Abstract
In the current study, the effects of the presence of symbiotic bacteria on the activity of the enzymes involved in An. stephensi resistance to temephos are evaluated for the first time. Four different strains (I. susceptible strain, II. resistant strain, III. resistant strain + antibiotic, and IV. resistant strain + bacteria) were considered in order to determine the possible effects of the symbiotic bacteria on their hosts’ resistance to temephos. The median values of all enzymes of susceptible strain were compared with those of other resistant strains. The results of this study indicated a direct relationship between the presence of bacteria in the symbiotic organs of An. stephensi and resistance to temephos. The profile of enzymatic activities in the resistant strain changed to a susceptible status after adding antibiotic. The resistance of An. stephensi to temephos could be completely broken artificially by removing their bacterial symbionts in a resistant population.
Keywords: Symbiotic bacteria, Anopheles stephensi, Insecticide resistance, Temephos
Background
Anopheles stephensi, as a sub-tropical species, is one of the most important vectors of human malaria throughout the Middle East and South Asian region, including the Indo-Pakistan subcontinent, with a westward extension through Iran and Iraq into the Middle East and Arabian Peninsula. This species is also considered to be the main malaria vector in the Persian Gulf area [1,2]. According to the previous studies, An. stephensi is the most prevalent anopheline species in the malarious areas of southern Iran [3].
Insects, as one of most ecologically successful animals on the earth, harbor various kinds of symbionts in their bodies due to their wide variety of diets. Regarding the hypothetical role of mosquitos’ bacterial symbionts in the biology and physiology of their hosts, several studies have been conducted for identification of the bacterial flora of different species of mosquitoes [4–8]. Accordingly, 68 genera of bacteria were detected and identified from An. stephensi in India, where Serratia marcescens as a commensal symbiont was the dominant species of field population [9].
Alongside the results of the aforementioned studies, which showed the rich flora of bacterial symbionts, their important role in their hosts’ nutrition and other physiological characteristics, such as reproduction, development, and protection against enemies, has been also suggested [10]. Moreover, various studies have examined the biological and physiological effects of insects’ symbiotic bacteria on their hosts. For instance, a previous study reported a higher level of resistance to parasitic wasps in phytophagous aphids due to the presence of Hamiltonella defensa, a facultative bacterium [11]. Yet, another research demonstrated that Buchnera aphidicola provided the essential amino acids for its hosts [12]. Also, some obligate endosymbiont bacteria, such as Wigglesworthia glossinidia were thought to prepare vitamins for their host; i.e. hematophagous tsetse flies [13]. Another well-studied obligate endosymbiotic bacterium, Wolbachia, directly influenced the development of mosquitoes and indirectly affected pathogen transmission [14,15]. Some other genera of bacteria, such as Pantoea, Acinetobacter, and Asia, are very common in mosquitoes [16]. Midgut, salivary glands, and reproductive organs are the most suitable places for the bacteria harboring and colonizing within mosquitos’ bodies [17].
Up to now, many studies have been conducted on bacterial microflora of mosquitoes in different parts of the world, most of which have been performed on malaria vectors [4–8,18]. Asaia was reported to be the dominant symbiotic bacteria of An. stephensi that have high stability in their host. The results also revealed that this bacterium could probably affect the vectorial capacity and physiology of the host [19].
Some studies showed a relationship between the presence of some specific bacteria in insects’ hosts and their ability to degrade some pesticides. It was found that the abundance of Wolbachia in Cx. pipiens is strongly influenced by the presence of insecticide resistance genes [20]. It is worth mentioning that Wolbachia has the ability to modify the resistance of its host to chemicals [21].
The results of some studies suggested a link between the presence of some bacteria with high frequency in Bemisia tabaci and their ability to detoxify some insecticides [22,23].
One of the most important studies about the role of symbiotic bacteria in insecticide resistance was carried out by Kikuchi et al. (2012), who introduced the idea ‘Symbiont-mediated insecticide resistance’ for the mechanism of insect resistance to pesticides. These researchers found that insecticide-degrading bacterial symbiont could be rapidly established in their hosts’ bodies, causing the resistance to some pests to certain insecticides. This phenomenon was confirmed in a stinkbug (Riptortus pedestris), which harbors some fenithrotion-resistant bacteria. Burkholderia is acquired from soil at different life stages. Fenitrothion-degrading Burkholderia strains establish a specific and beneficial symbiotic relationship with the host and finally lead to host’s resistance to fenitrothion [24].
Nowadays, despite numerous efforts, mosquito-borne diseases such as malaria, filariasis, yellow fever, dengue, and zika pose serious health problems, especially in developing countries. Vector control program is one of the best ways used in order to control these diseases. In this program, most emphasis is put on chemical pesticides such as temephos. Temephos is one of the most popular organophosphorus insecticides used worldwide, particularly for immature stages of mosquitoes. Control strategies based on chemical insecticides are not always practical. Because of the costs of these compounds, insecticide resistance, environmental and human health concerns, etc., other new methods should replace this strategy [25]. Therefore, understanding the factors affecting insect resistance to pesticides could be very critical. So far, no comprehensive studies have been done on the relationship between symbiotic bacteria of mosquitos and some host characteristics such as insecticide resistance. Hence, the present study aims to identify the effects of these symbionts on resistance of An. stephensi to temephos (Abate®), as one of the most widely used organophosphate pesticides.
Methods
Mosquito strains
Wild specimens of An. stephensi were collected from eight different areas in two most important malarious provinces of Iran (Hormozgan and Sistan-Baluchistan Provinces) using the standard dipping technique (350 ml dipper) for larval stages and hand catch method for adults [26]. The specimens were transferred alive to the laboratory and were then identified to species level using standard morphological key [27].
The field strains of An. stephensi were reared in the insectarium for further tests. A susceptible laboratory strain of An. stephensi (Beech-Lab from insectarium of Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences) was used to compare the susceptibility status of the field strains. This strain had been maintained in the laboratory without exposure to insecticides for more than 30 years.
Insecticide
Technical grade insecticide used in the present study was temephos 90% (Batch No: TEM/136-229) purchased from Levant Overseas Development Ltd., Argenteuil, France.
Selection process
The strain that showed the highest Resistance Ratio (RR) to temephos was preceded for selection pressure. This strain was selected for 5 generations by exposing late third or early fourth instars to the concentrations, which produced 50–70% mortality [28]. The selection was continued as long as a homogeneous resistant population with more than 10-fold RR was achieved.
Symbiotic bacteria of An. stephensi
Larvae were collected from larval habitats using the standard dipping technique. Fourth instar larvae of An. stephensi were selected for midgut microbiota analysis.
Before dissecting, the surfaces of larvae were completely sterilized with 70% ethanol in a sterile hood [6]. Under sterile conditions, the specimens were dissected individually and the midguts (30 pooled midguts for each breeding place) were washed and suspended in 500 μL of Brain Heart Infusion (BHI). Additionally, a 100 μL aliquot of the contents was serially diluted up to 10−6 and was plated onto the prepared media. The purified colonies were selected for molecular identification. DNA extraction was done using QIAGEN DNeasy Kit (Qiagen, Germany) according to the manufacturer’s instructions. The 16S rRNA universal primers of 16suF 5′-GAGTTTGATCCTGGCTCAG-0033′ and 16suR 5′-GTTACCTTGTTACGACTT-3′ were used to amplify about 1.5 Kilo Base (kb) partial sequence of the 16S rRNA gene from all of the DNA specimens [29]. The PCR conditions were set as the previous studies [7].
Identification of the isolated bacteria was based on sequence comparison to the Gen Bank and The Ribosomal Database Project (RDP-II) entries. Also, all the isolates were identified using classical phenotyping and biochemical methods [30].
Biochemical assays
In this study, 30 mosquito larvae from each strain (4 strains explained in study design) were assayed for α- and ß-esterases, Mixed Function Oxidase (MFO), Glutathione-S-Transferase (GST), insensitive acetylcholine esterase (iAChE), and Para Nitro Phenyl Acetate (PNPA)-esterase enzymes. To perform further statistical analyses, four replicates were performed for all enzymatic activity assays. Each larva was homogenized in 100 μL of potassium phosphate (KPO4) buffer (6.6 g dibasic potassium phosphate/1.7 g monobasic potassium phosphate/1000 mL distilled water (dH2O); pH7.2) and was then diluted to 2 mL with the same buffer. After that, each mosquito was analyzed in duplicate with100 μL of mosquito homogenate transferred to two wells on a 96-well flat-bottomed microtitration plate. Absorbance levels were measured spectrophotometrically with a microplate reader (ELX808 Ultra Microplate Reader BIO-TEK®) at the wavelengths indicated for each enzyme. Besides, the mean absorbance level was calculated based on the data for the two replicate wells per mosquito.
The procedures were followed based on slight modifications of a protocol from the Centers for Disease Control and Prevention [31]. The activities of all the enzymes were evaluated according to this protocol. The details of the procedures were described completely in a previous research [31]. The reagents and substrates for biochemical assays were provided by Sigma.
Data analyses of biochemical assays
Absorbance values obtained for mosquito replicates were corrected in relation to the volume of mosquito homogenates, the enzyme activity unit, and the total protein content of each mosquito [31]. Additionally, the means of enzyme activities for each An. stephensi larval strain was compared to those of susceptible strain (Beech-Lab) using unpaired t-test and Mann–Whitney test (p < 0.05). Moreover, Tukey’s Multiple Comparison Test, Kruskal–Wallis, and Dunn’s Multiple Comparison Test were employed to analyze the means of enzyme activities of all the stains.
The Beech-Lab 99th percentile was calculated for each enzyme and the percentage of specimens was calculated according to the enzymatic activity of the Beech-Lab 99th percentile. Enzyme activities were then classified as ‘altered’, ‘incipiently altered’, and ‘unaltered’ if the rate was >50%, between 15 and 50%, and <15%, respectively [32].
Study design
This part was designed in order to find the relationship between the presence of bacteria in mosquitoes and their ability to degrade temephos, a widely used organophosphate insecticide.
Doing so, the mean amounts of all the enzymes involved in insecticide resistance were compared in the following 4 populations:
-
(1)
Laboratory susceptible population (Beech-Lab strain = S);
-
(2)
Selected field population (resistant strain) without any additional agent (R);
-
(3)
Selected field population (resistant strain) + added Tetracycline antibiotic 0.5% (R+T) [33]; and
-
(4)
Selected field population (resistant strain) + added bacteria (1 CC suspension from each dominant genus of bacteria isolated from the gut of An. stephensi) to rearing trays (R+B).
In order to clarify the potential role of symbiotic bacteria in resistance of An. stephensi to temephos, the mean amounts of the enzymes obtained from all the populations were statistically analyzed using the SPSS ver.18 statistical software and GraphPad software.
Determination of the concentration of bacteria in the natural breeding places of mosquito larvae
As explained in the ‘study design’, bacteria should be added to a population of An. stephensi in order to find out the effects of extra bacteria on insecticide resistance in mosquitoes. For this purpose, first, we had to know the mean concentration of bacteria in their natural breeding places. Therefore, a number of water samples were collected from larval breeding places and the amounts of bacteria in these samples were determined by direct microscopic count method [34].
Antibiotic
One of the most important objectives pursued in the present work was to investigate the effects of adding antibiotics to the larval rearing environment under laboratory condition in the insectarium. In this context, it is required to select an antibiotic concentration with the least effect on the normal physiology of the mosquito. Therefore, we decided to apply the minimum concentration of antibiotic that is efficient to remove symbiotic bacteria from their hosts [35]. In this experiment, Tetracycline (>99%, Merck) with ≤0.05 mg/ml concentrations was added to the larval rearing trays.
Results
Among all the tested strains, Chabahar strain showed the highest RR to temephos (4.27 folds) and was then used for the selection process for further studies. The selection process continued for 15 months and after the 5th selection, a relatively homogenized resistance population of An. stephensi with a 15.82-fold RR at LC50 was obtained.
After isolation, the symbiotic bacteria isolated from the gut of the 4th instar larvae of An. stephensi were identified according to 16srRNA gene sequencing. Using PCR method, out of the 45 isolated colonies from the media, 40 species/strains of bacteria were identified most of which (30 species/strains) belonged to 4 genera, including Pseudomonas, Aeromonas, Exiguobacterium, and Microbacterium. These dominant genera of bacteria were selected and maintained at –80 ℃ Cryobank for further investigations.
As mentioned above, from different larval breeding places in the field, 20 water samples were collected and the amounts of bacteria in these samples were determined by direct microscopic count method. The mean number of bacteria in 1 cc was equal to 0.5 McFarland Standard (cells ≅ 1.5 × 108); thus, this concentration was added to the R+B population.
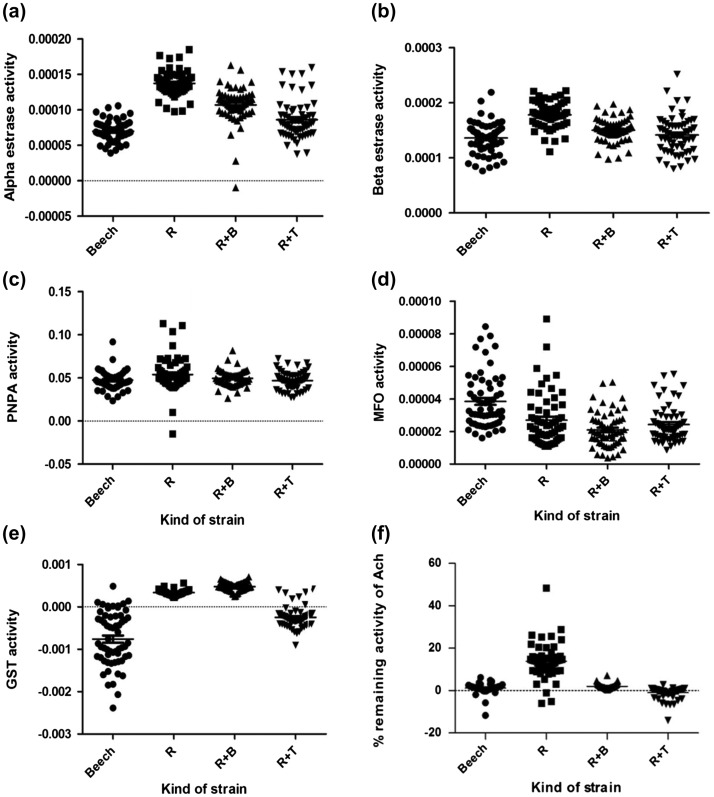
As stated in the ‘study design’, 4 different strains were considered in order to determine the possible effects of the symbiotic bacteria on their hosts’ resistance to insecticide. In this respect, the median value of all the enzymes involved in resistance to S was compared to that of the other three prepared resistant strains, including R, R+T, and R+B. The number of the mosquitoes assessed in each assay, the median values and percentage of strains (R, R+T, and R+B), and enzymatic activities in relation to S are presented in Tables 1 and 2. Besides, the classification profile of enzyme activity in the three resistant strains has been shown in Table 3. The activity levels of the enzymes in all the strains (R, R+T, R+B, and S) are graphically displayed in scatter plots (Figure 1).
Table 1.
Quantification of enzymatic activity of esterase in the four strains (S, R, R+T, and R+B) of An. stephensi.
| Strains | α-EST (nmol/mg ptn/min) |
ß-EST (nmol/mg ptn/min) |
PNPA-EST (Δabs/mg ptn/min) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Medianb | p99c | N | Median | p99 | N | Median | p99 | |
| Beech-Lab | 30 | 0.00006881 | 0.00010411 | 30 | 0.00014011 | 0.00020971 | 30 | 0.04560881 | 0.07969278 |
| N | Median | %>p99d | N | Median | %>p99 | N | Median | %>p99 | |
| R | 30 | 0.00013654 | 95 | 30 | 0.00017725 | 8.33 | 30 | 0.05132526 | 6.67 |
| R+B | 30 | 0.00010939 | 58.33 | 30 | 0.00014893 | 0 | 30 | 0.04944657 | 1.67 |
| R+T | 30 | 0.00008165 | 16.67 | 30 | 0.00014105 | 3.33 | 30 | 0.04698756 | 0 |
Number of tested mosquitoes.
Median value for each enzymatic activity.
99th percentile for Beech-Lab reference strain.
Percentage of mosquito specimen with activity above 99th percentile for Beech-Lab reference strain.
Table 2.
Quantification of enzymatic activity of MFO, GST, and iAChE in the four strains (S, R, R+T, and R+B) of An. stephensi.
| Strains | MFO (nmolcyt/mg ptn) |
GST (nmol/mg ptn/min) |
AChE (% activity) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Medianb | p99c | N | Median | p99 | N | Median | p99 | |
| Beech-Lab | 30 | 0.00003264 | 0.00008108 | 30 | –.00085944 | 0.00028138 | 30 | 1.40618583 | 5.36517282 |
| N | Median | %>p99d | N | Median | %>p99 | N | Median | %>p99 | |
| R | 30 | 0.00002279 | 1.67 | 30 | 0.00033844 | 86.67 | 30 | 14.16817118 | 90 |
| R+B | 30 | 0.00001932 | 0 | 30 | 0.00047489 | 96.67 | 30 | 1.66065793 | 1.67 |
| R+T | 30 | 0.00002123 | 0 | 30 | –.00027341 | 6.67 | 30 | –0.19493844 | 0 |
Number of tested mosquitoes.
Median value for each enzymatic activity.
99th percentile for Beech-Lab reference strain.
Percentage of mosquito specimen with activity above 99th percentile for Beech-Lab reference strain.
Table 3.
Profile of classification of enzyme activity in the three resistant strains of An. stephensi.
| Strains | Enzymes |
|||||
|---|---|---|---|---|---|---|
| α-EST | ß-EST | PNPA-EST | MFO | GST | AChE | |
| R | Altered | Unaltered | Unaltered | Unaltered | Altered | Altered |
| R+B | Altered | Unaltered | Unaltered | Unaltered | Altered | Unaltered |
| R+T | Incipiently altered | Unaltered | Unaltered | Unaltered | Unaltered | Unaltered |
Figure 1.
Activity profile of all tested enzymes (a: α-esterase enzyme, b: ß-esterase enzyme, c: PNPA-esterase enzyme, d: MFO enzyme, e: GST enzyme, f: Percentage of the remaining activity of AChE enzyme) in different strains under study.
The study results revealed a significant difference between S and all the tested resistant strains regarding α-EST activity levels (p < 0.0001). Moreover, R and R+B strains showed altered activity with >50% (95%) of the individuals recording activity above that of the 99th percentile of the Beech-Lab reference strain. R+T strain also showed incipiently altered activity (Tables 1 and 3).
The results also indicated a significant difference between R and some tested strains (S, R+B, and R+T) concerning ß-EST median activity levels (p < 0.0001). However, R+T and R+B were not significantly different from S in this regard. Also, no significant difference was found between R+B and R+T regarding the median activity levels of ß-EST. Based on the classification of activity profiles, all the tested resistant strains showed unaltered activity with <15% (8.33%) of the individuals recording activity above that of the 99th percentile of the Beech-Lab reference strain (Tables 1 and 3).
Based on the obtained results, a significant difference was observed between S and two of the tested strains (R and R+T) with respect to the median PNPA-EST activity level (p = 0.0139). Nevertheless, differences among the other tested strains were not statistically significant (p > 0.05). An unaltered profile of PNPA-EST was found in all the resistant strains (Tables 1 and 3).
In this study, a significant difference was found between S and all the three resistant strains (R, R+T, and R+B) with regard to MFO activity level (p < 0.0001). However, there was no significant difference between R and two of the tested strains (R+B and R+T) (p > 0.05) as well as between R+B and R+T. All the tested resistant strains showed unaltered activity with <15% of the individuals recording activity above that of the 99th percentile of the Beech-Lab reference strain (Tables 2 and 3).
The findings disclosed a significant difference among all the tested strains (p < 0.0001), except between S and R+T (p > 0.05), concerning the GST activity level. Based on the classification of activity profiles, R and R+B showed altered activity, while R+T revealed unaltered activity (Tables 2 and 3).
The activity level of AChE in the presence of propoxur was also significantly different among all the tested strains (p < 0.0001), except between S and R+B (p > 0.05). Moreover, AChE (90%) showed altered activity in R, but unaltered activity in the other resistant strains (R+B and R+T) (Tables 2 and 3).
Discussion
Despite the implementation of different prevention and control strategies, malaria is still one of the most important vector-borne diseases in Iran as well as in other countries around the world. An. stephensi is considered to be one of the most important vectors of malaria in the Middle East region, including Iran [36].
Although temephos (EC 50%), as one of the conventional larvicides in malaria vector control program, has been used in southern Iran for many years [37,38], this species is now almost susceptible to temephos from all the studied localities in Iran. Resistance to this organophosphate pesticide was reported from other malarious neighboring countries, such as India and Oman [39].
Regarding the development of resistant vectors to different pesticides in various parts of the world and existence of some limitations, such as restricted number of insecticides, understanding all effective factors in resistance of malaria vectors is highly recommended. Symbiotic species of vectors is one of these important factors, which has been less taken into account. Symbiotic bacteria are among the most effective organisms that have critical impacts on morphology, immunology, and physiology of their hosts, such as affecting the vectorial capacity and increasing the host’s tolerance of environment stresses.
Bacteria could establish in some symbiotic organs of mosquitoes, such as gut. So far, only a few studies have been published concerning the impact of bacterial symbionts on their hosts, especially malaria vectors [7,8,18]. In the current study, the effects of the presence of symbiotic bacteria on the activity of the enzymes involved in An. stephensi resistance to temephos were surveyed for the first time.
The results of this study clarified a drastic relationship between the presence of bacteria in the symbiotic organs of An. stephensi (one of the main malaria vectors) and the activity of detoxification enzymes. When the isolated bacteria from natural specimens were artificially added to the breeding trays of the resistant strain (R+B), no significant differences were observed between the enzymatic activities of R and R+B. Only for AChE enzyme, the activity profile was changed from altered to unaltered status. Such a change might be attributed to the decrease in mosquito fitness by adding extra bacteria to the rearing trays, which are limited places for mosquito larvae. Competition for food, oxygen, and place also presumably lead to a reduction of the insecticide detoxification ability. These results suggested that addition of bacteria to a natural population had no effect on the increase of detoxifying enzyme activities and could not enhance the resistance of An. stephensi to temephos.
On the other hand, completely different results were obtained with respect to R+T and drastic effects were observed after adding antibiotic to the rearing trays of the resistant strain. Accordingly, the profile of α-esterase activity was changed from altered to incipiently altered status. Also, GST and AChE activities changed from altered to unaltered status.
According to the results obtained regarding S and R, it can be concluded that the main enzymes involved in the mechanism of resistance of An. stephensi to temephos were α-esterase, GST, and AChE. Of note, these enzymes were dramatically reduced in R+T.
Furthermore, the profile of enzymatic activities in the resistant strain changed to susceptible status after adding antibiotic. Therefore, it can be concluded that presence of bacterial symbionts is critical for their hosts. Besides, the resistance of An. stephensi to temephos could be completely broken artificially by removing their bacterial symbionts in a resistant population. This phenomenon might result from the direct effects of symbionts on hydrolyzing and detoxifying of some organophosphate pesticides, such as temephos, in their hosts, inducing symbionts’ effects on production of detoxifying enzymes by their hosts. Another reason might be the effects of antibiotic on mosquitoes by suppressing or reducing the genes that are responsible for enzymes production. The present work is a preliminary study to investigate the role of midgut symbiotic bacteria in resistance of An. stephensi to a widely used organophosphate insecticide. Further studies in this field should be done in order to find out all the details and to provide more accurate comments on this issue.
Our findings support the possibility of the existence of a similar process for the recently introduced insecticide resistance mechanism (i.e. ‘Symbiont-mediated insecticide resistance’) in temephos resistant An. stephensi populations. In these mosquitoes, bacterial symbionts play a critical role in detoxifying organophosphate insecticides in their hosts’ bodies.
Conclusions
Considering the fact that the main mechanisms of temephos resistance in An. stephensi are enzymatic, simultaneous use of organophosphate insecticides with a synergist could be very useful for managing insecticides resistance in mosquitoes.
Overall, the results of the present study theoretically provided a novel idea for the management of pesticides resistance in vectors. This modern idea involves finding a specific antibiotic for insects and using it in larval breeding places of resistant mosquitoes, which could be very helpful for breaking down the resistance and preventing its spread in the populations. However, still further studies are required on this idea. In addition, we should discuss this issue cautiously because of some important consequences of excessive use of antibiotics and their unwanted side effects.
Abbreviations
- BHI
Brain Heart Infusion
- Kb
Kilo Base
- RDP-II
Ribosomal Database Project
- MFO
Mixed Function Oxidase
- GST
Glutathione-S-Transferase
- iAChE
Insensitive acetylcholine esterase
- PNPA
Para Nitro Phenyl Acetate-esterase enzymes
- KPO4
Potassium phosphate
- dH2O
Distilled water
- S
Susceptible strain
- R
Resistant strain
- R+T
Resistant strain + added Tetracycline
- R+B
Resistant strain + added bacteria
Authors’ contributions
AS participated in the design of the study, drafted the manuscript and carried out almost all parts of the study. HV participated in the design of the study, revised the paper and supervised the bioassay studies and other part of the study. MAO participated in the design of the study, performed the statistical analysis and interpretation of data. AAE supervised and interpreted the biochemical assays. ALC participated in bacteriology studies and helped to draft the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by the Tehran University of Medical Sciences (IR) [grant number 90-02-27-14091].
Acknowledgements
Authors are thankful to the assistance of vice-chancellorship for research and technology at Tehran University of Medical Sciences (The code number of project: 90-02-27-14091).
References
- [1].Oshaghi MA, Yaghoobi F, Vatandoost H, et al. Anopheles stephensi biological forms, geographical distribution, and malaria transmission in malarious regions in Iran. Pak J Biol Sci. 2006;9:294–298. [Google Scholar]
- [2].Hanafi-Bojd AA, Vatandoost H, Oshaghi MA, et al. Entomological and epidemiological attributes for malaria transmission and implementation of vector control in southern Iran. Acta Trop. 2012 Feb 29;121:85–92. 10.1016/j.actatropica.2011.04.017 [DOI] [PubMed] [Google Scholar]
- [3].Vatandoost H, Shahi H, Abai MR, et al. Larval habitats of main malaria vectors in Hormozgan province and their susceptibility to different larvicides. Southeast Asian J Trop Med Public Health. 2004;35:22–25. [PubMed] [Google Scholar]
- [4].Demaio J, Pumpuni CB, Kent M, et al. The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens, and Psorophora columbiae mosquitoes. Am J Trop Med Hyg. 1996 Feb;54:219–223. 10.4269/ajtmh.1996.54.219 [DOI] [PubMed] [Google Scholar]
- [5].Touré AM, Mackey AJ, Wang ZX, et al. Bactericidal effects of sugar-fed antibiotics on resident midgut bacteria of newly emerged anopheline mosquitoes (Diptera: Culicidae). J Med Entomol. 2000 Mar 1;37:246–249. 10.1093/jmedent/37.2.246 [DOI] [PubMed] [Google Scholar]
- [6].Pidiyar V, Kaznowski A, Narayan NB, et al. Aeromonas culicicola sp. nov., from the midgut of Culex quinquefasciatus. Int J Syst Evol Microbiol. 2002 Sep 1;52:1723–1728. [DOI] [PubMed] [Google Scholar]
- [7].Chavshin AR, Oshaghi MA, Vatandoost H, et al. Identification of bacterial microflora in the midgut of the larvae and adult of wild caught Anopheles stephensi: a step toward finding suitable paratransgenesis candidates. Acta Trop. 2012 Feb 29;121:129–134. 10.1016/j.actatropica.2011.10.015 [DOI] [PubMed] [Google Scholar]
- [8].Chavshin AR, Oshaghi MA, Vatandoost H, et al. Isolation and identification of culturable bacteria from wild Anopheles culicifacies, a first step in a paratransgenesis approach. Parasit Vectors. 2014 Sep 4;7:419. 10.1186/1756-3305-7-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rani A, Sharma A, Rajagopal R, et al. Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol. 2009 May 19;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Minard G, Mavingui P, Moro CV. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit Vectors. 2013 May 20;6:146. 10.1186/1756-3305-6-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Oliver KM, Russell JA, Moran NA, et al. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc Nat Acad Sci. 2003 Feb 18;100:1803–1807. 10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Douglas AE. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol. 1998 Jan;43:17–37. 10.1146/annurev.ento.43.1.17 [DOI] [PubMed] [Google Scholar]
- [13].Pais R, Lohs C, Wu Y, et al. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol. 2008 Oct 1;74:5965–5974. 10.1128/AEM.00741-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Calvitti M, Moretti R, Skidmore AR, et al. Wolbachia strain wPip yields a pattern of cytoplasmic incompatibility enhancing a Wolbachia-based suppression strategy against the disease vector Aedes albopictus. Parasit Vectors. 2012 Nov 12;5:254. 10.1186/1756-3305-5-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McMeniman CJ, Lane RV, Cass BN, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009 Jan 2;323:141–144. 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- [16].Moro CV, Tran FH, Raharimalala FN, et al. Diversity of culturable bacteria including Pantoea in wild mosquito Aedes albopictus. BMC Microbiol. 2013 Mar 27;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Noden BH, Vaughan JA, Pumpuni CB, et al. Mosquito ingestion of antibodies against mosquito midgut microbiota improves conversion of ookinetes to oocysts for Plasmodium falciparum, but not P. yoelii. Parasitol Int. 2011 Dec 31;60:440–446. 10.1016/j.parint.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Djadid ND, Jazayeri H, Raz A, et al. Identification of the midgut microbiota of An. stephensi and An. maculipennis for their application as a paratransgenic tool against malaria. PLoS One. 2011 Dec 6;6:e28484. 10.1371/journal.pone.0028484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Favia G, Ricci I, Damiani C, et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc Nat Acad Sci. 2007 May 22;104:9047–9051. 10.1073/pnas.0610451104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Berticat C, Rousset F, Raymond M, et al. High Wolbachia density in insecticide–resistant mosquitoes. Proc R Soc London B Biol Sci. 2002 Jul 7;269:1413–1416. 10.1098/rspb.2002.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Duron O, Labbé P, Berticat C, et al. High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution. 2006 Feb 1;60:303–314. 10.1111/evo.2006.60.issue-2 [DOI] [PubMed] [Google Scholar]
- [22].Ghanim M, Kontsedalov S. Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities. Pest Manag Sci. 2009 Sep 1;65:939–942. 10.1002/ps.v65:9 [DOI] [PubMed] [Google Scholar]
- [23].Kontsedalov S, Zchori-Fein E, Chiel E, et al. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag Sci. 2008 Aug 1;64:789–792. 10.1002/ps.v64:8 [DOI] [PubMed] [Google Scholar]
- [24].Kikuchi Y, Hayatsu M, Hosokawa T, et al. Symbiont-mediated insecticide resistance. Proc Nat Acad Sci. 2012 May 29;109:8618–8622. 10.1073/pnas.1200231109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soltani A, Vatandoost H, Jabbari H, et al. Field efficacy of expanded polystyrene and shredded waste polystyrene beads for mosquito control in artificial pools and field trials, Islamic Republic of Iran. EMHJ. 2012; 18: 1042–1048. [DOI] [PubMed] [Google Scholar]
- [26].Service MW, Townson H. The Anopheles vector In: Warrel DA, Gilles HM, editors. Essential malariology. 4th ed London: Arnold Publishers; 2002. p. 59–84. [Google Scholar]
- [27].Azari-Hamidian S, Harbach RE. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae). Zootaxa. 2009 Apr 20;2078:1–33. [Google Scholar]
- [28].Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000 Jan;45:371–391. 10.1146/annurev.ento.45.1.371 [DOI] [PubMed] [Google Scholar]
- [29].Weisburg WG, Barns SM, Pelletier DA, et al. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991 Jan 1;173:697–703. 10.1128/jb.173.2.697-703.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Garrity G, Staley JT, Boone DR, et al. Bergey’s manual of systematic bacteriology In: Brenner DJ, Krieg NR, editors. The Proteobacteria. Vol. 2. New York (NY): Springer Science & Business Media; 2006. Jul 25. p. 1. [Google Scholar]
- [31].Polson KA, Brogdon WG, Rawlins SC, et al. Characterization of insecticide resistance in Trinidadian strains of Aedes aegypti mosquitoes. Acta Trop. 2011 Jan 31;117:31–38. 10.1016/j.actatropica.2010.09.005 [DOI] [PubMed] [Google Scholar]
- [32].Malcolm CA, Hall LM. Cloning and characterization of a mosquito acetylcholinesterase gene. In: Hagedorn H, Hildebrand HJ, Kidwell M, Law J, editors. Molecular insect science. New York (NY): Springer; 1990. p. 57–66. [Google Scholar]
- [33].Wiwatanaratanabutr S, Kittayapong P. Effects of temephos and temperature on Wolbachia load and life history traits of Aedes albopictus. Med Vet Entomol. 2006 Sep 1;20:300–307. 10.1111/mve.2006.20.issue-3 [DOI] [PubMed] [Google Scholar]
- [34].Huang CC, Liau SM, Tsai WC, et al. Using direct epifluorescent microscopic count for rapid enumeration of viable yeast and bacteria in injured conditions. J Food Drug Anal. 2005 Apr 1;13:143–150. [Google Scholar]
- [35].Dobson SL, Rattanadechakul W. A novel technique for removing Wolbachia infections from Aedes albopictus (Diptera: Culicidae). J Med Entomol. 2001 Nov 1;38:844–849. 10.1603/0022-2585-38.6.844 [DOI] [PubMed] [Google Scholar]
- [36].Vatandoost H, Oshaghi MA, Abaie MR, et al. Bionomics of Anopheles stephensi Liston in the malarious area of Hormozgan province, southern Iran, 2002. Acta Trop. 2006 Feb 28;97:196–203. 10.1016/j.actatropica.2005.11.002 [DOI] [PubMed] [Google Scholar]
- [37].Soltani A, Vatandoost H, Oshaghi MA, et al. Baseline susceptibility of different geographical strains of Anopheles stephensi (Diptera: Culicidae) to temephos in malarious areas of Iran. J Arthropod Borne Dis. 2013 Jun 1;7(1):56–65. [PMC free article] [PubMed] [Google Scholar]
- [38].Soltani A, Vatandoost H, Oshaghi MA, et al. Resistance mechanisms of Anopheles stephensi (Diptera: Culicidae) to Temephos. J Arthropod Borne Dis. 2015 Jun;9(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- [39].Anderasen MH. Emerging resistance to temephos in Anopheles stephensi in the Al-Dhahira Region of Oman. Geneva: World Health Organization; 2006 May 6. p. 1–3. [Google Scholar]