Abstract
Slow fusion pore expansion could retain molecules within vesicles, enabling a chemical reaction that modifies secreted products.
Chromaffin cells are neuroendocrine cells that reside within the medulla of the adrenal glands. They release several hormones in amounts and proportions that vary greatly in response to homeostatic needs and to a wide range of challenges and stresses. In addition to catecholamines, the contents of chromaffin cell secretory granules include the prohormone chromogranin A, the hormone processing enzyme tissue plasminogen activator (tPA), and the tPA inhibitor plasminogen activator inhibitor (PAI). Products released from chromaffin cells have distal targets, including the vasculature, heart, and pancreas. In addition, local actions of chromaffin cell secretions provide feedback loops for local paracrine/autocrine actions. Different biological triggers elicit varied responses in chromaffin cells to regulate processes as diverse as blood pressure, blood clotting, angiogenesis, and metabolism in a coordinated manner. Elucidating the intricate control mechanisms that tune and orchestrate these diverse responses represents a major challenge to current research in physiology and endocrinology. In this issue of JGP, Bohannon et al. introduce a new concept to this field by suggesting that the pH rise within chromaffin cell granules that occurs when the fusion pore opens initiates a chemical reaction between tPA and PAI before these large proteins are released.
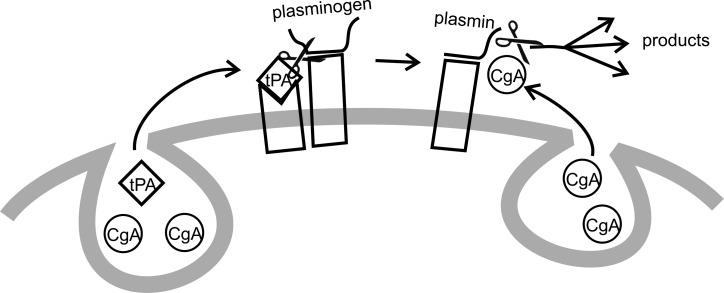
The autocrine control mechanisms of the adrenal medulla exploit proximity as part of their physiological strategy. For example, tPA released from chromaffin cells binds to the chromaffin cell surface, where its target, plasminogen, also binds (Bai et al., 2012). The close proximity of tPA and plasminogen enhances processing of plasminogen to plasmin by tPA (Fig. 1). Tethering recently generated plasmin at the surface of a chromaffin cell means that it is ready and waiting to process chromogranin A to several biologically active products as soon as it is released from chromaffin cells. Thus, two processing steps are enhanced by proximity. tPA can act locally within the adrenal medulla as illustrated in Fig. 1, or enter the circulation to regulate processes such as blood clotting. The limited space around chromaffin cells makes it easier for the processing enzyme to find its prohormone substrate.
Figure 1.
Hormone processing at or near the chromaffin cell surface. Chromaffin cell secretory vesicle cargo includes chromogranin A (CgA) and tPA, as well as PAI (not depicted). tPA is released during exocytosis and binds to the cell surface. Circulating plasminogen binds to the cell surface, and their close proximity accelerates processing of plasminogen to plasmin by tPA (left). Plasmin remains bound to the cell surface where it can process CgA (right) as it is released. The close proximity created by binding sites for the various signaling molecules accelerates processing (modified from Bai et al. [2012]).
The article by Bohannon et al. (2017) takes the localization of endocrine factors to a new level by suggesting that containment within the same vesicle enhances the inactivation of tPA by its inhibitor PAI. Because PAI is a suicide substrate of tPA, the inhibition is irreversible. Bohannon et al. (2017) show that individual vesicles contain both molecules but that their chemical reaction is prevented during storage by the low pH of the vesicle lumen. The authors show that raising the pH within vesicles leads to inactivation of tPA by PAI, even when there is no exocytosis. During exocytosis, the luminal pH of the vesicle rises as soon as the fusion pore opens. Protons have an exceptionally high mobility and can flow very easily, even through a narrow pore that completely blocks the passage of larger molecules. As a result, the pH of the vesicle lumen rises well before the efflux of any of the signaling molecules. Most signaling molecules escape through fusion pores in times of under 100 ms for small molecules or hundreds of milliseconds for larger molecules. However, tPA is different and remains confined within vesicles for several seconds. Bohannon et al. (2017) confirm this finding and further show that PAI lingers in vesicles much longer when the vesicles also contain tPA. They also use the polarization of membrane dye fluorescence to show that the membrane at the release site maintains the high curvature characteristic of omega-shaped narrow pores during tPA release. Thus, when a tPA-containing vesicle fuses with the plasma membrane, the fusion pore remains narrow for an unusually long time, keeping larger proteins within the vesicle.
Based on these observations, Bohannon et al. (2017) put forward the intriguing idea that the unusually long residence and close proximity of tPA and PAI within vesicles after the pH goes up creates a nanoscale chamber where the two molecules can chemically react. As a result, instead of tPA and PAI being released as distinct species, they emerge as a covalent complex lacking prohormone processing activity. It is a very reasonable idea; there should be enough time according to the kinetics of the reaction in vitro. The next step, which will probably be difficult, is showing that a fraction of secreted tPA is indeed inactivated by PAI. Until then, Bohannon et al. (2017) have planted the seed of a new and important idea. If tPA and PAI do indeed react before efflux from a vesicle, this represents a new level of localization between interacting endocrine signaling molecules. The localization is not quite extracellular and not quite intracellular, but in a novel highly transient compartment not previously thought to serve the function of bringing chemical reactants into close proximity.
This scenario suggests that it will be very difficult for tPA to escape in an active form. But as the authors note, chromaffin cells have been shown to release enzymatically active tPA (Parmer et al., 1997), so demonstrating and quantifying release of the inactivated species will be critical. Releasing tPA that has been inactivated by PAI would seem to have no functional role, unless the tPA–PAI complex has its own biological activity. Bohannon et al. (2017) discuss this possibility and summarize evidence to suggest this could be the case. Now there is strong motivation to explore possible signaling functions of the tPA–PAI complex further.
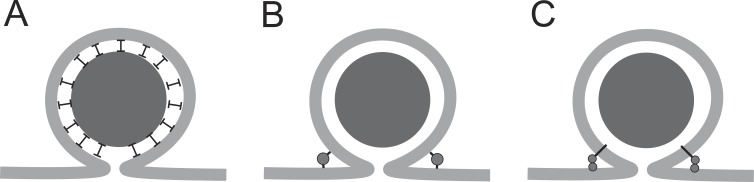
Things could become very interesting if fusion pore expansion, and hence the lifetime of the nanoscale reaction chamber, varies with stimulus conditions. This would provide a way for cells to regulate the proportions of active and inactivated species that are secreted. This would implicate fusion pore expansion as a biologically important variable in the control of hormone release from chromaffin cells. Fusion pores of tPA-containing vesicles have been shown to advance to a lipidic state that is narrow yet allows membrane labels to exit (Taraska and Almers, 2004). The fluorescence polarization data of Bohannon et al. (2017) support the presence of a highly curved lipid bilayer characteristic of a lipidic fusion pore. Thus, fusion pore expansion appears to stall after the transition from protein to lipid (Chang et al., 2017), suggesting that the metastable lipidic fusion pore can be stabilized. There are several ways in which this could occur. One possibility is that tPA could adhere to the inner surface of the vesicle (Fig. 2 A). The tPA could hold the membrane and dense core together, thus preserving the omega figure. This would suggest that tPA has an interacting partner in the vesicle membrane, and there are many possibilities (Wegrzyn et al., 2010) but no specific candidates. It is hard to think of how such a mechanism would support regulation of the process by a triggering stimulus, but if an interacting partner is found, a mechanism for regulation might become apparent.
Figure 2.
Possible mechanisms of limiting fusion pore expansion. (A) Adhesion between the dense core and the inner surface of the vesicle membrane would maintain the omega shape and oppose expansion of the fusion pore. (B) Dynamin forming a ring or collar around the fusion pore would resist expansion. (C) Synaptotagmin anchored in the vesicle membrane by its membrane-spanning α-helix and attached to the plasma membrane by its C2B domain would resist expansion.
Another possibility is that dynamin regulates the expansion of tPA-containing vesicles. Dynamin functions broadly in membrane trafficking, and several studies have shown that dynamin regulates fusion pore expansion (Graham et al., 2002; González-Jamett et al., 2010, 2013; Anantharam et al., 2011; Trouillon and Ewing, 2013). Dynamin could encircle the fusion pore and maintain its narrow neck (Fig. 2 B). However, it remains unclear how dynamin could act differently on different populations of vesicles. This would require that dynamin interact with specific molecules on the outer vesicle membrane surface, as well as sorting of the relevant vesicle membrane proteins to tPA-containing vesicles.
The synaptotagmins are a family of vesicle proteins that could also control pore expansion. Many of the 17 different isoforms of synaptotagmin have functions in endocrine release (Moghadam and Jackson, 2013). Synaptotagmins sort to different populations of vesicles within the same cell, and vesicles fuse with different kinetics depending on which isoforms are present (Zhang et al., 2011; Rao et al., 2017). In particular, synaptotagmin 7 has slow binding kinetics (Hui et al., 2005), and vesicles harboring synaptotagmin 7 discharge their content more slowly than vesicles harboring other isoforms (Zhang et al., 2011; Rao et al., 2017). Thus, if during secretory granule biogenesis synaptotagmin 7 preferentially sorts to tPA-containing vesicles, these vesicles would discharge more slowly. Synaptotagmin penetrates lipid bilayers (Chapman and Davis, 1998), and by penetrating the plasma membrane while remaining anchored by a transmembrane domain in the vesicle membrane, synaptotagmin could form a bridge that stabilizes an intermediate state of fusion (Fig. 2 C). A synaptotagmin that dissociates from membranes slowly could maintain this hourglass shape of a fusion pore. Furthermore, because synaptotagmins are the Ca2+ sensors for exocytosis, different Ca2+ signals could engage different synaptotagmin isoforms on a vesicle to grade the kinetics of fusion pore expansion. These ideas are quite speculative, but a growing body of work is providing strong motivation for efforts to explore connections between vesicle membrane proteins and secretory content.
What are the functions of this form of fusion pore–mediated regulation of hormone processing? If the tPA–PAI complex does in fact have biological activity, then regulating this inactivation step at the moment of release will allow chromaffin cells to release different hormones with different targets. Furthermore, when tPA escapes this inhibition, it will act first in the immediate vicinity of its release (Fig. 1). This would lead to local production of plasmin and local processing of chromogranin A. Because the chromogranin A cleavage products inhibit secretion (Bai et al., 2012), a chromaffin cell could regulate the termination of its response in a highly local and possibly cell-autonomous manner by releasing either active or inactive tPA.
Is this a general mechanism for regulating hormone processing? Do secretory granules serve as nanoscale reaction chambers in other contexts? Most vesicles contain multiple cargoes, and slowing the expansion of the fusion pore would keep them close together to enable a reaction under a pH that differs from that which prevails during storage. Chromogranin A and its processing enzymes are contained in the same vesicles (Seidah and Chrétien, 1999). Does a rapid change in luminal environment trigger chromogranin A processing? Can this occur in a nanoscale vesicular reaction chamber? Can the extent of the reaction be graded by fusion pore expansion? What other processing reactions can these ideas be applied to? Proneurotrophins are processed to neurotrophins and both molecules have distinct, generally opposing, signaling functions (Lu et al., 2005). So proneurotrophin processing by prohormone convertases serves a decisive role in the choice between neuronal death and growth. Regulation of this conversion during release would allow this choice to be made rapidly and locally.
The results reported by Bohannon et al. (2017), and their proposal that secretory vesicles can serve as nanoscale reaction chambers in which the reactants are in close proximity, raise many exciting possibilities and offer ideas that could broadly impact our understanding of how the endocrine system orchestrates and coordinates the delivery of multiple chemical signals to diverse targets.
Acknowledgments
Supported by NIH grant NS44057.
The author declares no competing financial interests.
Sharona E. Gordon served as editor.
References
- Anantharam A., Bittner M.A., Aikman R.L., Stuenkel E.L., Schmid S.L., Axelrod D., and Holz R.W.. 2011. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol. Biol. Cell. 22:1907–1918. 10.1091/mbc.E11-02-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai H., Nangia S., and Parmer R.J.. 2012. The plasminogen activation system and the regulation of catecholaminergic function. J. Biomed. Biotechnol. 2012:721657 10.1155/2012/721657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon K.P., Bittner M.A., Lawrence D.A., Axelrod D., and Holz R.W.. 2017. Slow fusion pore expansion creates a unique reaction chamber for co-packaged cargo. J. Gen. Physiol. 149 10.1085/jgp.201711842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.W., Chiang C.W., and Jackson M.B.. 2017. Fusion pores and their control of neurotransmitter and hormone release. J. Gen. Physiol. 149:301–322. 10.1085/jgp.201611724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.R., and Davis A.F.. 1998. Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J. Biol. Chem. 273:13995–14001. 10.1074/jbc.273.22.13995 [DOI] [PubMed] [Google Scholar]
- González-Jamett A.M., Báez-Matus X., Hevia M.A., Guerra M.J., Olivares M.J., Martínez A.D., Neely A., and Cárdenas A.M.. 2010. The association of dynamin with synaptophysin regulates quantal size and duration of exocytotic events in chromaffin cells. J. Neurosci. 30:10683–10691. 10.1523/JNEUROSCI.5210-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Jamett A.M., Momboisse F., Guerra M.J., Ory S., Báez-Matus X., Barraza N., Calco V., Houy S., Couve E., Neely A., et al. . 2013. Dynamin-2 regulates fusion pore expansion and quantal release through a mechanism that involves actin dynamics in neuroendocrine chromaffin cells. PLoS One. 8:e70638 10.1371/journal.pone.0070638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.E., O’Callaghan D.W., McMahon H.T., and Burgoyne R.D.. 2002. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc. Natl. Acad. Sci. USA. 99:7124–7129. 10.1073/pnas.102645099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E., Bai J., Wang P., Sugimori M., Llinas R.R., and Chapman E.R.. 2005. Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc. Natl. Acad. Sci. USA. 102:5210–5214. 10.1073/pnas.0500941102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Pang P.T., and Woo N.H.. 2005. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 6:603–614. 10.1038/nrn1726 [DOI] [PubMed] [Google Scholar]
- Moghadam P.K., and Jackson M.B.. 2013. The functional significance of synaptotagmin diversity in neuroendocrine secretion. Front. Endocrinol. (Lausanne). 4:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmer R.J., Mahata M., Mahata S., Sebald M.T., O’Connor D.T., and Miles L.A.. 1997. Tissue plasminogen activator (t-PA) is targeted to the regulated secretory pathway. Catecholamine storage vesicles as a reservoir for the rapid release of t-PA. J. Biol. Chem. 272:1976–1982. 10.1074/jbc.272.3.1976 [DOI] [PubMed] [Google Scholar]
- Rao T.C., Santana Rodriguez Z., Bradberry M.M., Ranski A.H., Dahl P.J., Schmidtke M.W., Jenkins P.M., Axelrod D., Chapman E.R., Giovannucci D.R., and Anantharam A.. 2017. Synaptotagmin isoforms confer distinct activation kinetics and dynamics to chromaffin cell granules. J. Gen. Physiol. 149:763–780. 10.1085/jgp.201711757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N.G., and Chrétien M.. 1999. Proprotein and prohormone convertases: a family of subtilases generating diverse bioactive polypeptides. Brain Res. 848:45–62. 10.1016/S0006-8993(99)01909-5 [DOI] [PubMed] [Google Scholar]
- Taraska J.W., and Almers W.. 2004. Bilayers merge even when exocytosis is transient. Proc. Natl. Acad. Sci. USA. 101:8780–8785. 10.1073/pnas.0401316101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillon R., and Ewing A.G.. 2013. Amperometric measurements at cells support a role for dynamin in the dilation of the fusion pore during exocytosis. ChemPhysChem. 14:2295–2301. 10.1002/cphc.201300319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn J.L., Bark S.J., Funkelstein L., Mosier C., Yap A., Kazemi-Esfarjani P., La Spada A.R., Sigurdson C., O’Connor D.T., and Hook V.. 2010. Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J. Proteome Res. 9:5002–5024. 10.1021/pr1003104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wu Y., Wang Z., Dunning F.M., Rehfuss J., Ramanan D., Chapman E.R., and Jackson M.B.. 2011. Release mode of large and small dense-core vesicles specified by different synaptotagmin isoforms in PC12 cells. Mol. Biol. Cell. 22:2324–2336. 10.1091/mbc.E11-02-0159 [DOI] [PMC free article] [PubMed] [Google Scholar]