Abstract
Toxoplasmosis is an infection caused by the intracellular protozoan parasite Toxoplasma gondii, and is associated with clinically significant infection in immunocompromised individuals. Vertical transmission during pregnancy can manifest as congenital toxoplasmosis in the neonate, and can have serious consequences. This review aims to describe the modalities for prophylaxis of toxoplasmosis in susceptible populations, and focuses on the following: (1) prophylaxis of congenital toxoplasmosis; (2) prophylaxis of toxoplasmosis in patients with HIV/AIDS; and (3) prophylaxis of toxoplasmosis in transplant recipients.
Keywords: Toxoplasmosis, prophylaxis, congenital, immunocompromised, HIV, transplant
Introduction
Toxoplasmosis is an infection caused by the intracellular protozoan parasite Toxoplasma gondii. In immunocompetent individuals, infection usually results in mild, self-limiting disease. However, severe disease can result in immunocompromised individuals. Vertical transmission during pregnancy occurs, and can manifest as congenital toxoplasmosis in the neonate. Prophylaxis is recommended for these two groups of individuals. With the recent increase in the worldwide prevalence of human immunodeficiency virus(HIV)/acquired immune deficiency syndrome (AIDS), there is renewed interest in treating and preventing toxoplasmosis. This review aims to describe the modalities for prophylaxis of toxoplasmosis in susceptible populations, and focuses on the following: (1) prophylaxis of congenital toxoplasmosis; (2) prophylaxis of toxoplasmosis in patients with HIV/AIDS; and (3) prophylaxis of toxoplasmosis in transplant recipients.
Methods
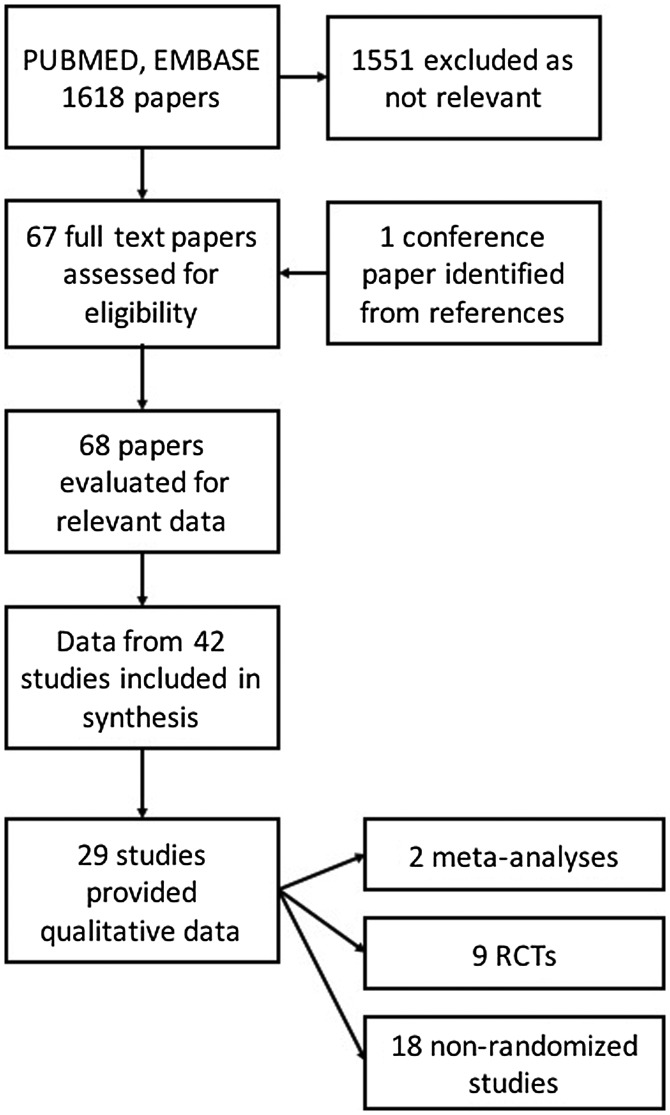
A search was carried out in PUBMED and EMBASE with the search terms ‘toxoplasmosis’ or ‘toxoplasmic’ or ‘toxoplasma’ or ‘toxoplasm*’ in the title field and prophylaxis in any field. The search terms were kept less specific in order to collect all relevant articles at the first screening. The search included all articles published from 1985 to 2017 with no restriction of language. Endnote X7 (Thomson Reuters, Carlsbad, CA 92011, USA) was used to filter the available papers. Bibliographies of cited literature were also searched. There were 1618 articles with the above search restrictions. Duplicates were removed. All abstracts were read independently by 3 authors (PW, CR, SR). Relevant articles were selected by a consensus of all authors, with verification by three authors (NLDS, DF, SR). Related or cited papers were also included. The final selection of articles included reviews, meta-analyses, randomized controlled trials, observational studies, case control studies and cohort studies related to the topic of prophylaxis in toxoplasmosis. A total of 68 potential papers were identified. Where meta-analyses summarised data, the original papers were not cited, but raw data was included in geographical mapping of data sources. The final number of papers cited in the review, after filtering was 43. Data on pharmacological prophylaxis for human toxoplasmosis were available in 29 papers (Figure 1).
Figure 1.
Selection of studies for review.
Characteristics of studies
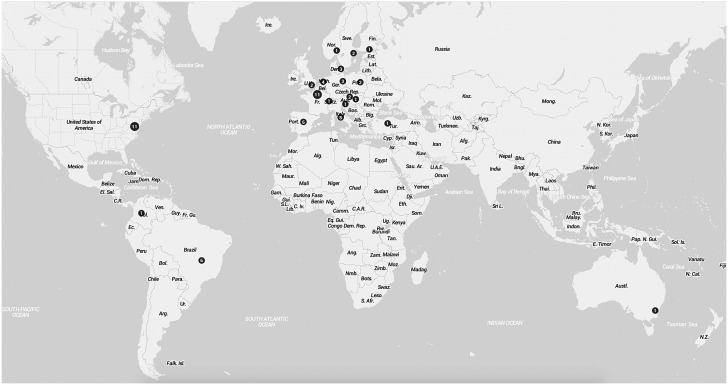
Countries from which toxoplasmosis prophylaxis efficacy data originate are shown in Figure 2. The majority of studies are from the United States of America and Europe. Of a total of 12570 patients, most were recruited in France (3234, 25.7%). Significantly, there were no studies from the African continent, which has the highest worldwide prevalence of HIV infection. There is marked variation in the genotype prevalence of Toxoplasma gondii. In Europe, the predominant genotypes are 1, 2 and 3, with the least pathogenic genotype 2 being the most prevalent [1]. On the other hand, in South America, there are many different genotypes, with none predominating, and the disease is more aggressive. Thus, data from Europe and North America may not be generalizable to other parts of the world. The characteristics and summary data from the 29 studies of pharmacological prophylaxis for toxoplasmosis are summarised in Table 1.
Figure 2.
World map showing countries of origin for data on toxoplasmosis prophylaxis. The numbers within the dots indicate the number of studies from each country. Map created using the online open source tool accessible at mapsdata.co.uk.
Table 1.
Studies providing clinical data on prophylactic regimens for toxoplasmosis in various situations.
| Year | Author | Type of study | Region | Patient group | Outcome measure | Drug regimens | No | Outcome |
|---|---|---|---|---|---|---|---|---|
| 2014 | Rodrigues et al. [11] | Cross-sectional | Brazil | Prenatal infection | Congenital toxoplasmosis | S | 247 | Reduction in severity of neonatal infection |
| 2007 | SYRACOT et al. [2] | Meta-analysis | Europe | Prenatal infection | Congenital toxoplasmosis | S, PS | 1438 | Reduction in maternal transmission. |
| 2015 | Valentini et al. [16] | Retrospective comparative | Italy | Prenatal infection | Congenital toxoplasmosis | S+TS vs.PS or S | 120 | S+TS superior to S, equal to PS |
| 2009 | Valentini et al. [15] | Retrospective single arm | Italy | Prenatal infection | Congenital toxoplasmosis | S+TS | 76 | Incidence of congenital toxoplasmosis 2.6%, |
| 2016 | Avci et al. [12] | Retrospective observational | Turkey | Prenatal infection | Congenital toxoplasmosis | S | 129 | Reduction in maternal transmission |
| 2010 | Cortina-Borja et al. [9] | Prospective observational | Europe | Prenatal infection | Severity of congenital toxoplasmosis | S, PS | 293 | Reduction in SNS or death |
| 2014 | Avelino et al. [10] | Prospective observational | Brazil | Prenatal infection | Severity of congenital toxoplasmosis | S | 246 | Reduction in maternal transmission. |
| 1999 | Ribera et al. [24] | Case control | Spain | HIV | TE | TS high vs. low dose | 521 | TS high dose superior, more adverse effects |
| 1997 | Bucher et al. [28] | Meta-analysis of RCTs | Europe | HIV | TE | TS vs. DP/D vs. aP/iP | 1484 | TS & DP equivalent, aP/iP inferior |
| 1996 | Klinker et al. [29] | Prospective observational | Germany | HIV | TE | P | 56 | TS in 1/56 |
| 1992 | Schneider et al. [21] | RCT | Netherlands | HIV | TE | TS vs. aP | 215 | TS superior to P, TS high vs. low dose similar |
| 1995 | Antinori et al. [26] | RCT | Italy | HIV | TE | TS vs. DP | 197 | TS and DP effective |
| 1995 | Opravil et al. [27] | RCT | Switzerland | HIV | TE | DP vs aP | 533 | DP superior to aP, higher adverse events |
| 1995 | Bozzette et al. [23] | RCT | USA | HIV | TE | TS vs. aP vs. D | 843 | TS and D superior to P when CD4<100/mm3 |
| 1995 | Durant et al. [33] | RCT | France | HIV | TE | aP vs. aP+R vs. R | 52 | R appears superior to aP alone |
| 1996 | Leport et al. [31] | RCT | France | HIV | TE | P vs. placebo | 554 | No difference |
| 1994 | Jacobson et al. [30] | RCT | USA | HIV | TE, death | P vs. placebo | 335 | Death rate higher with P, no dif in TE incidence |
| 1993 | Girard et al. [25] | RCT | France | HIV | Toxoplasma infection | DP vs. aP | 349 | PD superior to aP |
| 2001 | Schurmann et al. [32] | Prospective observational | Germany | HIV+previous PCP | TE | PS | 95 | PS safe. TE in 1/69 Toxoplasma positives |
| 1992 | Hardy et al. [22] | RCT | USA | HIV+previous PCP | TE | TS vs. aP | 310 | Inconclusive |
| 1992 | Carr et al. [20] | Retrospective comparative | Australia | HIV+previous PCP | TE | TS vs. aP/iP | 155 | TS superior to P |
| 2006 | Baran et al. [37] | Prospective observational | USA | Heart transplant | Toxoplasma infection | TS | 596 | Possible reduction in toxoplasma infection |
| 2001 | Montoya et al. [35] | Prospective observational | USA | Heart transplant | Toxoplasma infection | TS | 620 | Inconclusive |
| 2012 | Strabelli et al. [39] | Retrospective observational | Brazil | Heart transplant | Toxoplasma infection | P | 436 | Possible reduction in toxoplasma infection |
| 1989 | Wheghitt et al. [38] | Prospective observational | UK | Heart/lung transplant | Toxoplasma infection | P | 285 | Inconclusive |
| 2003 | Baden et al. [36] | Prospective observational | USA | Heart/lung transplant | Toxoplasma infection | TS | 250 | Possible reduction in toxoplasma infection |
| 1994 | Foot et al. [41] | Prospective observational | France | HSCT | Toxoplasma infection | PS | 69 | Bone marrow toxicity with P |
| 2016 | Conrad et al. [43] | Retrospective case control | France | HSCT | Toxoplasma infection | TS vs. A or PS | 588 | TS superior |
| 2015 | Mendorf et al. [42] | Retrospective observational | Germany | HSCT | Toxoplasma infection | TS, A, iP | 155 | TS or A possibly superior to iP |
Notes: TE-toxoplasmic encephalitis, PS-pyrimethamine+sulphadoxine, P-pyrimethamine, TS-trimethoprim+sulphamethoxazole, aP-aerosolized pentamidine, iP-intravenous pentamidine, S-spiramycin, D-dapsone, A-atovaquone, DP-dapsone+pyrimethamine, R-roxithromycin, HIV-human immunodeficiency virus infection, HSCT- hematopoietic stem cell transplant, PCP-Pneumocystis jiroveci pneumonia, RCT-randomized controlled study, SNS-serious neurological sequalae, USA-United States of America, UK- United Kingdom..
Prophylaxis of congenital toxoplasmosis
Toxoplasma infection during pregnancy can result in congenital toxoplasmosis. Congenital toxoplasmosis has a spectrum of manifestations, ranging from asymptomatic or mild neonatal infection to the classical triad of hydrocephalus, intracranial calcifications and choroidoretinitis. The more severe forms of the disease can result in permanent neurological impairment, seizures and blindness, often termed serious neurological sequalae or SNS. The risk of transmission and subsequent infection increases with advancing gestational age. However, the severity of disease is higher with seroconversion at an earlier gestational age [2].
Prevention of maternal infection
Based on available evidence from case control and retrospective cohort studies, exposure to raw or undercooked meat (lamb, mutton, pork), improper cleaning of utensils following preparation of raw meat, consumption of under-washed raw vegetables and fruits, exposure to soil, poor hand hygiene, and consumption of unfiltered water, have emerged as major risk factors [3–6]. Exposure to cat litter and travel to areas with high prevalence rates are also associated with maternal infection [3]. Prevention of maternal infection is foremost in the prevention of congenital toxoplasmosis, and the preventive principles are centered around the lifecycle of the parasite Toxoplasma gondii. Education of mothers on the prevention of toxoplasmosis has also been shown to play a significant role in the prevention of maternal infection [7].
Screening for congenital toxoplasmosis in pregnancy is recommended in high risk individuals (HIV positive/ immunosuppressed), or in patients with ultrasound evidence of congenital toxoplasmosis such as hydrocephalus, intracranial calcifications, intra-uterine growth retardation, and hepatospenomegaly [8].
Prenatal treatment for prevention of congenital toxoplasmosis
Prenatal treatment is offered to pregnant women with evidence of seroconversion in pregnancy but its efficacy is controversial. Evidence regarding the efficacy of prenatal treatment is primarily derived from a meta-analysis by the SYROCOT study group [2] and data from the EMSCOT trial [9]. The meta-analysis by the SYROCOT study group in 2007 regarding effectiveness of prenatal treatment in congenital toxoplasmosis included 26 observational prospective cohorts with a total of 1438 treated mothers identified by prenatal screening. Treatment was with spiramycin (S) or pyrimethamine+sulphonamide (PS). No randomized controlled clinical trials were available. This meta-analysis demonstrated only weak evidence of reduction in mother to child transmission when treatment was commenced at three weeks of seroconversion compared to eight weeks. (Adjusted odds ratio [OR] 0.48, 95% CI 0.28–0.80; p=0.05). The same meta-analysis concluded that prenatal treatment did not reduce the clinical severity of the illness (adjusted OR for treated vs. not treated 1.11, 95% CI 0.61–2.02). No statistically significant reduction in choroido-retinitis with prenatal treatment was found. However, the odds of intracranial lesions was reduced (OR = 0.9, CI 0.87–0.95). The findings of this meta-analysis question the efficacy of prenatal treatment.
Data from EMSCOT (European Multicenter Study on Congenital Toxoplasmosis) in 2010, which was a prospective observational cohort study of 293 subjects, reported significant reduction in serious neurological sequalae (SNS) of toxoplasmosis with prenatal treatment [9]. There was a statistically significant reduction in SNS (OR = 0.24 CI 0.07–0.71) with prenatal treatment after adjusting for gestational age. However, the number of cases of SNS in this study was low (23/293, 7.8%), and the results should be interpreted with caution.
Another prospective cohort study conducted from 2003 to 2011 in Brazil showed reduction in severity of neonatal infection with prenatal treatment [10]. The severe infection rate was 18.6% (13/70) in the treated group, whereas it was 60.7% (33/84) in the untreated group (OR=0.148 CI 0.03- 0.311). A community based cross sectional study was conducted in Brazil by the same group of investigators between 2004 and 2011 to assess whether prenatal treatment for toxoplasmosis interferes with detection of laboratory markers of congenial toxoplasmosis [11]. In this study, investigators also assessed the presence of clinical features and their severity in relation to prenatal treatment. However, in the design of this study, prenatal treatment was retrospectively analysed in newborns with Toxoplasma infection. Therefore, it is not possible to ascertain whether prenatal treatment prevents transmission of infection to the fetus. However, it was revealed that clinically evident congenital toxoplasmosis was less among the treated group. In the untreated group, 68.4% (13/19) were born with severe clinical infection whereas only 29.6% (8/27) of the treated group had clinical evidence of infection, and none had severe infection. However, the difference was not clinically significant (p = 0.096). The presence of hydrocephalus and optic neuropathy were significantly less in the treated group (p = 0.018 and 0.038 respectively).
In a multi-center retrospective study on the role of spiramycin for prevention of fetal toxoplasmosis, 55 out of 61 women with acute toxoplasmosis (based on serology) during pregnancy were given prophylactic spiramycin [12]. None of the newborns whose mothers were treated developed congenital toxoplasmosis. The other six patients refused treatment, and four were diagnosed to have fetal infection, and therapeutic termination was done. The other two had uncomplicated child birth with no congenital infection. This was, however, a non-randomised, retrospective study, with only a small number in the untreated group, which makes the validity of results questionable.
Prenatal treatments studied for the prevention of congenital toxoplasmosis include S and PS. The efficacy of these regimens are discussed below. There are no randomised studies on prenatal treatment of toxoplasmosis [13].
Prophylactic regimens
Spiramycin
Spiramycin (S) is a macrolide antibiotic which is thought to be concentrated in placental tissue and prevent transmission of infection to the fetus. Studies examining the efficacy and safety of S are scarce, with initial placental studies demonstrating some benefit [14]. In the two Brazilian studies mentioned above, prophylaxis with spiramycin was shown to be efficacious [10,11], although the safety profile was not assessed in these studies. However, the SYROCOT meta-analysis failed to demonstrate efficacy of spiramycin when compared to no treatment (OR = 0.68; 0.31–1.52).
Spiramycin vs. pyrimethamine and sulphonamide
Placental transmission of S is poor, hence PS has been used for treatment when there is demonstrable evidence of fetal infection. However, there is no evidence to demonstrate efficacy of PS alone, or superiority over S, as demonstrated by the SYROCOT study. PS has a higher side effect profile including bone marrow toxicity compared to S. Since the role of treatment itself has little evidence to support it, the decision to treat is largely a clinical one, especially considering the adverse effects of treatment. No recommendadtions can be reached without examining the effects of these medications in properly designed randomized controlled trials. However, such studies would pose many ethical dilemmas. The above issues have been raised by a Cochrane review which highlights the need of efforts to examine the cost effectiveness of screening and prevention programs for congenital toxoplasmosis [13].
Spiramycin with trimethoprim and sulphamethoxazole
There is limited evidence on the use of the spiramycin with trimethoprim+sulphamethoxazole (TS) combination in prenatal treatment. Data in this regard is available from a single arm retrospective study in 76 mothers with toxoplasmosis administered the above combination [15]. The incidence rate of congenital toxoplasmosis was 2.6%. Side effects were also minimal. However further investigation is required into use of this potentially efficacious and safe treatment option. Another retrospective analysis was conducted to compare S/TS combination vs. PS and spiramycin alone [16]. Out of 123 newborns (of 120 mothers), 43, 70 and 10 mothers of newborns were treated with S alone, S/TS, and PS respectively. The association between trimester of maternal infection and prenatal treatment with risk of transmission to the newborn were compared using odds ratio. After correcting for trimester using logistic regression, those treated with spiramycin alone showed increased odds of transmission compared to S/TS combination (OR = 4.368, 95% CI: 1.253–15.219). However, there was no significant reduction in odds when S/ TS was compared with PS (OR = 1.83, 95% CI: 0.184–18.274), although the sample size in the third group was too small to determine clinical significance. Overall, this study favours combination treatment with S/TS. Though the authors have claimed that there were no teratogenic effects observed with TS, details of assessment was not presented. Larger studies are needed to estimate risk-benefit ratio in this treatment strategy.
Other macrolides
Theoretical evidence exists for the use of azithromycin in prenatal treatment, as it has been shown to have benefit in prevention of ocular manifestations of congenital toxoplasmosis in animal models. This association should be studied further, as there may be a place for this drug particularly in immunocompromised individuals, in view of the adverse effects of PS [17].
Prophylaxis of toxoplasmosis in HIV patients
Reactivation of latent toxoplasmosis occurs in patients with AIDS, with devastating effects. Both primary and secondary prevention of toxoplasmosis in HIV patients is likely to be beneficial. Ideally, patients with HIV should undergo screening for toxoplasmosis at the time of diagnosis [18]. General recommendations for prevention should be instituted to all patients including those who are seronegative at the time of diagnosis. Central nervous system disease presenting with toxoplasmic encephalitis (TE) is the most common manifestation, especially in AIDS patients with a CD4 count <100/mm3. The greatest risk occurs when the CD4 count drops <50/mm3 [19]. Therefore, primary prophylaxis is indicated for AIDS patients with a CD4 count less <100/mm3 who are IgG positive for toxoplasmosis. Much of the evidence for primary prevention of TE is derived from clinical trials examining the treatment and prevention of Pneumocystis infection. TS, dapsone-pyrimethamine (DP) and atovaquone have been studied as potential options.
Prophylactic regimens for primary prophylaxis in HIV/AIDS
Trimethoprim and sulfamethoxazole vs. pentamidine
A retrospective study included patients with AIDS during a three-year period after primary episodes of Pneumocystis jiroveci pneumonia (PCP) [20]. Of these patients, 60 received low dose TS (trimethoprim 160 mg plus sulfamethoxazole 800 mg; one tablet twice daily, 2 days per week), and 95 patients received pentamidine (P) (aerosolized in 78 patients and intravenous in 17 patients). None of the patients in the TS group developed TE after a follow up period of 1153 days. In contrast, among patients in the P group, 12 of 36 (33%) seropositive patients developed TE, with a median time to TE of 460 days. The CD4 count at the beginning of prophylaxis did not appear to influence the risk of developing TE.
Further clinical trials have examined the efficacy of TS. In a multi-center trial, 215 HIV patients with no history of PCP and CD4 counts <200/mm3 were randomized to receive either aerosolized pentamidine (aP) once a month, 480 mg of TS once a day (trimethoprim 80 mg and sulfamethoxazole 400 mg), or 960 mg of TS once a day (160 mg and 800 mg) [21]. Only 3 cases of TE were diagnosed after a mean follow up duration of 288 days in the group given 480 mg of TS, and 277 days in the group given 960 mg of TS. The investigators concluded apparent superiority of TS over AP, with equal efficacy between the two TS dosing regimens. However, as the incidence of TE was low, the statistical power of these conclusions was low. This study also demonstrates a significant adverse effect profile of TS when compared to AP. Patients on higher doses of TS developed adverse effects earlier than those on lower doses. Major adverse effects reported were leucopenia, fever, rash and gastrointestinal disturbances.
Further weak evidence for the use of TS in TE is demonstrated in from an open-label study in 310 adults with recent PCP infection [22]. They were randomized to receive TS (trimethoprim 160 mg and sulfamethoxazole 800 mg once daily) or aP 300 mg once every four weeks. Four out of 154 (2.6%) developed TE with the TS regimen, and 6/156 (3.8%) with the aP regimen. Again, these numbers were too small to reach statistically worthwhile conclusions.
Similar conclusions are also derived from an open label randomized trial of three anti-Pneumocystis agents in patients with HIV [23]. A total of 843 participants were randomized to receive TS (one double-strength tablet 160 mg of trimethoprim and 800 mg of sulfamethoxazole twice daily), dapsone(D) (50 mg twice daily), or aP (300 mg every four weeks). A set protocol was used to alter the dose and type of medication in case of intolerance. The median time of follow up was 39 months, and a total of 24 cases of toxoplasmosis were diagnosed during this time period. Five cases of toxoplasmosis occurred in patients on TS, five in patients on dapsone and 14 in patients on aP. TS or D was superior to aP in patients with CD4 counts <100 mm3.
The question of dosing of TS is addressed by a case control study evaluating the dose of TS for TE prophylaxis [24]. Data was extracted from a cohort of 521 HIV infected patients. Twenty-seven (84.4%) of 32 case patients and 33 (51.6%) of 64 control patients received low doses of TS. The adjusted odds ratio (OR) was 9.36 (95% confidence interval [CI], 2.05–42.75). High doses appeared to be more effective than low doses but at the expense of higher adverse effects. This finding is in potential conflict with the findings of Schneider et al. [21] who did not find a dose related alteration of efficacy. Further studies have also examined an intermittent dosing schedule for TS (see below).
As is apparent from the data above, there is some evidence to support the use of TS as a first line agent in the primary prophylaxis of TE in HIV patients. These studies also demonstrate the superiority of TS over aP. Nonetheless, because of the low seroprevalence rate and incidence rate of toxoplasmosis in these trials, it is difficult to come to a clear conclusion.
Dapsone plus pyrimethamine vs. other drugs
Other prophylactic regimens studied include dapsone and pyrimethamine. A randomized study was carried out in 349 patients who had symptomatic HIV infection and CD4+counts below 200/mm3, with no history of PCP or symptomatic toxoplasmosis [25]. One group received DP (dapsone 50 mg per day and pyrimethamine 50 mg per week), and the other group received aP. Thirty-two out of 172 patients (18.6%) developed toxoplasmosis in the aP group compared to 19/173 (11%) in the DP group. The relative risk of developing symptomatic toxoplasmosis was 2.37 times higher in the AP group in patients seropositive to toxoplasmosis (95% CI, 1.3 to 4.4; P = 0.006). The adverse effect profile of DP was recorded to be higher than AP. This study showed that DP may be more efficacious for prophylaxis of toxoplasmosis compared to AP, but has a poorly tolerated adverse effect profile, which included hematological toxicity, skin rash and fever.
The efficacy of DP was examined against intermittent TS in a randomized, open label prospective study in 197 patients with HIV and CD4 counts <200/mm3 without previous PCP or TE [26]. Subjects were randomized to receive aP (aP 300 mg monthly), TS (trimethoprim 160 mg and sulfamethoxazole 800 mg every other day) or DP(dapsone 100 mg weekly and pyrimethamine 25 mg bi-weekly). Based on an intention to treat analysis, none of the patients in the DP group developed TE, while rates were 34.7 per 100 person-years in the aP group and 2.5 per 100 person-years in the TS group (aP vs. TS: p = 0.01; AP vs. DP: p = 0.004). This trial demonstrated the efficacy of intermittent TS and DP in primary prophylaxis of TE.
Another trial evaluated weekly DP vs. aP [27]. In this trial, 533 patients with symptomatic HIV and/or CD4 <200/mm3 were randomized into two groups. One group received DP and the other aP. Fourteen cases of TE were reported in the DP group, compared to 20 cases in the aP group, with only 2 cases occurring during actual treatment in the DP group. This trial also reports a poor adverse effect profile of DP, with 30% of patients showing poor tolerance.
A meta-analysis on primary prophylaxis for TE in patients with HIV was performed by Bucher et al. [28] in 1997, of 22 randomized controlled trials. A total of 1484 patients were treated with TS, 1548 patients with DP or dapsone, 1800 patients with aP, and 38 patients with P and aP. This meta-analysis concluded that for the prevention of toxoplasmosis, TS was equivalent to DP, with higher efficacy when compared to AP (DP vs. aP risk ratio 0.72 [95% CI, 0.54–0.97], TS vs. aP 0.78 [95% CI, 0.55–1.11], TS vs. DP 1.17 [95% CI, 0.68–2.04]) [28]. The investigators did not find any difference in comparison between high and low doses of TS. Further subgroup analysis demonstrated significant reduction of mortality in patients on TS vs. DP 0.43(95% CI, 0.21–0.88) in patients with CD4 counts <100/mm3. Safety profiles of the above treatment options were also examined, with drug limiting toxicity noted in 477 (31.5%) of the patients on TS, 460 (29.7%) of patients on DP, and 123 (6.8%) of patients on aP. The findings of this meta-analysis suggest that TS is more efficacious when used as a first line option for prevention of TE. In patients intolerant of TS, DP seem to be a rational choice. However further studies should be designed to optimize dosage regimens, taking into consideration their adverse effect profiles.
Pyrimethamine/pyrimethamine plus sulphadoxine
A few other less popular prophylactic regimens have also been examined. These include pyrimethamine (P) monotherapy and PS. In one study, prophylaxis for TE with P (50 mg daily) was evaluated in 56 patients with advanced HIV, of whom 38/56 were at high risk for toxoplasmic encephalitis (CD4+counts <200/mm3, and seropositive to Toxoplasma gondii) [29]. The overall treatment period was 697 months (mean 12.5, SD ± 12.1). During prophylaxis, only one patient developed toxoplasmic encephalitis, and four patients discontinued treatment due to adverse reactions. This study was not a randomized trial and the results should be interpreted with caution. To address the deficiencies of this study, a double blind randomized clinical trial was carried out to compare the efficacy of P vs. placebo, in patients on prophylaxis for PCP [30]. This trial included patients with HIV, CD4 count <200/mm3, or prior AIDS defining opportunistic infection, who were seropositive to Toxoplasma IgG, but had no evidence of TE. The death rate was higher for patients receiving pyrimethamine, compared to placebo (relative risk [RR] 2.5; 95% confidence interval [CI], 1.3–4.8; p = 0.006) after adjusting for other factors affecting survival; however there was no difference in the risk of developing TE (RR 1.3, 95% CI, 0.35–5.01; p = 0.68). A further randomized controlled clinical trial comparing P and placebo was carried out in 554 HIV patients seropositive for TG with CD4 <200/mm [31]. The findings of this trial demonstrate no difference in the incidence of TE between the pyrimethamine and placebo groups, with a similar survival rate. In summary, pyrimethamine monotherapy does not demonstrate efficacy or safety for the primary prevention of TE in patients with HIV.
Based on the use of PS in the prevention of congenital toxoplasmosis, efforts have been made to evaluate its role in TE prophylaxis. The efficacy and safety of twice weekly PS was evaluated for primary prevention of TE in 95 patients with HIV with successfully treated PCP, in a single arm open label study [32]. Of 69 patients positive for anti-Toxoplasma IgG antibodies, one (1.5%) developed cerebral lesions compatible with toxoplasmic encephalitis after 50 months. The reported safety profile of the regimen was also reported to be favourable. However, this apparently efficacious regimen requires further evaluation.
Other macrolides
Preliminary data is available from a randomized trial in 52 patients with HIV regarding the role of roxithromycin (R) in the primary prevention of TE [33]. Five out of 18 patients treated with aP, 1/17 patients treated with aP+roxithromycin, and none of 17 patients treated with roxithromycin alone developed cerebral toxoplasmosis (p = 0.038). There are no studies of spiramycin for prevention of toxoplasmosis in HIV patients.
In summary, TS and DP are the most efficacious options for the primary prophylaxis of TE in patients infected with HIV. Further characterization of dosing regimens is required. Promising evidence also is available for R and PS monotherapy. There have been no adequately powered studies on the use of atorvaquone in the primary prophylaxis of TE.
Secondary prophylaxis
Current Center for Disease Control (CDC) guidelines suggest that PS prophylaxis should be administered to patients with an episode of TE to prevent relapses [18]. Treatment with HAART has been shown to reduce relapse rates in patients on pyrimethamine-based secondary prophylaxis [34]. Alternatives include P and clindamycin/atovaquone.
Prophylaxis for Toxoplasmosis in solid organ transplantation
Toxoplasmosis is a rare but devastating infection following organ transplantation. Patients undergoing cardiac transplantation are at the highest risk, as Toxoplasma cysts can be transmitted in the transplanted muscle tissue from seropositive donors. Thus, most data available is from experiences with cardiac transplantation, with some evidence in patients with bone marrow transplantation. No controlled trials are available.
Heart transplant
There is limited evidence that TS may prevent toxoplasmosis in patients undergoing heart transplantation. Evidence for this is largely from cohorts given TS to prevent PCP. In a descriptive study on infectious complications among 620 consecutive heart transplant patients, the authors concluded that there was insufficient data to recommend TS for prophylaxis against toxoplasmosis [35]. Observational, uncontrolled studies have shown that in heart transplant patients, TS appeared to reduce the risk of developing symptomatic toxoplasmosis [36,37].
P has also been evaluated in prophylaxis for toxoplasmosis in cardiac transplantation, although evidence is very limited. A study in 1989 evaluated 217 out of 250 heart and 33 out of 35 heart and lung transplant recipients for Toxoplasma infection [38]. Prophylaxis with pyrimethamine was introduced when T. gondii antibody negative transplant recipients had a T. gondii antibody positive donor. Four (57%) patients who were not offered prophylaxis acquired primary T. gondii infection compared to 2 (14%) receiving P prophylaxis. A retrospective study in 436 adult patients receiving cardiac transplantation reported that six patients developed disseminated toxoplasmosis [39]. None of these patients were on prophylactic medication. After commencing routine P prophylaxis in 1996, no cases of toxoplasmosis were detected up to 2012. Further studies are required, in high volume cardiac transplantation centers, to investigate these findings further.
Hematopoetic stem cell transplantation
Toxoplasmosis is a rare infection associated with hematopoietic stem cell transplantation (HSCT). Risk factors for developing toxoplasmosis following HSCT are: allogeneic HSCT with R (+), HSCT with cord cells, GVHD, history of previous clinical toxoplasmosis and use of corticosteroids for prolonged periods or in high doses [40].
Apart from general measures for prophylaxis of toxoplasmosis, drug treatment with P has been studied in observational studies. Very limited evidence is available. In one study of 69 patients who were administered weekly P over a follow up period of 21 months since the time of established engraftment, no instances of toxoplasmosis were recorded [41]. However, bone marrow toxicity with P was reported, requiring cessation of therapy in 4/69 patients, and interruption of therapy in 8/69 patients. Another retrospective study involving 155 patients who received HSCT investigated the role of TS and atovaquone as possible prophylactic agents against toxoplasmosis [42]. Of 141 patients who received TS as first line treatment, 16 were changed to other treatment options (13 to atovaquone, 3 to aerosolized pentamidine) due to drug intolerance. Eight patients received atovaquone and six patients received aP as first line treatment. None of the patients who received continuous TS or atovaquone developed active toxoplasmosis. Two patients who were converted to pentamidine from TS and atovaquone due to intolerance developed active cerebral toxoplasmosis. This provides supportive evidence that TS or atovaquone maybe superior as prophylaxis against toxoplasmosis in patients who underwent HSCT. However, due to the heterogenous study population with differences in Toxoplasma serostatus, other immunosuppressive treatment, and conversion of treatment at different stages, evidence from this study cannot be taken as conclusive evidence.
A retrospective observational case control study on a population of 588 patients who had allogenic HSCT to assess risk factors for toxoplasmosis was conducted [43]. Out of 588 patients 23 who developed toxoplasmosis and 46 controls who did not develop toxoplasmosis and had similar donor characteristics were selected. The risk of toxoplasmosis was compared with the regimen of prophylaxis and other factors. TS was taken as ‘effective treatment’ and atovaquone or PS were considered as possibly effective prophylactic regimens. Out of 23 cases 19 were not on treatment and 3 were on effective treatment and other on possibly effective treatment (21.7% on treatment). Thirty-two out of 46 controls were on treatment (69.5%). Lack of ‘effective treatment’ was strongly associated with toxoplasmosis occurrence (OR = 11.95, 95% CI: 3.04–46.88). Association of risk with possible effective treatment vs. effective treatment did not show statistical significance (OR = 6.28, 95% CI: 0.75- 52.82). Overall the study supports prophylactic treatment with TS, but the efficacy of alternative treatments is unclear, partly due to small numbers of patients treated with those regimens.
Conclusions
-
(1)
General life style modifications are important for prevention, and are complimentary to the pharmacological modalities in the prevention of toxoplasmosis in high risk groups.
-
(2)
The efficacy of prenatal treatment for congenital toxoplasmosis is controversial; current regimens used commonly include S and PS.
-
(3)
Primary prophylaxis of TE in patients with HIV should be commenced in patients with Toxoplasma seropositivity with a CD4 count <100/mm3. Currently used agents include trimethoprim – sulfamethoxazole (TS), and dapsone – pyrimethamine (DP)
-
(4)
Evidence is limited on the role of Toxoplasma prevention in organ transplantation, with possible efficacy in the use of TS and P.
Disclosure statement
All authors declare no conflict of interest.
Contributions
SR and PW conceived the paper. PW, SR and NLDS searched the literature. All authors were involved in selection and critical review of the papers for the review. PW, CR and SR wrote the first draft. PW, DF and SR finalised the manuscript.
References
- [1].Shwab EK, Zhu XQ, Majumdar D, et al. . Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. 2014;141(04):453–461. 10.1017/S0031182013001844 [DOI] [PubMed] [Google Scholar]
- [2].SYROCOT (Systematic Review on Congenital Toxoplasmosis) study group, Thiebaut R, Leproust S, et al. . Effectiveness of prenatal treatment for congenital toxoplasmosis: a meta-analysis of individual patients’ data. Lancet. 2007;369(9556):115–122. [DOI] [PubMed] [Google Scholar]
- [3].Baril L, Ancelle T, Goulet V, et al. . Risk factors for Toxoplasma infection in pregnancy: a case-control study in France. Scand J Infect Dis. 1999;31(3):305–309. [DOI] [PubMed] [Google Scholar]
- [4].Cook AJ, Gilbert RE, Buffolano W, et al. . Sources of toxoplasma infection in pregnant women: European multicentre case-control study. European research network on congenital toxoplasmosis. BMJ. 2000;321(7254):142–147. 10.1136/bmj.321.7254.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kapperud G, Jenum PA, Stray-Pedersen B, et al. . Risk factors for toxoplasma gondii infection in pregnancy: results of a prospective case-control study in Norway. Am J Epidemiol. 1996;144(4):405–412. 10.1093/oxfordjournals.aje.a008942 [DOI] [PubMed] [Google Scholar]
- [6].Bobic B, Nikolic A, Djurkovic-Djakovic O. Identification of risk factors for infection with Toxoplasma gondii in Serbia as a basis of a program for prevention of congenital toxoplasmosis. Srp Arh Celok Lek. 2003;131(3–4):162–167. 10.2298/SARH0304162B [DOI] [PubMed] [Google Scholar]
- [7].Foulon W, Naessens A, Lauwers S, et al. . Impact of primary prevention on the incidence of toxoplasmosis during pregnancy. Obstet Gynecol. 1988;72(3 Pt 1):363–366. [PubMed] [Google Scholar]
- [8].Paquet C, Yudin MH. Toxoplasmosis in pregnancy: prevention, screening, and treatment. J Obstet Gynaecol Can. 2013;35(1):78–79. 10.1016/S1701-2163(15)31053-7 [DOI] [PubMed] [Google Scholar]
- [9].Cortina-Borja M, Tan HK, Wallon M, et al. . Prenatal treatment for serious neurological sequelae of congenital toxoplasmosis: an observational prospective cohort study. PLoS Med. 2010;7(10):e1000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Avelino MM, Amaral WN, Rodrigues IM, et al. . Congenital toxoplasmosis and prenatal care state programs. BMC Infect Dis. 2014;14:41 10.1186/1471-2334-14-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rodrigues IM, Costa TL, Avelar JB, et al. . Assessment of laboratory methods used in the diagnosis of congenital toxoplasmosis after maternal treatment with spiramycin in pregnancy. BMC Infect Dis. 2014;14:349 10.1186/1471-2334-14-349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Avci ME, Arslan F, Ciftci S, et al. . Role of spiramycin in prevention of fetal toxoplasmosis. J Matern Fetal Neonatal Med. 2016;29(13):2073–2076. 10.3109/14767058.2015.1074998 [DOI] [PubMed] [Google Scholar]
- [13].Peyron F, Wallon M, Liou C, et al. . Treatments for toxoplasmosis in pregnancy. Cochrane Database Syst Rev. 2000(2):CD001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Couvreur J, Desmonts G, Thulliez P. Prophylaxis of congenital toxoplasmosis. effects of spiramycin on placental infection. J Antimicrob Chemother. 1988;22 Suppl B:193–200. 10.1093/jac/22.Supplement_B.193 [DOI] [PubMed] [Google Scholar]
- [15].Valentini P, Annunziata ML, Angelone DF, et al. . Role of spiramycin/cotrimoxazole association in the mother-to-child transmission of toxoplasmosis infection in pregnancy. Eur J Clin Microbiol Infect Dis. 2009;28(3):297–300. 10.1007/s10096-008-0612-5 [DOI] [PubMed] [Google Scholar]
- [16].Valentini P, Buonsenso D, Barone G, et al. . Spiramycin/cotrimoxazole versus pyrimethamine/sulfonamide and spiramycin alone for the treatment of toxoplasmosis in pregnancy. J Perinatol. 2015;35(2):90–94. 10.1038/jp.2014.161 [DOI] [PubMed] [Google Scholar]
- [17].Lopes CD, Silva NM, Ferro EA, et al. . Azithromycin reduces ocular infection during congenital transmission of toxoplasmosis in the calomys callosus model. J Parasitol. 2009;95(4):1005–1010. 10.1645/GE-1765.1 [DOI] [PubMed] [Google Scholar]
- [18].Kaplan JE, Benson C, Holmes KH, et al. . Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207; quiz CE1-4. [PubMed] [Google Scholar]
- [19].Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327(23):1643–1648. 10.1056/NEJM199212033272306 [DOI] [PubMed] [Google Scholar]
- [20].Carr A, Tindall B, Brew BJ, et al. . Low-dose trimethoprim-sulfamethoxazole prophylaxis for toxoplasmic encephalitis in patients with AIDS. Ann Intern Med. 1992;117(2):106–111. 10.7326/0003-4819-117-2-106 [DOI] [PubMed] [Google Scholar]
- [21].Schneider MM, Hoepelman AI, Schattenkerk JK, et al. . A controlled trial of aerosolized pentamidine or trimethoprim-sulfamethoxazole as primary prophylaxis against Pneumocystis carinii pneumonia in patients with human immunodeficiency virus infection. The Dutch AIDS Treatment Group. N Engl J Med. 1992;327(26):1836–1841. 10.1056/NEJM199212243272603 [DOI] [PubMed] [Google Scholar]
- [22].Hardy WD, Feinberg J, Finkelstein DM, et al. . A controlled trial of trimethoprim-sulfamethoxazole or aerosolized pentamidine for secondary prophylaxis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trials Group Protocol 021. N Engl J Med. 1992;327(26):1842–1848. 10.1056/NEJM199212243272604 [DOI] [PubMed] [Google Scholar]
- [23].Bozzette SA, Finkelstein DM, Spector SA, et al. . A randomized trial of three antipneumocystis agents in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. N Engl J Med. 1995;332(11):693–699. 10.1056/NEJM199503163321101 [DOI] [PubMed] [Google Scholar]
- [24].Ribera E, Fernandez-Sola A, Juste C, et al. . Comparison of high and low doses of trimethoprim-sulfamethoxazole for primary prevention of toxoplasmic encephalitis in human immunodeficiency virus-infected patients. Clin Infect Dis. 1999;29(6):1461–1466. 10.1086/313515 [DOI] [PubMed] [Google Scholar]
- [25].Girard PM, Landman R, Gaudebout C, et al. . Dapsone-pyrimethamine compared with aerosolized pentamidine as primary prophylaxis against Pneumocystis carinii pneumonia and toxoplasmosis in HIV infection. The PRIO Study Group. N Engl J Med. 1993;328(21):1514–1520. 10.1056/NEJM199305273282102 [DOI] [PubMed] [Google Scholar]
- [26].Antinori A, Murri R, Ammassari A, et al. . Aerosolized pentamidine, cotrimoxazole and dapsone-pyrimethamine for primary prophylaxis of Pneumocystis carinii pneumonia and toxoplasmic encephalitis. AIDS. 1995;9(12):1343–1350. 10.1097/00002030-199512000-00007 [DOI] [PubMed] [Google Scholar]
- [27].Opravil M, Hirschel B, Lazzarin A, et al. . Once-weekly administration of dapsone/pyrimethamine vs. aerosolized pentamidine as combined prophylaxis for pneumocystis carinii pneumonia and toxoplasmic encephalitis in human immunodeficiency virus-infected patients. Clin Infect Dis. 1995;20(3):531–541. 10.1093/clinids/20.3.531 [DOI] [PubMed] [Google Scholar]
- [28].Bucher HC, Griffith L, Guyatt GH, et al. . Meta-analysis of prophylactic treatments against pneumocystis carinii pneumonia and toxoplasma encephalitis in hiv-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15(2):104–114. 10.1097/00042560-199706010-00002 [DOI] [PubMed] [Google Scholar]
- [29].Klinker H, Langmann P, Richter E. Pyrimethamine alone as prophylaxis for cerebral toxoplasmosis in patients with advanced HIV infection. Infection. 1996;24(4):324–327. 10.1007/BF01743370 [DOI] [PubMed] [Google Scholar]
- [30].Jacobson MA, Besch CL, Child C, et al. . Primary prophylaxis with pyrimethamine for toxoplasmic encephalitis in patients with advanced human immunodeficiency virus disease: results of a randomized trial. Terry Beirn Community Programs for Clinical Research on AIDS. J Infect Dis. 1994;169(2):384–394. 10.1093/infdis/169.2.384 [DOI] [PubMed] [Google Scholar]
- [31].Leport C, Chene G, Morlat P, et al. . Pyrimethamine for primary prophylaxis of toxoplasmic encephalitis in patients with human immunodeficiency virus infection: a double-blind, randomized trial. ANRS 005-ACTG 154 Group Members. Agence Nationale de Recherche sur le SIDA. AIDS Clinical Trial Group. J Infect Dis. 1996;173(1):91–97. 10.1093/infdis/173.1.91 [DOI] [PubMed] [Google Scholar]
- [32].Schurmann D, Bergmann F, Albrecht H, et al. . Twice-weekly pyrimethamine–sulfadoxine effectively prevents Pneumocystis carinii pneumonia relapse and toxoplasmic encephalitis in patients with AIDS. J Infect. 2001;42(1):8–15. 10.1053/jinf.2000.0772 [DOI] [PubMed] [Google Scholar]
- [33].Durant J, Hazime F, Carles M, et al. . Prevention of Pneumocystis carinii pneumonia and of cerebral toxoplasmosis by roxithromycin in HIV-infected patients. Infection. 1995;23(S1):S33–S38. 10.1007/BF02464958 [DOI] [PubMed] [Google Scholar]
- [34].Connolly MP, Goodwin E, Schey C, et al. . Toxoplasmic encephalitis relapse rates with pyrimethamine-based therapy: systematic review and meta-analysis. Pathog Glob Health. 2017;111(1):31–44. 10.1080/20477724.2016.1273597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Montoya JG, Giraldo LF, Efron B, et al. . Infectious complications among 620 consecutive heart transplant patients at stanford university medical center. Clin Infect Dis. 2001;33(5):629–640. 10.1086/cid.2001.33.issue-5 [DOI] [PubMed] [Google Scholar]
- [36].Baden LR, Katz JT, Franck L, et al. . Successful toxoplasmosis prophylaxis after orthotopic cardiac transplantation with trimethoprim-sulfamethoxazole. Transplantation. 2003;75(3):339–343. 10.1097/01.TP.0000044864.99398.F1 [DOI] [PubMed] [Google Scholar]
- [37].Baran DA, Alwarshetty MM, Alvi S, et al. . Is toxoplasmosis prophylaxis necessary in cardiac transplantation? long-term follow-up at two transplant centers. J Heart Lung Transplant. 2006;25(11):1380–1382. 10.1016/j.healun.2006.08.001 [DOI] [PubMed] [Google Scholar]
- [38].Wreghitt TG, Hakim M, Gray JJ, et al. . Toxoplasmosis in heart and heart and lung transplant recipients. J Clin Pathol. 1989;42(2):194–199. 10.1136/jcp.42.2.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Strabelli TM, Siciliano RF, Vidal Campos S, et al. . Toxoplasma gondii myocarditis after adult heart transplantation: successful prophylaxis with pyrimethamine. J Trop Med. 2012;2012:853562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Payá E, Noemí I, Tassara R, et al. . Prophylaxis against Toxoplasma gondii disease in pediatric and adult patients undergoing solid organ and hematopoietic stem cells transplantation. Rev Chilena Infectol. 2012;29(Suppl 1):37–39. 10.4067/S0716-10182012000500007 [DOI] [PubMed] [Google Scholar]
- [41].Foot AB, Garin YJ, Ribaud P, et al. . Prophylaxis of toxoplasmosis infection with pyrimethamine/sulfadoxine (Fansidar) in bone marrow transplant recipients. Bone Marrow Transplant. 1994;14(2):241–245. [PubMed] [Google Scholar]
- [42].Mendorf A, Klyuchnikov E, Langebrake C, et al. . Atovaquone for prophylaxis of toxoplasmosis after allogeneic hematopoietic stem cell transplantation. Acta Haematol. 2015;134(3):146–154. 10.1159/000380757 [DOI] [PubMed] [Google Scholar]
- [43].Conrad A, Le Maréchal M, Dupont D, et al. . A matched case-control study of toxoplasmosis after allogeneic haematopoietic stem cell transplantation: still a devastating complication. Clin Microbiol Infect. 2016;22(7):636–641. 10.1016/j.cmi.2016.04.025 [DOI] [PubMed] [Google Scholar]