funding for VHF surveillance in Africa, with the roll-out of disease surveillance policy frameworks such as the WHO International Health Regulations (IHR2005) and Integrated Disease Surveillance and Response (IDSR), and especially following the severity and slow global response to the 2013–2016 EVD epidemic in West Africa [86]. As a result, a fuller picture of the endemic distribution of LF and LASV has started to emerge.
Although the current broadening geographical range of reported LF is unlikely to be a true range expansion, regional environmental changes may impact LF ecology and epidemiology in several ways (Figure 4). West Africa has experienced profound environmental, socioeconomic and demographic shifts in recent decades, including population growth, land use change (including deforestation, mining and commercial agricultural expansion), bushmeat extraction, urbanization and growing transport connectivity [10,87]. The region is also forecast to experience strong climate change effects, impacting crop yields alongside other important ecosystem services [88]. Such anthropogenic processes are thought to be major drivers of zoonotic and vector-borne disease [89]. Ecological niche modelling has identified key environmental variables correlated with LF outbreaks, in particular rainfall, human population density and rice yields [38,66]. Future climate projections for West Africa involve warmer temperatures and increased rainfall, which are predicted to increase the climatic suitability for M. natalensis across much of the region [38]. Most land use forecasts predict extensive cultivated land expansion, both for subsistence and commercial export agriculture (e.g. oil palm) [10,90]. This could drive increases in human LASV exposure by expanding suitable habitat for M. natalensis, facilitating the spread of LASV between geographically separate rodent populations, or driving shifts in ecological community composition towards higher densities of generalist small mammals (including Mastomys) in more intensively managed land (dilution effects) [91,92]. Such land use homogenization has been hypothesized as a driver of EBOV emergence in West Africa [11]. Alternatively, it has been proposed that expansion of mechanized commercial agriculture might reduce M. natalensis populations [93], thereby decreasing LASV exposure, but at the cost of reducing community access to land for subsistence farming [94]. A useful but challenging future step with policy relevance would be to evaluate the trade-offs associated with different land use futures, incorporating zoonotic disease burden alongside other ecosystem services with poverty, human health and conservation relevance [95].
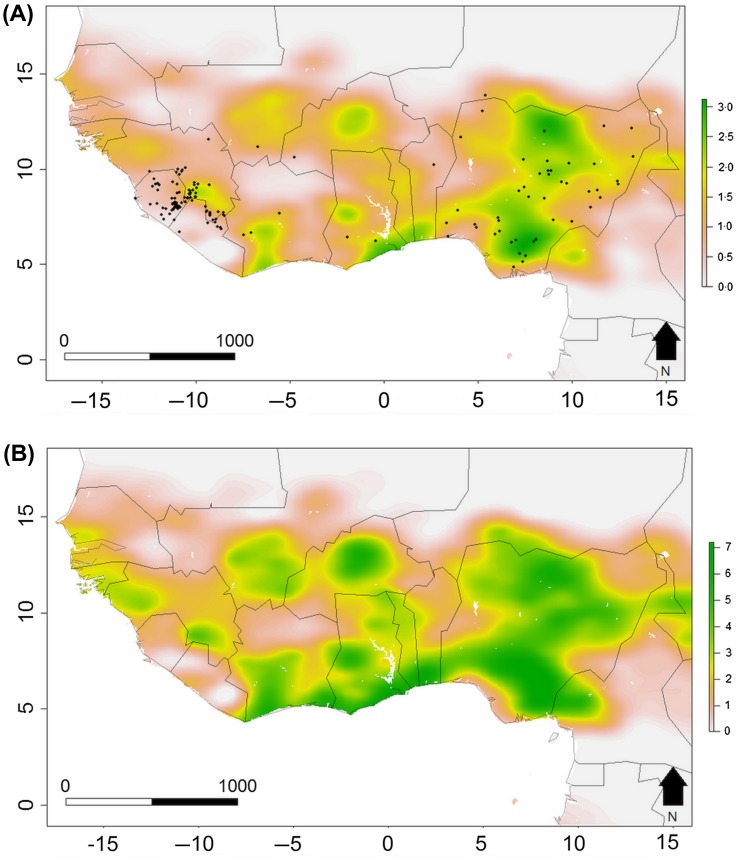
Figure 4.
Present and predicted future distribution of Lassa fever risk in West Africa, estimated from environmental-mechanistic models.
Colour scales show predicted annual LASV spillover events per grid cell for (A) present day and (B) 2070 under a projected scenario of land use change, human population growth and climate change (RCP 6.5). Annual LASV spillover events are predicted using a spatially-explicit epidemiological model parameterized with M. natalensis distribution and human population data, reported in [38]. (Figure reproduced from [38], © 2016 The Authors, under CC BY 4.0 license https://creativecommons.org/licenses/by/4.0/).
The ability to predict the effects of these environmental drivers depends on unpicking how different anthropogenic processes impact viral transmission. Prospective field-based studies into the ecology and epidemiology of LASV in human-dominated ecosystems, including longitudinal monitoring of rodents and humans over months and years, are required to elucidate the spatial and seasonal dynamics of disease with less detection bias. The environmental and behavioral routes of rodent-to-human LASV transmission can be identified through community-based studies. Ecological and epidemiological modelling is also required to evaluate the impacts of different drivers on transmission, inform disease management and enable forecasting. For example, LF was recently used as a case study system for novel, One Health-informed modelling approaches [14,59]. A recent study developed a theoretical framework, based on a generalization of Poisson processes, to jointly model zoonotic spillover and onward human-to-human transmission, in order to evaluate the effects of biological, ecological and social parameters on transmission outcomes [65]. Modelling Kenema General Hospital LF data as a case study, their results again suggested that seasonal variation in infection risk may underpin the observed distribution of hospitalized cases. Another recent analysis developed a large-scale, spatially-explicit compartmental model to evaluate the impacts of future climate, land-use and socioeconomic scenarios on human LASV infections (Figure 4(A)) [38]. Their results suggested that climate change and population growth may lead to a doubling of LASV infections by 2070 (Figure 4(B)). Such studies make many simplifying assumptions, and given the gaps in knowledge of the present-day LASV ecology and distribution, any resulting predictions involve large amounts of uncertainty. Nonetheless, the advantage of such process-based frameworks is in enabling diverse information to be incorporated into model parameters, including both biological (e.g. host abundance, immunity, infection prevalence) and socio-ecological factors (e.g. land use practices, rodent consumption, age-related variation in exposure risk, human movement) [96–98]. This creates the potential to move away from small-scale and correlative risk mapping towards a predictive system dynamics approach that considers zoonotic disease dynamics as a function of multiple interacting environmental and social drivers.
Recent multidisciplinary case studies of LF in Sierra Leone have emphasized that such integrated knowledge can inform interventions to reduce exposure to the viral reservoir, such as land management practices, food storage and rodent control [10,59]. LF is potentially a good model system for operationalizing and assessing the outcomes of such a One Health-informed approach, since it is tractable to study; its single primary host species and high prevalence in rodent populations make ongoing monitoring more practically viable than for most other VHFs. There is potential for insights into LF management to be applied to ecologically similar rodent-borne viruses such as New World arenaviruses and hantaviruses [99,100]. However, as a mainly single host system, any insights into LF may be less relevant to more complex disease systems with multiple reservoir, amplifying host or vector species which each respond differently to environmental drivers (e.g. yellow fever, EBOV, chikungunya) [101]. Since LASV is much less transmissible between people than viruses such as EBOV, it may also be less useful as an epidemiological model for epidemic VHFs, where single spillover events can lead to extended chains of human–human transmission.
Summary and outstanding questions
In this review we have shown that past biases in LF research have produced an incomplete picture of the disease’s true incidence and distribution in West Africa, with consequences for medical treatment and disease management. We therefore close by emphasizing several critical knowledge gaps that must be filled to improve LF prevention and forecasting. These are: (1) Establishing the true spatial and seasonal distribution of LASV in rodent populations across West Africa, and how these correlate with observed spatiotemporal trends in human LF infections (both acute and asymptomatic). This will require longitudinal studies of LASV seroprevalence in people and rodents throughout the predicted geographic range of the virus, alongside improved disease monitoring of at-risk human populations inside and outside the known LF-endemic range. (2) Unpicking the mechanisms of zoonotic LASV transmission, including behaviors and local practices (e.g. housing, land use, rodent consumption) that enhance infection risk. (3) Identifying geographical variation and genetic correlates of LASV strain virulence and human and rodent susceptibility to LASV infection. Are certain virus lineages more pathogenic than others, and does innate resistance to infection vary across human and rodent subpopulations in West Africa, and how do these affect understanding of who is most at risk? (4) Identifying the barriers (biological, geographical and/or ecological) that limit LASV to M. natalensis populations in West and Central Africa.
The growing attention on LF by global health institutions, such as its recent prioritization for vaccine development, is a positive trend, especially given the consistent impacts of the disease on many districts that were also severely affected by the 2013–2016 Ebola epidemic. Nonetheless, a continued shift in institutional understanding is required towards treating LF as a high-burden neglected disease rather than an epidemic threat. The situation should be improved by ongoing improvements in diagnostics and surveillance, which may provide the data required for a more robust assessment of the true burden of LF on communities in West Africa. Simultaneously, further unpicking the complex ecological drivers of LASV dynamics will enable improved forecasting and targeted medical and environmental interventions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Natural Environment Research Council (NERC) [grant number NE-J001570-1].
Supplemental data
The data-set and sources for spatial distribution and history of Lassa fever and Lassa virus in West Africa (used to produce Figure 1) are hosted on Figshare (http://figshare.com/s/0ac8547b7147e400c140).
Supplementary Material
Acknowledgements
The authors thank James Koninga and Donald Grant (Kenema General Hospital), Alie Kamara, Alhaji Brima Gogra and Morrison Lahai (Njala University), Kelsey Confreda (Tulane University), Melissa Leach (University of Sussex), and Duke Rogers (Brigham Young University) for discussion and comments on the previous versions of the manuscript.
References
- 1.Richmond JK, Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ [Internet]. 2003;327(7426):1271–1275. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC286250/. 10.1136/bmj.327.7426.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brosh-Nissimov T. Lassa fever: another threat from West Africa. Disaster Mil Med [Internet]. 2016;2(1):1087 Available from: https://disastermilitarymedicine.biomedcentral.com/articles/10.1186/s40696-016-0018-3 10.1186/s40696-016-0018-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services 2015 Annual report of the federal select agent program [Internet]. 2016. Available from: https://www.selectagents.gov/annualreport2015.html [Google Scholar]
- 4.McCormick JB, Webb PA, Krebs JW, et al. . A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis. 1987;155(3):437–444. 10.1093/infdis/155.3.437 [DOI] [PubMed] [Google Scholar]
- 5.WHO World Health Organization: list of blueprint priority diseases (revised January 2017) [Internet]. 2017. [cited [cited 2017 Aug 10]]. Available from: https://www.who.int/blueprint/priority-diseases/en/ [Google Scholar]
- 6.Rottingen J, Gouglas D, Feinberg M, et al. . New vaccines against epidemic infectious diseases. New Engl J Med. 2017;376:1–3. [DOI] [PubMed] [Google Scholar]
- 7.Sogoba N, Rosenke K, Adjemian J, et al. . Lassa virus seroprevalence in Sibirilia Commune, Bougouni district, Southern Mali. Emerg Infect Dis. 2016;22(4):657–663. 10.3201/eid2204.151814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmerich P, Thome-Bolduan C, Drosten C, et al. . Reverse ELISA for IgG and IgM antibodies to detect Lassa virus infections in Africa. J Clin Virol. 2006;37(4):277–281. 10.1016/j.jcv.2006.08.015 [DOI] [PubMed] [Google Scholar]
- 9.Akoua-Koffi C, Ter Meulen J, Legros D, et al. . Detection of anti-Lassa antibodies in the Western Forest area of the Ivory Coast. Med Trop. 2006;66(5):465–468. [PubMed] [Google Scholar]
- 10.Wilkinson A. Beyond biosecurity: the politics of Lassa fever in Sierra Leone In: Bardosh K, editor. One health: science, politics and zoonotic disease in Africa. London (NY): Routledge; 2016. p. 117–138. [Google Scholar]
- 11.Wallace RG, Gilbert M, Wallace R, et al. . Did Ebola emerge in West Africa by a policy-driven phase change in agroecology? Environ Plan A. 2014;46(11):2533–2542. 10.1068/a4712com [DOI] [Google Scholar]
- 12.Bardosh K, Leach M, Galaz V. The limits of rapid response: Ebola and structural violence in West Africa In: Bardosh K, editor. One health: science, politics and zoonotic disease in Africa. London (NY): Routledge; 2016. p. 74–94. [Google Scholar]
- 13.Kelly JD, Barrie MB, Ross RA, et al. . Housing equity for health equity: a rights-based approach to the control of Lassa fever in post-war Sierra Leone. BMC Int Health Hum Rights [Internet]. 2013;13(2):1–6. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3562201&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scoones I, Jones KE, Lo Iacono G, et al. . Integrative modelling for one health: pattern, process and participation. Philos Trans R Soc B. 2017;372:20160164. 10.1098/rstb.2016.0164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bausch DG, Demby AH, Coulibaly M, et al. . Lassa Fever in Guinea: I. Epidemiology of human disease and clinical observations. Vector Borne Zoonotic Dis. 2001;1(4):269–281. 10.1089/15303660160025903 [DOI] [PubMed] [Google Scholar]
- 16.McCormick JB, King IJ, Webb PA, et al. . A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis [Internet]. 1987;155(3):445–455. DOI: 10.1093/infdis/155.3.445 [DOI] [PubMed] [Google Scholar]
- 17.Yun NE, Walker DH. Pathogenesis of Lassa fever. Viruses. 2012;4(10):2031–2048. 10.3390/v4102031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paessler S, Walker DH. Pathogenesis of the viral hemorrhagic fevers. Annu Rev Pathol Mech Dis [Internet]. 2013;8(1):411–440. Available from: http://www.annualreviews.org/doi/abs/10.1146/annurev-pathol-020712-164041. 10.1146/annurev-pathol-020712-164041 [DOI] [PubMed] [Google Scholar]
- 19.Price ME, Fisher-Hoch SP, Craven RB, et al. . A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. BMJ [Internet]. 1988;297(6648):584–587. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1834487. 10.1136/bmj.297.6648.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaffer JG, Grant DS, Schieffelin JS, et al. . Lassa fever in post-conflict Sierra Leone. PLoS Negl Trop Dis. 2014;8(3):e2748. 10.1371/journal.pntd.0002748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummins D, McCormick JB, Bennett D. Acute sensorineural deafness in Lassa fever. JAMA. 1990;264(16):2093–2096. 10.1001/jama.1990.03450160063030 [DOI] [PubMed] [Google Scholar]
- 22.Kernéis S, Koivogui L, Magassouba N, et al. . Prevalence and risk factors of Lassa seropositivity in inhabitants of the forest region of Guinea: a cross-sectional study. PLoS Negl Trop Dis. 2009;3(11):e548. 10.1371/journal.pntd.0000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Hearn AE, Voorhees MA, Fetterer DP, et al. . Serosurveillance of viral pathogens circulating in West Africa. Virol J [Internet]. 2016;13:163.. Available from: https://virologyj.biomedcentral.com/articles/10.1186/s12985-016-0621-4%5Cnhttp://virologyj.biomedcentral.com/articles/10.1186/s12985-016-0621-4. 10.1186/s12985-016-0621-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sogoba N, Feldmann H, Safronetz D.. Lassa fever in West Africa: evidence for an expanded region of endemicity. Zoonoses Public Health. 2012;59(Suppl.2):43–47. 10.1111/zph.2012.59.issue-s2 [DOI] [PubMed] [Google Scholar]
- 25.Bowen MD, Rollin PE, Ksiazek TG, et al. . Genetic diversity among Lassa virus strains. J Virol. 2000;74(15):6992–7004. 10.1128/JVI.74.15.6992-7004.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen KG, Shylakhter I, Tabrizi S, et al. . Genome-wide scans provide evidence for positive selection of genes implicated in Lassa fever. Philos Trans R Soc Lond B Biol Sci [Internet]. 2012;367(1590):868–877. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22312054. 10.1098/rstb.2011.0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zapata JC, Goicochea M, Nadai Y, et al. . Genetic variation in vitro and in vivo of an attenuated Lassa vaccine candidate. J Virol [Internet]. 2014;88(6):3058–3066. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3957910. 10.1128/JVI.03035-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grant-Klein RJ, Altamura LA, Schmaljohn CS. Progress in recombinant DNA-derived vaccines for Lassa virus and filoviruses. Virus Res [Internet]. 2011;162(1–2):148–161. Available from: https://doi.org/10.1016/j.virusres.2011.09.005 10.1016/j.virusres.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 29.Hastie KM, Zandonatti MA, Kleinfelter LM, et al. . Structural basis for antibody-mediated neutralization of Lassa virus. Science (80-). 2017;356(6341):923–928. 10.1126/science.aam7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder LF, Amukele T. Medical laboratories in sub-Saharan Africa that meet international quality standards. Am J Clin Pathol. 2014;141(6):791–795. 10.1309/AJCPQ5KTKAGSSCFN [DOI] [PubMed] [Google Scholar]
- 31.Raabe V, Koehler J.. Laboratory diagnosis of Lassa fever. J Clin Microbiol. 2017;(April):1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormick JB, King IJ, Webb PA, et al. . Lassa fever: effective therapy with ribavirin. N Engl J Med. 1986;314(1):20–26. 10.1056/NEJM198601023140104 [DOI] [PubMed] [Google Scholar]
- 33.Khan SH, Goba A, Chu M, et al. . New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antiviral Res. 2008;78(1):103–115. 10.1016/j.antiviral.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 34.Gire SK, Stremlau M, Andersen KG, et al. . Emerging disease or diagnosis? Science [Internet]. 2012;338(6108):750–752. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23139320. [DOI] [PubMed] [Google Scholar]
- 35.Asogun DA, Adomeh DI, Ehimuan J, et al. . Molecular diagnostics for Lassa fever at Irrua specialist teaching hospital, Nigeria: lessons learnt from two years of laboratory operation. PLoS Negl Trop Dis. 2012;6(9):e1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monath TP, Newhouse VF, Kemp GE, et al. . Lassa virus isolation from Mastomys natalensis rodents during an epidemic in Sierra Leone. Science (80-) [Internet]. 1974;185(4147):263–265. Available from: https://www.sciencemag.org/cgi/doi/10.1126/science.185.4147.263 10.1126/science.185.4147.263 [DOI] [PubMed] [Google Scholar]
- 37.Granjon L. Mastomys natalensis (errata version published in 2017). IUCN Red List Threat Species. 2016;e.T12868A115107375. [Google Scholar]
- 38.Redding DW, Moses LM, Cunningham AA, et al. . Environmental-mechanistic modelling of the impact of global change on human zoonotic disease emergence: a case study of Lassa fever. Methods Ecol Evol. 2016;7(6):646–655. 10.1111/2041-210X.12549 [DOI] [Google Scholar]
- 39.Makundi RH, Massawe AW, Mulungu LS. Reproduction and population dynamics of Mastomys natalensis Smith, 1834 in an agricultural landscape in the Western Usambara Mountains, Tanzania. Integr Zool. 2007;2(4):233–238. 10.1111/inz.2007.2.issue-4 [DOI] [PubMed] [Google Scholar]
- 40.Fichet-Calvet E, Lecompte E, Koivogui L, et al. . Fluctuation of abundance and Lassa virus prevalence in Mastomys natalensis in Guinea, West Africa. Vector-Borne Zoonotic Dis [Internet]. 2007;7(2):119–128. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17627428. 10.1089/vbz.2006.0520 [DOI] [PubMed] [Google Scholar]
- 41.Fichet-Calvet E, LeCompte E, Koivogui L, et al. . Reproductive characteristics of Mastomys natalensis and Lassa virus prevalence in Guinea, West Africa. Vector-Borne Zoonotic Dis [Internet]. 2008;8(1):41–48. Available from: http://www.liebertonline.com/doi/abs/10.1089/vbz.2007.0118. 10.1089/vbz.2007.0118 [DOI] [PubMed] [Google Scholar]
- 42.Demby AH, Inapogui A, Kargbo K, et al. . Lassa fever in Guinea II: distribution and prevalence of Lassa virus infection in small mammals. Vector Borne Zoonotic Dis. 2001;1(4):283–297. 10.1089/15303660160025912 [DOI] [PubMed] [Google Scholar]
- 43.Borremans B, Leirs H, Gryseels S, et al. . Presence of Mopeia virus, an African arenavirus, related to biotope and individual rodent host characteristics: implications for virus transmission. Vector-Borne Zoonotic Dis [Internet]. 2011;11(8):1125–1131. Available from: http://www.liebertonline.com/doi/abs/10.1089/vbz.2010.0010. 10.1089/vbz.2010.0010 [DOI] [PubMed] [Google Scholar]
- 44.Garba M, Dalecky A, Kadaoure I, et al. . Spatial segregation between invasive and native commensal rodents in an urban environment: a case study in Niamey, Niger. PLoS ONE. 2014;9(11):e110666. 10.1371/journal.pone.0110666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katakweba AAS, Mulungu LS, Eiseb SJ, et al. . Prevalence of haemoparasites, leptospires and coccobacilli with potential for human infection in the blood of rodents and shrews from selected localities in Tanzania, Namibia and Swaziland. Afr Zool [Internet]. 2012;47(1):119–127. Available from: http://africanzoology.journals.ac.za/pub/article/view/678/761. [Google Scholar]
- 46.McCauley DJ, Salkeld DJ, Young HS, et al. . Effects of land use on plague (Yersinia pestis) activity in rodents in Tanzania. Am J Trop Med Hyg. 2015;92(4):776–783. 10.4269/ajtmh.14-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halliday JEB, Knobel DL, Agwanda B, et al. . Prevalence and diversity of small mammal-associated Bartonella species in rural and urban Kenya. PLoS Negl Trop Dis. 2015;9(3):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gryseels S, Rieger T, Oestereich L, et al. . Gairo virus, a novel arenavirus of the widespread Mastomys natalensis: genetically divergent, but ecologically similar to Lassa and Morogoro viruses. Virology [Internet]. 2015;476:249–256. Available from: https://doi.org/10.1016/j.virol.2014.12.011 10.1016/j.virol.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 49.Fichet-Calvet E, Ölschläger S, Strecker T, et al. . Spatial and temporal evolution of Lassa virus in the natural host population in Upper Guinea. Sci Rep [Internet]. 2016;6:670.. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4766397&tool=pmcentrez&rendertype=abstract. 10.1038/srep21977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sluydts V, Davis S, Mercelis S, et al. . Comparison of multimammate mouse (Mastomys natalensis) demography in monoculture and mosaic agricultural habitat: implications for pest management. Crop Prot [Internet]. 2009;28(8):647–654. Available from: https://doi.org/10.1016/j.cropro.2009.03.018 10.1016/j.cropro.2009.03.018 [DOI] [Google Scholar]
- 51.Walker DH, Wulff H, Lange JV, et al. . Comparative pathology of Lassa virus infection in monkeys, guinea pigs, and Mastomys natalensis. Bull World Health Organ. 1975;52:523–534. [PMC free article] [PubMed] [Google Scholar]
- 52.Mariën J, Borremans B, Gryseels S, et al. . No measurable adverse effects of Lassa, Morogoro and Gairo arenaviruses on their rodent reservoir host in natural conditions. Parasit Vectors [Internet]. 2017;10(210):1–11. Available from: http://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-017-2146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borremans B, Vossen R, Becker-Ziaja B, et al. . Shedding dynamics of Morogoro virus, an African arenavirus closely related to Lassa virus, in its natural reservoir host Mastomys natalensis. Sci Rep [Internet]. 2015;5(April):56 Available from: /pmc/articles/PMC4448520/?report=abstract 10.1038/srep10445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mariën J, Borremans B, Gryseels S, et al. . Arenavirus dynamics in experimentally and naturally infected rodents. Ecohealth. 2017:1–11. [online advance publication]. [DOI] [PubMed] [Google Scholar]
- 55.Fichet-Calvet E, Becker-Ziaja B, Koivogui L, et al. . Lassa serology in natural populations of rodents and horizontal transmission. Vector-Borne Zoonotic Dis [Internet]. 2014;14(9):665–674. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4170823. 10.1089/vbz.2013.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gryseels S, Baird SJE, Borremans B, et al. . When viruses don’t go viral: the importance of host phylogeographic structure in the spatial spread of arenaviruses. PLOS Pathog [Internet]. 2017;13(1):e1006073 Available from: https://dx.plos.org/10.1371/journal.ppat.1006073 10.1371/journal.ppat.1006073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olayemi A, Cadar D, Magassouba N, et al. . New hosts of the Lassa virus. Sci Rep [Internet]. 2016;6:75.. Available from: http://www.nature.com/srep/2016/160503/srep25280/full/srep25280.html. 10.1038/srep25280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ter Meulen J, Lukashevich I, Sidibe K, et al. . Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of lassa virus in the Republic of Guinea. Am J Trop Med Hyg. 1996;55(6):661–666. 10.4269/ajtmh.1996.55.661 [DOI] [PubMed] [Google Scholar]
- 59.Grant C, Lo Iacono, Giovanni Dzingirai V, et al. . Moving interdisciplinary science forward: integrating participatory modelling with mathematical modelling of zoonotic disease in Africa. Infect Dis Poverty [Internet]. 2016;5(17):1–12 Available from: https://doi.org/10.1186/s40249-016-0110-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dzingirai V, Bett B, Bukachi S, et al. . Zoonotic diseases: who gets sick, and why? Explorations from Africa. Crit Public Health [Internet]. 2016;1596(Feb):1–14. Available from: http://www.tandfonline.com/doi/full/10.1080/09581596.2016.1187260. [Google Scholar]
- 61.Bonwitt J, Sáez AM, Lamin J, et al. . At home with Mastomys and Rattus : human-rodent interactions and potential for primary transmission of Lassa virus in domestic spaces. Am J Trop Med Hyg [Internet]. 2017;96(4):16–0675. Available from: http://www.ajtmh.org/lookup/doi/10.4269/ajtmh.16-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonwitt J, Kelly AH, Ansumana R, et al. . Rat-atouille: a mixed method study to characterise rodent hunting and consumption in the context of Lassa fever. EcoHealth. 2016;13(2):234–247. 10.1007/s10393-016-1098-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonner PC, Schmidt WP, Belmain SR, et al. . Poor housing quality increases risk of rodent infestation and Lassa fever in refugee camps of Sierra Leone. Am J Trop Med Hyg. 2007;77(1):169–175. [PubMed] [Google Scholar]
- 64.Kamara A, Koroma BM, Gogra AB. Seasonal changes in vegetation and land use in Lassa fever-prone areas (Kenema and Kailahun districts) in eastern Sierra Leone. Nat Res [Internet]. 2015;6:450–456. Available from: http://file.scirp.org/Html/. [Google Scholar]
- 65.Lo Iacono G, Cunningham AA, Fichet-Calvet E, et al. . A unified framework for the infection dynamics of zoonotic spillover and spread. PLoS Negl Trop Dis. 2016;10(9):e0004957. 10.1371/journal.pntd.0004957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fichet-Calvet E, Rogers DJ. Risk maps of Lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3(3):e388. 10.1371/journal.pntd.0000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Monath TP, Mertens PE, Patton R, et al. . A hospital epidemic of Lassa fever in Zorzor, Liberia, March–April 1972. Am J Trop Med Hyg. 1973;22(6):773–779. 10.4269/ajtmh.1973.22.773 [DOI] [PubMed] [Google Scholar]
- 68.Carey JM, White HA, Fom AL, et al. . An outbreak of Lassa fever on the Jos plateau, Nigeria, in January–February 1970. A preliminary report. Am J Trop Med Hyg. 1970;19(4):695–696. 10.4269/ajtmh.1970.19.695 [DOI] [PubMed] [Google Scholar]
- 69.Lo Iacono G, Cunningham AA, Fichet-Calvet E, et al. . Using modelling to disentangle the relative contributions of zoonotic and anthroponotic transmission: the case of Lassa fever. PLoS Negl Trop Dis. 2015;9(1):e3398. 10.1371/journal.pntd.0003398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andersen KG, Shapiro BJ, Matranga CB, et al. . Clinical sequencing uncovers origins and evolution of Lassa virus. Cell. 2015;162(4):738–750. 10.1016/j.cell.2015.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dzotsi EK, Ohene S-A, Asiedu-Bekoe F, et al. . The first cases of Lassa fever in Ghana. Ghana Med J [Internet]. 2012;46(3):166–170. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3645162&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 72.Safronetz D, Lopez JE, Sogoba N, et al. . Detection of Lassa virus, Mali. Emerg Infect Dis. 2010;16(7):1123–1126. 10.3201/eid1607.100146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manning JT, Forrester N, Paessler S. Lassa virus isolates from Mali and the Ivory Coast represent an emerging fifth lineage. Front Microbiol. 2015;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Safronetz D, Sogoba N, Lopez JE, et al. . Geographic distribution and genetic characterization of Lassa virus in sub-Saharan Mali. PLoS Negl Trop Dis. 2013;7(12):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peterson AT, Moses LM, Bausch DG. Mapping transmission risk of Lassa fever in West Africa: the importance of quality control, sampling bias, and error weighting. PLoS ONE. 2014;9(8):e100711. 10.1371/journal.pone.0100711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mylne AQN, Pigott DM, Longbottom J, et al. . Mapping the zoonotic niche of Lassa fever in Africa. Trans R Soc Trop Med Hyg. 2015;109(8):483–492. 10.1093/trstmh/trv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Emonet S, Lemasson JJ, Gonzalez JP, et al. . Phylogeny and evolution of old world arenaviruses. Virology. 2006;350(2):251–257. 10.1016/j.virol.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 78.Coulibaly-N’Golo D, Allali B, Kouassi SK, et al. . Novel arenavirus sequences in Hylomyscus sp. and Mus (Nannomys) setulosus from Cote d’Ivoire: implications for evolution of arenaviruses in Africa. PLoS ONE. 2011;6(6):e20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leski TA, Stockelman MG, Moses LM, et al. . Sequence variability and geographic distribution of Lassa Virus. Sierra Leone. Emerg Infect Dis. 2015;21(4):609–618. 10.3201/eid2104.141469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Russo IM, Sole C, Barbato M, et al. . Landscape determinants of fine-scale genetic structure of a small rodent in a heterogeneous landscape (Hluhluwe-iMfolozi Park, South Africa). Sci Rep [Internet]. 2016;6(29168):1–14. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4942783/pdf/srep29168.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gryseels S, Goüy de Bellocq J, Makundi R, et al. . Genetic distinction between contiguous urban and rural multimammate mice in Tanzania despite gene flow. J Evol Biol. 2016;29(10):1952–1967. 10.1111/jeb.2016.29.issue-10 [DOI] [PubMed] [Google Scholar]
- 82.Lalis A, Leblois R, Lecompte E, et al. . The impact of human conflict on the genetics of Mastomys natalensis and Lassa virus in West Africa. PLoS ONE. 2013;7(5):e37068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peel AJ, Sargan DR, Baker KS, et al. . Continent-wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nat Commun [Internet]. 2013. January;4:2770 Available from: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3836177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colangelo P, Verheyen E, Leirs H, et al. . A mitochondrial phylogeographic scenario for the most widespread African rodent, Mastomys natalensis. Biol J Linn Soc. 2013;108(4):901–916. 10.1111/bij.2013.108.issue-4 [DOI] [Google Scholar]
- 85.World Health Organisation: Lassa fever – Benin, Togo and Burkina Faso [Internet]. 2017. [cited [cited 2017 Jun 28]]. Available from: https://www.who.int/csr/don/10-march-2017-lassa-fever-benin-togo-burkina-faso/en/ [Google Scholar]
- 86.Coltart CEM, Lindsey B, Ghinai I, et al. . The Ebola outbreak, 2013-2016: old lessons for new epidemics. Philos Trans R Soc B. 2017;372(1721):2013–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Norris K, Asase A, Collen B, et al. . Biodiversity in a forest-agriculture mosaic – the changing face of West African rainforests. Biol Conserv [Internet]. 2010;143(10):2341–2350. Available from: https://doi.org/10.1016/j.biocon.2009.12.032 [Google Scholar]
- 88.Roudier P, Sultan B, Quirion P, et al. . The impact of future climate change on West African crop yields: what does the recent literature say? Glob Environ Chang [Internet]. 2011;21(3):1073–1083. Available from: https://doi.org/10.1016/j.gloenvcha.2011.04.007 [Google Scholar]
- 89.Patz JA, Daszak P, Tabor GM, et al. . Unhealthy landscapes: policy recommendations on land use change and infectious disease emergence. Environ Health Perspect. 2004;112(10):1092–1098. 10.1289/ehp.6877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ahmed KF, Wang G, You L, et al. . Potential impact of climate and socioeconomic changes on future agricultural land use in West Africa. Earth Syst Dyn. 2016;7(1):151–165. 10.5194/esd-7-151-2016 [DOI] [Google Scholar]
- 91.Keesing F, Belden LK, Daszak P, et al. . Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature [Internet]. 2010;468(7324):647–652. Available from: https://doi.org/10.1038/nature09575 10.1038/nature09575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bordes F, Blasdell K, Morand S. Transmission ecology of rodent-borne diseases: new frontiers. Integr Zool. 2015;10(5):424–435. 10.1111/inz2.2015.10.issue-5 [DOI] [PubMed] [Google Scholar]
- 93.Massawe AW, Rwamugira W, Leirs H, et al. . Do farming practices influence population dynamics of rodents? A case study of the multimammate field rats, Mastomys natalensis. Tanzania. Afr J Ecol. 2007;45(3):293–301. 10.1111/aje.2007.45.issue-3 [DOI] [Google Scholar]
- 94.Moses LM, Kamara A, Gogra AB, et al. . Lassa fever case study: situation analysis Sierra Leone, Dynamic Drivers of Disease in Africa Consortium. Brighton: STEPS Centre; 2012. [Google Scholar]
- 95.Kilpatrick AM, Salkeld DJ, Titcomb G, et al. . Conservation of biodiversity as a strategy for improving human health and well-being. Philos Trans R Soc B Biol Sci. 2017;372:20160131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buckee CO, Tatem AJ, Metcalf CJE. Seasonal population movements and the surveillance and control of infectious diseases. Trends Parasitol [Internet]. 2017;33(1):10–20. Available from: https://doi.org/10.1016/j.pt.2016.10.006 10.1016/j.pt.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perez-Saez J, Mande T, Ceperley N, et al. . Hydrology and density feedbacks control the ecology of intermediate hosts of schistosomiasis across habitats in seasonal climates. Proc Natl Acad Sci [Internet]. 2016;113(23):6427–6432. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1602251113. 10.1073/pnas.1602251113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stensgaard AS, Booth M, Nikulin G, et al. . Combining process-based and correlative models improves predictions of climate change effects on schistosoma mansoni transmission in eastern Africa. Geospat Health. 2016;11(1S). [DOI] [PubMed] [Google Scholar]
- 99.Yan L, Fang LQ, Huang HG, et al. . Landscape elements and Hantaan virus-related hemorrhagic fever with renal syndrome, People’s Republic of China. Emerg Infect Dis. 2007;13(9):1301–1306. 10.3201/eid1309.061481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Charrel RN, De Lamballerie X. Zoonotic aspects of arenavirus infections. Vet Microbiol. 2010;140(3-4):213–220. 10.1016/j.vetmic.2009.08.027 [DOI] [PubMed] [Google Scholar]
- 101.Alexander KA, Lewis BL, Marathe M, et al. . Modeling of wildlife-associated zoonoses: applications and caveats. Vector Borne Zoonotic Dis [Internet]. 2012 Dec;12(12):1005–1018. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3525896. 10.1089/vbz.2012.0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.