Abstract
In this review we provide an analysis of the biochemistry of peroxynitrite and tyrosine nitration. Peroxynitrite is the product of the diffusion-controlled reaction between superoxide (O2•-) and nitric oxide (•NO). This process is in competition with the enzymatic dismutation of O2•- and the diffusion of •NO across cells and tissues and its reaction with molecular targets (e.g. guanylate cyclase). Understanding the kinetics and compartmentalization of the O2•- / •NO interplay is critical to rationalize the shift of •NO from a physiological mediator to a cytotoxic intermediate. Once formed, peroxynitrite (ONOO- and ONOOH; pKa = 6,8) behaves as a strong one and two-electron oxidant towards a series of biomolecules including transition metal centers and thiols. In addition, peroxynitrite anion can secondarily evolve to secondary radicals either via its fast reaction with CO2 or through proton-catalyzed homolysis. Thus, peroxynitrite can participate in direct (bimolecular) and indirect (through secondary radical intermediates) oxidation reactions; through these processes peroxynitrite can participate as cytotoxic effector molecule against invading pathogens and/or as an endogenous pathogenic mediator. Peroxynitrite can cause protein tyrosine nitration in vitro and in vivo. Indeed, tyrosine nitration is a hallmark of the reactions of •NO-derived oxidants in cells and tissues and serves as a biomarker of oxidative damage. Protein tyrosine nitration can mediate changes in protein structure and function that affect cell homeostasis. Tyrosine nitration in biological systems is a free radical process that can be promoted either by peroxynitrite-derived radicals or by other related •NO-dependent oxidative processes. Recently, mechanisms responsible of tyrosine nitration in hydrophobic biostructures such as membranes and lipoproteins have been assessed and involve the parallel occurrence and connection with lipid peroxidation. Experimental strategies to reveal the proximal oxidizing mechanism during tyrosine nitration in given pathophysiologically-relevant conditions include mapping and identification of the tyrosine nitration sites in specific proteins.
Keywords: Free radicals, Oxidants, Nitric oxide, Peroxynitrite and tyrosine nitration
Graphical abstract
Highlights
-
•
Peroxynitrite is a reactive peroxide formed from superoxide and nitric oxide radicals.
-
•
Peroxynitrite is a strong one- and two-electron oxidant.
-
•
Protein tyrosine nitration requires free radical reactions.
-
•
3-Nitrotyrosine is a biomarker of nitroxidative stress.
-
•
Nitration of key protein tyrosines leads to changes in protein function.
1. Introduction
Oxidants and free radical species are physiologically and continuously formed in cells [1], [2], and can participate in redox signaling [3], [4]; however, under pathological conditions the formation of these species may significantly increase and mediate oxidative damage of different biomolecules such as lipids, sugars, DNA and proteins [5], [6], [7].
At a cellular level there are several sources of oxidants and free radical species, mainly based on the action of different enzymes present in plasma membrane (lipoxygenase, prostaglandin synthase, NADPH oxidase), mitochondrial electron transport chain (NADH dehydrogenase, ubiquinone), peroxisomes (oxidases and flavoproteins), endoplasmic reticulum and nuclear membrane (cytochrome P450 and cytochrome b5) and other enzymes such as oxidoreductases (xanthine oxidase, myeloperoxidase, P450 enzymes) or soluble heme-proteins (hemoglobin, myoglobin, cytochrome c). Lipid peroxidation in membranes is an important source of free radical species. Importantly, the family of nitric oxide synthases (NOS), generate the relatively stable and signal transducing free radical, nitric oxide (•NO), and NADPH oxidases family (NOX) among other enzymes produce superoxide radical anion (O2•-) [8], [9]. Finally, oxidants can be formed by the action of environmental factors such as, high energy irradiation, air pollutants, toxic chemicals and drug metabolism.
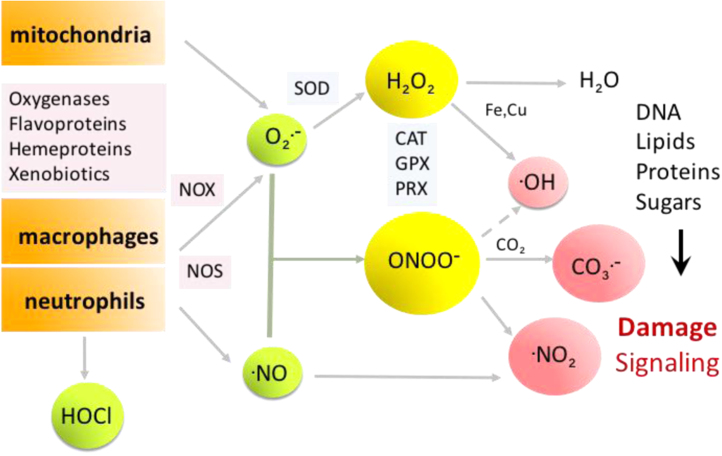
By the action of these enzymatic and non-enzymatic pathways several biologically-relevant oxidants can be formed including O2•-, hydrogen peroxide (H2O2) hypochlorite (HOCl) and hypobromide (HOBr), singlet oxygen (1O2), hydroxyl radical (•OH), peroxynitrite (ONOO-), nitrogen dioxide (•NO2) and carbonate radical (CO3•-) among others (Fig. 1). The production of these species can be enhanced in the presence of redox-active transition metal centers such as Fe3+, Cu2+, which in turn can potentiate the effect of oxidants (e.g. catalysis of nitration reactions) [1], [2].
Fig. 1.
Main sources of oxidants and the O2•- /•NO interplay. Free radicals can be formed by a variety of intra and extracellular sources. The simultaneous generation of nitric oxide and superoxide radicals yields peroxynitrite. Both, peroxynitrite and its secondary oxidants can mediate oxidative modifications in biomolecules. Antioxidant enzyme systems such as SOD, or peroxiredoxins (PRX) participate in controlling steady state levels of peroxynitrite. Other peroxidatic systems include catalase (CAT) and glutathione peroxidase (GPX).
In this chapter, we will focus on the interplay that exists between the •NO and O2•- routes for the generation of strongly oxidizing and nitrating species, which are connected through the formation of peroxynitrite (Fig. 1).
Several enzymatic antioxidant systems are present in cells to catabolize oxidants such as superoxide dismutase (SOD) [10], [11], which catalyzes superoxide dismutation to hydrogen peroxide, glutathione peroxidases (GPX), catalase (CAT) [12] and peroxiredoxins (PRX), which altogether remove hydrogen peroxide, peroxynitrite and lipid peroxides in various cellular compartments [13], [14], [15]. In the context of the O2•-/•NO interplay, SODs inhibit the formation of peroxynitrite and cytosolic and mitochondrial peroxiredoxins typically have an extraordinary catalytic ability to reduce it to nitrite (NO2-) [16].
2. Nitric oxide, superoxide and peroxynitrite
Nitric oxide (•NO), a relatively stable free radical formed in vivo, is a pleiotropic regulator and effector of the immune, cardiovascular and nervous system. In the early 80 s, the chemical nature of this molecule was still unknown, and its effects were ascribed to the endothelium-derived relaxing factor (EDRF) (for a review see [6]). At the end of that decade, EDRF identity was finally elucidated, and defined as •NO by Moncada et al. and Ignarro et al. [17], [18].
Nitric oxide can play regulatory functions by acting on vascular tone and plasticity [19], as a mediator at central nervous system and by inhibiting platelet aggregation [20]. It also can act as a protective agent by its antioxidant activity, mainly as a chain termination agent in lipid peroxidation reactions, inhibition of leukocyte adhesion and antimicrobial action [20]. In addition, •NO may play a deleterious role inhibiting enzyme function, promoting DNA damage or participating in pro-oxidant processes through the formation of •NO-derived oxidants. The action of •NO as a signaling molecule or cytotoxic agent largely depends its concentration and the redox environment present at the time. Most of •NO-derived toxicity in the context of oxidative stress conditions is due to the formation of •NO-derived oxidants, which are further more reactive than •NO itself [21].
Nitric oxide is synthetized by a family of enzymes called nitric oxide synthases (NOS) from l-arginine, NADPH, O2 and substrates FAD and FMN, using tetrahydrobiopterin (BH4) and calmodulin as cofactors [22], [23]. Three NOS isoforms have been isolated and characterized: neuronal NOS (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS) [22], [23], [24]. The presence of a mitochondrial NOS has been described, however, there is still no agreement in the presence of this isoform [25].
The chemistry of •NO dictates much of its biological activity [5], [6], [7], [20]. Nitric oxide may undergo autoxidation reactions in the presence of oxygen, leading to •NO2 formation, a strong oxidizing and nitrating agent (E°' •NO2/NO2- = 0.99 V). However, under normal conditions this is a rather slow termolecular process (k = 2.8 × 106 M−2 s−1) [26]. Nitrogen dioxide, may further react with •NO, forming dinitrogen trioxide (N2O3), an unstable species which mainly mediates nitrosation and deamination reactions [7]. In addition, •NO2 is present in pollution and cigarette smoke, playing an important role in environmental toxicity [27]. Autoxidation reactions are usually favored in hydrophobic environments such as lipoproteins and membranes, where •NO and O2 concentration may be higher than in aqueous solutions [28], [29].
Nitric oxide, is neither a strong oxidant (Eo' •NO/NO- = 0.39 V) nor a strong reductant (Eo' •NO/NO+ = 1.21 V) [30], [31] and therefore is not reactive with most of the biological molecules in spite of its radical nature. Nitric oxide does react fast with oxygen-, carbon-, sulfur- and nitrogen-centered radicals, such as O2•-, •OH, tyrosyl (Tyr•), thiyl (RS•), •NO2 and peroxyl (ROO•) radicals.
In the reaction with ROO•, nitrosated and nitrated lipids can be formed, in radical-radical termination reactions, accounting this way for some of its antioxidant properties [32].
Importantly, •NO has direct signaling effects by its reversible reaction with metal complexes of enzymes forming heme-nitrosyl complexes. This is the case of the reaction with guanylate cyclase (i.e. stimulates formation of cGMP) [33] or cytochrome aa3 (i.e. inhibits oxygen binding) [34]. An important •NO pathway in the vasculature, is its reaction with oxyhemoglobin (Hb2+-O2) and formation of methemoglobin (Hb3+) and NO3-. Due to the large amounts of Hb2+-O2 in red blood cells, this is an important intravascular reaction and sink for •NO produced in tissues [6].
Due to its hydrophobicity, small size and neutral condition, •NO has the ability of freely diffusing towards membranes, and it can react far away from its site of formation (100 µm – 1 mm) having quite long half life compared to other free radicals (1–10 s) [24].
Superoxide radical is a short-lived free radical, which may act either as an oxidant (E°’ O2•-/ H2O2 = 0.94 V) or reductant (Eo' O2/O2•- = 0.33 V). In cells, superoxide is formed by the action of several enzymes such as oxygenases, flavoproteins, xanthine oxidase, NADPH oxidases, uncoupled NOS and Complex I and III of the mitochondrial electron transport chain, among others [10], [11].
Under oxidative conditions, the interplay between the formation pathways of •NO and O2•- will play an important role in the mediation of cellular toxicity. In particular, the fast reaction of •NO with O2•- leading to the formation of peroxynitrite (ONOO-), which in turn will promote oxidation and nitration reactions affecting different biomolecules [7] (Fig. 2).
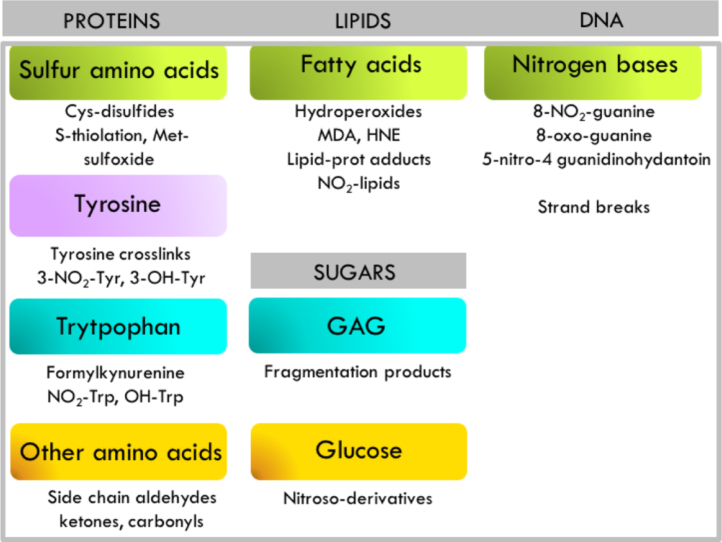
Fig. 2.
Representative peroxynitrite-mediated oxidative modifications of biomolecules and its products. Modifications on proteins, lipids, sugars and DNA include those directly mediated by peroxynitrite or indirectly by peroxynitrite-derived radicals [7].
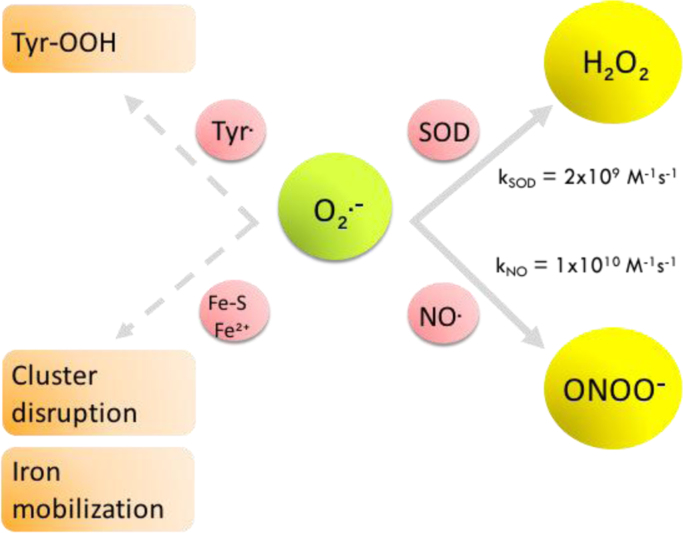
A central point to consider when invoking peroxynitrite as a mediator of oxidative effects of O2•- and •NO, relates to the kinetic competitiveness of the peroxynitrite formation reaction in biological systems. Indeed, once O2•- is formed, it may undergo the SOD-catalyzed dismutation reaction to H2O2 (kSOD = 2 × 109 M−1 s−1) [35], or react with •NO (kNO = 1 × 1010 M−1 s−1) [36] in a potentially faster reaction when •NO concentration increases. Therefore, in quantitative terms, the fate of O2•- radicals will mainly depend on the competition between two reactions: i) superoxide dismutation and ii) peroxynitrite formation; the ratio of this competition is relevant to determine the switch from signaling pathways of •NO to oxidative damage [37]. Still, it is important to consider that small amounts of O2•- will have secondary targets such as Fe-S centers present in proteins and enzymes (e.g. aconitase, producing cluster disruption) [38], free radicals (e.g. Tyr• leading to the formation of tyrosine hydroperoxide (Tyr-OOH) [39]), or transition metals present in low molecular weight complexes or proteins (e.g. O2•- favors iron release form ferritin) [40], which can disturb cell/tissue homeostasis due to the promotion of undesired transition metal mobilization and reactions (Fig. 3).
Fig. 3.
Fates of superoxide in the presence of nitric oxide. Competition between O2•- dismutation and ONOO- formation is shown. Alternative reactions of O2•- are indicated with dashed arrows.
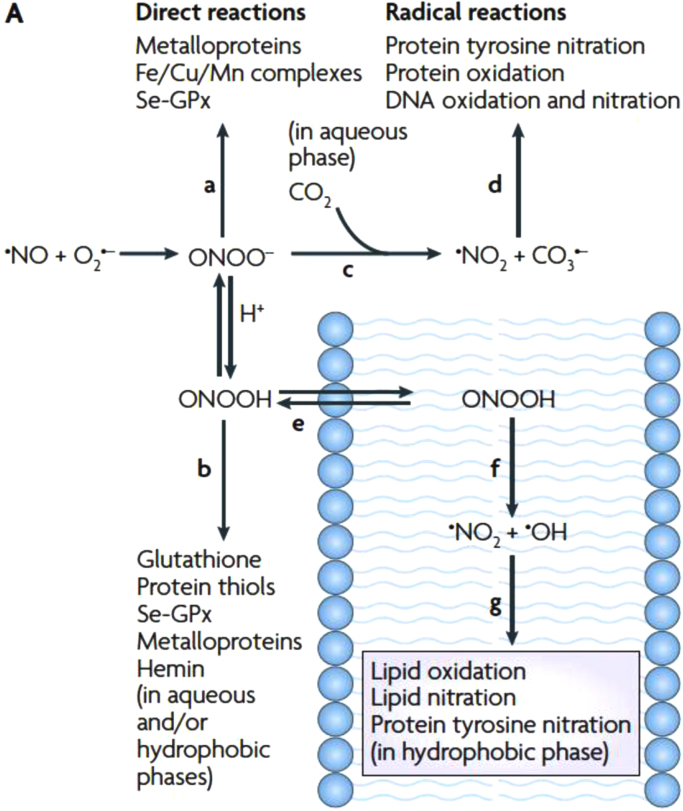
Peroxynitrite (ONOO-/ONOOH, pKa = 6.8) is a powerful and short-lived (half life ca. 10 ms) [41] oxidant formed in vivo, that can directly react with different biomolecules by one (i.e. reaction with transition metals) or two-electron (i.e. reaction with thiols) oxidations [42]. In addition, it can decompose by homolysis of peroxynitrous acid (ONOOH), to •NO2 and •OH radicals (in 30% yield), species that can further participate in nitration/oxidation reactions [41], [43], [44], a process that is relatively slow in biological systems [45]. One of the most important reactions of ONOO- in biological systems is its reaction with carbon dioxide (CO2), in equilibrium with bicarbonate (HCO3-) [46], and the concomitant formation of •NO2 and CO3•- radicals (in 35% yield), highly oxidant species that can in turn mediate oxidative damage to biomolecules [47]. An important aspect to take into account, is that peroxynitrite can mediate oxidations in aqueous phases (i.e. with, thiols, metalloproteins and CO2), or very importantly, freely diffuse through membranes (ONOOH), and react via its secondary radicals with lipids and proteins present in the hydrophobic milieu (Fig. 4) [48]. This may have particular relevance in lipid peroxidation reactions as will be discussed next.
Fig. 4.
Fates of Peroxynitrite. Peroxynitrite can mediate direct or indirect reactions via its derived radicals, either in the aqueous phase or within membranes, promoting modifications in lipids and proteins.
Reproduced from [48].
3. Peroxynitrite-mediated reactions
3.1. Direct oxidation reactions
3.1.1. Thiols
One important peroxynitrite-mediated oxidation is its reaction with low molecular-weight or protein thiols, as described by Radi in 1991, who showed the reaction of peroxynitrite with free cysteine (k = 5900 M−1 s−1) [49], or the bovine serum albumim (BSA) thiol (Cys34) (k = 2800 M−1 s−1) at pH 7.4 and 37 °C. Later on, the reaction constant of peroxynitrite and human serum albumin (HSA) thiol was determined (k = 3800 M−1 s−1) at pH 7.4 and 37 °C [50].
Peroxynitrite reacts directly with thiols in a two-electron oxidation process involving a nucleophilic attack of the thiolate to the protonated form of peroxynitrite, ONOOH. The reaction yields nitrite and sulfenic acid (RSOH), which can in turn react with another thiol forming a disulfide (RSSR) [51] or become stabilized in special environments such as HSA [52]. In addition, peroxynitrite-derived radicals may react with thiols in one-electron oxidation reactions, yielding thiyl radicals (RS•), an oxidizing radical that can recombine with another RS• radical to form disulfides (RSSR), or initiate oxygen-dependent chain reactions that can produce thiyl-peroxyl radicals (RSOO•) and sulfinyl radicals (RSO•), among other products. It is important to consider that peroxynitrite chemistry leads to the formation of further oxidant species that can in turn mediate cellular damage.
In addition to cysteine, a few amino acids can be directly oxidized by peroxynitrite, namely methionine (k = 1.7–1.8 × 102 M−1 s−1) [53] and tryptophan (k = 37 M−1s−1) [54], [55].
Peroxynitrite does not directly react with tyrosine residues, however peroyxnitrite-derived radicals •OH, •NO2 and CO3•- play a critical role on tyrosine oxidation / nitration reactions, that will be described in the following section. Phenylalanine and histidine can also be modified by the action of peroxynitrite-derived radicals.
The oxidation of thiols and other amino acids by peroxynitrite may have an important impact on protein structure and function [41].
3.1.2. Metals
Peroxynitrite can react with transition metal centers such as iron, copper and manganese ions, forming part of proteins or low molecular weight complexes. The oxidation may take place through one-electron pathways yielding NO2- (e.g. heme proteins) or through two-electron reactions yielding •NO2 and the oxidized metal [37]. Some metal complexes can promote the isomerization of ONOO- to nitrate (NO3-). In enzymes such as myeloperoxidase (MPO), peroxynitrite reacts through a one-electron process with the iron, yielding •NO2 and an oxo-ferryl compound, also a powerful oxidant. In the presence of reductants such as glutathione or ascorbic acid, these oxidizing intermediates may be reduced to NO2- and the initial oxidation state of the metal center [41], [45].
3.2. Indirect oxidation reactions
3.2.1. Other biomolecules
Peroxynitrite-derived radicals, •OH, •NO2 can react in addition to amino acids, with lipids, sugars and nitrogen bases [7].
After ONOOH homolysis, •NO2 and •OH radical can readily initiate lipid peroxidation by one-electron abstraction on allylic hydrogen atoms in double bonds of unsaturated fatty acids [56]. The reaction of •NO and •NO2 with lipids, may lead to the formation of a variety of modified oxidized and nitrated fatty acids [57].
Peroxynitrite-derived radicals can also react with sugars present in monosaccharides (e.g. glucose) or polysaccharides (e.g. glycosaminoglycanes) [58]. The reaction of peroxynitrite with glycosaminoglycanes (GAGs) will result in extense polymer and fragmentation (hyaluronan, chondroitin and heparin, dermatan and heparan sulfates) [59]. DNA bases can also be affected by peroxynitrite-derived radicals in nitration and oxidation reactions. Peroxynitrite can promote guanine oxidation and nitration, being 8-oxo-guanine and 8-nitro-guanine main products of these reactions. Hydroxyl radical leads to DNA strands breaks. In addition, other reactive nitrogen species such as trioxide nitrogen may promote DNA deamination reactions [60], [61] (Fig. 2).
3.2.2. Carbon dioxide
The reaction of peroxynitrite and carbon dioxide, present at high concentrations (1.3 mM, in equilibrium with 25 mM bicarbonate anion) is one of the most important in biological systems. This reaction occurs through a nucleophilic addition of ONOO- to CO2 (k = 4.6 × 104 M−1 s−1) [62], [63], [64], [65] yielding a transient species, nitroso-peroxocarbonate (ONOOCO2-), which will decompose homolytically yielding •NO2 and CO3•- radicals. These radicals are short-lived one-electron oxidants that can promote themselves nitro-oxidative damage [45].
4. The case of protein tyrosine nitration
As mentioned above, peroxynitrite can mediate oxidative modifications in a variety of biomolecules. An important aspect of peroxy-nitrite-mediated toxicity is its capability of promoting protein tyrosine nitration, a covalent oxidative posttranslational modification mediated by nitric oxide-derived oxidants such as •NO2 and ONOO-.
Protein tyrosine nitration could result in dramatic changes in protein structure and can affect protein function either by a loss-(e.g. MnSOD, actin, prostacyclin synthase and tyrosine hydroxylase), or by a gain-of-a previously inexistent function (e.g. cytochrome c, fibrinogen, protein kinase, glutathione S-transferase and apoA1) [66]. It may alter phosphorylation cascades, or induce immunological responses by the generation of antibodies against nitrated proteins [66], [67], [68], [69].
4.1. Nitration as a free radical process
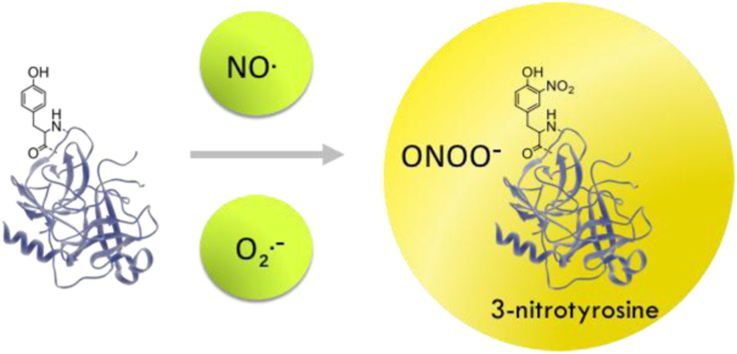
The nitration of protein tyrosine residues constitutes the substitution of a hydrogen by a nitro group (-NO2) in the position 3 of the phenolic ring, being 3-nitrotyrosine (3-NT) the product of this reaction.
Protein tyrosine nitration is a free radical-mediated pathway, which involves the intermediate formation of Tyr• radical from tyrosine [21], [69]. There are several oxidants that can perform the one-electron oxidation of tyrosine in biological systems, •OH, •NO2, CO3•- radicals, oxo-metal compounds (O=MnIV) and compounds I and II of hemoperoxidases, such as MPO. Moreover, we have recently demonstrated a “connection reaction” in membranes between lipid peroxidation and tyrosine oxidation, by which lipid-derived radicals, peroxyl (LOO•) [70] and alkoxyl (LO•) [71] can oxidize tyrosine to Tyr• in membranes, therefore promoting nitration reactions [67], [70], [72], [73].
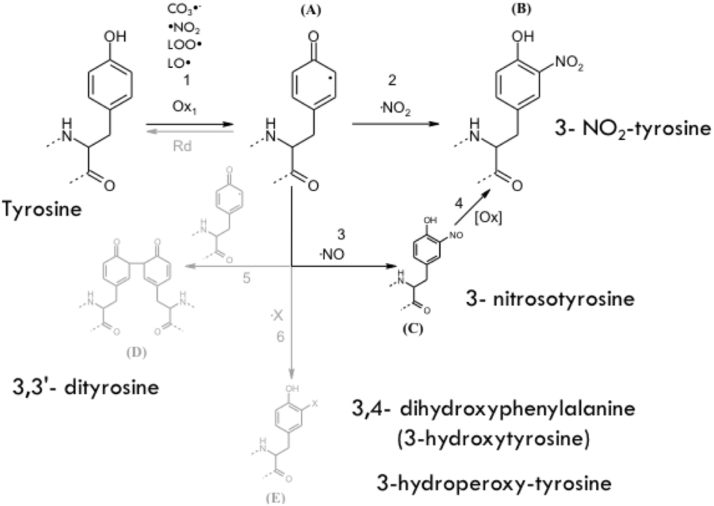
Once Tyr• radical is formed it can have different fates, one of which in the context of peroxynitrite-dependent reaction is the diffusion-controlled reaction with •NO2 to yield 3-NT (Fig. 5). In addition to this product, other oxidation products can be formed. The combination of two Tyr• will lead to the formation of the dimerized form, 3,3′-dityrosine, the addition of •OH will yield 3,4-dihydroxyphenylalanine (DOPA) (also known as 3-hydroxytyrosine), and the reaction with O2•- radical produces tyrosine hydroperoxide. In addition, Tyr• can react with •NO, forming 3-nitrosotyrosine, which will further evolve to 3-NT formation [21], [69] (Fig. 5).
Fig. 5.
Tyrosine oxidation pathways. Tyrosine can be oxidized by several oxidants to yield Tyr• (1), leading to 3-nitrotyrosine formation in the presence of •NO2 (2); nitrosotyrosine in reaction with •NO; 3,3′-dityrosine by combination with another Tyr• radical (5) or 3,4′-dihydroxyphenylalanine (DOPA) by a hydroxylation reaction, (X = OH), or 3-hydroperoxytyrosine by its reaction with O2•- (X = OOH) (6).
Modified from [67].
It is important to consider that tyrosine oxidation reactions can be reversed by the repair of Tyr• radical (i.e. reducing it back to tyrosine), by the action of reductants such as glutathione or ascorbate, or through intramolecular electron transfer reactions with other amino acids, such as cysteine [74], [75].
4.2. Peroxynitrite-dependent and independent tyrosine nitration mechanisms
Protein tyrosine nitration is consistently observed in several pathologies such as cardiovascular disease [76], neurodegeneration [77], [78], [79], inflammation and cancer [80]. Indeed, protein 3-NT is established as a biomarker of oxidative stress in vivo, being revealed as a strong biomarker and predictor of disease onset and progression.
In addition to peroxynitrite, protein tyrosine nitration can occur by the action of enzymes such as MPO in the presence of NO2- and H2O2, a process that plays an important role during neutrophil degranulation in inflammation sites. In this tyrosine nitration mechanism, the MPO reaction with H2O2 yields compound I, a high oxidation state oxo-heme complex that oxidizes tyrosine to Tyr• radical and NO2- to •NO2, generating the proper combination of reagents to yield 3-NT [81]. Additional biological mechanisms of tyrosine nitration involve the action of NO2- under acidic conditions as observed in the gastric lumen [82], [83].
4.3. Nitration selectivity
Protein tyrosine nitration is a low yield process and is highly selective, since neither all proteins can become nitrated nor all tyrosine-residues within a particular protein can undergo nitration. Usually, in whole tissue/cell only 1–5 over 10,000 tyrosine residues become nitrated [21]. However, in some specific proteins, tyrosine nitration yields are large and responsible of structural and functional changes [21], [68], [69].
Several physicochemical factors control tyrosine nitration either in aqueous or hydrophobic environments, however, there is not a general rule to anticipate tyrosine nitration selectivity and each protein has to be specifically-analyzed.
The nitration of a particular tyrosine residue will depend on several factors including, protein structure, nitration mechanism, and the environment were the tyrosine residue is located [66], [67], [68], [84], [85].
4.3.1. Protein structure
Usually in aqueous solution and solvent-exposed tyrosine residues, nitration is enhanced in the presence of CO2, transition metal centers and the proximity of charged amino acids (through hydrogen-bonding) or binding sites for hemeperoxidases [68]. The presence of nearby turn-inducing amino acids such as proline and glycine usually favors nitration of nearby tyrosine residues as observed in RNAase A and lysozyme [84], [85].
The existence of a consensus sequence for tyrosine nitration has been proposed by some authors [84], however it has not been really demonstrated, and the secondary and tertiary structures and solvent accessibility appear to be the most important factors affecting tyrosine nitration selectivity.
However, the presence of some amino acids in the close proximity of tyrosine residues do affect nitration. This is the case of cysteine which can inhibit nitration due to intramolecular-electron transfer reactions between Tyr and Cys residues, as shown by studies in model peptides and proteins such as Fe-SOD present in T. cruzi [75], [86]. Nearby methionine residues could in turn, promote nitration also by intramolecular electron transfer reactions between Tyr and Met residues [87]. In addition, the presence of charged amino acids usually favor nitration reactions due the formation of hydrogen bonds. On the other hand, the presence of nearby positively-charged amino acids such as arginine, may inhibit tyrosine nitration due to electrostatic forces (e.g. Y20 and R21 in lysozyme) [85].
The location of the tyrosine residue within the protein turns to be critical in the possibility of a tyrosine residue in being nitrated. Buried tyrosine residues usually cannot accommodate the voluminous nitro group (-NO2) by a steric effect, while exposure of the aromatic ring of the tyrosine to the protein surface should facilitate the reaction. Solvent-exposed tyrosine residues are particularly sensitive in the presence of CO2 as observed for Y48, Y74 and Y97 of cytochrome c [88].
The redox environment of the particular tyrosine residue will determine as well the possibilities of nitration. The presence of endogenous antioxidants such as glutathione [45], [74], ascorbate [89], [90] and uric acid [45], [91] will inhibit nitration reactions by the consumption of oxidizing and nitrating species such as •NO2.
4.3.2. Nitration mechanism
In some cases, proximity of transition metals centers to tyrosine become the key selectivity element. The presence of a transition metal center may site-specifically direct nitration, as observed in Mn-SOD which is nitrated by peroxynitrite specifically on Y34 in active site, located 5 Å away for manganese atom [92], [93], [94] and Y192 in apoA1 located in the MPO-binding site region [95].
Nitration of Mn-SOD in Y34 present in the access channel for superoxide, will block the entrance of the channel and generates an electrostatic repulsion effect (as the pKa of the –OH group of tyrosine drops from pH 10.5 to 7–7.5 upon nitration) promoting the inactivation of the enzyme [68], [69].
In addition, tyrosine nitration may promote a gain of function, such is the case of the cytocrome c which after nitration displays a peroxidatic activity. Indeed, the nitration of solvent-exposed (Y74), triggers a conformational change on cytocrome c, resulting in an alternative conformation, which affects its normal electron transport function, and enhances a peroxidatic activity [69], [96].
In general, •NO2 tends to modify solvent-exposed tyrosines while transition metals can provide regio-selectivity for the nitration of internal or buried tyrosine residues.
4.3.3. Cellular and redox environments
In hydrophobic environments (i.e. membranes and lipoproteins), the physicochemical factors controlling nitration reactions may differ from those observed in aqueous solution, mainly due to the high concentration of unsaturated fatty acids, which may outcompete for the free radical species, the exclusion of antioxidants usually present in polar environments, such as glutathione (GSH), which usually acts inhibiting nitration due to its fast reaction with •NO2 (k = 5.3 × 106 M−1 s−1) [97], and the diffusion of •NO and •NO2, that easily diffuse towards lipid milieu, and partition in a favored manner compared to aqueous phases [28], [29]. In membranes, factors as interaction with water molecules, oxygen concentration and lipid-soluble antioxidants, in addition to phospholipid composition, should also be taken into account [68]. In these compartments, lipid peroxidation reactions may fuel nitration/oxidation pathways, since there is a contribution of lipid-derived radicals (i.e. LOO• and LO•) in the generation of Tyr•, due to the one-electron oxidation of tyrosine (Eq. (1)) [67], [70], [72].
| (1) |
While oxygen does not react with tyrosine or tyrosyl radicals, it becomes a critical factor in the modulation of tyrosine nitration in membranes [70], [72], via its participation in the propagation phase of lipid peroxidation, so the physicochemical properties of the milieu will also directly impact on tyrosine nitration [67]. Hydrophobic environments such as membranes or lipoproteins will limit the diffusion of charged reactive species (i.e. CO3•-) which are potent oxidants in the aqueous phase [98]. In addition there is an exclusion of hydrophilic anti-nitrating agents, such as glutathione, which reacts fast with •NO2. On the other hand, nitrating species such as •NO and •NO2, will easily diffuse and concentrate in hydrophobic media, favoring nitration pathways [99].
Changes in pH will influence peroxynitrite-dependent nitration yields [73] and acidic conditions favor nitrite-dependent nitration pathways as observed in pepsin [82].
5. Conclusions
Understanding the conditions that facilitate the biological formation of peroxynitrite, its preferential reactions with biomolecules and the main oxidation products generated require comprehension of kinetic, mechanistic and physico-chemical elements. Moreover, the redox processes that lead to protein tyrosine nitration are intimately associated with decay pathways of peroxynitrite, including the notable reaction with CO2, that simultaneously generate oxidizing and nitrating species. Peroxynitrite can be considered an unusual peroxide due to its intrinsic instability in biological systems, the coexistence of the anionic and protonated forms at physiological pH with distinctive reactivities and its capacity to readily trigger free radical processes. Among the latter, protein tyrosine nitration constitutes a hallmark of the actions of peroxynitrite and related •NO-derived oxidants in cells and tissues. While the biochemical routes leading to the formation of 3-NT in various cellular and extracellular compartments have been mostly settled, its impact in protein structure and function in vivo still requires substantially more research. The well-established association of protein tyrosine nitration to several pathologies, and even with the aging process, opens opportunities to unravel whether this oxidative posttranslational modification in specific proteins (and residues) can disrupt cell homeostasis. The fundamentals provided in this review may assist for stimulating further experimental designs, sample analysis and therapeutic developments directed to assess the role of peroxynitrite and protein tyrosine nitration in human disease and aging.
Acknowledgements
This work was supported by grants of Universidad de la República (Espacio Interdisciplinario and CSIC) and Agencia Nacional de Investigación e Innovación (FCE_2014_104233). Additional support was obtained from PEDECIBA and from Ridaline through Fundación Manuel Pérez, Uruguay. The authors are grateful to Federation of European Biochemical Societies (FEBS) for supporting their participation in the FEBS Advanced Lecture Courses 2016 on Redox Regulation of Metabolic Processes in which lectures related to this review were presented.
Contributor Information
Silvina Bartesaghi, Email: sbartesa@fmed.edu.uy.
Rafael Radi, Email: rradi@fmed.edu.uy.
References
- 1.Crapo J.D. J. Appl. Physiol. 1994;77:2022–2056. [Google Scholar]
- 2.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 3.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 5.A. Aicardo, D.M. Martinez, N. Campolo, S. Bartesaghi, R. Radi, Biochemistry of nitric oxide and peroxynitrite: sources, targets and biological implications, in: N.S. Dhalla (Series Ed.), Advances in Biochemistry in Health and Disease, in: A. Boveris, R. Gelpi, J.J. Poderoso (Guest Eds.), Biochemistry of oxidative stress. Physiopathology and clinical aspects, Springer, New York, 2016, pp. 49–77 〈http://www.springer.com/la/book/9783319458649〉.
- 6.N. Subelzu, S. Bartesaghi, A. De Bem, R. Radi, Oxidative Inactivation of Nitric Oxide and Peroxynitrite Formation in the Vasculature, in: S. Andreescu, M. Hempel (Eds.), Oxidative Stress: Diagnostics and Therapy, vol. 2, ACS Books (Symposium Series), 2015, pp. 91–145 (Chapter 4).
- 7.Bartesaghi S., Romero N., Radi R. Nitric oxide and derived oxidants. In: Pantapoulos K., Schipper H.M., editors. Principiles of Free Radical Biomedicine. Nova Science Publishers, Inc.; 2011. [Google Scholar]
- 8.Babior B.M., Lambeth J.D., Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 9.Dang P.M., Cross A.R., Quinn M.T., Babior B.M. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67PHOX and cytochrome b558 II. Proc. Natl. Acad. Sci. USA. 2002;99:4262–4265. doi: 10.1073/pnas.072345299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridovich I. Superoxide dismutases. Annu. Rev. Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 11.Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J. Biol. Chem. 1989;264:7761–7764. [PubMed] [Google Scholar]
- 12.Radi R. Biological antioxidant defenses. Toxicol. Ind. Health. 1993;9:53–62. doi: 10.1177/0748233793009001-206. [DOI] [PubMed] [Google Scholar]
- 13.Trujillo M., Ferrer-Sueta G., Radi R. Kinetic studies on peroxynitrite reduction by peroxiredoxins. Methods Enzymol. 2008;441:173–196. doi: 10.1016/S0076-6879(08)01210-X. [DOI] [PubMed] [Google Scholar]
- 14.Trujillo M., Ferrer-Sueta G., Thomson L., Flohe L., Radi R. Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite. Subcell. Biochem. 2007;44:83–113. doi: 10.1007/978-1-4020-6051-9_5. [DOI] [PubMed] [Google Scholar]
- 15.Zeida A., Reyes A.M., Lichtig P., Hugo M., Vazquez D.S., Santos J., Gonzalez Flecha F.L., Radi R., Estrin D.A., Trujillo M. Molecular basis of hydroperoxide specificity in peroxiredoxins: the case of AhpE from mycobacterium tuberculosis. Biochemistry. 2015;54:7237–7247. doi: 10.1021/acs.biochem.5b00758. [DOI] [PubMed] [Google Scholar]
- 16.Trujillo M., Alvarez B., Radi R. One- and two-electron oxidation of thiols: mechanisms, kinetics and biological fates. Free Radic. Res. 2016;50:150–171. doi: 10.3109/10715762.2015.1089988. [DOI] [PubMed] [Google Scholar]
- 17.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer R.M., Ferrige A.G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 19.Gewaltig M.T., Kojda G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc. Res. 2002;55:250–260. doi: 10.1016/s0008-6363(02)00327-9. [DOI] [PubMed] [Google Scholar]
- 20.R. Radi, G. Ferrer-Sueta, H. Rubbo, Nitric Oxide, Place Published, 2000.
- 21.Radi R. Vol. 101. 2004. Nitric oxide, oxidants, and protein tyrosine nitration; pp. 4003–4008. (Proc. Natl. Acad. Sci. USA). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ignarro L.J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989;3:31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- 23.Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 24.Ignarro L.J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev. Pharmacol. Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 25.Giulivi C., Poderoso J.J., Boveris A. Production of nitric oxide by mitochondria. J. Biol. Chem. 1998;273:11038–11043. doi: 10.1074/jbc.273.18.11038. [DOI] [PubMed] [Google Scholar]
- 26.Nottingham W.C., Sutter J.R. Kinetics of the oxidation of nitric oxide by clorine and oxgyen in nonaqueous media. Int. J. Chem. Kinet. 1986;18:1289–1302. [Google Scholar]
- 27.Gamon L.F., Wille U. Oxidative damage of biomolecules by the environmental pollutants NO2* and NO3*. Acc. Chem. Res. 2016;49:2136–2145. doi: 10.1021/acs.accounts.6b00219. [DOI] [PubMed] [Google Scholar]
- 28.Moller M., Botti H., Batthyany C., Rubbo H., Radi R., Denicola A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J. Biol. Chem. 2005;280:8850–8854. doi: 10.1074/jbc.M413699200. [DOI] [PubMed] [Google Scholar]
- 29.Moller M.N., Lancaster J.R., Denicola Jr, A. The interaction of reactive oxygen and nitrogen species with membranes. In: Matalon S., editor. Vol. 61. Academic Press Inc.; 2008. pp. 1–262. (Free Radical Effects on Membranes). Current Topics in Membranes. [Google Scholar]
- 30.Koppenol W.H., Moreno J.J., Pryor W.A., Ischiropoulos H., Beckman J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 31.Radi R. Reactions of nitric oxide with metalloproteins. Chem. Res. Toxicol. 1996:828–835. doi: 10.1021/tx950176s. [DOI] [PubMed] [Google Scholar]
- 32.Padmaja S., Huie R.E. The reaction of nitric oxide with organic peroxyl radicals. Biochem. Biophys. Res. Commun. 1993;195:539–544. doi: 10.1006/bbrc.1993.2079. [DOI] [PubMed] [Google Scholar]
- 33.Denninger J.W., Marletta M.A. Guanylate cyclase and the.NO/cGMP signaling pathway. Biochim. Biophys. Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 34.Cleeter M.W., Cooper J.M., Darley-Usmar V.M., Moncada S., Schapira A.H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. IFEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 35.Klug D., Rabani J., Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J. Biol. Chem. 1972;247:4839–4842. [PubMed] [Google Scholar]
- 36.Kissner R., Nauser T., Bugnon P., Lye P.G., Koppenol W.H. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem. Res. Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer-Sueta G., Radi R. Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem. Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 38.Gardner P.R., Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J. Biol. Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 39.Nagy P., Kettle A.J., Winterbourn C.C. Superoxide-mediated formation of tyrosine hydroperoxides and methionine sulfoxide in peptides through radical addition and intramolecular oxygen transfer. J. Biol. Chem. 2009;284:14723–14733. doi: 10.1074/jbc.M809396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolann B.J., Ulvik R.J. On the limited ability of superoxide to release iron from ferritin. Eur. J. Biochem. 1990;193:899–904. doi: 10.1111/j.1432-1033.1990.tb19415.x. [DOI] [PubMed] [Google Scholar]
- 41.Radi R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013;288:26464–26472. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radi R., Peluffo G., Alvarez M.N., Naviliat M., Cayota A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001;30:463–488. doi: 10.1016/s0891-5849(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 43.Radi R. Kinetic analysis of reactivity of peroxynitrite with biomolecules. Methods Enzymol. 1996;269:354–366. doi: 10.1016/s0076-6879(96)69036-3. [DOI] [PubMed] [Google Scholar]
- 44.Radi R., Denicola A., Alvarez B., Ferrer G., Rubbo H. The biological chemistry of peroxynitrite. In: Ignarro L.J., editor. Nitric Oxide Biology and Pathobiology. Academic Press; Los Angeles, California: 2000. pp. 57–82. [Google Scholar]
- 45.Carballal S., Bartesaghi S., Radi R. Kinetic and mechanistic considerations to assess the biological fate of peroxynitrite. Biochim. Et. Biophys. Acta (BBA)-General. Subj. 2014;1840:768–780. doi: 10.1016/j.bbagen.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radi R., Denicola A., Freeman B.A. Peroxynitrite reactions with carbon dioxide-bicarbonate. Methods Enzymol. 1999;301:353–367. doi: 10.1016/s0076-6879(99)01099-x. [DOI] [PubMed] [Google Scholar]
- 47.Radi R. Peroxynitrite reactions and diffusion in biology. Chem. Res. Toxicol. 1998;11:720–721. doi: 10.1021/tx980096z. [DOI] [PubMed] [Google Scholar]
- 48.Szabo C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 49.Radi R., Beckman J.S., Bush K.M., Freeman B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 50.Alvarez B., Ferrer-Sueta G., Freeman B.A., Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J. Biol. Chem. 1999;274:842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 51.Trujillo M., Radi R. Peroxynitrite reaction with the reduced and the oxidized forms of lipoic acid: new insights into the reaction of peroxynitrite with thiols. Arch. Biochem. Biophys. 2002;397:91–98. doi: 10.1006/abbi.2001.2619. [DOI] [PubMed] [Google Scholar]
- 52.Carballal S., Radi R., Kirk M.C., Barnes S., Freeman B.A., Alvarez B. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry. 2003;42:9906–9914. doi: 10.1021/bi027434m. [DOI] [PubMed] [Google Scholar]
- 53.Pryor W.A., Jin X., Squadrito G.L. One- and two-electron oxidations of methionine by peroxynitrite. Proc. Natl. Acad. Sci. USA. 1994;91:11173–11177. doi: 10.1073/pnas.91.23.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez B., Radi R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids. 2003;25:295–311. doi: 10.1007/s00726-003-0018-8. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez B., Rubbo H., Kirk M., Barnes S., Freeman B.A., Radi R. Peroxynitrite-dependent tryptophan nitration. Chem. Res Toxicol. 1996;9:390–396. doi: 10.1021/tx950133b. [DOI] [PubMed] [Google Scholar]
- 56.Pryor W.A., Lightsey J.W. Mechanisms of nitrogen dioxide reactions: initiation of lipid peroxidation and the production of nitrous acid. Science. 1981;214:435–437. doi: 10.1126/science.214.4519.435. [DOI] [PubMed] [Google Scholar]
- 57.Rubbo H., Radi R., Trujillo M., Telleri R., Kalyanaraman B., Barnes S., Kirk M., Freeman B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 58.Kennett E.C., Davies M.J. Glycosaminoglycans are fragmented by hydroxyl, carbonate, and nitrogen dioxide radicals in a site-selective manner: implications for peroxynitrite-mediated damage at sites of inflammation. Free Radic. Biol. Med. 2009;47:389–400. doi: 10.1016/j.freeradbiomed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Kennett E.C., Rees M.D., Malle E., Hammer A., Whitelock J.M., Davies M.J. Peroxynitrite modifies the structure and function of the extracellular matrix proteoglycan perlecan by reaction with both the protein core and the heparan sulfate chains. Free Radic. Biol. Med. 2010;49:282–293. doi: 10.1016/j.freeradbiomed.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Douki T., Cadet J. Peroxynitrite mediated oxidation of purine bases of nucleosides and isolated DNA. Free Radic. Res. 1996;24:369–380. doi: 10.3109/10715769609088035. [DOI] [PubMed] [Google Scholar]
- 61.Kennedy L.J., Moore K., Caulfield Jr, J.L., Tannenbaum S.R., Dedon P.C. Quantitation of 8-oxoguanine and strand breaks produced by four oxidizing agents. Chem. Res Toxicol. 1997;10:386–392. doi: 10.1021/tx960102w. [DOI] [PubMed] [Google Scholar]
- 62.Bonini M.G., Radi R., Ferrer-Sueta G., Ferreira A.M., Augusto O. Direct EPR detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J. Biol. Chem. 1999;274:10802–10806. doi: 10.1074/jbc.274.16.10802. [DOI] [PubMed] [Google Scholar]
- 63.Denicola A., Freeman B.A., Trujillo M., Radi R. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch. Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 64.Goldstein S., Czapski G. The effect of bicarbonate on oxidation by peroxynitrite: implications for its biological activity. Inorg. Chem. 1997;36:5113–5117. [Google Scholar]
- 65.Lymar S.V., Hurst J.K. CO2-Catalyzed one-electron oxidations by peroxynitrite: properties of the reactive intermediate. Inorg. Chem. 1998;37:294–301. [Google Scholar]
- 66.Souza J.M., Peluffo G., Radi R. Protein tyrosine nitration--functional alteration or just a biomarker? Free Radic. Biol. Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Bartesaghi S., Ferrer-Sueta G., Peluffo G., Valez V., Zhang H., Kalyanaraman B., Radi R. Protein tyrosine nitration in hydrophilic and hydrophobic environments. Amino Acids. 2007;32:501–515. doi: 10.1007/s00726-006-0425-8. [DOI] [PubMed] [Google Scholar]
- 68.Batthyany C., Bartesaghi S., Mastrogiovanni M., Lima A., Demicheli V., Radi R. Tyrosine-nitrated proteins: proteomic and bioanalytical aspects. Antioxid Redox Signal. 2016 doi: 10.1089/ars.2016.6787. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radi R. Protein tyrosine nitration: biochemical mechanisms and structural basis of its functional effects. Acc. Chem. Res. 2012;46:550–559. doi: 10.1021/ar300234c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bartesaghi S., Wenzel J., Trujillo M., Lopez M., Joseph J., Kalyanaraman B., Radi R. Lipid peroxyl radicals mediate tyrosine dimerization and nitration in membranes. Chem. Res. Toxicol. 2010 doi: 10.1021/tx900446r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Folkes L.K., Bartesaghi S., Trujillo M., Radi R., Wardman P. Kinetics of oxidation of tyrosine by a model alkoxyl radical. Free Radic. Res. 2012 doi: 10.3109/10715762.2012.695868. [DOI] [PubMed] [Google Scholar]
- 72.Bartesaghi S., Herrera D., Martinez D.M., Petruk A., Demicheli V., Trujillo M., Marti M.A., Estrin D.A., Radi R. Tyrosine oxidation and nitration in transmembrane peptides is connected to lipid peroxidation. Arch. Biochem. Biophys. 2017;622:9–25. doi: 10.1016/j.abb.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 73.Bartesaghi S., Valez V., Trujillo M., Peluffo G., Romero N., Zhang H., Kalyanaraman B., Radi R. Mechanistic studies of peroxynitrite-mediated tyrosine nitration in membranes using the hydrophobic probe N-t-BOC-l-tyrosine tert-butyl ester. Biochemistry. 2006;45:6813–6825. doi: 10.1021/bi060363x. [DOI] [PubMed] [Google Scholar]
- 74.Folkes L.K., Trujillo M., Bartesaghi S., Radi R., Wardman P. Kinetics of reduction of tyrosine phenoxyl radicals by glutathione. Arch. Biochem. Biophys. 2011;506:242–249. doi: 10.1016/j.abb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Petruk A.A., Bartesaghi S., Trujillo M., Estrin D.A., Murgida D., Kalyanaraman B., Marti M.A., Radi R. Molecular basis of intramolecular electron transfer in proteins during radical-mediated oxidations: computer simulation studies in model tyrosine-cysteine peptides in solution. Arch. Biochem. Biophys. 2012;525:82–91. doi: 10.1016/j.abb.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feletou M., Vanhoutte P.M. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am. J. Physiol. Heart Circ. Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 77.Baillet A., Chanteperdrix V., Trocme C., Casez P., Garrel C., Besson G. The role of oxidative stress in amyotrophic lateral sclerosis and Parkinson's disease. Neurochem. Res. 2010;35:1530–1537. doi: 10.1007/s11064-010-0212-5. [DOI] [PubMed] [Google Scholar]
- 78.Giasson B.I., Duda J.E., Murray I.V., Chen Q., Souza J.M., Hurtig H.I., Ischiropoulos H., Trojanowski J.Q., Lee V.M. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 79.Good P.F., Hsu A., Werner P., Perl D.P., Olanow C.W. Protein nitration in Parkinson's disease. J. Neuropathol. Exp. Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 80.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Campolo N., Bartesaghi S., Radi R. Metal-catalyzed protein tyrosine nitration in biological systems. Redox Rep. 2014;19:221–231. doi: 10.1179/1351000214Y.0000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rocha B.S., Gago B., Barbosa R.M., Lundberg J.O., Mann G.E., Radi R., Laranjinha J. Pepsin is nitrated in the rat stomach, acquiring antiulcerogenic activity: a novel interaction between dietary nitrate and gut proteins. Free Radic. Biol. Med. 2013;58:26–34. doi: 10.1016/j.freeradbiomed.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 83.Rocha B.S., Gago B., Pereira C., Barbosa R.M., Bartesaghi S., Lundberg J.O., Radi R., Laranjinha J. Dietary nitrite in nitric oxide biology: a redox interplay with implications for pathophysiology and therapeutics. Curr. Drug Targets. 2011;12:1351–1363. doi: 10.2174/138945011796150334. [DOI] [PubMed] [Google Scholar]
- 84.Bayden A.S., Yakovlev V.A., Graves P.R., Mikkelsen R.B., Kellogg G.E. Factors influencing protein tyrosine nitration--structure-based predictive models. Free Radic. Biol. Med. 2011;50:749–762. doi: 10.1016/j.freeradbiomed.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Souza J.M., Daikhin E., Yudkoff M., Raman C.S., Ischiropoulos H. Factors determining the selectivity of protein tyrosine nitration. Arch. Biochem Biophys. 1999;371:169–178. doi: 10.1006/abbi.1999.1480. [DOI] [PubMed] [Google Scholar]
- 86.Martinez A., Peluffo G., Petruk A.A., Hugo M., Pineyro D., Demicheli V., Moreno D.M., Lima A., Batthyany C., Duran R., Robello C., Marti M.A., Larrieux N., Buschiazzo A., Trujillo M., Radi R., Piacenza L. Structural and molecular basis of the peroxynitrite-mediated nitration and inactivation of Trypanosoma cruzi iron-superoxide dismutases (Fe-SODs) A and B: disparate susceptibilities due to the repair of Tyr35 radical by Cys83 in Fe-SODB through intramolecular electron transfer. J. Biol. Chem. 2014;289:12760–12778. doi: 10.1074/jbc.M113.545590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang H., Zielonka J., Sikora A., Joseph J., Xu Y., Kalyanaraman B. The effect of neighboring methionine residue on tyrosine nitration and oxidation in peptides treated with MPO, H2O2, and NO2(-) or peroxynitrite and bicarbonate: role of intramolecular electron transfer mechanism? Arch. Biochem. Biophys. 2009;484:134–145. doi: 10.1016/j.abb.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Batthyany C., Souza J.M., Duran R., Cassina A., Cervenansky C., Radi R. Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry. 2005;44:8038–8046. doi: 10.1021/bi0474620. [DOI] [PubMed] [Google Scholar]
- 89.Gebicki J.M., Nauser T., Domazou A., Steinmann D., Bounds P.L., Koppenol W.H. Reduction of protein radicals by GSH and ascorbate: potential biological significance. Amino Acids. 2010;39:1131–1137. doi: 10.1007/s00726-010-0610-7. [DOI] [PubMed] [Google Scholar]
- 90.Hunter E.P., Desrosiers M.F., Simic M.G. The effect of oxygen, antioxidants, and superoxide radical on tyrosine phenoxyl radical dimerization. Free Radic. Biol. Med. 1989;6:581–585. doi: 10.1016/0891-5849(89)90064-6. [DOI] [PubMed] [Google Scholar]
- 91.Simic M., Jovanovic S. Antioxidation mechanisms of uric acid. J. Am. Chem. Soc. 1989;111 (5718-5182) [Google Scholar]
- 92.MacMillan-Crow L.A.C., Kerby J.P., Beckman J.D., Thompson J.S. J.A, Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allgrafts. Proc. Natl. Acad. Sci. USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quijano C., Hernandez-Saavedra D., Castro L., McCord J.M., Freeman B.A., Radi R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J. Biol. Chem. 2001;276:11631–11638. doi: 10.1074/jbc.M009429200. [DOI] [PubMed] [Google Scholar]
- 94.Yamakura F., Taka H., Fujimura T., Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]
- 95.Shao B., Bergt C., Fu X., Green P., Voss J.C., Oda M.N., Oram J.F., Heinecke J.W. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J. Biol. Chem. 2005;280:5983–5993. doi: 10.1074/jbc.M411484200. [DOI] [PubMed] [Google Scholar]
- 96.Abriata L.A., Cassina A., Tortora V., Marin M., Souza J.M., Castro L., Vila A.J., Radi R. Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption. Nuclear magnetic resonance and optical spectroscopy studies. J. Biol. Chem. 2009;284:17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ford E., Hughes M.N., Wardman P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic. Biol. Med. 2002;32:1314–1323. doi: 10.1016/s0891-5849(02)00850-x. [DOI] [PubMed] [Google Scholar]
- 98.Romero N., Peluffo G., Bartesaghi S., Zhang H., Joseph J., Kalyanaraman B., Radi R. Incorporation of the hydrophobic probe N-t-BOC-l-tyrosine tert-butyl ester to red blood cell membranes to study peroxynitrite-dependent reactions. Chem. Res. Toxicol. 2007;20:1638–1648. doi: 10.1021/tx700142a. [DOI] [PubMed] [Google Scholar]
- 99.Signorelli S., Moller M.N., Coitino E.L., Denicola A. Nitrogen dioxide solubility and permeation in lipid membranes. Arch. Biochem. Biophys. 2011;512:190–196. doi: 10.1016/j.abb.2011.06.003. [DOI] [PubMed] [Google Scholar]