Abstract
A recent systematic literature and meta-analysis reported relative efficacy of trimethoprim-sulfamethoxazole (TMP-SMX) for the treatment of toxoplasmic encephalitis (TE) in HIV-infected adults. Here, we estimated relapse rates during secondary prophylaxis with TMP-SMX, and further explored differences in relapse rates prior to introduction of highly active antiretroviral therapy (HAART) and the widespread adoption of HAART. A systematic search of PubMed, Embase, and Cochrane Central Register of Controlled Trials yielded 707 studies whereby 663 were excluded after abstract screening, and 38 were excluded after full review leaving 6 studies for extraction. We performed double data extraction with a third-party adjudicator. Study designs varied with only one randomized study, four prospective cohorts and one retrospective cohort. Relapse rates were transformed using the Freeman-Tukey method and pooled using both fixed-effect and random-effects meta-analysis models. The TMP-SMX relapse rate was 16.4% (95% CI = 6.2% to 30.3%) based on random-effects models. When the disaggregated pre-HAART studies (n = 4) were included, the relapse rate was 14.9% (random effects; 95% CI = 3.7% to 31.9%). Analysis of two post-HAART studies indicated a relapse rate of 19.2% (random effects; 95% CI = 2.8% to 45.6%). Comparing the relapse rates between pre- and post-HAART studies were contrary to what might be expected based on known benefits of HAART therapy in this population. Nevertheless, cautious interpretation is necessary considering the heterogeneity of the included studies and a limited number of subjects receiving TMP-SMX reported in the post-HAART era.
Keywords: Trimethoprim-sulfamethoxazole, secondary prevention, toxoplasmic encephalitis, cerebral toxoplasmosis, secondary prophylaxis, meta-analysis, HIV, relapse rates
Background
A recent systematic literature and meta-analysis reported by Hernandez et al. [1], reported relative efficacy of trimethoprim-sulfamethoxazole (TMP-SMX) for the treatment of toxoplasmic encephalitis (TE) in patients with AIDS [1]. Building on the work of Hernandez et al. [1],we quantifiedTE relapse rates during secondary prophylaxis with TMP-SMX. Defining relapse rates is an important element of patient management as it represents additional mortality and morbidity and costs for the healthcare system, as well as hospitals. This is particularly challenging in scenarios where rehospitalization within defined periods of time often are not reimbursed by insurers. Therefore,rehospitalization costs are paid by the hospital without reimbursement.
The aim of this study was to conduct a systematic literature review and meta-analysis to estimate relapse rates associated with TMP-SMX in secondary prophylaxis following improvement of an episode of TE. We further assessed relapse rates in the pre- and post- highly active antiretroviral therapy (HAART) era.
Methods
Search strategy
The study was conducted following PRISMA guidelines [2]. PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched up to November 11, 2016. The search strings for the three databases are available as supplementary data (Appendix S1). To be eligible for inclusion, studies had to include datafindings on patients with HIV infection or AIDS, receiving secondary prophylaxis with TMP-SMX after resolution of a prior TE event.Studies needed to report on relapse outcomes during maintenance treatment and be published in peer-reviewed journals in English, Spanish, Portuguese, German or French. Studies in primary prophylaxis were excluded. Review articles, letters to the editor (not being research letters), comments, case reports, and preclinical studies were also excluded. Study eligibility for inclusion was assessed in duplicate by two independent reviewers, and in cases of discrepancy,a third reviewer was consulted to reach consensus.
Data extraction
Data extraction was performedin duplicate on all included studies to limit human error. A third reviewer resolved discrepancies in extracted data between the two independent reviewers. Non-English studies were translated and extracted. Extracted data included: first author, publication year, study design, demographics, acute and maintenance treatment regimens, number of patients evaluated for relapse, incidence of relapse, and time of follow-up.HAART status of studies was determined by the period of patient enrollment in the study (not publication date), either before or after 1996, and the reported background anti-retroviral therapy administered to patients. The meta-analysis consisted of six studies representing 235 patients receiving TMP-SMX as a secondary prophylaxis for TE (Table 1).
Table 1.
Summary of study characteristics.
| First author (year) | Study Design | Demographics | TMP-SMX based therapy |
No. patients evaluated for relapse, n | Relapse, n (%) | Follow-up | |
|---|---|---|---|---|---|---|---|
| Acute | Maintenance | ||||||
|
Pre-HAART | |||||||
| Torre (1998) [5] | Randomized | N = 77 | TMP-SMX | TMP-SMX | 37 | 1 (3%) | 4 months |
| Trimethoprim (10 mg/kg/day) plus sulfamethoxazole (50 mg/kg/day) | Trimethoprim (5 mg/kg/day) plus sulfamethoxazole (25 mg/kg/day) | ||||||
| Male: 57 (75%) | |||||||
| Mean age: 33.2 yrs (±5.6) | |||||||
| TMP-SMXn = 40 | |||||||
| Male: 28 (70.0%) | |||||||
| Mean age: 34.0 yrs (±6.4) | |||||||
| Torre (1998) [10] | Retrospective cohort | N = 71 | TMP-SMZ | TMP-SMZ | 71 | 5 (7%) | 9 months |
| TMP (10 mg/kg/day), plus SMX (50 mg/kg/day) | TMP (5 mg/kg/day), plus SMX (25 mg/kg/day) | ||||||
| Male: 58 (81.7%) | |||||||
| Mean age: 30.5 yrs ±4.9 | |||||||
| Smadja (1998) [9] | Open, prospective trial | N = 18 | TMP-SMX | TMP-SMX | 17 | 7 (41%) | n.r. |
| Male: 11 (61%) | First 48 h: TMP (640 mg/day) plus SMX (3.2 g/day) | TMP (160 mg/day), plus SMX (800 mg/day) | |||||
| Median age: 39 yrs | |||||||
| Next two weeks: TMP (480 mg/day) plus SMX (2.4 g/day) | |||||||
| Until disappearance of active lesions: TMP (320 mg/day) plus SMX (1.6 g/day) | |||||||
| Chaddha (1999) [8] | Prospective | N = 11 | PYR ± TMP-SMX | TMP-SMX | 10 | 2 (20%) | 3–6 months |
| TMP (160 mg/day), plus SMX (800 mg/day) | |||||||
| Male: 10 (91%) | PYR (200 mg loading dose followed by 75 mg/day) plus TMP (20 mg/kg/day) plus SMX (100 mg/kg/day) | ||||||
| Mean age: 32 ± 4 yrs | |||||||
|
Post-HAART | |||||||
| Duval (2004) [7] | Prospective cohort | N = 17 | NR | TMP-SMX | 17 | 1 (6%) | 31 months |
| TMP (160 mg/BID), plus SMX (800 mg/BID) | |||||||
| Male: 13 | |||||||
| Beraud (2009) [6] | Observational prospective cohort | N = 83 | TMP-SMX | TMP-SMX | 83 | 25 (30%) | Mean 36.1 ± 36.9 months |
| Male: 56 (67.5%) | TMP (10–50 mg/kg/day) plus SMX (50–250 mg/kg/day) | TMP (160 mg/day), plus SMX (800 mg/day) | |||||
| Mean age: 39.8 ± 11.0 yrs | |||||||
n.r., not reported; PYR, pyrimethamine; TMP-SMX, trimethoprim plus sulfamethoxazole.
Quality assessment
The study quality assessment tools of the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) for quality assessment of Observational Cohort and Cross-Sectional Studies and Controlled Intervention Studies were used to assess the quality of included studies [3].
Statistical analyses
The primary outcome was rate of relapse associated with TMP-SMX maintenance therapy. Relapse rates were transformed using the Freeman-Tukey method and pooled using both fixed-effect (inverse variance method) and random-effects (DerSimonian and Laird method) meta-analysis models. The estimate of heterogeneity was taken from the inverse variance model and was quantified by the I2 consistency score.The I2 describes the percentage variation across studies attributed to heterogeneity rather than chance. High I2 values (over 75%) suggests considerable heterogeneity between studies. Scores over 50% are moderate and over 75% suggest the need to consider estimates from random-effects models [4]. The choice to present fixed or random effects results was based on heterogeneity determined by I2.The analysis was performed using StatsDirect (Altrincham, UK) statistical program version 2.8.0 (27 October 2013). To simplify interpretation independent proportions were converted to percentages reflecting relapse rates.
Results
Characteristics of studies
From the initial 707identified studies, 663 were excluded based onabstract screening and removal of duplicates. An additional38were rejectedafter full text review forthe following reasons: being an abstract only (n = 5), not administering a TMP-SMX based regimen (n = 15), not providing relapse rates (n = 5), being an epidemiology or a switching study (n = 3), not evaluating TE (n = 1), being a primary prophylaxis study (n = 3), being a review article (n = 2), not being published in English, French, Spanish, German or Portuguese (n = 2), andinclusion of inappropriate patient population (n = 2).
Study designs varied with only one randomized study, four prospective cohortsand one retrospective cohort [5,6,7,8,9,10]. No substantial differences were found in administered doses of TMP-SMX for both the acute and maintenance treatment phase across studies, except for Chaddha et al. [8] in which patients received a combination of TMP-SMX plus pyrimethamine. Four studies were either fully or partly performed in the pre-HAART era (n = 135) and two studies were conducted entirely in the post-HAART era (n = 100). The follow-up for pre-HAART studies was considerably shorter than the follow up for post-HAART [pre-HAART: 3 – 9 months; post-HAART 31 – 36 months].
Quality assesment
The quality of all (n = 6)included studies wasrated as fair, with a risk of bias due to lack of blinding, neverthelessthe reported relapse outcomes were considered of sufficient qualityto be included in the meta-analysis.
Meta-analyses
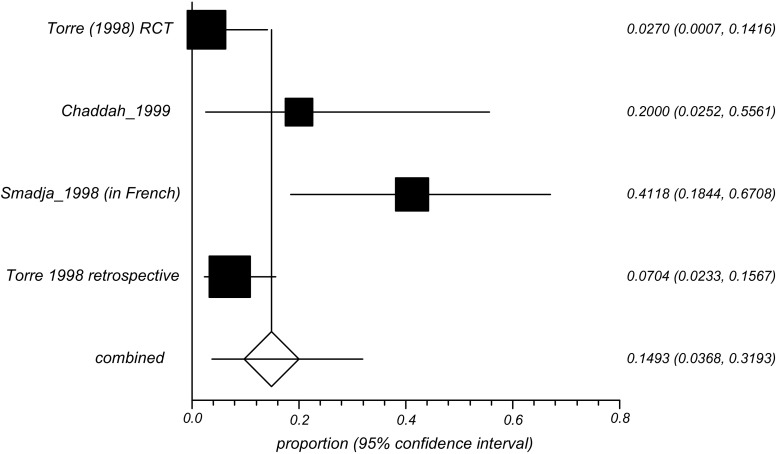
The forest plot of the six included studies illustrates the range of relapse rates observed in the analysis Figure 1.
Figure 1.
Forest Plot from the combined analysis of pre- and post-HAART studies.
The combined TMP-SMX relapse rate was 16.4% (95%CI = 6.2% to 30.3%) using a random-effects models. In a sub analysis in which only the pre-HAART studies (n = 4) were included, the relapse rate was14.9% (random effects; 95% CI = 3.7% to 31.9%) with I2 = 79.4% (95% CI = 11.7% to 90.4%). Analysis of two post-HAART studies indicated a relapse rate of 19.2% (random effects; 95% CI = 2.8% to 45.6%), I2 values unassessable due to limited number of studies (n = 2) Table 2.
Table 2.
TMP-SMX maintenance therapy relapse rates based on proportions meta-analysis for aggregate Pre- and Post-HAART and individual HAART periods.
| Relapse rate Random effects (95% CI) | I² | |
|---|---|---|
| Pre-and Post HAART combined (6 studies) | 16.4% | 83% |
| (6.2% to 30.3%) | (58.3% to 90.4%) | |
| Pre-HAART (4 studies) | 14.9% | 79.4% |
| (3.7% 31.9%) | (11.7% to 90.4%) | |
| Post-HAART (2 studies) | 19.2% | N/A* |
| (2.8% to 45.6%) |
Not assessable, limited studies included.
Discussion
Pyrimethamine plus sulfadiazine is the preferred regimen in secondary prophylaxis and pyrimethamine plus clindamycin is the preferred second-line treatment in most guidelines. TMP-SMX and other antibiotic alone and in combination are listed as alternative regimens [11,12,13].
A previous investigation using a similar protocol to the work described here reported pyrimethamine-based regimens relapse rates of 19.2% and 11.1% for fixed-effects models including 1,596 subjects during pre-HAART and post-HAART treatment eras, respectively [14].The present study identified data of six studies including 235 patients and a relapse rate of 16.2%. Although the total number of included subjects in Connolly et al. 2017 is higher than the present study, likely reflecting the licensed indication of pyrimethamine for the treatment of toxoplasmosis, the previous study also showed significant heterogeneity across studies. Considering the two studies use independent proportions test we can not directly compare the rates of relapse under different prophylaxis schemes. Future high quality randomised studies should be conducted compare the efficacy of pyrimethamine and TMP-SMX.
As reported here, the disaggregated HAART analysis did not follow the expected order in that pre-HAART relapse rates were not observed to be higher than the post-HAART which is contrary as one would expect HAART to confer benefits. We attribute this to the heterogeneity in the clinical study data based on I2 scores and a limited number of post-HAART evaluations using TMP-SMX (n = 100). In addition, although not included in the present systematic review and meta-analysis, poor adherence to TMP-SMX secondary prophylaxis seem to be the most probable cause of TE relapse [6]. Although it is tempting to compare relapse rates between interventions, as these studies represent independent assessments, a direct comparison is inappropriate here. The present analysis identified high I2 value (83%) with large interval (CI = 58.3% to 90.4%) reflecting a considerable heterogeneity among the included studies, which makes it difficult to draw conclusions. On this basis we have presented the random effect results.
Similar to the analysis reported by Hernandez et al. [1], we included a wide range of study designs in our analysis to reflect real-world outcomes for the management of secondary prophylaxis [1]. Because the focus of our analysis was on relapse rates, it was necessary that studies reported follow-up over the maintenance period and reported relapse outcomes. We believe this is an added dimension to the results reported by Hernandez., and can inform relapse rates for TMP-SMX but cautious interpretation is necessary due to the heterogeneity observed. Larger randomized clinical trials are important to obtain a conclusive answer regarding relapse rates associated with TMP-SMX for secondary prophylaxis of HIV-related TE.
Disclosure statement
The sponsor had no influence on study design or writing of the manuscript. AVH and JEV report no conflicts of interest. All authors provided final approval of the submitted manuscript. The work described here will be used to fulfil doctoral requirements for GH.
Funding
MPC and GH received funding for the statistical analysis by Turing Pharmaceuticals.
References
- [1].Hernandez AV, Thota P, Pellegrino D, et al. A systematic review and meta-analysis of the relative efficacy and safety of treatment regimens for HIV-associated cerebral toxoplasmosis: is trimethoprim-sulfamethoxazole a real option? HIV Med. 2016;18:1–10. [DOI] [PubMed] [Google Scholar]
- [2].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;6: e1–e34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- [3].NIH National Heart, Lung and Blood Institute Study quality assessment tools [cited [cited 2017, March 30]]. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools.
- [4].Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. [cited [cited 2017 Mar 30]]. Available from: http://handbook.cochrane.org. [Google Scholar]
- [5].Torre D, Casari S, Speranza F, et al. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Italian Collaborative Study Group. Antimicrob Agents Chemother. 1998;42(6):1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beraud G, Pierre-Francois S, Foltzer A, et al. Cotrimoxazole for treatment of cerebral toxoplasmosis: an observational cohort study during 1994-2006. Am J Trop Med Hyg [Internet]. 2009;80(4):583–7. 0 [PubMed] [Google Scholar]
- [7].Duval X, Pajot O, Le Moing V, et al. Maintenance therapy with cotrimoxazole for toxoplasmic encephalitis in the era of highly active antiretroviral therapy. AIDS. 2004; 18: 1342–1344. 10.1097/00002030-200406180-00016 [DOI] [PubMed] [Google Scholar]
- [8].Chaddha DS, Kalra SP, Singh AP, et al. Toxoplasmic Encephalitis in acquired immunodeficiency syndrome. J Assoc Physicians India. 1999;47:680–684. [PubMed] [Google Scholar]
- [9].Smadja D, Fournerie P, Cabre P, et al. Efficacy and tolerance of cotrimoxazole for the treatment of toxoplasmic encephalitis in AIDS patients. Presse Med. 1998;27(26):1315–1320. [PubMed] [Google Scholar]
- [10].Torre D, Speranza F, Marteganie R, Zeroli C, Banfi M, Airoldi M. A retrospective study of treatment of cerebral Toxoplasmosis in AIDS patients with Trimethoprim-sulphamethoxazole. J Infect. 1998;37:15–18. 10.1016/S0163-4453(98)90217-1 [DOI] [PubMed] [Google Scholar]
- [11].Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. [cited [cited 2017, April 20]]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- [12].European AIDS Clinical Society Guidelines. Version 8.2, 2017 [cited [cited 2017, April 20]]. Available from: http://www.eacsociety.org/files/guidelines_8.2-english.pdf.
- [13].Nelson M, Dockrell D, Edwards S, et al. British HIV Association and British Infection Association guidelines for the treatment of opportunistic infection in HIV-seropositive individuals 2011. HIV Med. 2011;12(Suppl 2):1–5. 10.1111/hiv.2011.12.issue-s2 [DOI] [PubMed] [Google Scholar]
- [14].Connolly MP, Goodwin E, Schey C, et al. Toxoplasmic encephalitis relapse rates with pyrimethamine-based therapy: systematic review and meta-analysis. Pathog Glob Heal. 2017;111:31–44. 10.1080/20477724.2016.1273597 [DOI] [PMC free article] [PubMed] [Google Scholar]